Protein Induced by Vitamin K Absence II: A Potential Biomarker to Differentiate Pancreatic Ductal Adenocarcinoma from Pancreatic Benign Lesions and Predict Vascular Invasion
Abstract
1. Introduction
2. Materials and Methods
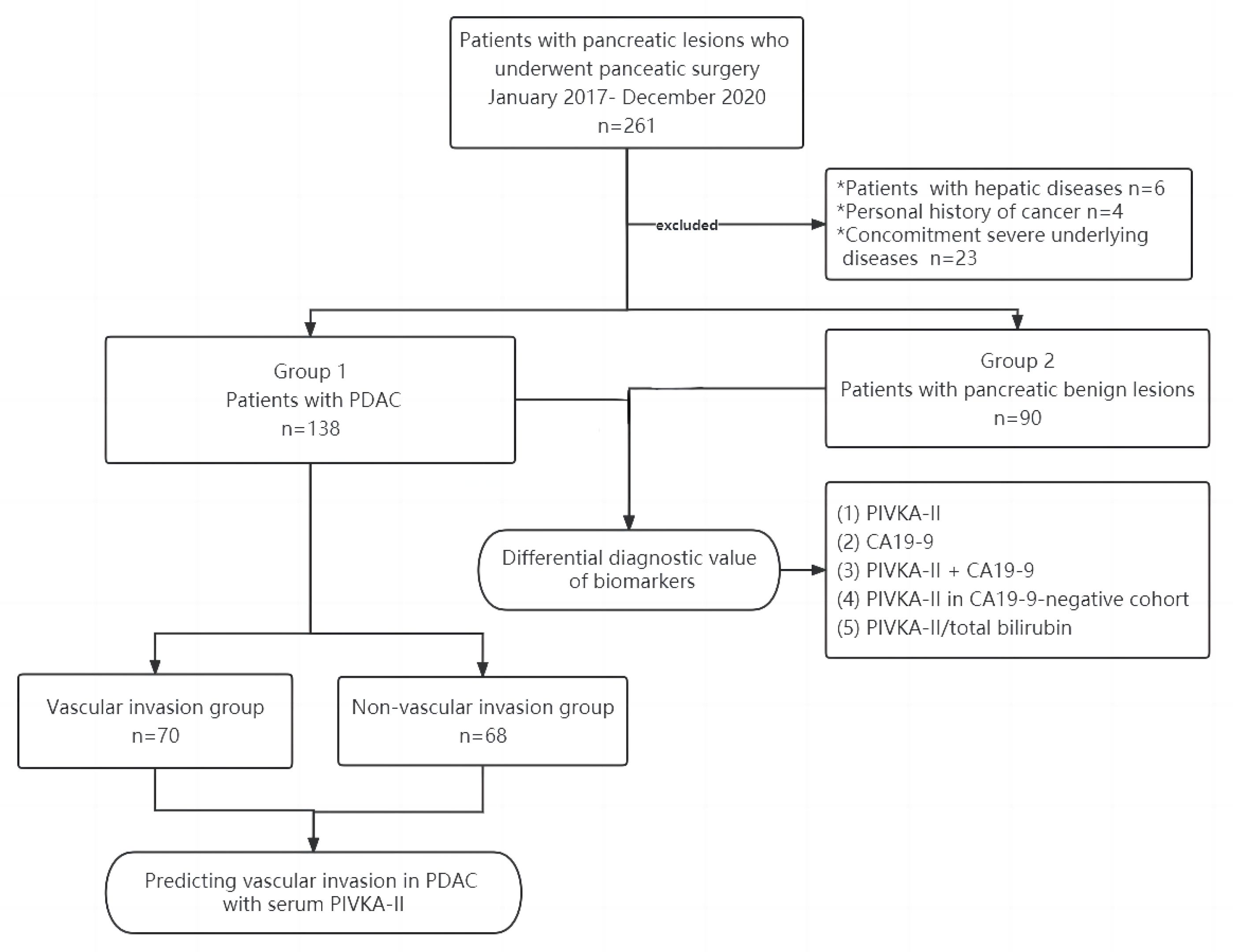
2.1. Study Design and Population
2.2. Measurements of PIVKA-II
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
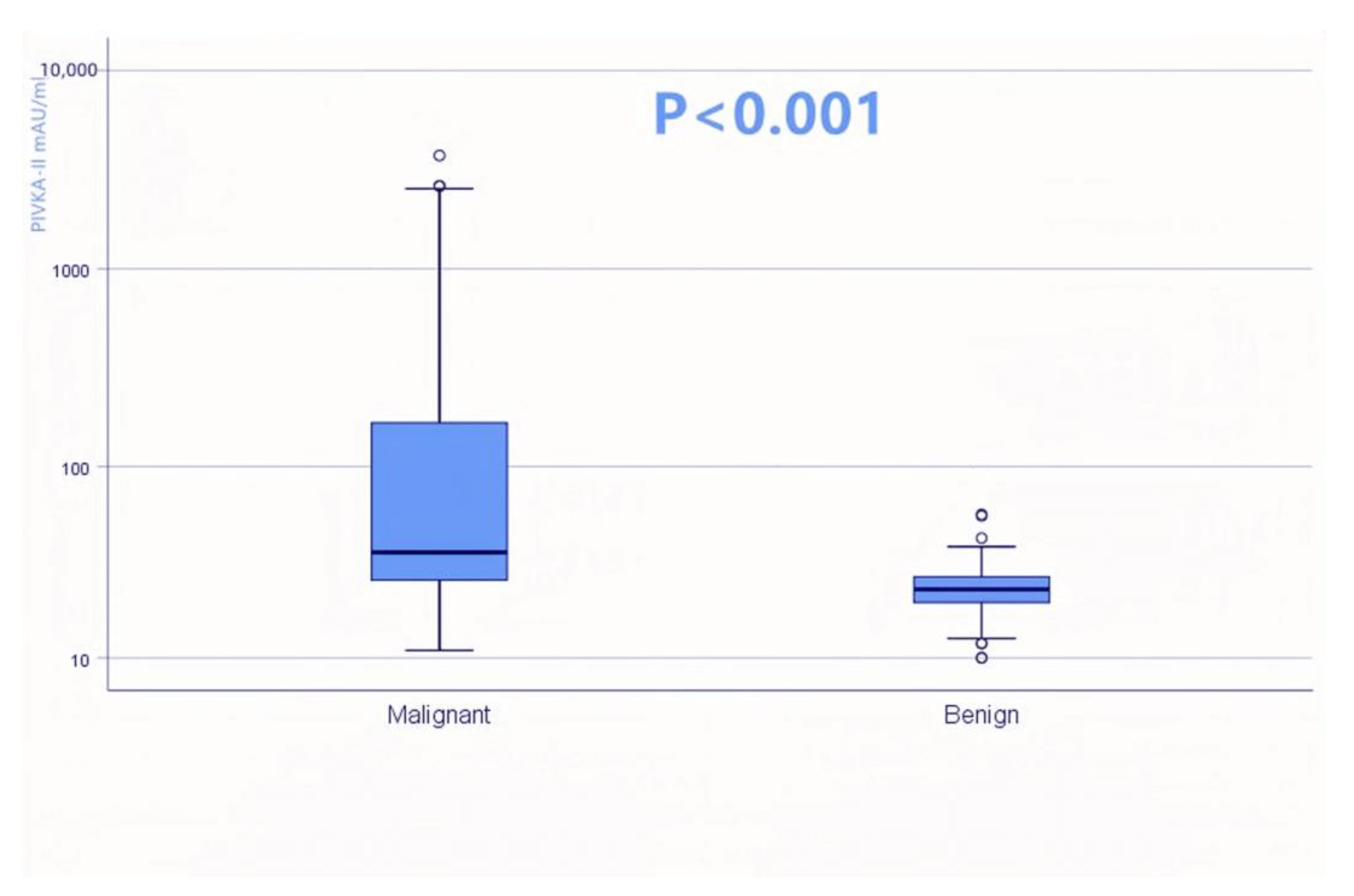
3.2. Differential Diagnostic Value of PIVKA-II in PDAC
3.3. Comparison of Differential Diagnostic Value of PIVKA-II, CA19-9, and Their Combination in PDAC
3.4. Differential Diagnostic Value of PIVKA-II in CA19-9-Negative Cohort
3.5. Differential Diagnostic Value of Adjusted PIVKA-II in PDAC
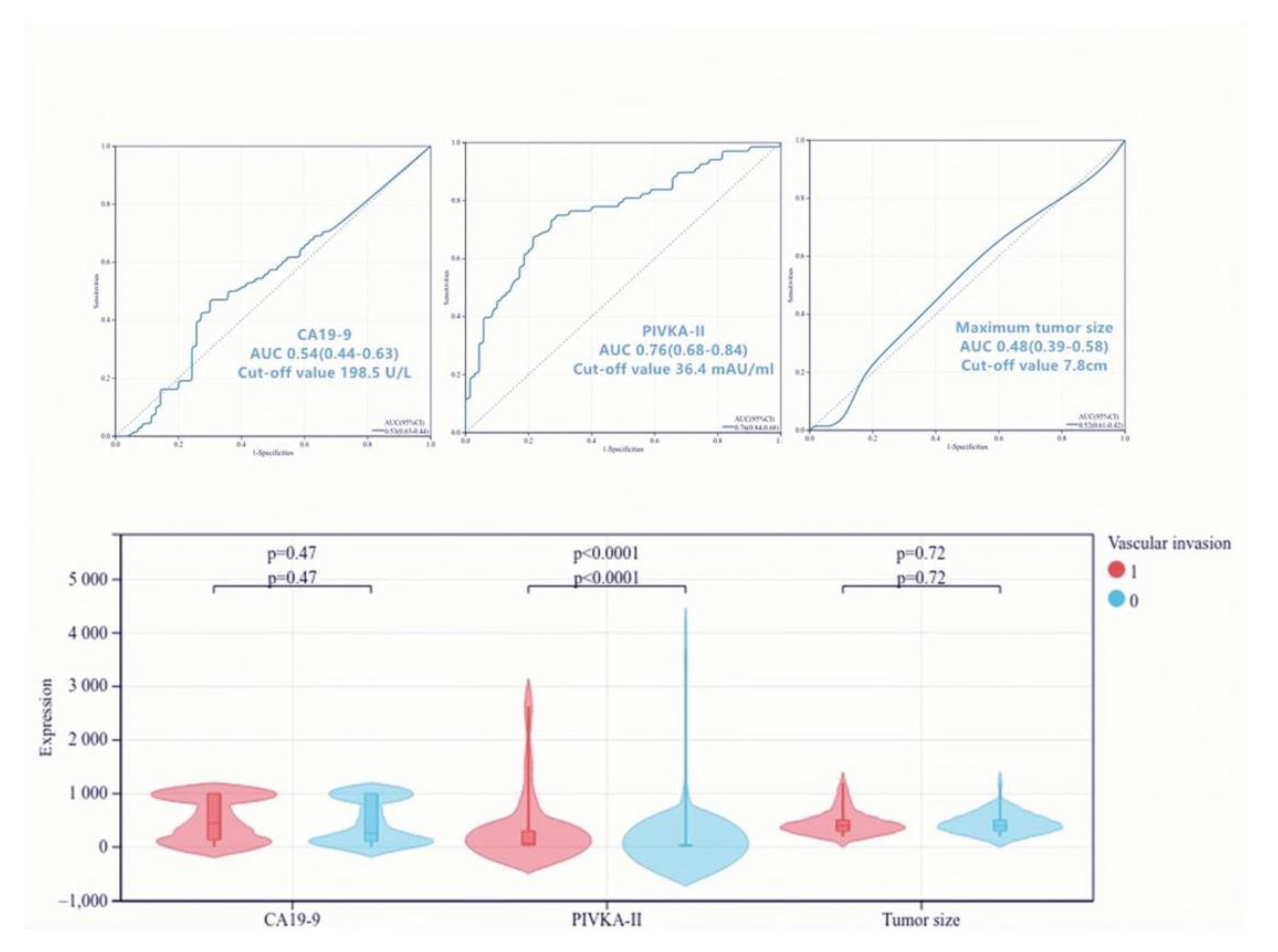
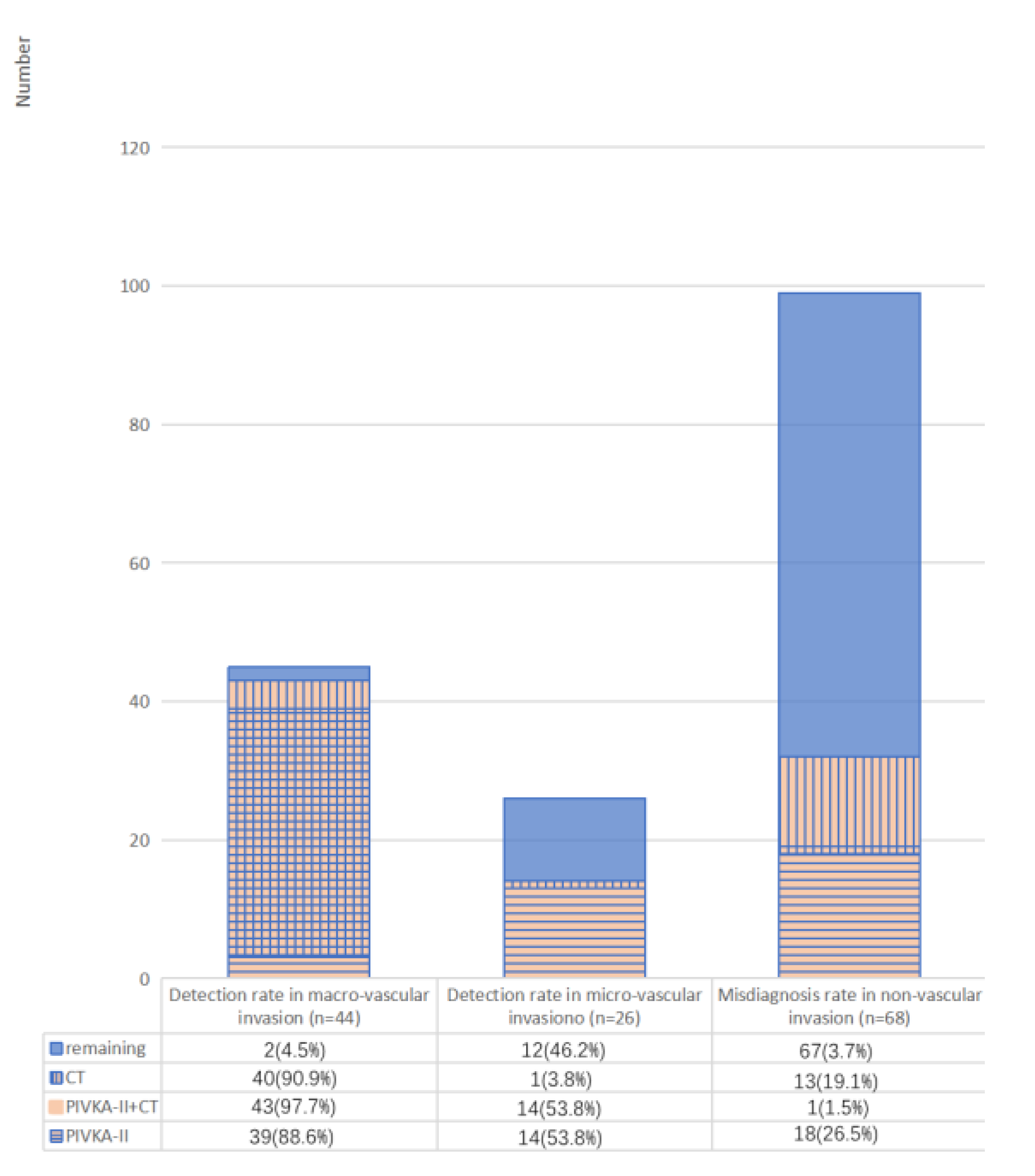
3.6. Predictive Value of PIVKA-II for Vascular Invasion in PDAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Cloyd, J.M. Surgery for pancreatic cancer: Recent progress and future directions. Hepatobiliary Surg. Nutr. 2021, 10, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. The lancet. Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Blackford, A.L.; Canto, M.I.; Klein, A.P.; Hruban, R.H.; Goggins, M. Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis. J. Natl. Cancer Inst. 2020, 112, 1162–1169. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e381. [Google Scholar] [CrossRef]
- Liu, C.; Deng, S.; Jin, K.; Gong, Y.; Cheng, H.; Fan, Z.; Qian, Y.; Huang, Q.; Ni, Q.; Luo, G.; et al. Lewis antigen-negative pancreatic cancer: An aggressive subgroup. Int. J. Oncol. 2020, 56, 900–908. [Google Scholar] [CrossRef]
- Tonack, S.; Jenkinson, C.; Cox, T.; Elliott, V.; Jenkins, R.E.; Kitteringham, N.R.; Greenhalf, W.; Shaw, V.; Michalski, C.W.; Friess, H.; et al. iTRAQ reveals candidate pancreatic cancer serum biomarkers: Influence of obstructive jaundice on their performance. Br. J. Cancer 2013, 108, 1846–1853. [Google Scholar] [CrossRef]
- de Icaza, E.; López-Cervantes, M.; Arredondo, A.; Robles-Díaz, G. Likelihood ratios of clinical, laboratory and image data of pancreatic cancer: Bayesian approach. J. Eval. Clin. Pract. 2009, 15, 62–68. [Google Scholar] [CrossRef]
- Liebman, H.A. Isolation and characterization of a hepatoma-associated abnormal (des-gamma-carboxy)prothrombin. Cancer Res. 1989, 49, 6493–6497. [Google Scholar]
- Tang, W.; Kokudo, N.; Sugawara, Y.; Guo, Q.; Imamura, H.; Sano, K.; Karako, H.; Qu, X.; Nakata, M.; Makuuchi, M. Des-gamma-carboxyprothrombin expression in cancer and/or non-cancer liver tissues: Association with survival of patients with resectable hepatocellular carcinoma. Oncol. Rep. 2005, 13, 25–30. [Google Scholar] [PubMed]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Poté, N.; Cauchy, F.; Albuquerque, M.; Voitot, H.; Belghiti, J.; Castera, L.; Puy, H.; Bedossa, P.; Paradis, V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J. Hepatol. 2015, 62, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Kemik, A.S.; Kemik, O.; Purisa, S.; Tuzun, S. Serum des-gamma-carboxyprothrombin in patients with pancreatic head adenocarcinoma. Bratisl. Lek. Listy 2011, 112, 552–554. [Google Scholar]
- Tartaglione, S.; Pecorella, I.; Zarrillo, S.R.; Granato, T.; Viggiani, V.; Manganaro, L.; Marchese, C.; Angeloni, A.; Anastasi, E. Protein Induced by Vitamin K Absence II (PIVKA-II) as a potential serological biomarker in pancreatic cancer: A pilot study. Biochem. Med. 2019, 29, 020707. [Google Scholar] [CrossRef]
- Tartaglione, S.; Mancini, P.; Viggiani, V.; Chirletti, P.; Angeloni, A.; Anastasi, E. PIVKA-II: A biomarker for diagnosing and monitoring patients with pancreatic adenocarcinoma. PLoS ONE 2021, 16, e0251656. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Lu, Z.; Liu, Y.; Kong, J.; Liu, J. Progression of Prothrombin Induced by Vitamin K Absence-II in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 726213. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ichihara, T.; Nakao, A.; Sakamoto, J.; Takagi, H.; Nagura, H. High serum levels of DUPAN2 antigen and CA19-9 in pancreatic cancer: Correlation with immunocytochemical localization of antigens in cancer cells. Hepato-Gastroenterology 1988, 35, 128–135. [Google Scholar]
- Wong, J.C.; Raman, S. Surgical resectability of pancreatic adenocarcinoma: CTA. Abdom. Imaging 2010, 35, 471–480. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Li, H.; Pan, W.; Xu, L.; Yin, D.; Cheng, S.; Zhao, F. Prognostic Significance of Microvascular Invasion in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930545. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Cheng, Y.N.; Cui, S.X.; Zhong, J.L.; Ward, S.G.; Sun, L.R.; Chen, M.H.; Kokudo, N.; Tang, W.; Qu, X.J. Des-gamma-carboxy prothrombin stimulates human vascular endothelial cell growth and migration. Clin. Exp. Metastasis 2009, 26, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch. Pathol. Lab. Med. 2021, 145, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaheri, F.N.; Alhamdani, M.S.S.; Bauer, A.S.; Giese, N.; Büchler, M.W.; Hackert, T.; Hoheisel, J.D. Blood biomarkers for differential diagnosis and early detection of pancreatic cancer. Cancer Treat. Rev. 2021, 96, 102193. [Google Scholar] [CrossRef]
- Chang, J.C.; Kundranda, M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2017, 18, 667. [Google Scholar] [CrossRef]
- Humphris, J.L.; Chang, D.K.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Jones, M.D.; Colvin, E.K.; Nagrial, A.; Chin, V.T.; Chantrill, L.A.; et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 1713–1722. [Google Scholar] [CrossRef]
- Le Pendu, J.; Marionneau, S.; Cailleau-Thomas, A.; Rocher, J.; Le Moullac-Vaidye, B.; Clément, M. ABH and Lewis histo-blood group antigens in cancer. APMIS Acta Pathol. Microbiol. Et Immunol. Scand. 2001, 109, 9–31. [Google Scholar] [CrossRef]
- Vestergaard, E.M.; Hein, H.O.; Meyer, H.; Grunnet, N.; Jørgensen, J.; Wolf, H.; Orntoft, T.F. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin. Chem. 1999, 45, 54–61. [Google Scholar]
- Su, S.B.; Qin, S.Y.; Chen, W.; Luo, W.; Jiang, H.X. Carbohydrate antigen 19-9 for differential diagnosis of pancreatic carcinoma and chronic pancreatitis. World J. Gastroenterol. 2015, 21, 4323–4333. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, Z.; Liu, W.; You, L.; Zhou, L.; Wang, C.; Lou, W.; Sun, B.; Miao, Y.; Liu, X.; et al. Plasma miRNAs Effectively Distinguish Patients With Pancreatic Cancer From Controls: A Multicenter Study. Ann. Surg. 2016, 263, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Prassas, I.; Dimitromanolakis, A.; Brand, R.E.; Serra, S.; Diamandis, E.P.; Blasutig, I.M. Validation of biomarkers that complement CA19.9 in detecting early pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 5787–5795. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Inoue, T.; Fukusato, T. Protein induced by vitamin K absence or antagonist II-producing gastric cancer. World J. Gastrointest. Pathophysiol. 2010, 1, 129–136. [Google Scholar] [CrossRef]
- Mayerle, J.; Kalthoff, H.; Reszka, R.; Kamlage, B.; Peter, E.; Schniewind, B.; González Maldonado, S.; Pilarsky, C.; Heidecke, C.D.; Schatz, P.; et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut 2018, 67, 128–137. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Kochman, M.L.; Lewis, J.D.; Kadish, S.; Morris, J.B.; Rosato, E.F.; Ginsberg, G.G. Endosonography is superior to angiography in the preoperative assessment of vascular involvement among patients with pancreatic carcinoma. J. Clin. Gastroenterol. 2001, 32, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Castells, A.; Ayuso, C.; Ayuso, J.R.; de Caralt, M.T.; Ginès, M.A.; Real, M.I.; Gilabert, R.; Quintó, L.; Trilla, A.; et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: Prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am. J. Gastroenterol. 2004, 99, 492–501. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Wolff, R.A. Adjuvant or Neoadjuvant Therapy in the Treatment in Pancreatic Malignancies: Where Are We? Surg. Clin. N. Am. 2018, 98, 95–111. [Google Scholar] [CrossRef]
- Chen, V.L.; Sharma, P. Role of Biomarkers and Biopsy in Hepatocellular Carcinoma. Clin. Liver Dis. 2020, 24, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.X.; Zhang, Y.S.; Chu, J.H.; Song, Z.Y.; Qu, X.J. Des-gamma-carboxy prothrombin (DCP) antagonizes the effects of gefitinib on human hepatocellular carcinoma cells. Cell. Physiol. Biochem. 2015, 35, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Shiraha, H.; Ueda, N.; Takaoka, N.; Nakanishi, Y.; Matsuo, N.; Tanaka, S.; Nishina, S.; Suzuki, M.; Takaki, A.; et al. Des-gamma-carboxyl prothrombin-promoted vascular endothelial cell proliferation and migration. J. Biol. Chem. 2007, 282, 8741–8748. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; La Vaccara, V.; Fiore, M.; Farolfi, T.; Ramella, S.; Angeletti, S.; Coppola, R.; Caputo, D. CA19.9 Serum Level Predicts Lymph-Nodes Status in Resectable Pancreatic Ductal Adenocarcinoma: A Retrospective Single-Center Analysis. Front. Oncol. 2021, 11, 690580. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lee, S.; Lee, H.S.; Bang, S.; Han, K.; Park, M.S. Retrospective Evaluation of Treatment Response in Patients with Nonmetastatic Pancreatic Cancer Using CT and CA 19-9. Radiology 2022, 303, 548–556. [Google Scholar] [CrossRef]
- Koom, W.S.; Seong, J.; Kim, Y.B.; Pyun, H.O.; Song, S.Y. CA 19-9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1148–1154. [Google Scholar] [CrossRef]


| Characteristic | Group 1 n = 138 | Group 2 n = 90 | p | ||
|---|---|---|---|---|---|
| Age, years, median (IQR) | 62 (52–67) | 60 (50–66) | p = 0.352 | ||
| Sex, male, n (%) | 91 (65.9%) | 55 (60.0%) | p = 0.457 | ||
| Total bilirubin, mg/dL, median (IQR) | 23.6 (13.3–185.4) | 12.5 (9.3–15.9) | p < 0.001 | ||
| Type of resection PD n (%) | 100 (72.5%) | 33 (36.7%) | p < 0.001 | ||
| CA19-9 U/L median (IQR) | 379.3 (114.1–1000) | 10.6 (6.8–15.7) | p < 0.001 | ||
| PIVKA-II mAU/mL, median (IQR) | 36.4 (26.1–166.8) | 23.3 (19.9–27.2) | p < 0.001 | ||
| AST IU/L median (IQR) | 39.5 (21–111.5) | 20 (16.3–24) | p < 0.001 | ||
| ALT IU/L median (IQR) | 55 (17–185.8) | 16 (11.3–23) | p < 0.001 | ||
| Albumin (g/dL) mean ± SD | 3.6 ± 0.1 | 3.9 ± 0.1 | p = 0.027 | ||
| WBC × 109/L median (IQR) | 5.64 (4.8–7.2) | 5.7 (4.9–6.5) | p = 0.696 | ||
| CP n (%) | 14 (10.1%) | 17 (18.9%) | p = 0.060 | ||
| Variables | Group 1 (n%) | Group 2 (n%) |
|---|---|---|
| Location (pancreatic head) | 100 (72.5%) | 33 (36.67%) |
| Differentiated grade (poor), | 36 (26.1%) | NA |
| Lymphatic metastasis | 57 (41.3%) | NA |
| Vascular invasion | 7069 (50.70%) | NA |
| Macro- | 44 (31.9%) | NA |
| Micro- | 26 (18.8%) | NA |
| Vascular resection and or reconstruction | 54 (39.1%) | NA |
| Tumor size *, >4 cm | 84 (60.9%) | 42 (46.67%) |
| Staging, T4NxM0 + | 10 (7.2%) | NA |
| Vascular resection and or reconstruction | 54 (39.1%) | NA |
| R0 resection # | 92 (66.7%) | NA |
| Variables | Sensitivity | Specificity | AUC (95%CI) | PPV | NPV |
|---|---|---|---|---|---|
| PIVKA-II | 68.1% | 83.3% | 0.787 (0.730–0.845) | 86.2% | 63.0% |
| CA19-9 | 83.3% | 94.4% | 0.906 (0.864–0.948) | 95.8% | 78.7% |
| PIVKA-II + CA19-9 | 87.7% | 94.4% | 0.945 (0.916–0.974) | 96.0% | 83.3% |
| PIVKA-II/ Total bilirubin | 66.7% | 79.2% | 0.749 (0.686–0.813) | 82.9% | 60.7% |
| Group 1 | Group 2 | Total | p | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| PIVKA-II(+) | 15 | 14 | 29 | <0.001 | 65.2% | 83.5% |
| PIVKA-II(−) | 8 | 71 | 79 | |||
| Total | 23 | 85 | 108 |
| Characteristic | Vascular Invasion | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Positive n = 70 | Negative n = 68 | p Value | OR (95% CI) | p Value | OR (95% CI) | |
| PIVKA-II, >I36.4 mAU/mL, n | 51 | 18 | <0.001 | 7.46 (3.51–15.84) | <0.001 | 0.07 (0.03–0.21) |
| Age, ≥70 years, n | 8 | 12 | 0.300 | 0.60 (0.23–2.58) | NA | NA |
| Sex, male, n | 47 | 44 | 0.763 | 1.12 (0.56–2.25) | NA | NA |
| CA19-9. >198.5 U/L, n | 49 | 36 | 0.039 | 2.07 (1.03–4.17) | 0.73 | 0.85 (0.35–2.10) |
| Albumin <3.2 g/dl, n | 22 | 30 | 0.124 | 0.58 (0.29–1.16) | NA | NA |
| Tumor size * >7.8 cm, n | 9 | 2 | 0.032 | 4.87 (1.01–23.43) | 0.10 | 0.19 (0.03–1.39) |
| Location, pancreatic head, n | 54 | 46 | 0.212 | 1.61 (0.76–3.43) | NA | NA |
| Pathologic differentiation, poorly, n | 20 | 16 | 0.500 | 1.30 (0.61–2.79) | NA | NA |
| Imaging findings, positive, n | 41 | 13 | <0.001 | 5.98 (2.77–12.91) | <0.001 | 0.09 (0.03–0.26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, G.; Zhang, Y.; Cui, Y.; Liu, J. Protein Induced by Vitamin K Absence II: A Potential Biomarker to Differentiate Pancreatic Ductal Adenocarcinoma from Pancreatic Benign Lesions and Predict Vascular Invasion. J. Clin. Med. 2023, 12, 2769. https://doi.org/10.3390/jcm12082769
Yang Y, Li G, Zhang Y, Cui Y, Liu J. Protein Induced by Vitamin K Absence II: A Potential Biomarker to Differentiate Pancreatic Ductal Adenocarcinoma from Pancreatic Benign Lesions and Predict Vascular Invasion. Journal of Clinical Medicine. 2023; 12(8):2769. https://doi.org/10.3390/jcm12082769
Chicago/Turabian StyleYang, Yang, Guangbing Li, Yu Zhang, Yunfeng Cui, and Jun Liu. 2023. "Protein Induced by Vitamin K Absence II: A Potential Biomarker to Differentiate Pancreatic Ductal Adenocarcinoma from Pancreatic Benign Lesions and Predict Vascular Invasion" Journal of Clinical Medicine 12, no. 8: 2769. https://doi.org/10.3390/jcm12082769
APA StyleYang, Y., Li, G., Zhang, Y., Cui, Y., & Liu, J. (2023). Protein Induced by Vitamin K Absence II: A Potential Biomarker to Differentiate Pancreatic Ductal Adenocarcinoma from Pancreatic Benign Lesions and Predict Vascular Invasion. Journal of Clinical Medicine, 12(8), 2769. https://doi.org/10.3390/jcm12082769








