Pre-Transplant Total Lymphocyte Count Determines Anti-Thymocyte Globulin Exposure, Modifying Graft-versus-Host Disease Incidence and Post-Transplant Thymic Restoration: A Single-Center Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
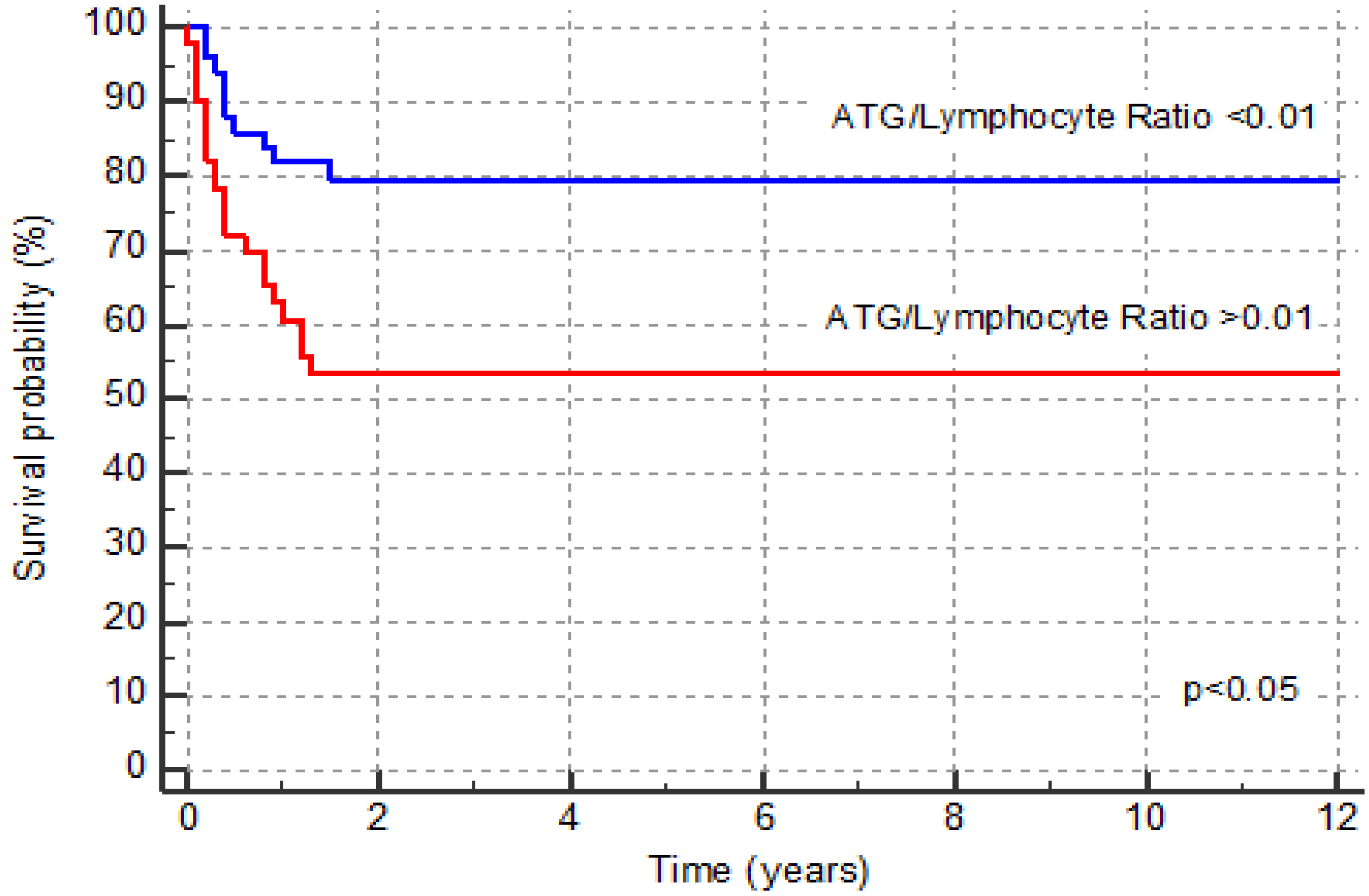
3.1. GVHD Incidence
3.2. Survival and Relapse
3.3. Hematopoietic Recovery and Virus Reactivation
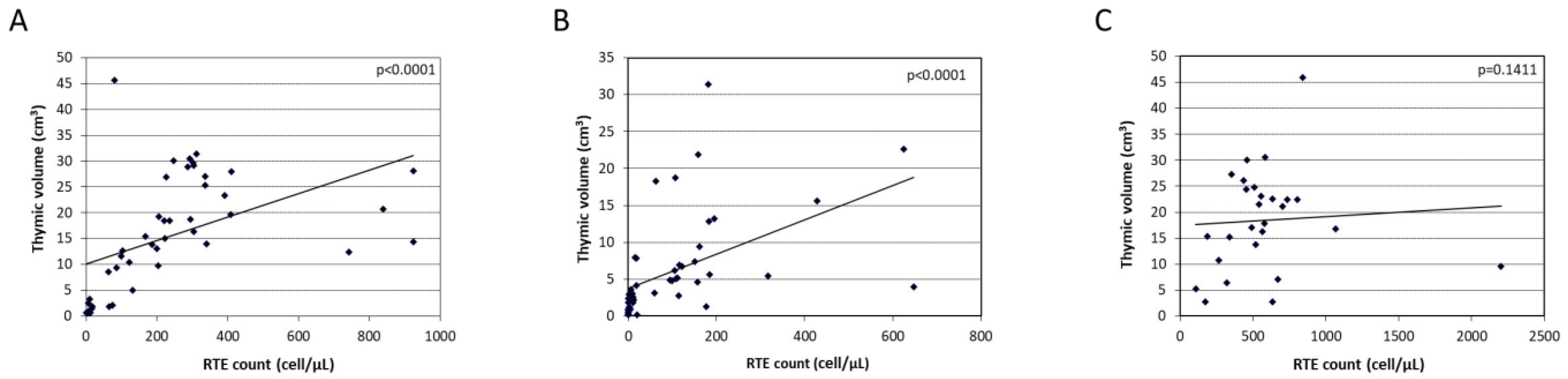
3.4. T-Cell and Thymic Volume Reconstitution Following HSCT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaucha-Prazmo, A.; Gozdzik, J.; Debski, R.; Drabko, K.; Sadurska, E.; Kowalczyk, J. Transplant-related mortality and survival in children with malignancies treated with allogeneic hematopoietic stem cell transplantation. A multicenter analysis. Pediatr. Transplant. 2018, 22, e13158. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-Host Disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Saliba, R.M.; Couriel, D.R.; Giralt, S.; Rondon, G.; Okoroji, G.-J.; Rashid, A.; Champlin, R.E.; Alousi, A.M. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant. 2012, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.O.; Mineishi, S. State-of-the-art acute and chronic GVHD treatment. Int. J. Hematol. 2015, 101, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamani, K.; Russell, J.A.; Daly, A.; Stewart, D.; Savoie, L.; Duggan, P.; Storek, J. Prognosis of grade 3–4 acute GVHD continues to be dismal. Bone Marrow Transplant. 2013, 48, 1359–1361. [Google Scholar] [CrossRef]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Soiffer, R.J.; Kim, H.T.; McGuirk, J.; Horwitz, M.E.; Johnston, L.; Patnaik, M.M.; Rybka, W.; Artz, A.; Porter, D.L.; Shea, T.C.; et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti–T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease–Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J. Clin. Oncol. 2017, 35, 4003–4011. [Google Scholar] [CrossRef]
- Walker, I.; Panzarella, T.; Couban, S.; Couture, F.; Devins, G.; Elemary, M.; Gallagher, G.; Kerr, H.; Kuruvilla, J.; Lee, S.J.; et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2015, 17, 164–173. [Google Scholar] [CrossRef]
- Bacigalupo, A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: Efficacy and side effects. Bone Marrow Transplant. 2005, 35, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Bacigalupo, A.; Lamparelli, T.; Bruzzi, P.; Guidi, S.; Alessandrino, P.E.; Di Bartolomeo, P.; Oneto, R.; Bruno, B.; Barbanti, M.; Sacchi, N.; et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001, 98, 2942–2947. [Google Scholar] [CrossRef]
- Kröger, N.; Solano, C.; Wolschke, C.; Bandini, G.; Patriarca, F.; Pini, M.; Nagler, A.; Selleri, C.; Risitano, A.; Messina, G.; et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2016, 374, 43–53. [Google Scholar] [CrossRef]
- Bosch, M.; Dhadda, M.; Hoegh-Petersen, M.; Liu, Y.; Hagel, L.M.; Podgorny, P.; Ugarte-Torres, A.; Khan, F.M.; Luider, J.; Auer-Grzesiak, I.; et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012, 14, 1258–1275. [Google Scholar] [CrossRef] [Green Version]
- Theurich, S.; Fischmann, H.; Chakupurakal, G.; Shimabukuro-Vornhagen, A.; Chemnitz, J.M.; Holtick, U.; Rothe, A.; Scheid, C.; Hallek, M.; Skoetz, N.; et al. Anti-thymocyte globulins for post-transplant graft-versus-host disease prophylaxis—A systematic review and meta-analysis. Crit. Rev. Oncol. 2013, 88, 178–186. [Google Scholar] [CrossRef]
- Podgorny, P.J.; Ugarte-Torres, A.; Liu, Y.; Williamson, T.S.; Russell, J.A.; Storek, J. High Rabbit-Antihuman Thymocyte Globulin Levels Are Associated with Low Likelihood of Graft-vs-Host Disease and High Likelihood of Posttransplant Lymphoproliferative Disorder. Biol. Blood Marrow Transplant. 2010, 16, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, F.; Bernardo, M.E.; Bertaina, A.; Rognoni, C.; Comoli, P.; Rovelli, A.; Pession, A.; Fagioli, F.; Favre, C.; Lanino, E.; et al. Efficacy of two different doses of rabbit anti-T-lymphocyte globulin to prevent graft-versus-host disease in children with haematological malignancies transplanted from an unrelated donor: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1126–1136. [Google Scholar] [CrossRef]
- Dabas, R.; Lee, R.; Servito, M.T.; Dharmani-Khan, P.; Modi, M.; van Slyke, T.; Luider, J.; Durand, C.; Larratt, L.; Brandwein, J.; et al. Antithymocyte Globulin at Clinically Relevant Concentrations Kills Leukemic Blasts. Biol. Blood Marrow Transplant. 2016, 22, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Admiraal, R.; van Kesteren, C.; Jol-van der Zijde, C.M.; van Tol, M.J.D.; Bartelink, I.H.; Bredius, R.G.M.; Boelens, J.J.; Knibbe, C.A.J. Population pharmacokinetic modeling of thymoglobulin® in children receiving allogeneic-hematopoietic cell transplantation: Towards improved survival through individualized dosing. Clin. Pharmacokinet. 2015, 54, 435–446. [Google Scholar] [CrossRef]
- Call, S.K.; Kasow, K.A.; Barfield, R.; Madden, R.; Leung, W.; Horwitz, E.; Woodard, P.; Panetta, J.C.; Baker, S.; Handgretinger, R.; et al. Total and Active Rabbit Antithymocyte Globulin (rATG; Thymoglobulin) Pharmacokinetics in Pediatric Patients Undergoing Unrelated Donor Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2009, 15, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Admiraal, R.; Lindemans, C.A.; van Kesteren, C.; Bierings, M.B.; Versluijs, A.B.; Nierkens, S.; Boelens, J.J. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood 2016, 128, 2734–2741. [Google Scholar] [CrossRef] [Green Version]
- Admiraal, R.; van Kesteren, C.; Jol-van der Zijde, C.M.; Lankester, A.C.; Bierings, M.B.; Egberts, T.C.G.; van Tol, M.J.D.; Knibbe, C.A.J.; Bredius, R.G.M.; Boelens, J.J. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: A multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015, 2, e194–e203. [Google Scholar] [CrossRef]
- Velardi, E.; Clave, E.; Arruda, L.C.M.; Benini, F.; Locatelli, F.; Toubert, A. The role of the thymus in allogeneic bone marrow transplantation and the recovery of the peripheral T-cell compartment. Semin. Immunopathol. 2021, 43, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Janeczko-Czarnecka, M.; Rybka, B.; Ryczan-Krawczyk, R.; Kałwak, K.; Ussowicz, M. Thymic activity in immune recovery after allogeneic hematopoietic stem cell transplantation in children. Central Eur. J. Immunol. 2020, 44, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Maximova, N.; Schillani, G.; Simeone, R.; Maestro, A.; Zanon, D. Comparison of Efficacy and Safety of Caspofungin versus Micafungin in Pediatric Allogeneic Stem Cell Transplant Recipients: A Retrospective Analysis. Adv. Ther. 2017, 34, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Armand, P.; Kim, H.T.; Logan, B.R.; Wang, Z.; Alyea, E.P.; Kalaycio, M.E.; Maziarz, R.T.; Antin, J.H.; Soiffer, R.J.; Weisdorf, D.J.; et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarelli, G.; Clift, R.A.; Galimberti, M.; Polchi, P.; Angelucci, E.; Baronciani, D.; Giardini, C.; Andreani, M.; Manna, M.; Nesci, S.; et al. Marrow transplantation for patients with thalassemia: Results in class 3 patients. Blood 1996, 87, 2082–2088. [Google Scholar] [CrossRef] [Green Version]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Lee, S.J.; Vogelsang, G.; Flowers, M.E. Chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2003, 9, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Admiraal, R.; Nierkens, S.; de Witte, M.A.; Petersen, E.J.; Fleurke, G.J.; Verrest, L.; Belitser, S.V.; Bredius, R.G.M.; Raymakers, R.A.P.; Knibbe, C.A.J.; et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: A multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017, 4, e183–e191. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Chen, H.; Savani, B.N.; Greer, J.; Kassim, A.A.; Engelhardt, B.G.; Goodman, S.; Sengsayadeth, S.; Chinratanalab, W.; Jagasia, M. Optimizing Antithymocyte Globulin Dosing for Unrelated Donor Allogeneic Hematopoietic Cell Transplantation Based on Recipient Absolute Lymphocyte Count. Biol. Blood Marrow Transplant. 2018, 24, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Admiraal, R.; van Kesteren, C.; Nierkens, S.; Boelens, J.J. Antithymocyte globulin: Importance of good clinical pharmacological practice. J. Allergy Clin. Immunol. 2016, 138, 633. [Google Scholar] [CrossRef] [Green Version]
- Seidel, M.G.; Fritsch, G.; Matthes-Martin, S.; Lawitschka, A.; Lion, T.; Pötschger, U.; Rosenmayr, A.; Fischer, G.; Gadner, H.; Peters, C. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J. Pediatr. Hematol. 2005, 27, 532–536. [Google Scholar] [CrossRef]
- Jamani, K.; Dabas, R.; Kangarloo, S.B.; Prokopishyn, N.L.; Luider, J.; Dharmani-Khan, P.; Khan, F.M.; Daly, A.; Storek, J. Rabbit Antithymocyte Globulin Serum Levels: Factors Impacting the Levels and Clinical Outcomes Impacted by the Levels. Biol. Blood Marrow Transplant. 2019, 25, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Scordo, M.; Bhatt, V.; Hilden, P.; Smith, M.; Thoren, K.; Cho, C.; Shah, G.L.; Maloy, M.A.; Papadopoulos, E.B.; Jakubowski, A.A.; et al. Standard Antithymocyte Globulin Dosing Results in Poorer Outcomes in Overexposed Patients after Ex Vivo CD34+ Selected Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1526–1535. [Google Scholar] [CrossRef]
- MacMillan, M.L.; Weisdorf, D.J.; Brunstein, C.G.; Cao, Q.; DeFor, T.E.; Verneris, M.R.; Blazar, B.R.; Wagner, J.E. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: Analysis of risk factors. Blood 2009, 113, 2410–2415. [Google Scholar] [CrossRef] [Green Version]
- Finke, J.; Bertz, H.; Schmoor, C.; Veelken, H.; Behringer, D.; Wäsch, R.; Kunzmann, R.; Heidecker, L.; Lang, H.; Meyer-König, U.; et al. Allogeneic bone marrow transplantation from unrelated donors using in vivo anti-T-cell globulin. Br. J. Haematol. 2000, 111, 303–313. [Google Scholar]
- Wolschke, C.; Zabelina, T.; Ayuk, F.; Alchalby, H.; Berger, J.; Klyuchnikov, E.; Pein, U.-M.; Schumacher, S.; Amtsfeld, G.; Adjallé, R.; et al. Effective prevention of GVHD using in vivo T-cell depletion with anti-lymphocyte globulin in HLA-identical or -mismatched sibling peripheral blood stem cell transplantation. Bone Marrow Transplant. 2014, 49, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Bryant, A.; Mallick, R.; Huebsch, L.B.; Allan, D.S.; Atkins, H.; Anstee, G.; Bence-Bruckler, I.; Hamelin, L.; Hodgins, M.; Sabloff, M.; et al. Low-Dose Anti-Thymocyte Globulin for Graft-Versus-Host-Disease Prophylaxis in Matched Unrelated Allogeneic Hematopoietic Stem Cell Transplant. Blood 2016, 128, 5782. [Google Scholar] [CrossRef]
- Finke, J.; Bethge, W.A.; Schmoor, C.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009, 10, 855–864. [Google Scholar] [CrossRef]
- Remberger, M.; Ringdén, O.; Hägglund, H.; Svahn, B.-M.; Ljungman, P.; Uhlin, M.; Mattsson, J. A high antithymocyte globulin dose increases the risk of relapse after reduced intensity conditioning HSCT with unrelated donors. Clin. Transplant. 2013, 27, E368–E374. [Google Scholar] [CrossRef]
- Socié, G.; Schmoor, C.; Bethge, W.A.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. Chronic graft-versus-host disease: Long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti–T-cell globulin ATG-Fresenius. Blood 2011, 117, 6375–6382. [Google Scholar] [CrossRef] [Green Version]
- Kröger, N.; Zabelina, T.; Krüger, W.; Renges, H.; Stute, N.; Rischewski, J.; Sonnenberg, S.; Ayuk, F.; Tögel, F.; Schade, U.; et al. In vivo T cell depletion with pretransplant anti-thymocyte globulin reduces graft-vs-host disease without increasing relapse in good risk myeloid leukemia patients after stem cell transplantation from matched related donors. Bone Marrow Transplant. 2002, 29, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storek, J.; Geddes, M.; Khan, F.; Huard, B.; Helg, C.; Chalandon, Y.; Passweg, J.R.; Roosnek, E. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin. Immunopathol. 2008, 30, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, K.; Blazar, B.R.; Wagner, J.E.; Agura, E.; Hill, B.J.; Smogorzewska, M.; Koup, R.A.; Betts, M.R.; Collins, R.H.; Douek, D.C. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood 2001, 97, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Toubert, A.; Glauzy, S.; Douay, C.; Clave, E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: Never say never again. Tissue Antigens 2012, 79, 83–89. [Google Scholar] [CrossRef]
- Krenger, W.; Blazar, B.R.; Holländer, G.A. Thymic T-cell development in allogeneic stem cell transplantation. Blood 2011, 117, 6768–6776. [Google Scholar] [CrossRef] [Green Version]
- Kaebisch, E.M.; Cho, M.-Y.; Oh, Y.-S.; Olfe, L.I.; Szyska, M.; Becker, S.C.; Reinke, P.; Volk, H.-D.; Gillissen, B.; Bullinger, L.; et al. Cytotoxic Effects of Rabbit Anti-thymocyte Globulin Preparations on Primary Human Thymic Epithelial Cells. Transplantation 2019, 103, 2234–2244. [Google Scholar] [CrossRef]

| Pre-Transplant Characteristics | Study Group (ATG Exposed) | Control Group (ATG Unexposed) | p-Value |
|---|---|---|---|
| Number of patients (%) | 102 (100) | 69 (100) | |
| Sex: | |||
| Male (%) | 60 (58.9) | 42 (60.9) | 0.97 |
| Female (%) | 42 (41.1) | 27 (39.1) | 0.98 |
| Age at transplant, years, mean (± SD) | 8.25 (5.32) | 9.03 (5.8) | 0.86 |
| Underlying disease, number (%): | |||
| Acute lymphoblastic leukemia | 39 (38.2) | 25 (36.2) | 0.87 |
| Acute myeloid leukemia | 19 (18.6) | 14 (20.3) | 0.81 |
| Myelodysplastic syndrome | 16 (15.6) | 13 (18.8) | 0.63 |
| Solid tumor | 5 (4.9) | 2 (2.9) | 0.62 |
| Nonmalignant disorders | 23 (22.5) | 15 (21.7) | 1 |
| Disease risk index, number (%): | |||
| Low | 25 (24.5) | 18 (26.1) | 0.84 |
| Intermediate | 33 (32.4) | 22 (31.9) | 1 |
| High | 28 (27.4) | 19 (27.5) | 1 |
| Very high | 16 (15.7) | 10 (14.5) | 0.79 |
| Myeloablative conditioning, number (%): | |||
| MCHT-based | 70 (68.6) | 44 (63.8) | 0.8 |
| TBI-based | 32 (31.4) | 25 (36.2) | 0.66 |
| Graft source, number (%): | |||
| Bone marrow | 78 (76.5) | 52 (75.4) | 0.95 |
| Peripheral blood stem cells | 18 (17.6) | 13 (18.8) | 0.81 |
| Umbilical cord blood | 6 (5.9) | 4 (5.8) | 1 |
| Allogeneic donor type, number (%): | |||
| Matched related donor | 6 (5.9) | 69 (100) | <0.001 |
| Matched unrelated donor | 71 (69.6) | - | |
| Haploidentical donor | 25 (24.5) | - | |
| Follow-up, weeks, median (range) | 113 (2–679) | 120 (4–703) | 0.24 |
| Variables | ATG/Lymphocyte Ratio < 0.01 (n = 51) | ATG/Lymphocyte Ratio > 0.01 (n = 51) | p-Value |
|---|---|---|---|
| Disease risk index, number (%): | |||
| Low/Intermediate | 27 (53.0) | 31 (60.8) | 0.59 |
| High/Very high | 24 (47.0) | 20 (39.2) | 0.54 |
| Total ATG dose, mg/kg, patients (%): | |||
| <5 | 11 (42.3) | 15 (57.7) | 0.578 |
| 5–10 | 17 (48.6) | 18 (51.4) | 0.9 |
| >10 | 23 (56.1) | 18 (43.9) | 0.58 |
| Lymphocyte count before ATG, cells × 102/μL, mean (±SD) | 16.04 (13.2) | 1.49 (1.3) | <0.001 |
| Total ATG dose, mg/kg, mean (±SD) | 6 (3.8) | 13.2 (7.3) | <0.001 |
| CD4 day +100, cells/μL, mean (±SD) | 330 (187) | 127 (97) | 0.0018 |
| CD4 > 500 cells/μL, days, median (range) | 130 (0 *–1095) | 199 (0*–450) | 0.0431 |
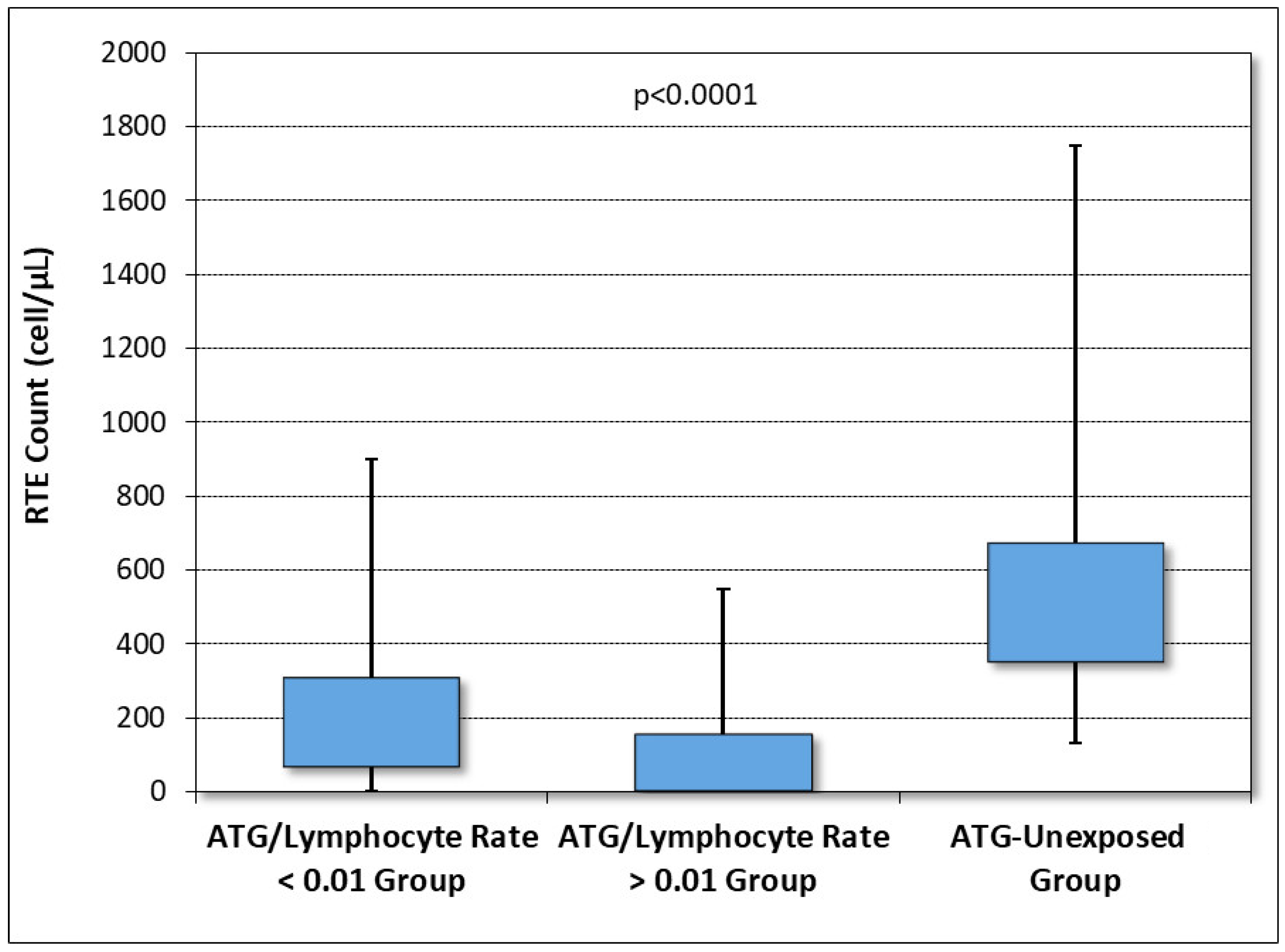
| Recent thymic migrants + 1 year, cells/μL, median (range) | 206 (0 *–924) | 59 (0*–647) | <0.001 |
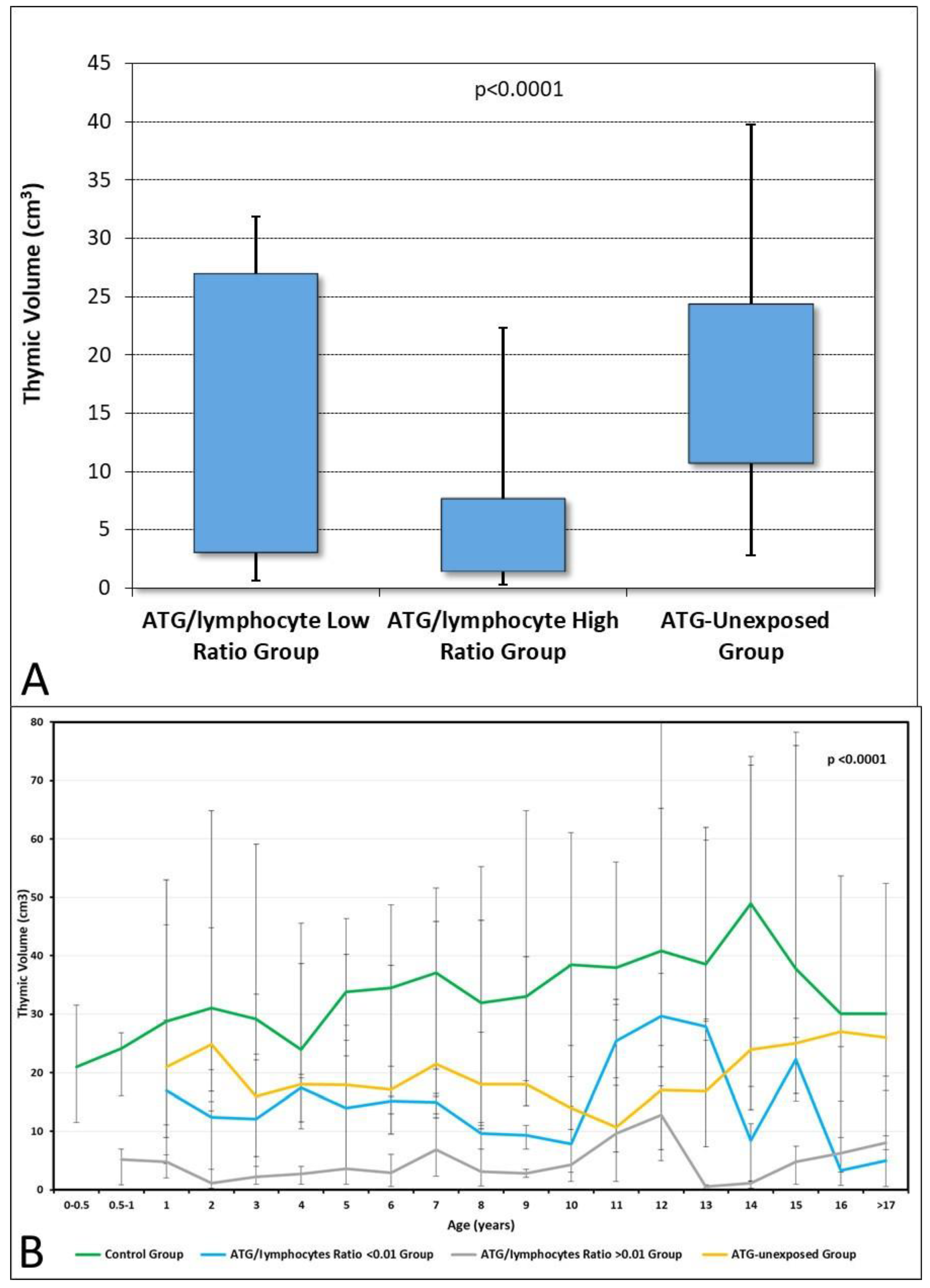
| Thymic volume + 1 year, cm3, median (range) | 14.7 (0.4–45.7) | 4.5 (0.2–31.4) | <0.001 |
| Acute GVHD (any grade), number (%): | 29 (59.0) | 7 (16.6) | <0.001 |
| Grade I-II | 17 (34.4) | 4 (9.5) | <0.001 |
| Grade III-IV | 12 (24.5) | 3 (7.1) | <0.05 |
| Chronic GVHD, number (%) | 9 (18.4) | 2 (4.8) | 0.0965 |
| Virus infection/reactivation, number (%): | 28 (56.0) | 38 (74.0) | 0.06 |
| CMV | 20 (39.2) | 31 (60.7) | 0.0437 |
| EBV | 9 (17.6) | 14 (27.5) | 0.34 |
| Adenovirus | 5 (9.8) | 5 (9.8) | 1 |
| <3 episodes | 14 (27.0) | 14 (27.0) | 1 |
| 3–5 episodes | 10 (19.6) | 15 (29.4) | 0.35 |
| >5 episodes | 5 (8.8) | 9 (17.6) | 0.38 |
| Endothelial damage-related complications, number (%) ** | 3 (5.9) | 9 (17.6) | 0.12 |
| Off immunosuppression at 1 year, number (%) | 26 (63.0) | 32 (91.0) | <0.001 |
| Overall survival, number (%) | 41 (80.4) | 30 (58.0) | 0.0313 |
| Event-free survival, number (%) | 33 (64.0) | 24 (47.0) | 0.11 |
| Non-relapse mortality, number (%): | 7 (13.1) | 17 (33.0) | 0.0357 |
| GVHD | 0 | 1 (2.0) | - |
| VOD | 1 (2.0) | 3 (5.9) | 0.6 |
| Infection | 3 (5.9) | 8 (15.6) | 0.2 |
| Other | 3 (5.9) | 5 (9.8) | 0.7 |
| Relapse-related mortality, number (%) | 3 (5.9) | 5 (9.8) | 0.7 |
| Overall Survival | ||
|---|---|---|
| Variable | Hazard Ratio (95% Cl) | p |
| ATG/Lym Low < 0.01 | 1 | |
| ATG/Lym High > 0.01 | 2.4 (1–5.4) | 0.03 |
| LLA | 1 | |
| LMA/MDS | 0.32 (0.08–1) | 0.06 |
| Solid Tumor | 1.19 (0.2–5.4) | 0.81 |
| Non Oncological Disease | 0.46 (0.13–1.6) | 0.22 |
| TBI-based | 1 | |
| Chemo-based | 1.18 (0.67–3) | 0.87 |
| BM | 1 | |
| PBSC | 0.81 (0.27–2.3) | 0.55 |
| CB | 0.46 (0.6–3.6) | 0.46 |
| MUD | 1 | |
| Haploidentical | 1.2 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasso, A.G.; Simeone, R.; Maestro, A.; Zanon, D.; Maximova, N. Pre-Transplant Total Lymphocyte Count Determines Anti-Thymocyte Globulin Exposure, Modifying Graft-versus-Host Disease Incidence and Post-Transplant Thymic Restoration: A Single-Center Retrospective Study. J. Clin. Med. 2023, 12, 730. https://doi.org/10.3390/jcm12020730
Grasso AG, Simeone R, Maestro A, Zanon D, Maximova N. Pre-Transplant Total Lymphocyte Count Determines Anti-Thymocyte Globulin Exposure, Modifying Graft-versus-Host Disease Incidence and Post-Transplant Thymic Restoration: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2023; 12(2):730. https://doi.org/10.3390/jcm12020730
Chicago/Turabian StyleGrasso, Antonio Giacomo, Roberto Simeone, Alessandra Maestro, Davide Zanon, and Natalia Maximova. 2023. "Pre-Transplant Total Lymphocyte Count Determines Anti-Thymocyte Globulin Exposure, Modifying Graft-versus-Host Disease Incidence and Post-Transplant Thymic Restoration: A Single-Center Retrospective Study" Journal of Clinical Medicine 12, no. 2: 730. https://doi.org/10.3390/jcm12020730






