Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
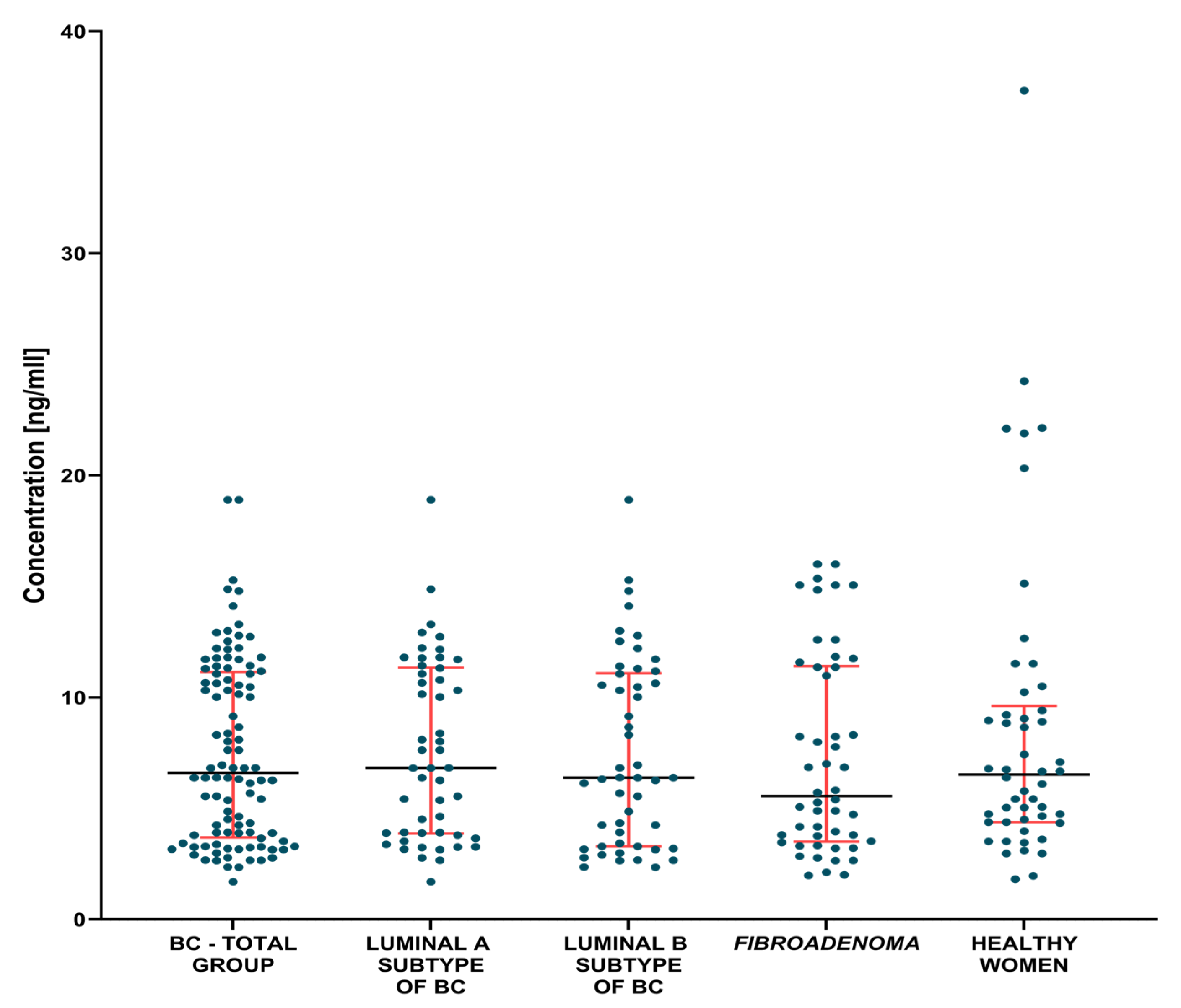
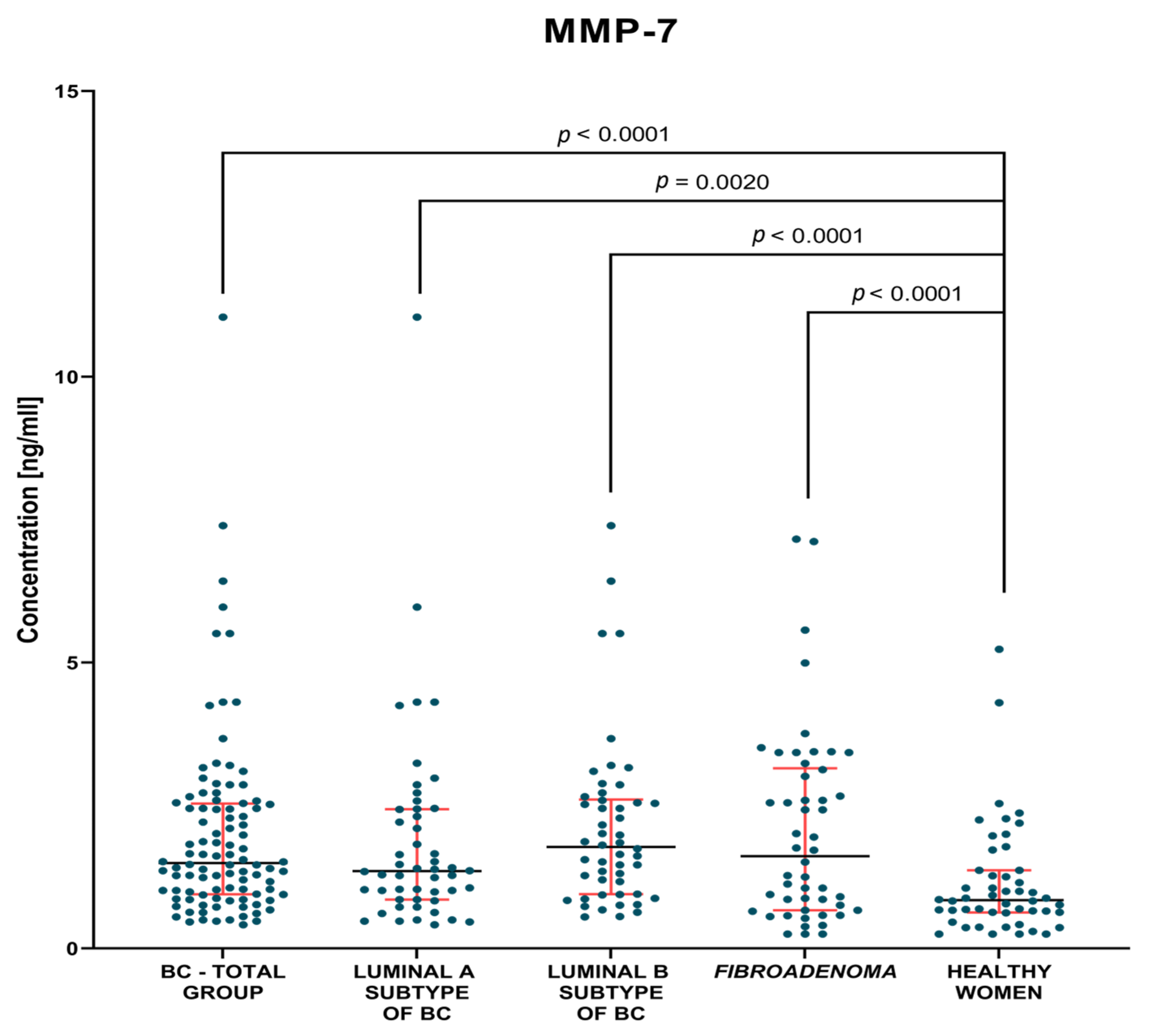
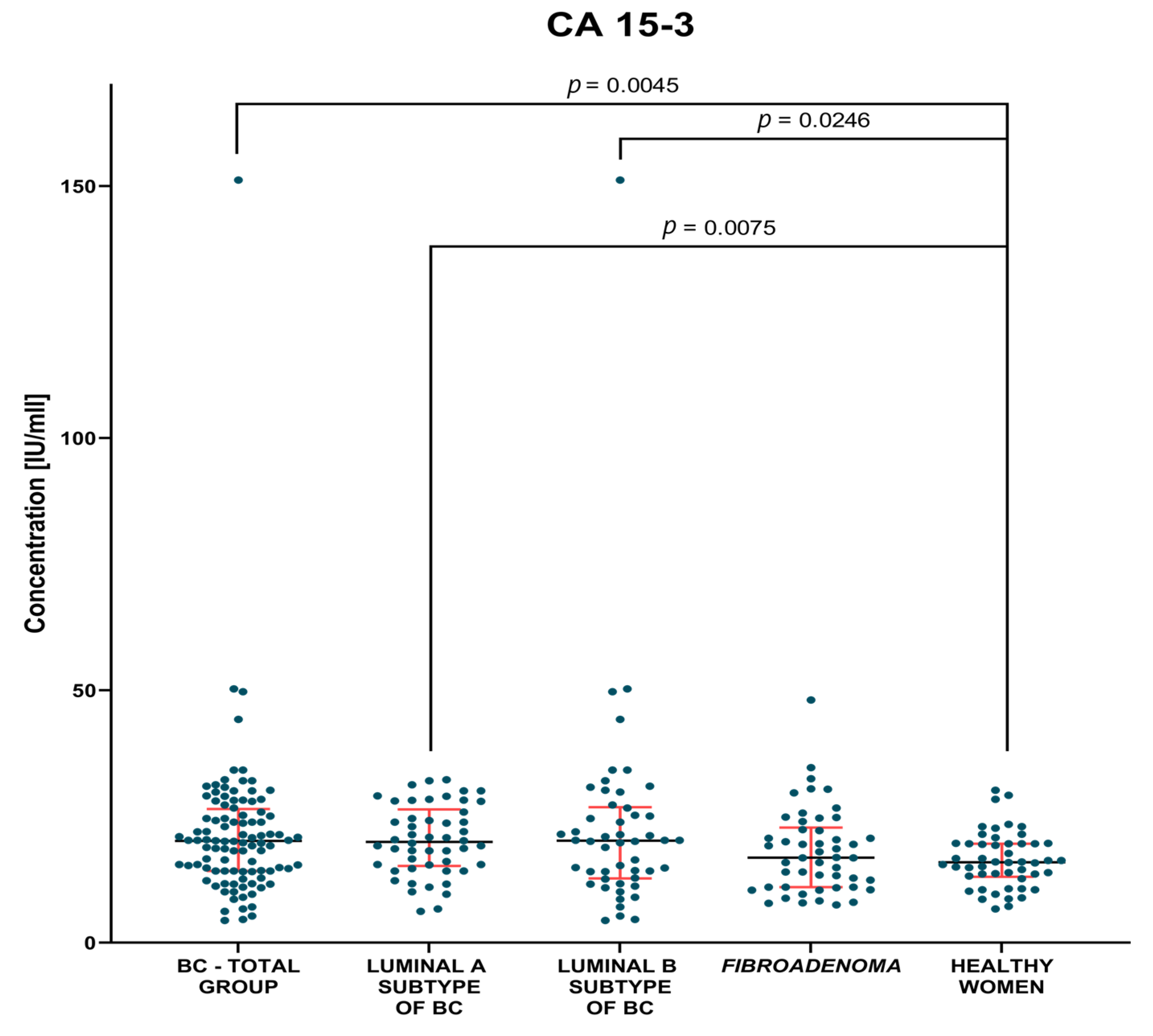
3.1. Plasma Concentration of Tested Parameters
3.1.1. Preoperative Groups of Patients
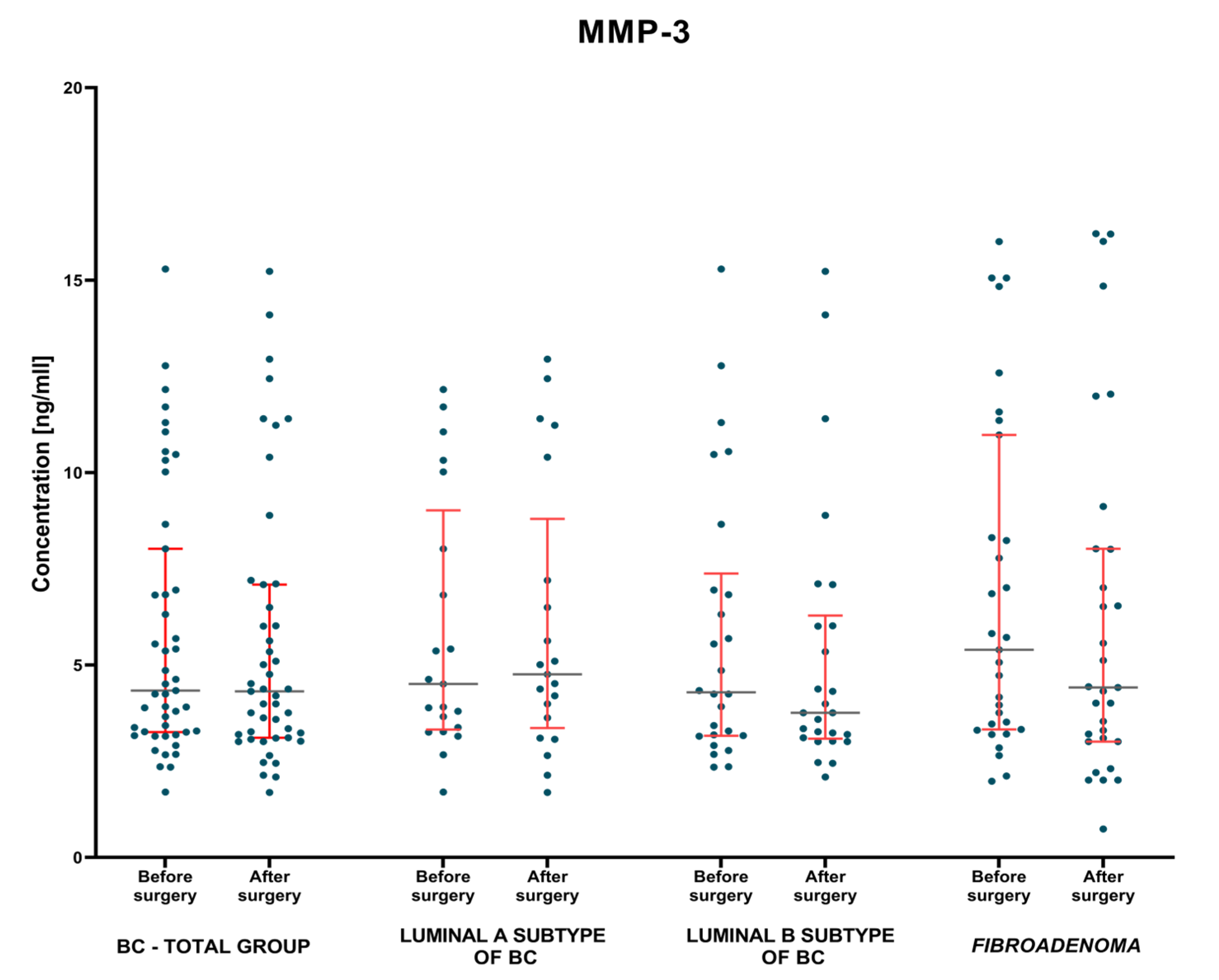
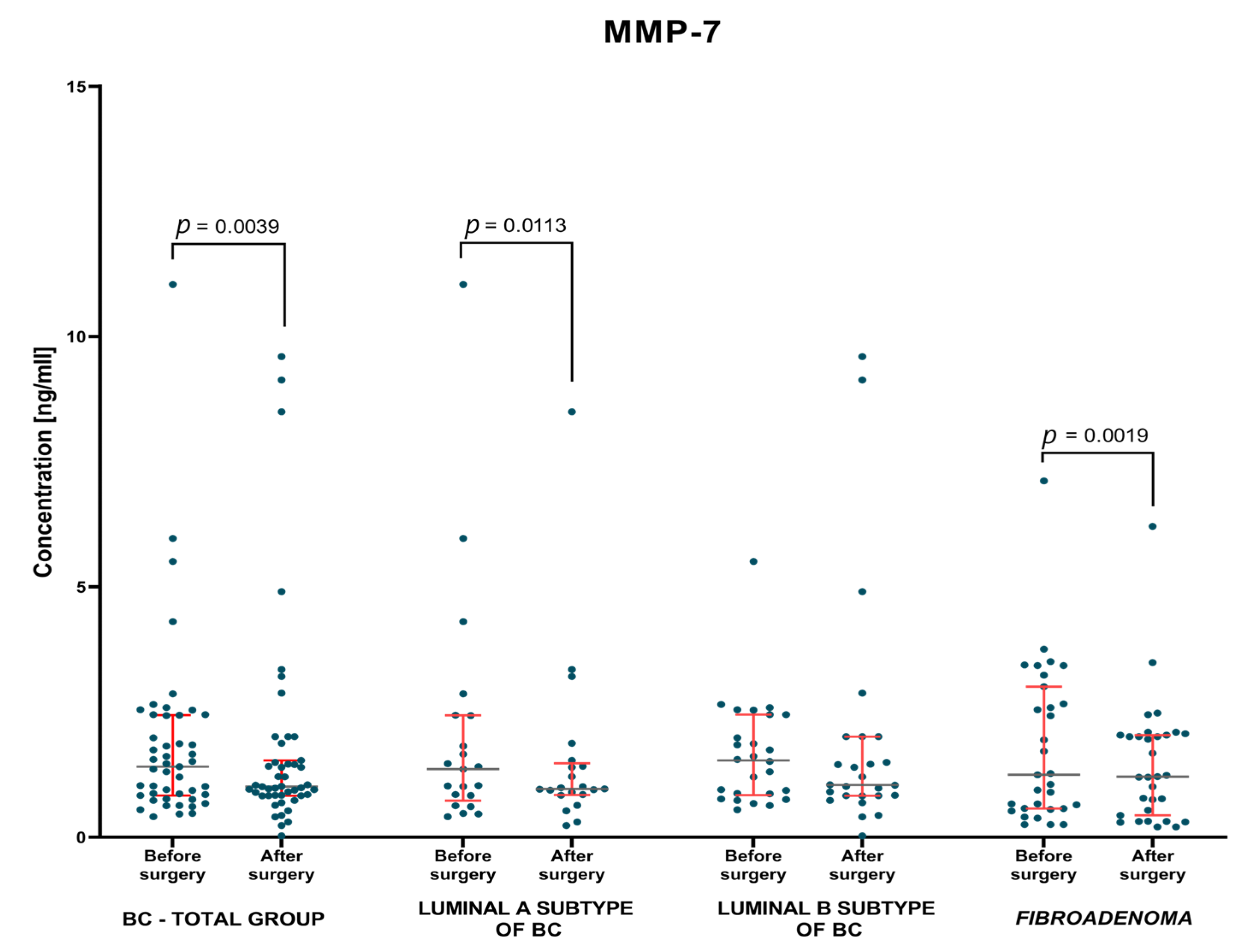
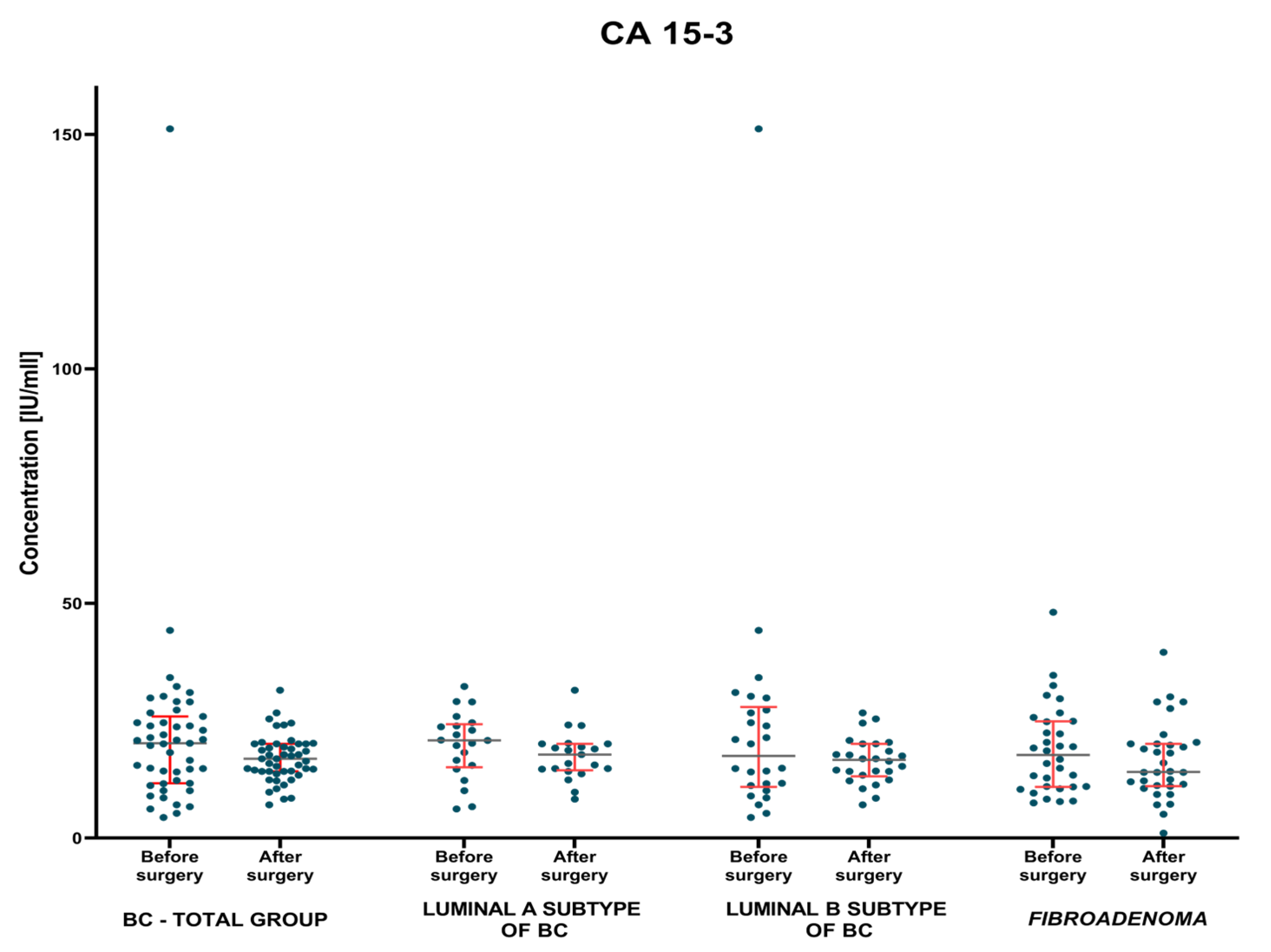
3.1.2. Postoperative Group of Patients—Analysis of Matched Pre- to Postoperative Pairs
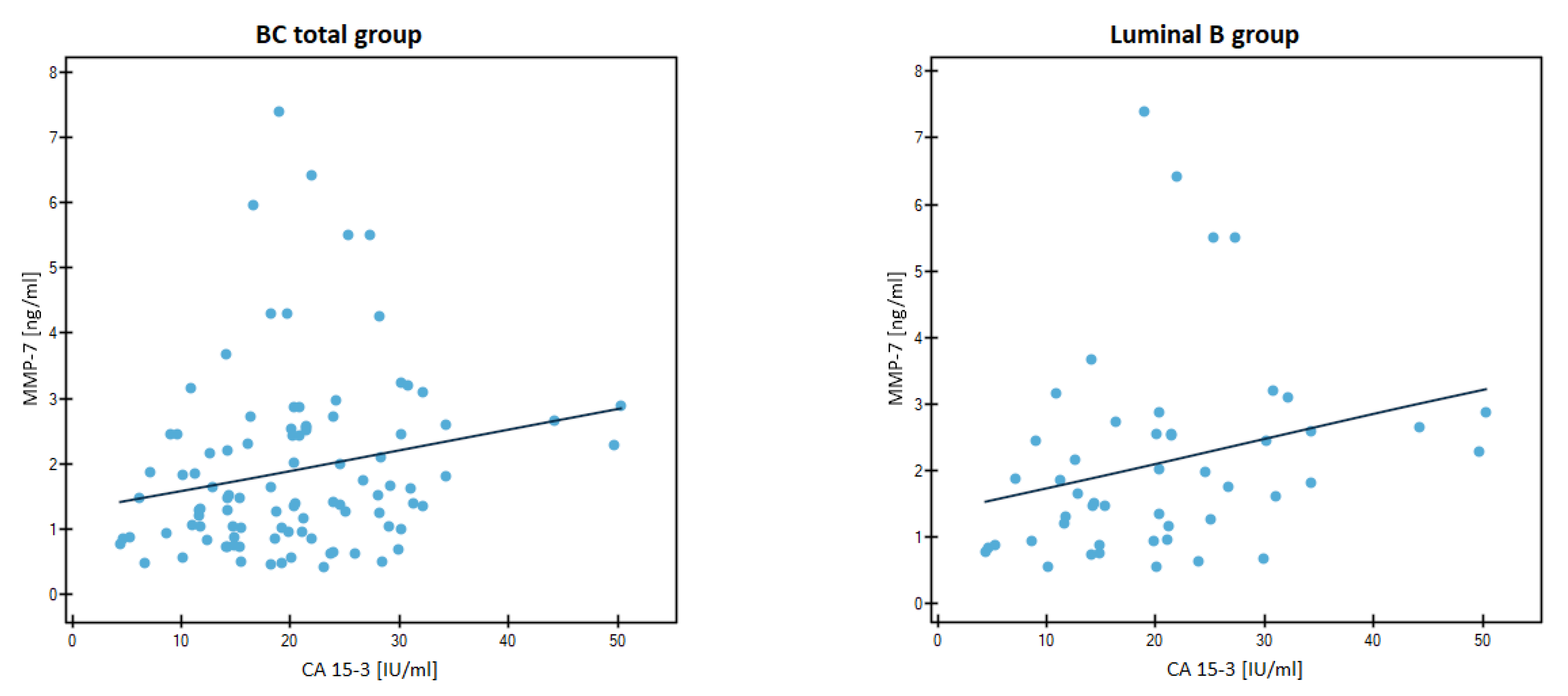
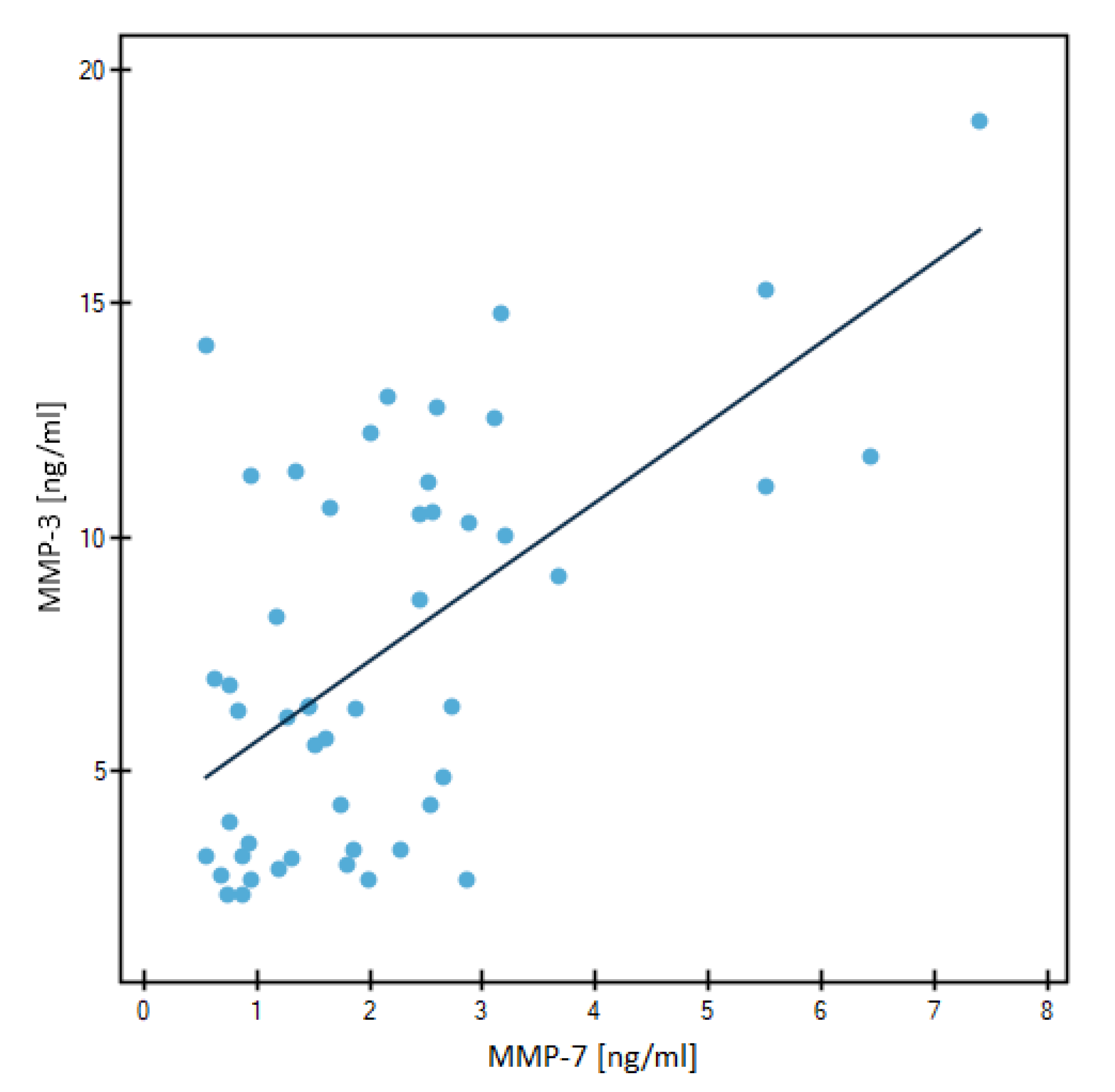
3.2. Evaluation of Correlation by Spearman’s Method
3.3. Diagnostic Criteria of MMP-3, MMP-7 and CA 15-3
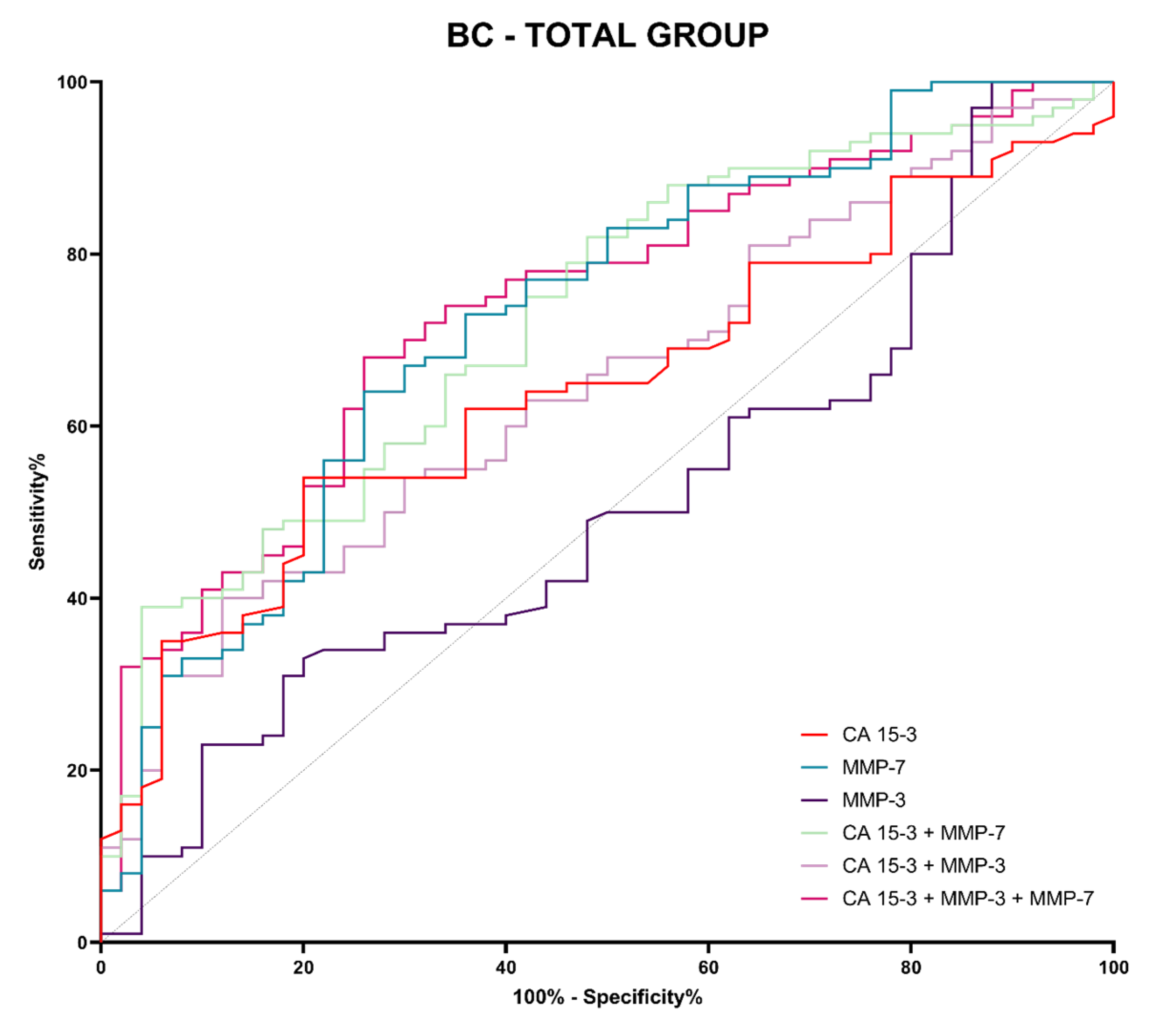
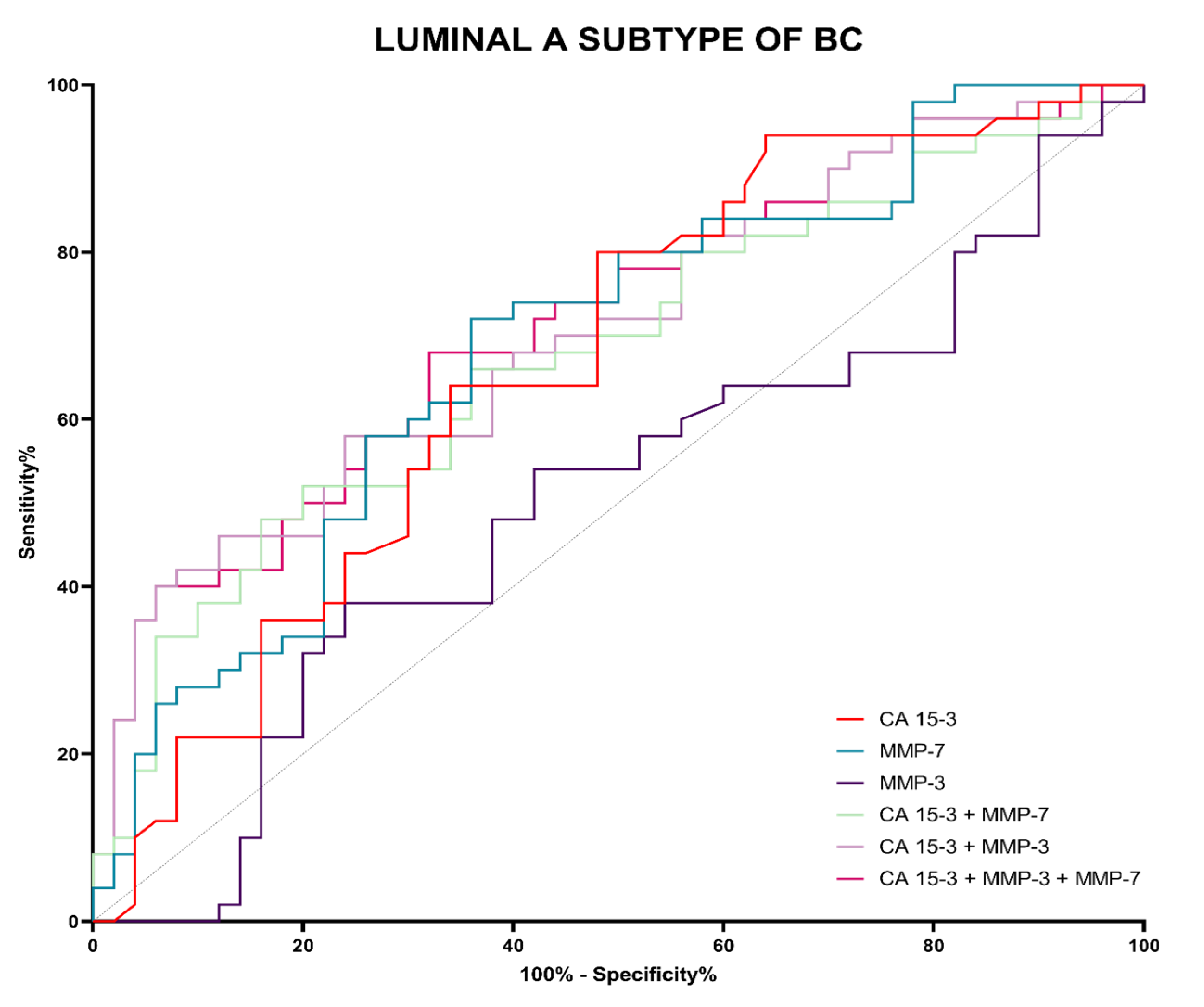
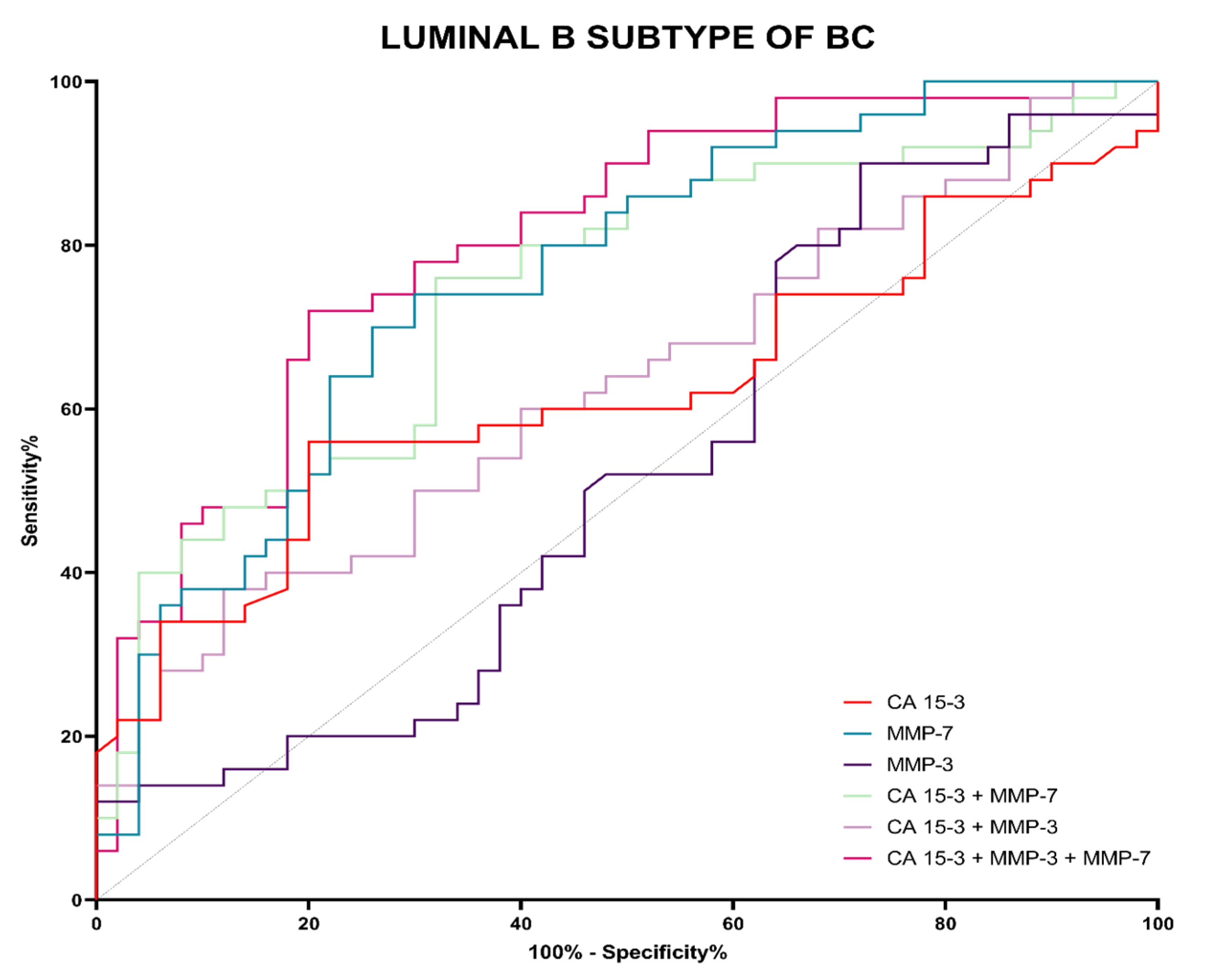
3.4. Evaluation of the Diagnostic Power of Tests (ROC Function)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollán, M. Epidemiology of breast cancer in young women. Breast Cancer Res. Treat. 2010, 123, 3–6. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rezaianzadeh, A.; Jalali, M.; Maghsoudi, A.; Mokhtari, A.M.; Azgomi, S.H.; Dehghani, S.L. The overall 5-year survival rate of breast cancer among Iranian women: A systematic review and meta-analysis of published studies. Breast Dis. 2017, 37, 63–68. [Google Scholar] [CrossRef]
- Jones, T.; Duquette, D.; Underhill, M.; Ming, C.; Mendelsohn-Victor, K.E.; Anderson, B.; Milliron, K.J.; Copeland, G.; Janz, N.K.; Northouse, L.L.; et al. Surveillance for cancer recurrence in long-term young breast cancer survivors randomly selected from a statewide cancer registry. Breast Cancer Res. Treat. 2018, 169, 41–152. [Google Scholar] [CrossRef]
- Chen, L.; Linden, H.M.; Anderson, B.O.; Li, C.I. Trends in 5-year survival rates among breast cancer patients by hormone receptor status and stage. Breast Cancer Res. Treat. 2014, 147, 609–616. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Aspects. Med. 2008, 29, 290–308. [Google Scholar] [CrossRef]
- Roy, R.; Morad, G.; Jedinak, A.; Moses, M.A. Metalloproteinases and their roles in human cancer. Anat. Rec. 2020, 303, 1557–1572. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Niczyporuk, M.; Ławicki, S. Matrilysins and Stromelysins in Pathogenesis and Diagnostics of Cancers. Cancer Manag. Res. 2020, 12, 10949–10964. [Google Scholar] [CrossRef]
- Suhaimi, S.A.; Chan, S.C.; Rosli, R. Matrix Metallopeptidase 3 Polymorphisms: Emerging genetic Markers in Human Breast Cancer Metastasis. J. Breast Cancer 2020, 23, 1–9. [Google Scholar] [CrossRef]
- AbdRaboh, N.R.; Bayoumi, F.A. Gene polymorphism of matrix metalloproteinases 3 and 9 in breast cancer. Gene Rep. 2016, 5, 151–156. [Google Scholar] [CrossRef]
- Banik, D.; Netherby, C.S.; Bogner, P.N.; Abrams, S.I. MMP3-mediated tumor progression is controlled transcriptionally by a novel IRF8-MMP3 interaction. Oncotarget 2015, 6, 15164–15179. [Google Scholar] [CrossRef]
- Sipos, F.; Germann, T.M.; Wichmann, B.; Galamb, O.; Spisák, S.; Krenács, T.; Tulassay, Z.; Molnár, B.; Műzes, G. MMP3 and CXCL1 are potent stromal protein markers of dysplasia-carcinoma transition in sporadic colorectal cancer. Eur. J. Cancer Prev. 2014, 23, 336–343. [Google Scholar] [CrossRef]
- Hassan, S.A.; Hammam, O.; Htwe, T.T.; Ghanem, H.Z. Possible diagnostic/prognostic role of survivin and MMP3 in breast cancer disease. J. Multidiscip. Eng. Sci. Technol. 2015, 2, 3121–3127. [Google Scholar]
- Jiang, W.G.; Davies, G.; Martin, T.A.; Parr, C.; Watkins, G.; Mason, M.D.; Mokbel, K.; Mansel, R.E. Targeting matrilysin and its impact on tumor growth in vivo: The potential implications in breast cancer therapy. Clin. Cancer Res. 2005, 11, 6012–6019. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, P.; Chen, J.; Deng, Y.; Huang, C. Landscape Analysis of Matrix Metalloproteinases Unveils Key Prognostic Markers for Patients with Breast Cancer. Front. Genet. 2022, 12, 809600. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Hass, R. Extracellular signals in young and aging breast epithelial cells and possible connections to age-associated breast cancer development. Mech. Ageing Dev. 2011, 132, 213–219. [Google Scholar] [CrossRef]
- Gonzalez, L.O.; Corte, M.D.; Vazquez, J.; Junquera, S.; Sanchez, R.; Viña, A.; Rodriguez, J.C.; Lamelas, M.L.; Vizoso, F. Study of matrix metalloproteinases and their tissue inhibitors in ductal in situ carcinomas of the breast. Histopathology 2008, 53, 403–415. [Google Scholar] [CrossRef]
- Cao, P.L.; Wang, K.; Wang, C.B.; Li, X.F.; Yang, Z.; Yang, Z.; Wang, H.Y. Expressions of FOXC1 and MMP-7 in molecular subtypes of breast cancer and their association with clinicopathological characteristics. Zhejiang Da Xue Xue Bao. Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2014, 43, 406–412. [Google Scholar]
- Davidson, B.; Stavnes, H.T.; Hellesylt, E.; Hager, T.; Zeppa, P.; Pinamonti, M.; Wohlschlaeger, J. MMP-7 is a highly specific negative marker for benign and malignant mesothelial cells in serous effusions. Hum. Pathol. 2016, 47, 104–108. [Google Scholar] [CrossRef]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma Concentrations of Matrilysins MMP-7 and MMP-26 as Diagnostic Biomarkers in Breast Cancer. J. Clin. Med. 2021, 10, 1436. [Google Scholar] [CrossRef]
- Acar, A.; Onan, A.; Coskun, U.; Under, A.; Bagriacik, U.; Atalay, F.; Unsal, D.K.; Guner, H. Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med. Oncol. 2008, 25, 279–283. [Google Scholar] [CrossRef]
- Zohny, S.F.; Fayed, S.T. Clinical utility of circulating matrix metalloproteinase-7 (MMP-7), CC chemokine ligand 18 (CCL18) and CC chemokine ligand 11 (CCL11) as markers for diagnosis of epithelial ovarian cancer. Med. Oncol. 2010, 27, 1246–1253. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Gacuta, E.; Zajkowska, M.; Głażewska, E.K.; Osada, J.; Szmitkowski, M.; Chrostek, L.; Dąbrowska, M.; Ławicki, S. Plasma levels of MMP-7 and TIMP-1 in laboratory diagnostics and differentiation of selected histological types of epithelial ovarian cancers. J. Ovarian. Res. 2017, 10, 39. [Google Scholar] [CrossRef]
- Obokata, A.; Watanabe, J.; Nishimura, Y.; Arai, T.; Kawaguchi, M.; Kuramoto, H. Significance of matrix metalloproteinase-7 [correction of matrix metalloproteinase-2], -11 and tissue inhibitor of metalloproteinase-1 expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res. 2007, 27, 95–105. [Google Scholar]
- Chen, W.; Huang, S.; Shi, K.; Yi, L.; Liu, Y.; Liu, W. Prognostic Role of Matrix Metalloproteinases in Cervical Cancer: A Meta-Analysis. Cancer Control 2021, 28, 10732748211033743. [Google Scholar] [CrossRef]
- Wu, S.H.; Zhang, J.; Li, Y.; Li, J.M. Expression of ETV5 and MMP-7 in early stage cervical squamous cell carcinoma and its role in lymphatic metastasis. Ai Zheng Aizheng Chin. J. Cancer 2006, 25, 315–339. [Google Scholar]
- Zhu, L.; Zheng, X.; Du, Y.; Xing, Y.; Xu, K.; Cui, L. Matrix metalloproteinase-7 may serve as a novel biomarker for cervical cancer. Onco. Targets Ther. 2018, 11, 4207–4220. [Google Scholar] [CrossRef]
- Somiari, S.B.; Somiari, R.I.; Heckman, C.M.; Olsen, C.H.; Jordan, R.M.; Russell, S.J.; Shriver, C.D. Circulating MMP2 and MMP9 in breast cancer—Potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. Int. J. Cancer 2006, 119, 403–411. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, X.; Liu, Y.; Cao, W.; Zhang, F.; Zhang, S.; Li, H.; Ning, L.; Fu, L.; Niu, Y.; et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer 2008, 8, 83. [Google Scholar] [CrossRef]
- Boström, P.; Söderström, M.; Vahlberg, T.; Söderström, K.O.; Roberts, P.J.; Carpén, O.; Hirsimäki, P. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer 2011, 11, 348. [Google Scholar] [CrossRef]
- Patel, S.; Sumitra, G.; Koner, B.C.; Saxena, A. Role of serum matrix metalloproteinase-2 and -9 to predict breast cancer progression. Clin. Biochem. 2011, 44, 869–872. [Google Scholar] [CrossRef]
- Song, J.; Su, H.; Zhou, Y.Y.; Guo, L.L. Prognostic value of matrix metalloproteinase 9 expression in breast cancer patients: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 1615–1621. [Google Scholar] [CrossRef]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: Uses and limitation as a biomarker for breast cancer. Clin. Chim. Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Moll, S.A.; Wiertz, I.A.; Vorselaars, A.D.; Zanen, P.; Ruven, H.J.; van Moorsel, C.H.; Grutters, J.C. Serum biomarker CA 15-3 as predictor of response to antifibrotic treatment and survival in idiopathic pulmonary fibrosis. Biomarks Med. 2020, 14, 997–1007. [Google Scholar] [CrossRef]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 188. [Google Scholar] [CrossRef]
- Argote Camacho, A.X.; González Ramírez, A.R.; Pérez Alonso, A.J.; Rejón García, J.D.; Olivares Urbano, M.A.; Torné Poyatos, P.; Ríos Arrabal, S.; Núñez, M.I. Metalloproteinases 1 and 3 as Potential Biomarkers in Breast Cancer Development. Int. J. Mol. Sci. 2021, 22, 9012. [Google Scholar] [CrossRef]
- McGowan, P.M.; Duffy, M.J. Matrix metalloproteinase expression and outcome in patients with breast cancer: Analysis of a published database. Ann. Oncol. 2008, 19, 1566–1572. [Google Scholar] [CrossRef]
- Kim, G.E.; Lee, J.S.; Choi, Y.D.; Lee, K.H.; Lee, J.H.; Nam, J.H.; Choi, C.; Kim, S.S.; Park, M.H.; Yoon, J.H.; et al. Expression of matrix metalloproteinases and their inhibitors in different immunohistochemical-based molecular subtypes of breast cancer. BMC Cancer 2014, 14, 959. [Google Scholar] [CrossRef]
- Zhang, M.; Teng, X.D.; Guo, X.X.; Li, Z.G.; Han, J.G.; Yao, L. Expression of tissue levels of matrix metalloproteinases and their inhibitors in breast cancer. Breast 2013, 22, 330–334. [Google Scholar] [CrossRef]
- Benson, C.S.; Babu, S.D.; Radhakrishna, S.; Selvamurugan, N.; Ravi Sankar, B. Expression of matrix metalloproteinases in human breast cancer tissues. Dis. Markers 2013, 34, 395–405. [Google Scholar] [CrossRef]
- Mehner, C.; Miller, E.; Nassar, A.; Bamlet, W.R.; Radisky, E.S.; Radisky, D.C. Tumor cell expression of MMP3 as a prognostic factor for poor survival in pancreatic, pulmonary, and mammary carcinoma. Genes Cancer 2015, 6, 480–489. [Google Scholar] [CrossRef]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Machaliński, B.; Menkiszak, J.; Sompolska-Rzechuła, A. Suitability assessment of baseline concentration of MMP3, TIMP3, HE4 and CA125 in the serum of patients with ovarian cancer. J. Ovarian Res. 2018, 11, 1. [Google Scholar] [CrossRef]
- Motyka, J.; Gacuta, E.; Kicman, A.; Kulesza, M.; Ławicki, P.; Ławicki, S. Plasma Levels of CXC Motif Chemokine 1 (CXCL1) and Chemokine 8 (CXCL8) as Diagnostic Biomarkers in Luminal A and B Breast Cancer. J. Clin. Med. 2022, 11, 6694. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Gacuta, E.; Zbucka-Krętowska, M.; Ławicki, P.; Szmitkowski, M.; Lemancewicz, A.; Motyka, J.; Kobus, A.; Chorąży, M.; Paniczko, M.; et al. Plasma Levels and Diagnostic Utility of VEGF in a Three-Year Follow-up of Patients with Breast Cancer. J. Clin. Med. 2021, 10, 5452. [Google Scholar] [CrossRef]
- Balkhi, S.; Mashayekhi, F.; Salehzadeh, A.; Saedi, H.S. Matrix metalloproteinase (MMP)-1 and MMP-3 gene variations affect MMP-1 and -3 serum concentration and associates with breast cancer. Mol. Biol. Rep. 2020, 47, 9637–9644. [Google Scholar] [CrossRef]
- Padala, C.; Tupurani, M.A.; Puranam, K.; Gantala, S.; Shyamala, N.; Kondapalli, M.S.; Gundapaneni, K.K.; Mudigonda, S.; Galimudi, R.K.; Kupsal, K.; et al. Synergistic effect of collagenase-1 (MMP1), stromelysin-1 (MMP3) and gelatinase-B (MMP9) gene polymorphisms in breast cancer. PLoS ONE 2017, 12, e0184448. [Google Scholar] [CrossRef]
- Slattery, M.L.; John, E.; Torres-Mejia, G.; Stern, M.; Lundgreen, A.; Hines, L.; Giuliano, A.; Baumgartner, K.; Herrick, J.; Wolff, R.K. Matrix metalloproteinase genes are associated with breast cancer risk and survival: The Breast Cancer Health Disparities Study. PLoS ONE 2013, 8, e63165. [Google Scholar] [CrossRef]
- Aroner, S.A.; Rosner, B.A.; Tamimi, R.M.; Tworoger, S.S.; Baur, N.; Joos, T.O.; Hankinson, S.E. Plasma matrix metalloproteinase 1, 3, and 7 levels and breast cancer risk in the Nurses’ Health study. Cancer Causes Control 2014, 25, 1717–1723. [Google Scholar] [CrossRef]
- Nakopoulou, L.; Giannopoulou, I.; Gakiopoulou, H.; Liapis, H.; Tzonou, A.; Davaris, P.S. Matrix metalloproteinase-1 and -3 in breast cancer: Correlation with progesterone receptors and other clinicopathologic features. Hum. Pathol. 1999, 30, 436–442. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Zurita, M.; Del Moral, R.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Arrebola, J.P.; González, A.R.; León, J.; Antonio Marchal, J.; et al. Matrix metalloproteases and TIMPs as prognostic biomarkers in breast cancer patients treated with radiotherapy: A pilot study. J. Cell. Mol. Med. 2020, 24, 139–148. [Google Scholar] [CrossRef]
- Dey, N.; Young, B.; Abramovitz, M.; Bouzyk, M.; Barwick, B.; De, P.; Leyland-Jones, B. Differential activation of Wnt-β-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PLoS ONE 2013, 8, e77425. [Google Scholar] [CrossRef]
- Sizemore, S.T.; Sizemore, G.M.; Booth, C.N.; Thompson, C.L.; Silverman, P.; Bebek, G.; Abdul-Karim, F.W.; Avril, S.; Keri, R.A. Hypomethylation of the MMP7 promoter and increased expression of MMP7 distinguishes the basal-like breast cancer subtype from other triple-negative tumors. Breast Cancer Res. Treat. 2014, 146, 25–40. [Google Scholar] [CrossRef]
- Katunina, A.I.; Gershtein, E.S.; Ermilova, V.D.; Tereshkina, I.V.; Nazarenko, A.Y.; Tyleuova, A.A.; Dvorova, E.K.; Karabekova, Z.K.; Gritskevich, M.V.; Berezov, T.T. Matrix metalloproteinases 2, 7, and 9 in tumors and sera of patients with breast cancer. Bull. Exp. Biol. Med. 2011, 151, 359–362. [Google Scholar] [CrossRef]
- Mahmoud, R.; Ordóñez-Morán, P.; Allegrucci, C. Challenges for Triple Negative Breast Cancer Treatment: Defeating Heterogeneity and Cancer Stemness. Cancers 2022, 14, 4280. [Google Scholar] [CrossRef]
| Group Size | Age | |||
|---|---|---|---|---|
| Median | Range | |||
| Breast cancer groups | BC—total | 100 | 59 | 45–85 |
| Lum A | 50 | 56 | 46–65 | |
| Lum B | 50 | 59 | 48–85 | |
| Control groups | Fibroadenoma | 50 | 58 | 45–85 |
| Healthy women | 50 | 50 | 44–64 | |
| MMP-3 [ng/mL] | MMP-7 [mg/mL] | CA 15-3 [IU/mL] | |
|---|---|---|---|
| Breast Cancer Groups | |||
| BC—total | 6.61 1.7–18.9 | 1.49 0.41–11.05 | 20.16 4.4–151.2 |
| Lum A | 6.83 1.7–18.9 | 1.35 0.41–11.05 | 19.98 6.2–32.3 |
| Lum B | 6.38 2.35–18.9 | 1.78 0.55–7.40 | 20.2 4.4–151.2 |
| Control Groups | |||
| Fibroadenoma | 5.56 1.98–16.01 | 1.62 0.25–7.16 | 16.85 7.5–48.1 |
| Healthy women | 6.53 1.81–37.33 | 0.84 0.25–5.23 | 15.95 6.7–30.2 |
| Surgical Treatment | MMP-3 [ng/mL] | MMP-7 [ng/mL] | CA 15-3 [IU/mL] | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Breast cancer group | ||||||
| BC—total | 4.34 1.7–15.29 | 4.32 1.69–15.23 | 1.41 0.41–11.05 | 1.01 0.03–9.60 | 20.22 4.4–151.2 | 16.9 7.1–31.5 |
| Lum A | 4.51 1.7–12.16 | 4.76 1.69–12.95 | 1.36 0.41–11.05 | 0.97 0.234–8.5 | 20.8 6.2–32.3 | 17.8 8.3–31.5 |
| Lum B | 4.30 2.35–15.29 | 3.76 2.09–15.23 | 1.54 0.55–5.51 | 1.045 0.03–9.60 | 17.5 4.4–151.2 | 16.65 7.1–26.7 |
| Control group | ||||||
| Fibroadenoma | 5.4 1.98–16.01 | 4.42 0.74–16.21 | 1.25 0.254–7.12 | 1.21 0.21–6.21 | 17.7 7.5–48.1 | 14.1 1.07–39.6 |
| MMP-3 | MMP-7 | ||||
|---|---|---|---|---|---|
| Breast cancer groups | BC -total | CA 15-3 | r | −0.0028 | 0.2583 |
| p | 0.9779 | 0.0102 | |||
| MMP-3 | r | - | 0.1333 | ||
| p | - | 0.1861 | |||
| Lum A | CA 15-3 | r | −0.2116 | 0.1757 | |
| p | 0.1401 | 0.2222 | |||
| MMP-3 | r | - | −0.1634 | ||
| p | - | 0.2569 | |||
| Lum B | CA 15-3 | r | 0.1527 | 0.3701 | |
| p | 0.2897 | 0.0082 | |||
| MMP-3 | r | - | 0.4901 | ||
| p | - | 0.0003 | |||
| Control groups | Fibroadenoma | CA 15-3 | r | 0.1435 | −0.1225 |
| p | 0.3200 | 0.3968 | |||
| MMP-3 | r | - | 0.2216 | ||
| p | - | 0.1220 | |||
| Healthy women | CA 15-3 | r | −0.0572 | 0.0922 | |
| p | 0.6934 | 0.5241 | |||
| MMP-3 | r | - | 0.2412 | ||
| p | - | 0.0915 | |||
| Tested Parameter | Diagnostic Criteria [%] | Breast Cancer Patients | ||
|---|---|---|---|---|
| Luminal A Subgroup | Luminal B HER2- Negative Subgroup | Total Group | ||
| MMP-3 | SE | 32 | 34 | 33 |
| SP | 80 | 80 | 80 | |
| PPV | 62 | 63 | 77 | |
| NPV | 54 | 55 | 37 | |
| MMP-7 | SE | 58 | 70 | 64 |
| SP | 74 | 74 | 74 | |
| PPV | 69 | 73 | 83 | |
| NPV | 64 | 71 | 51 | |
| CA 15-3 | SE | 52 | 56 | 54 |
| SP | 80 | 80 | 80 | |
| PPV | 72 | 74 | 84 | |
| NPV | 63 | 65 | 47 | |
| MMP-3 + CA 15-3 | SE | 62 | 74 | 68 |
| SP | 64 | 64 | 64 | |
| PPV | 63 | 69 | 79 | |
| NPV | 63 | 72 | 50 | |
| MMP-7 + CA 15-3 | SE | 74 | 82 | 78 |
| SP | 60 | 60 | 60 | |
| PPV | 65 | 67 | 80 | |
| NPV | 70 | 77 | 58 | |
| MMP-3 + MMP-7 + CA 15-3 | SE | 94 | 86 | 90 |
| SP | 14 | 14 | 14 | |
| PPV | 52 | 50 | 68 | |
| NPV | 70 | 50 | 41 | |
| Tested Parameter | AUC | SE | 95% C.I. (AUC) | p (AUC = 0.5) |
|---|---|---|---|---|
| Breast cancer total group | ||||
| CA 15-3 | 0.6443 | 0.0451 | 0.5558–0.7327 | 0.0040 |
| MMP-3 | 0.5096 | 0.0500 | 0.4116–0.6076 | 0.8482 |
| MMP-7 | 0.7250 | 0.0443 | 0.6382–0.8119 | 0.0001 |
| MMP-3 + CA 15-3 | 0.6468 | 0.0457 | 0.5570–0.7365 | 0.2709 |
| MMP-7 + CA 15-3 | 0.7246 | 0.0428 | 0.6406–0.8085 | 0.0009 |
| MMP-3 + MMP-7 + CA 15-3 | 0.7380 | 0.0418 | 0.6560–0.8200 | 0.0006 |
| Luminal A subgroup | ||||
| CA 15-3 | 0.6672 | 0.0546 | 0.5600–0.7743 | 0.0040 |
| MMP-3 | 0.5040 | 0.0591 | 0.3881–0.6198 | 0.9450 |
| MMP-7 | 0.6888 | 0.0532 | 0.5845–0.7931 | 0.0011 |
| MMP-3 + CA 15-3 | 0.6776 | 0.0539 | 0.5718–0.7833 | 0.5705 |
| MMP-7 + CA 15-3 | 0.7052 | 0.0519 | 0.6033–0.8070 | 0.0207 |
| MMP-3 + MMP-7 + CA 15-3 | 0.7124 | 0.0516 | 0.6112–0.8135 | 0.0513 |
| Luminal B HER2-negative subgroup | ||||
| CA 15-3 | 0.6214 | 0.0579 | 0.5078–0.7349 | 0.0364 |
| MMP-3 | 0.5232 | 0.0591 | 0.4073–0.6391 | 0.6893 |
| MMP-7 | 0.7612 | 0.0476 | 0.6679–0.8545 | 0.0001 |
| MMP-3 + CA 15-3 | 0.6284 | 0.0559 | 0.5187–0.7380 | 0.3090 |
| MMP-7 + CA 15-3 | 0.7432 | 0.0498 | 0.6454–0.8409 | 0.0006 |
| MMP-3 + MMP-7 + CA 15-3 | 0.8040 | 0.0437 | 0.7182–0.8898 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ławicki, P.; Malinowski, P.; Motyka, J.; Ławicki, M.; Kicman, A.; Kulesza, M.; Gacuta, E.; Guszczyn, T.; Januszkiewicz, M.; Zbucka-Krętowska, M.; et al. Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients. J. Clin. Med. 2023, 12, 2618. https://doi.org/10.3390/jcm12072618
Ławicki P, Malinowski P, Motyka J, Ławicki M, Kicman A, Kulesza M, Gacuta E, Guszczyn T, Januszkiewicz M, Zbucka-Krętowska M, et al. Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients. Journal of Clinical Medicine. 2023; 12(7):2618. https://doi.org/10.3390/jcm12072618
Chicago/Turabian StyleŁawicki, Paweł, Paweł Malinowski, Joanna Motyka, Michał Ławicki, Aleksandra Kicman, Monika Kulesza, Ewa Gacuta, Tomasz Guszczyn, Marcin Januszkiewicz, Monika Zbucka-Krętowska, and et al. 2023. "Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients" Journal of Clinical Medicine 12, no. 7: 2618. https://doi.org/10.3390/jcm12072618
APA StyleŁawicki, P., Malinowski, P., Motyka, J., Ławicki, M., Kicman, A., Kulesza, M., Gacuta, E., Guszczyn, T., Januszkiewicz, M., Zbucka-Krętowska, M., & Ławicki, S. (2023). Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients. Journal of Clinical Medicine, 12(7), 2618. https://doi.org/10.3390/jcm12072618






