The Importance of New EBMT Criteria on the Diagnosis of Veno-Occlusive Liver Disease in Children
Abstract
:1. Introduction
2. Material and Methods
2.1. Statistical Methods
2.2. Characteristics of Study Cohort
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazarbachi, A.H.; Al Hamed, R.; Labopin, M.; Halaburda, K.; Labussiere, H.; Bernasconi, P.; Schroyens, W.; Gandemer, V.; Schaap, N.P.; Loschi, M.; et al. Underdiagnosed veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) as a major cause of multi-organ failure in acute leukemia transplant patients: An analysis from the EBMT Acute Leukemia Working Party. Bone Marrow Transplant. 2021, 56, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, F.; Barbato, F.; Ravaioli, F.; Sessa, M.; Defrancesco, I.; Arpinati, M.; Cavo, M.; Colecchia, A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairo, M.S.; Cooke, K.R.; Lazarus, H.M.; Chao, N. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br. J. Haematol. 2020, 190, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br. J. Haematol. 2015, 168, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kernan, N.A.; Grupp, S.; Smith, A.R.; Arai, S.; Triplett, B.; Antin, J.H.; Lehmann, L.; Shore, T.; Ho, V.T.; Bunin, N.; et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br. J. Haematol. 2018, 181, 816–827. [Google Scholar] [CrossRef]
- Richardson, P.; Aggarwal, S.; Topaloglu, O.; Villa, K.F.; Corbacioglu, S. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transplant. 2019, 54, 1951–1962. [Google Scholar] [CrossRef] [Green Version]
- Szmit, Z.; Gorczyńska, E.; Mielcarek-Siedziuk, M.; Ussowicz, M.; Owoc-Lempach, J.; Kałwak, K. Veno-occlusive disease in children and adolescents after hematopoietic stem cell transplantation: Did the Modified Seattle Criteria fit the characteristics of pediatric population? Adv. Clin. Exp. Med. 2020, 29, 339–344. [Google Scholar] [CrossRef]
- Szmit, Z.; Gorczynska, E.; Król, A.; Ussowicz, M.; Mielcarek-Siedziuk, M.; Olejnik, I.; Panasiuk, A.; Kałwak, K. Introduction of new pediatric EBMT criteria for VOD diagnosis: Is it time-saving or money-wasting?: Prospective evaluation of pediatric EBMT criteria for VOD. Bone Marrow Transplant. 2020, 55, 2138–2146. [Google Scholar] [CrossRef]
- Dhir, A.; Wadhwa, A.; Haines, H.; Chewning, J.; Murthy, S.; Kim, J.; Beatty, L.; Mixon, E.; Fowler, K.B.; Gage, J.; et al. Retrospective Application of Sinusoidal Obstruction Syndrome/Veno-occlusive Disease Diagnostic Criteria in a Pediatric Hematopoietic Stem Cell Transplant Cohort. J. Pediatr. Hematol. Oncol. 2022, 44, E343–E348. [Google Scholar] [CrossRef]
- Yoon, J.H.; Choi, C.W.; Won, J.H. Hepatic sinusoidal obstruction syndrome/ veno-occlusive disease after hematopoietic cell transplantation: Historical and current consider-ations in Korea. Korean J. Intern. Med. 2021, 36, 1261–1280. [Google Scholar] [CrossRef]
- Mahadeo, K.M.; Bajwa, R.; Abdel-Azim, H.; Lehmann, L.E.; Duncan, C.; Zantek, N.; Vittorio, J.; Angelo, J.; McArthur, J.; Schadler, K.; et al. Diagnosis, grading, and treatment recommendations for children, adolescents, and young adults with sinusoidal obstructive syndrome: An international expert position statement. Lancet Haematol. 2020, 7, e61–e72. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Díaz-Beyá, M.; Rosiñol, L.; Martínez, C.; Fernández-Avilés, F.; Rovira, M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol. Blood Marrow Transplant. 2011, 17, 1713–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbacioglu, S.; Jabbour, E.J.; Mohty, M. Risk Factors for Development of and Progression of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2019, 25, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, P.G.; Carreras, E.; Iacobelli, M.; Nejadnik, B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018, 2, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015, 50, 781–789. [Google Scholar] [CrossRef]
- Tewari, P.; Wallis, W. Manifestations and Management of Veno-occlusive Disease/Sinusoidal Obstruction Syndrome in the Era of Contemporary Therapies—Hematology & Oncology. Clin. Adv. Hematol. Oncol. 2017, 15, 130–139. [Google Scholar]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Corbacioglu, S.; Richardson, P.G. Defibrotide for children and adults with hepatic veno-occlusive disease post hematopoietic cell transplantation. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 885–898. [Google Scholar] [CrossRef] [Green Version]
- Corbacioglu, S.; Kernan, N.A.; Pagliuca, A.; Ryan, R.J.; Tappe, W.; Richardson, P.G. Incidence of Anicteric Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome and Outcomes with Defibrotide following Hematopoietic Cell Transplantation in Adult and Pediatric Patients. Biol. Blood Marrow Transplant. 2020, 26, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.J.; Lee, K.S.; Beschorner, W.E.; Vogel, V.G.; Grochow, L.B.; Braine, H.G.; Vogelsang, G.B.; Sensenbrenner, L.L.; Santos, G.W.; Saral, R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987, 44, 778–783. [Google Scholar] [CrossRef]
- Ragoonanan, D.; Khazal, S.J.; Wang, J.; Payne, A.; Kohorst, M.; Harden, A.; Tewari, P.; Petropoulos, D.; Shoberu, B.; Kebriaei, P.; et al. Improved detection of sinusoidal obstructive syndrome using pediatric-AYA diagnostic criteria and severity grading. Bone Marrow Transplant. 2021, 56, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S. The coming of age of the pediatric EBMT criteria. Bone Marrow Transplant. 2021, 56, 767–768. [Google Scholar] [CrossRef]
- Embaby, M.M.; Rangarajan, H.G.; Abu-Arja, R.; Auletta, J.J.; Stanek, J.; Pai, V.; Nicol, K.K.; Bajwa, R.S. Refractory Thrombocytopenia Is a Valid Early Diagnostic Criteria for Hepatic Veno-Occlusive Disease in Children. Biol. Blood Marrow Transplant. 2020, 26, 546–552. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Hinds, M.S.; Fisher, L.D.; Schoch, H.G.; Wolford, J.L.; Banaji, M.; Hardin, B.J.; Shulman, H.M.; Clift, R.A. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann. Intern. Med. 1993, 118, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Deleve, L.D.; Wang, X.; Tsai, J.; Kanel, G.; Strasberg, S.; Tokes, Z.A. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology 2003, 125, 882–890. [Google Scholar] [CrossRef]
- Ravaioli, F.; Colecchia, A.; Alemanni, L.V.; Vestito, A.; Dajti, E.; Marasco, G.; Sessa, M.; Pession, A.; Bonifazi, F.; Festi, D. Role of imaging techniques in liver veno-occlusive disease diagnosis: Recent advances and literature review. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 463–484. [Google Scholar] [CrossRef]
- Faraci, M.; Bertaina, A.; Luksch, R.; Calore, E.; Lanino, E.; Saglio, F.; Prete, A.; Menconi, M.; De Simone, G.; Tintori, V.; et al. Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease after Autologous or Allogeneic Hematopoietic Stem Cell Transplantation in Children: A retrospective study of the Italian Hematology-Oncology Association-Hematopoietic Stem Cell Transplantation Group. Biol. Blood Marrow Transplant. 2019, 25, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Nauffal, M.; Kim, H.T.; Richardson, P.G.; Soiffer, R.J.; Antin, J.H.; Cutler, C.; Nikiforow, S.; Gooptu, M.; Koreth, J.; Romee, R.; et al. Defibrotide: Real-world management of veno-occlusive disease/sinusoidal obstructive syndrome after stem cell transplant. Blood Adv. 2022, 6, 181–188. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Topaloglu, O.; Aggarwal, S. A Systematic Review and Meta-Analysis of Studies of Defibrotide Prophylaxis for Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Clin. Drug Investig. 2022, 42, 465–476. [Google Scholar] [CrossRef]
- Kammersgaard, M.B.; Kielsen, K.; Heilmann, C.; Ifversen, M.; Müller, K. Assessment of the proposed EBMT pediatric criteria for diagnosis and severity grading of sinusoidal obstruction syndrome. Bone Marrow Transplant. 2019, 54, 1406–1418. [Google Scholar] [CrossRef]
- Hildebrandt, G.C.; Chao, N. Endothelial cell function and endothelial-related disorders following haematopoietic cell transplantation. Br. J. Haematol. 2020, 190, 508–519. [Google Scholar] [CrossRef]
- Mahadeo, K.; Bajwa, R. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. J. Pediatr. Intensive Care 2014, 3, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, S.; Michonneau, D.; Sicre de Fontbrune, F.; Sutra del Galy, A.; Xhaard, A.; Robin, M.; Peffault de Latour, R.; Socie, G. Allogeneic reactivity-mediated endothelial cell complications after HSCT: A plea for consensual definitions. Blood Adv. 2019, 3, 2424–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruutu, T.; Eriksson, B.; Remes, K.; Juvonen, E.; Volin, L.; Remberger, M.; Parkkali, T.; Hägglund, H.; Ringdén, O. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood 2002, 100, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Bohte, A.E.; Dierselhuis, M.P.; van Noesel, M.M.; Lequin, M.H. Imaging features of hepatic sinusoidal obstruction syndrome or veno-occlusive disease in children. Pediatr. Radiol. 2022, 52, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Schechter, T.; Perez-Albuerne, E.; Lin, T.F.; Irwin, M.S.; Essa, M.; Desai, A.V.; Frangoul, H.; Yanik, G.; Dupuis, L.L.; Jacobsohn, D.; et al. Veno-occlusive disease after high-dose busulfan-melphalan in neuroblastoma. Bone Marrow Transplant. 2020, 55, 531–537. [Google Scholar] [CrossRef]
- Lankester, A.C.; Albert, M.H.; Booth, C.; Gennery, A.R.; Güngör, T.; Hönig, M.; Morris, E.C.; Moshous, D.; Neven, B.; Schulz, A.; et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transplant. 2021, 56, 2052–2062. [Google Scholar] [CrossRef]
- Ansari, M.; Théoret, Y.; Rezgui, M.A.; Peters, C.; Mezziani, S.; Desjean, C.; Vachon, M.F.; Champagne, M.A.; Duval, M.; Krajinovic, M.; et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematopoietic stem cell transplantation. Drug Monit. 2014, 36, 93–99. [Google Scholar] [CrossRef]
- Yakushijin, K.; Atsuta, Y.; Doki, N.; Yokota, A.; Kanamori, H.; Miyamoto, T.; Ohwada, C.; Miyamura, K.; Nawa, Y.; Kurokawa, M.; et al. Sinusoidal obstruction syndrome after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors and outcomes. Bone Marrow Transplant. 2016, 51, 403–409. [Google Scholar] [CrossRef]
- Stanworth, S.J.; Navarrete, C.; Estcourt, L.; Marsh, J. Platelet refractoriness—Practical approaches and ongoing dilemmas in patient management. Br. J. Haematol. 2015, 171, 297–305. [Google Scholar] [CrossRef]
- Kleinman, S.; Silvergleid, A.; Tirnauer, J.S. Refractoriness to Platelet Transfusion Therapy—UpToDate. 2021. Available online: https://www.uptodate.com/contents/refractoriness-to-platelet-transfusion-therapy (accessed on 31 December 2021).
- Rebulla, P. Formulae for the definition of refractoriness to platelet transfusion. Transfus. Med. 1993, 3, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Leclère, J.; Auperin, A.; Bourhis, J.H.; Hartmann, O.; Valteau-Couanet, D.; Benhamou, E.; Bosq, J.; Ibrahim, A.; Girinski, T.; et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: Value of gray-scale and Doppler US in 100 patients. Radiology 1997, 204, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Kahata, K.; Hayase, E.; Shigematsu, A.; Sato, M.; KudoNishida, M.; Kahata, K.; Hayase, E.; Shigematsu, A.; Sato, M.; et al. Novel Ultrasonographic Scoring System of Sinusoidal Obstruction Syndrome after Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 1896–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkan, S.G.; Pata, C.; Şekuri, A.; Çınar, Y.; Özkan, H.A. Transient elastography of liver: Could it be a guide for diagnosis and management strategy in hepatic veno-occlusive disease (sinusoidal obstruction syndrome)? Transfus. Apher. Sci. 2022, 61, 103370. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Colecchia, A.; Duarte, R.F.; Bonifazi, F.; Ravaioli, F.; Bourhis, J.H. Imaging in Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome. Biol. Blood Marrow Transplant. 2020, 26, 1770–1779. [Google Scholar] [CrossRef]
- Strouse, C.; Zhang, Y.; Zhang, M.J.; DiGilio, A.; Pasquini, M.; Horowitz, M.M. Risk Score for Development of Veno-Occlusive Disease After Allogeneic Hematopoietic Cell Transplant. Biol. Blood Marrow Transplant. 2018, 24, 2072. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Penack, O.; Terzer, T.; Schult, D.; Majer-Lauterbach, J.; Radujkovic, A.; Blau, I.W.; Bullinger, L.; Muller-Tidow, C.; Dreger, P.; et al. Predicting sinusoidal obstruction syndrome after allogeneic stem cell transplantation with the EASIX biomarker panel. Haematologica 2021, 106, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. Hematopoietic Stem Cell Transplantation and Cellular Therapies The EBMT Handbook; Springer: Berlin/Heidelberg, Germany, 2019; pp. 373–702. [Google Scholar]
- Lia, G.; Giaccone, L.; Leone, S.; Bruno, B. Biomarkers for Early Complications of Endothelial Origin After Allogeneic Hematopoietic Stem Cell Transplantation: Do They Have a Potential Clinical Role? Front. Immunol. 2021, 12, 641427. [Google Scholar] [CrossRef]
- Luft, T.; Dreger, P.; Radujkovic, A. Endothelial cell dysfunction: A key determinant for the outcome of allogeneic stem cell transplantation. Bone Marrow Transplant. 2021, 56, 2326–2335. [Google Scholar] [CrossRef]
| Presence of ≥2 of the Following Criteria (Excluding Other Potential Diagnoses): |
|---|
|
| Criteria | Original Seattle Criteria | Modified Seattle Criteria | Baltimore Criteria |
|---|---|---|---|
| Time after HCT | Before D+30 | Before D+20 | Before D+21 |
| Number of symptoms for diagnosis | ≥2 | ≥2 | Hyperbilirubinemia and ≥1 of the following symptoms |
| Symptoms | Hyperbilirubinemia | Hyperbilirubinemia >34 µmol/L (>2 mg/dL) and ≥1 another symptom | Hyperbilirubinemia >34 µmol/L (>2 mg/dL) |
| Hepatomegaly and right upper quadrant pain of liver origin | Hepatomegaly or right upper quadrant painof liver origin | Hepatomegaly | |
| Ascites +/− Unexplained weight gain | Unexplained weight gain >2% baseline due to fluid accumulation in the body | Ascites | |
| Weight gain ≥5% baseline |
| Severity Grading Classification of HVOD | Criteria |
|---|---|
| Mild disease | No apparent adverse effect from liver disease. No medications for diuresis of excessive fluid or for hepatic pain. Completely reversible signs, symptoms, and laboratory abnormalities. |
| Moderate disease (≥1 criteria) | Adverse effect from liver disease. Need to sodium restriction and diuretics to minimize signs of fluid excess (edema, ascites, cardiopulmonary congestion) or medication to alleviate pain from hepatomegaly. Complete resolution of all signs of liver damage (a return of weight to baseline, a decrease in liver size, and a decrease in total serum bilirubin to <34.2 µmol/L (2 mg/dL). |
| Severe disease (2 criteria) | Adverse effect from liver disease. No resolution of signs, symptoms and laboratory values before D+100. Death. |
| Severity Grading of the Disease | Mild (Grade I) | Moderate (Grade II) | Severe (Grade III) | Very Severe (Grade IV) | Death |
|---|---|---|---|---|---|
| CTCAE | 1 | 2 | 3 | 4 | 5 |
| Liver enzymes (AST, ALT, GLDH) | ≤2× normal | >2 a ≤5× normal | >5× normal | ||
| Bilirubin (mg/dL) | <2 | ≥2 | |||
| Bilirubin (µmol/L) | <34 | ≥34 | |||
| Coagulopathy (not responsive to vitamin K administration, INR value ) | <1.5 | 1.5–1.9 | >2 | Need for replacement of coagulation factors | |
| Ascites | Mild (minimal fluid by liver, spleen, or pelvis) | Moderate (<1 cm fluid) | Severe (fluid in all three regions, with fluid collection >1 cm in at least 2 regions) | Need for paracentesis (external derivation of ascites) | |
| Weight gain (from baseline) | 2–5% | 5–10% despite diuretic use | >10% | Persistent rise | |
| Renal function score | KDIGO 1: serum creatinine 1.5–1.9 × baseline or ≥26.5 mmol/L (≥0.3 mg/dL) increase or urine output <0.5 mL/kg/h for 6–12 h | KDIGO 2: serum creatinine 2.0–2.9 × baseline or urine output <0.5 mL/kg/h for ≥12 h | KDIGO 3: serum creatinine 3.0 × baseline or increase in serum creatinine ≥353.6 mmol/L (≥4.0 mg/dL) or initiation of renal replacement therapy or decrease in eGFR to <35 mL/min per 1.73 m2 (patients <18 years) or urine output <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h (patients <18 years) | Need for renal replacement therapy | |
| Encephalopathy | CAPD <9 | CAPD ≥9 | |||
| Persistent RT | <3 days | 3–7 days | >7 days | ||
| Pulmonary function (need for oxygen therapy) | <2 L | >2 L | Non-invasive/invasive mechanical ventilation | Invasive mechanical ventilation | |
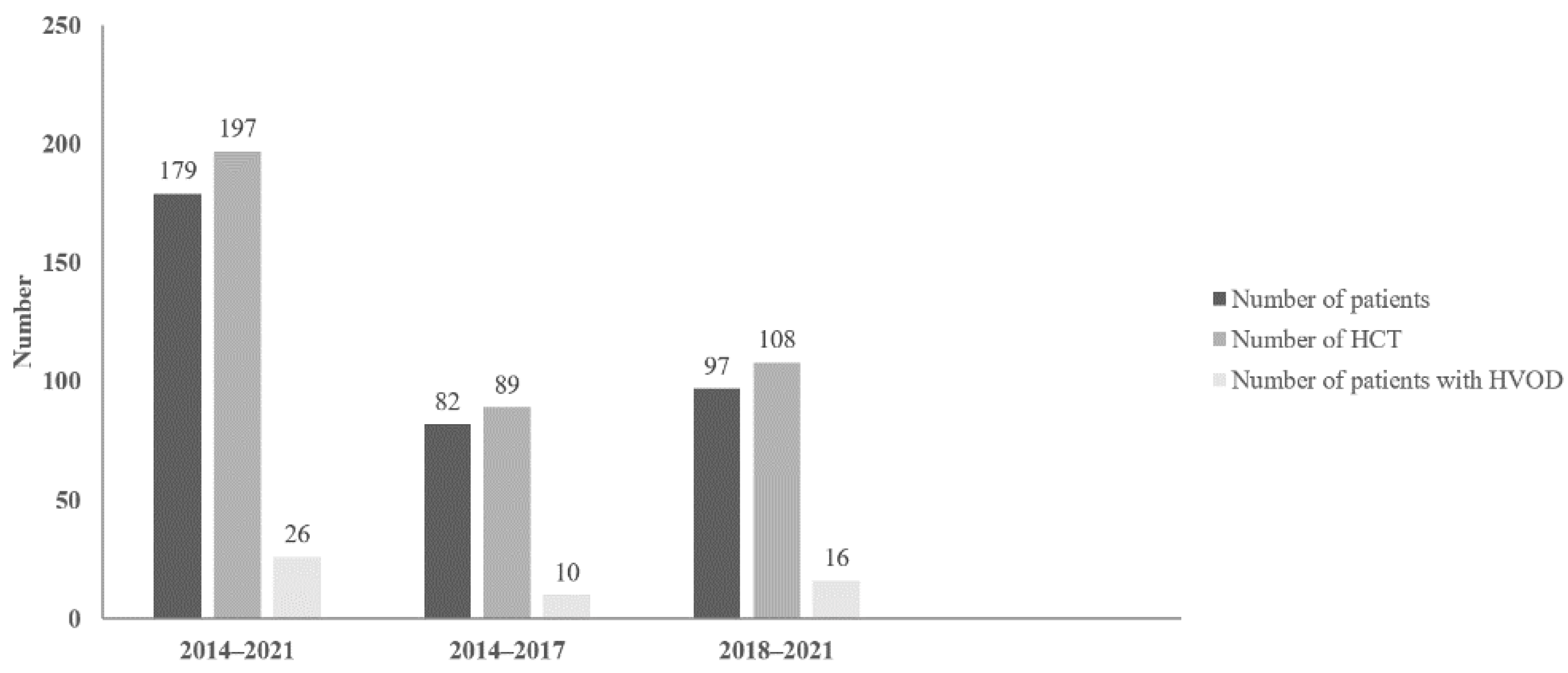
| 2014–2017 | 2018–2021 | |
|---|---|---|
| Number of patients | 82 | 97 |
| Number of HCT | 89 | 108 |
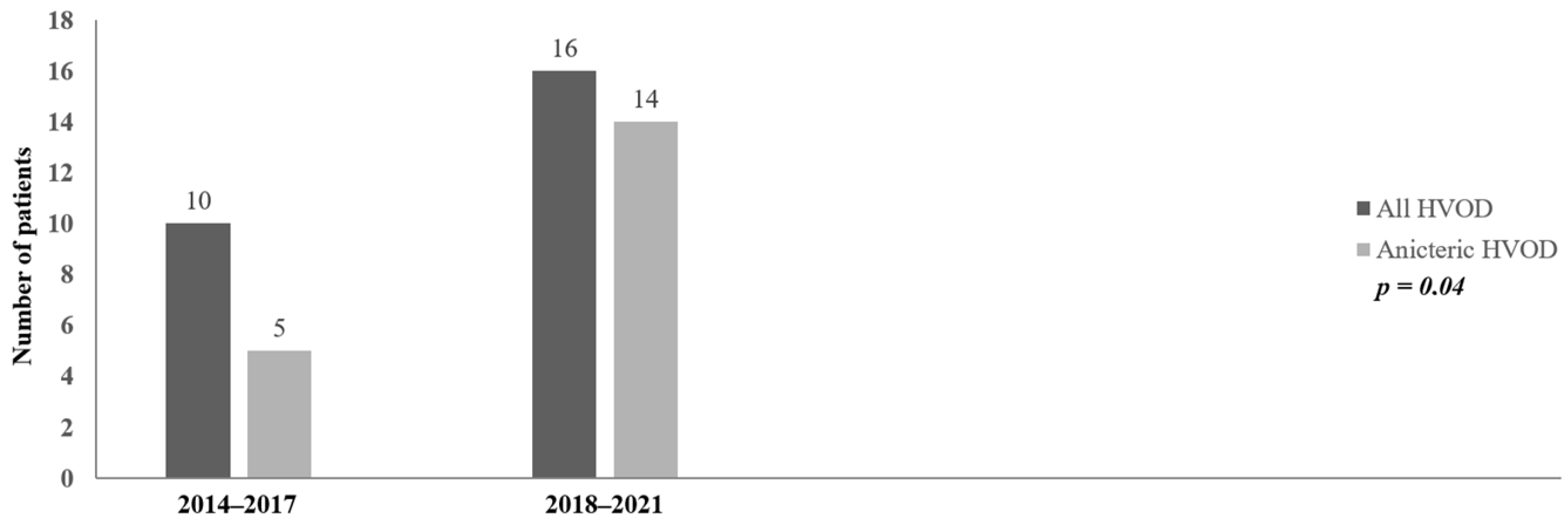
| Number of HVOD patients | 10 | 16 |
| Gender of HVOD patients | ||
| Male | 3 (30%) | 6 (37.5%) |
| Female | 7 (70%) | 10 (62.5%) |
| Age of HVOD patients (years) | 1.2–19 (median: 10.3) | 1.6–16 (median: 5) |
| Diagnosis of HVOD patients | ||
| B-ALL | 4 (40%) | 1 (6.2%) |
| T-ALL | 0 | 1 (6.2%) |
| MDS | 1 (10%) | 2 (12.5%) |
| AML | 0 | 2 (12.5%) |
| JMML | 1 (10%) | 1 (6.2%) |
| MBL | 0 | 1 (6.2%) |
| NBL | 1 (10%) | 8 (50%) |
| WAS | 1 (10%) | 0 |
| CAMT | 1 (10%) | 0 |
| VSAA | 1 (10%) | 0 |
| Type of HCT in HVOD patients | ||
| autologous | 1 (10%) | 9 (56.2%) |
| allogeneic | 9 (90%) | 7 (43.8%) |
| MUD | 8 | 7 |
| MSD | 1 | 0 |
| Source of HSC in HVOD patients | ||
| PBSC | 6 (60%) | 12 (75%) |
| BM | 3 (30%) | 4 (25%) |
| BM + UCB | 1 (10%) | 0 |
| Conditioning regimen in HVOD patients | ||
| MAC | 7 (70%) | 14 (87.5%) |
| RIC | 3 (30%) | 2 (12.5%) |
| Monitored Parameters | 2014–2017 | 2018–2021 | Statistical Comparison |
|---|---|---|---|
| Incidence of HVOD | 10 (11.2%) | 16 (14.8%) | Chi-square = 0.55 p = 0.46 |
| Symptoms and signs at diagnosis | |||
| RT | 9 (90%) | 13 (81.2%) | |
| Weight gain | 8 (80%) | 7 (43.7%) | |
| Hepatomegaly | 10 (100%) | 12 (75%) | |
| Ascites | 8 (80%) | 14 (87.5%) | |
| Elevation of serum bilirubin level (>34 µmol/L = 2 mg/dL or increase of serum bilirubin level from baseline during first three days following) | 6 (60%) | 9 (56.2%) | |
| US signs of HVOD at diagnosis | |||
| Classical US | 10 (100%) | 15 (93.7%) | |
| Doppler US | 5 (50%) | 13 (81.2%) | |
| Time of HVOD diagnosis post-HCT | 6–35 days (median: 15.6 days) | 2–23 days (median: 15.7 days) | U = 74 p = 0.75 |
| Early HVOD | 9 (90%) | 13 (81.2%) | |
| Late-onset HVOD (according to D+21 after HCT) | 1 (10%) | 3 (18.7%) | |
| Anicteric HVOD at diagnosis | 5 (50%) | 14 (87.5%) | Chi-square = 4.40 p = 0.04 |
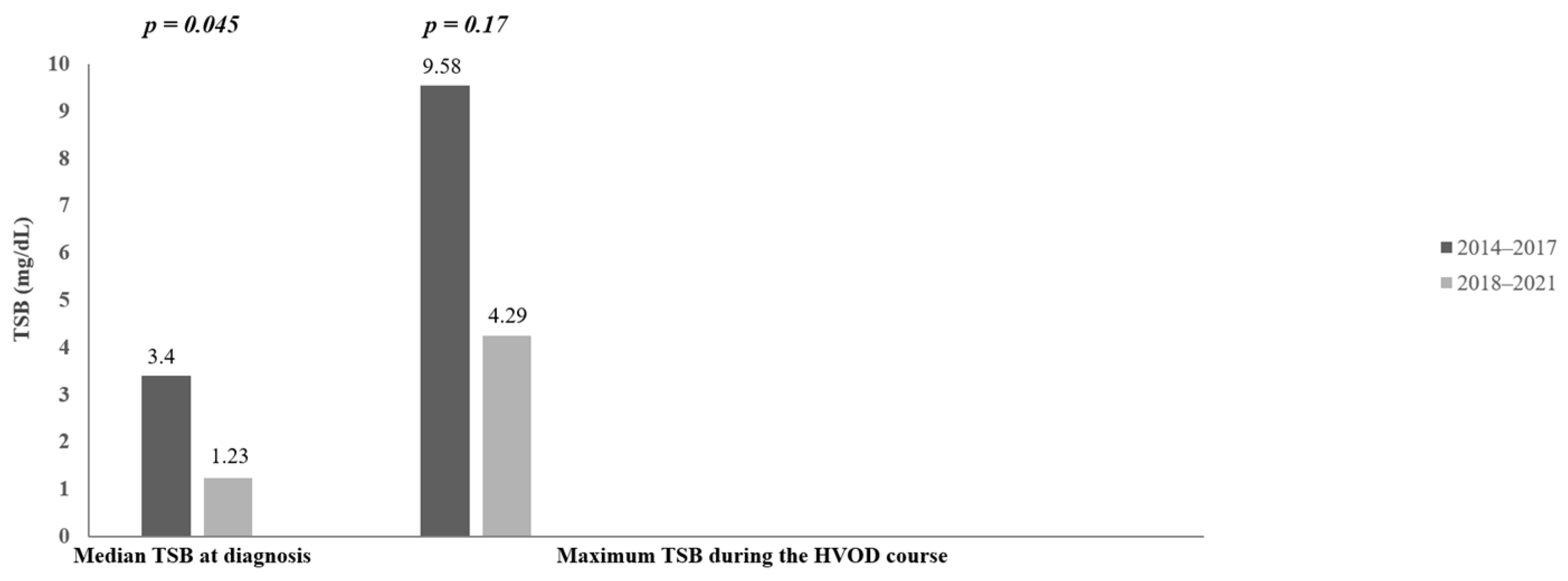
| Serum bilirubin concentration at diagnosis | 7.2–234.6 µmol/L = 0.42–13.72 mg/dL (median: 58.2 µmol/L = 3.4 mg/dL) | 2.9–87.4 µmol/L = 0.17–5.11 mg/dL (median: 21 µmol/L = 1.23 mg/dL) | U = 42 p = 0.045 |
| Maximum serum bilirubin concentration during the HVOD course | 7.2–544.1 µmol/L = 0.42–31.82 mg/dL (median: 163.9 µmol/L = 9.58 mg/dL) | 17.7–520 µmol/L = 1.04–30.41 mg/dL (median: 73.3 µmol/L = 4.29 mg/dL) | U = 53 p = 0.17 |
| Severity grade of HVOD | |||
| I | 2 (20%) | 5 (31.2%) | |
| II | 5 (50%) | 4 (25%) | |
| III | 3 (30%) | 1 (6.2%) | |
| IV | - | 6 (37.5%) | |
| MOD/MOF | |||
| Coagulopathy | 5 (50%) | 4 (25%) | |
| Oxygen inhalation therapy | 5 (50%) | 2 (12.5%) | |
| Ventilation support | 3 (30%) | 1 (6.2%) | |
| Renal insufficiency | 2 (20%) | 1 (6.2%) | |
| Encephalopathy | 3 (30%) | 2 (12.5%) | |
| Duration of defibrotid treatment | 5–43 days (median: 21.7 days) | 7–30 days (median: 15.6 days) | U = 73.5 p = 0.73 |
| CR of HVOD with defibrotid treatment | 7 (70%) | 15 (93.7%) | |
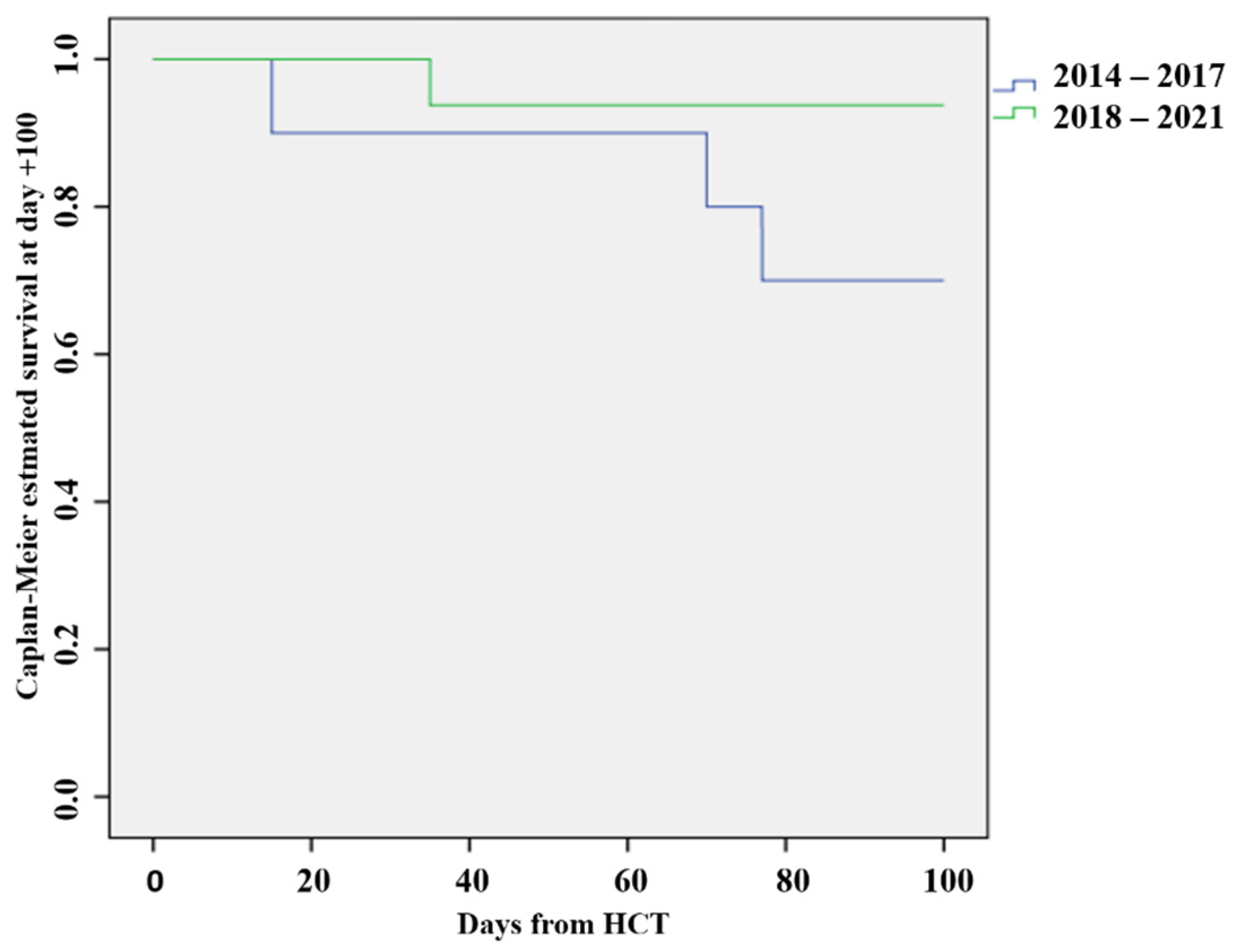
| OS D+100 | 7 (70%) | 15 (93.7%) | Chi-square = 2.67 p = 0.10 |
| Mortality D+100 | 3 (30%) | 1 (6.2%) | Chi-square = 2.67 p = 0.10 |
| Length of hospitalization | 30–134 days (median: 73.1 days) | 39–88 days (median: 59.6 days) | U = 68.5 p = 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Füssiová, M.; Švec, P.; Horáková, J.; Sedláček, P.; Rohoň, P.; Celec, P.; Boďová, I.; Adamčáková, J.; Sýkora, T.; Dobšinská, V.; et al. The Importance of New EBMT Criteria on the Diagnosis of Veno-Occlusive Liver Disease in Children. J. Clin. Med. 2023, 12, 826. https://doi.org/10.3390/jcm12030826
Füssiová M, Švec P, Horáková J, Sedláček P, Rohoň P, Celec P, Boďová I, Adamčáková J, Sýkora T, Dobšinská V, et al. The Importance of New EBMT Criteria on the Diagnosis of Veno-Occlusive Liver Disease in Children. Journal of Clinical Medicine. 2023; 12(3):826. https://doi.org/10.3390/jcm12030826
Chicago/Turabian StyleFüssiová, Mária, Peter Švec, Júlia Horáková, Petr Sedláček, Peter Rohoň, Peter Celec, Ivana Boďová, Jaroslava Adamčáková, Tomáš Sýkora, Veronika Dobšinská, and et al. 2023. "The Importance of New EBMT Criteria on the Diagnosis of Veno-Occlusive Liver Disease in Children" Journal of Clinical Medicine 12, no. 3: 826. https://doi.org/10.3390/jcm12030826






