Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
1. Introduction
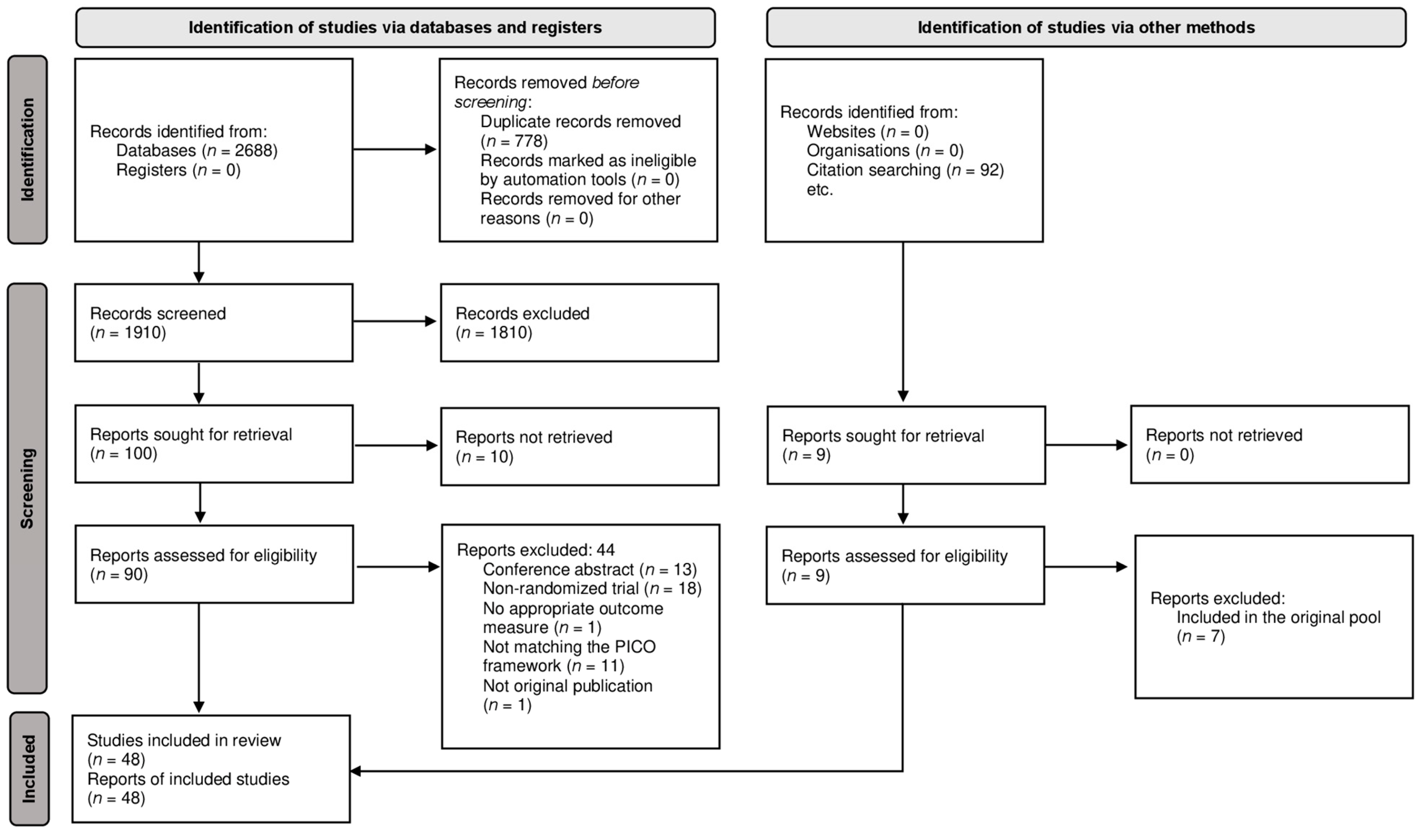
2. Materials and Methods
- P—Adult patients with chronic wounds;
- I—Platelet-rich plasma (PRP) treatment;
- C—Conventional ulcer therapy;
- O—Primary outcome: change in wound size (complete closure, reduction of wound area, healing rate); secondary outcomes: healing time, infection, pain, adverse events, amputation, recurrence, and quality of life.
3. Results
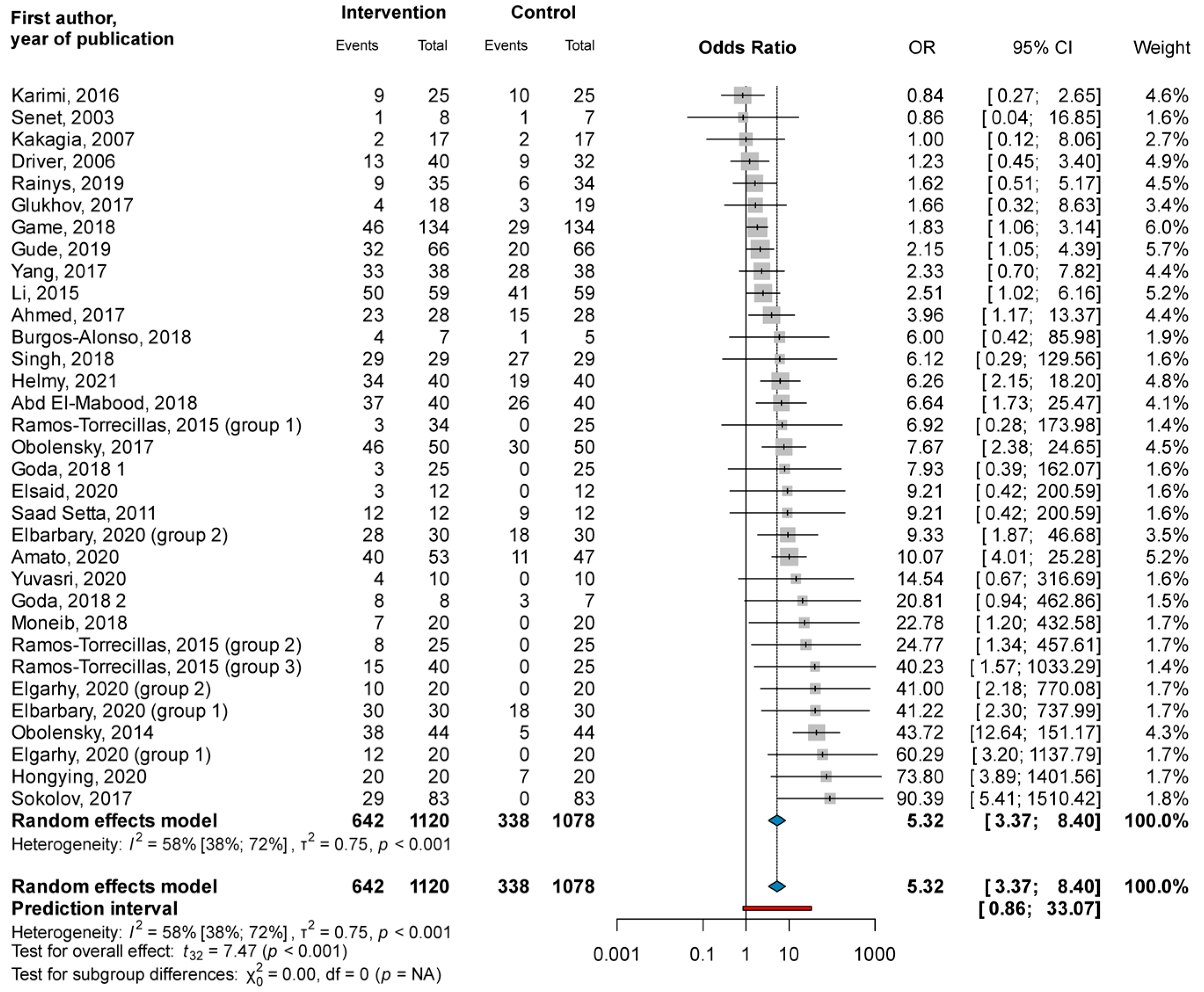
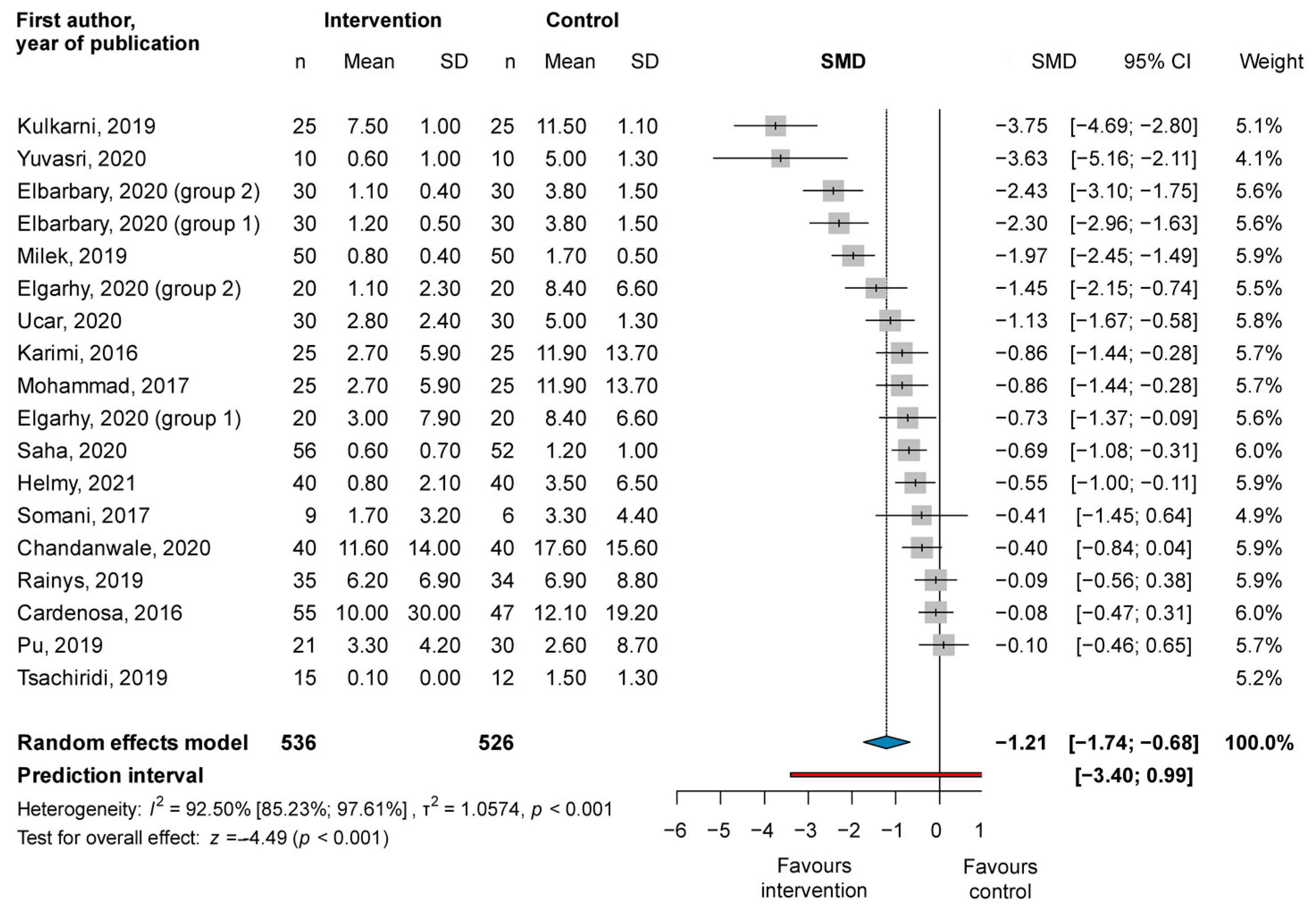
3.1. Primary Outcome
3.1.1. Complete Closure
3.1.2. Reduction of Wound Area
3.2. Secondary Outcomes
3.3. Risk of Bias Assessment
3.4. Quality of Evidence
3.5. Publication Bias
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Research
4.3. Implications for Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapp, S.; Miller, C.; Santamaria, N. The quality of life of people who have chronic wounds and who self-treat. J. Clin. Nurs. 2018, 27, 182–192. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Derm. 2016, 74, 589–605, quiz 5–6. [Google Scholar] [CrossRef]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Derm. 2016, 74, 607–625, quiz 25–26. [Google Scholar] [CrossRef]
- Hesseler, M.J.; Shyam, N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J. Am. Acad. Derm. 2019, 81, 834–846. [Google Scholar] [CrossRef]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef]
- Qu, W.; Wang, Z.; Hunt, C.; Morrow, A.S.; Urtecho, M.; Amin, M.; Shah, S.; Hasan, B.; Abd-Rabu, R.; Ashmore, Z.; et al. The effectiveness and safety of platelet-rich plasma for chronic wounds: A systematic review and meta-analysis. Mayo Clin. Proc. 2021, 96, 2407–2417. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2022.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Iorio, A.; Spencer, F.A.; Falavigna, M.; Alba, C.; Lang, E.; Burnand, B.; McGinn, T.; Hayden, J.; Williams, K.; Shea, B.; et al. Use of grade for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 2015, 350, h870. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Schwarzer, G. Meta: General Package for Meta-Analysis, Version 6.0-0; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Cuijpers, P.; Furukawa, T.; Ebert, D.D. dMetar: Companion R Package for the Guide Doing Meta-Analysis in R; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the mantel-haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Thompson, S.G.; Turner, R.M.; Warn, D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001, 10, 375–392. [Google Scholar] [CrossRef]
- The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009.
- Sweeting, M.J.; Sutton, A.J.; Lambert, P.C. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat. Med. 2004, 23, 1351–1375. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with r: A Hands-On Guide, 1st ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J.A.C. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Abd El-Mabood, E.A.; Ali, H.E. Platelet-rich plasma versus conventional dressing: Does this really affect diabetic foot wound-healing outcomes? Egypt. J. Surg. 2018, 37, 16–26. [Google Scholar] [CrossRef]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef]
- Alamdari, N.M.; Sha, A.; Mirmohseni, A.; Besharat, S. Evaluation of the efficacy of platelet-rich plasma on healing of clean diabetic foot ulcers: A randomized clinical trial in Tehran, Iran. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Amato, B.; Farina, M.A.; Campisi, S.; Ciliberti, M.; di Donna, V.; Florio, A.; Grasso, A.; Miranda, R.; Pompeo, F.; Farina, E.; et al. Cgf treatment of leg ulcers: A randomized controlled trial. Open Med. 2020, 14, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Aguirre, J.J.; Algorta, J.; Ayerdi, E.; Cabezas, A.I.; Orive, G.; Andia, I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Alonso, N.; Lobato, I.; Hernandez, I.; Sebastian, K.S.; Rodriguez, B.; March, A.G.; Perez-Salvador, A.; Arce, V.; Garcia-Alvarez, A.; Gomez-Fernandez, M.C.; et al. Autologous platelet-rich plasma in the treatment of venous leg ulcers in primary care: A randomised controlled, pilot study. J. Wound Care 2018, 27, S20–S24. [Google Scholar] [CrossRef]
- Cardenosa, M.E.; Dominguez-Maldonado, G.; Cordoba-Fernandez, A. Efficacy and safety of the use of platelet-rich plasma to manage venous ulcers. J. Tissue Viability 2017, 26, 138–143. [Google Scholar] [CrossRef]
- Chandanwale, K.A.; Mahakalkar, C.C.; Kothule, A.K.; Khithani, D.V. Management of wounds of peripheral arterial disease using platelet rich plasma. J. Evol. Med. Dent. Sci. 2020, 9, 2239–2245. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; Abbade, L.P.F.; Miot, H.A.; Ferreira, R.R.; Deffune, E. Pilot study of homologous platelet gel in venous ulcers. An. Bras. Dermatol. 2017, 92, 499–504. [Google Scholar] [CrossRef]
- Driver, V.R.; Hanft, J.; Fylling, C.P.; Beriou, J.M. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Manag. 2006, 52, 68–70, 72, 74 passim. [Google Scholar]
- Elbarbary, A.H.; Hassan, H.A.; Elbendak, E.A. Autologous platelet-rich plasma injection enhances healing of chronic venous leg ulcer: A prospective randomised study. Int. Wound J. 2020, 17, 992–1001. [Google Scholar] [CrossRef]
- Elgarhy, L.H.; El-Ashmawy, A.A.; Bedeer, A.E.; Al-Bahnasy, A.M. Evaluation of safety and efficacy of autologous topical platelet gel vs platelet rich plasma injection in the treatment of venous leg ulcers: A randomized case control study. Dermatol. Ther. 2020, 33, e13897. [Google Scholar] [CrossRef]
- Elsaid, A.; El-Said, M.; Emile, S.; Youssef, M.; Khafagy, W.; Elshobaky, A. Randomized controlled trial on autologous platelet-rich plasma versus saline dressing in treatment of non-healing diabetic foot ulcers. World J. Surg. 2020, 44, 1294–1301. [Google Scholar] [CrossRef]
- Game, F.; Jeffcoate, W.; Tarnow, L.; Jacobsen, J.L.; Whitham, D.; Harrison, E.F.; Ellender, S.J.; Fitzsimmons, D.; Londahl, M.; LeucoPatch, I.I.T.T. Leucopatch system for the management of hard-to-heal diabetic foot ulcers in the uk, denmark, and sweden: An observer-masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, A.A.; Aralova, M.V. The study of the effectiveness of the drug combination of collagen and platelet-rich plasma for the regional treatment of venous ulcers. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 2258–2263. [Google Scholar]
- Goda, A.A. Autogenous leucocyte-rich and platelet-rich fibrin for the treatment of leg venous ulcer: A randomized control study. Egypt. J. Surg. 2018, 37, 316–321. [Google Scholar] [CrossRef]
- Goda, A.A.; Metwally, M.; Ewada, A.; Ewees, H. Platelet-rich plasma for the treatment of diabetic foot ulcer: A randomized, double-blind study. Egypt. J. Surg. 2018, 37, 178–184. [Google Scholar] [CrossRef]
- Gude, W.; Hagan, D.; Abood, F.; Clausen, P. Aurix gel is an effective intervention for chronic diabetic foot ulcers: A pragmatic randomized controlled trial. Adv. Ski. Wound Care 2019, 32, 416–426. [Google Scholar] [CrossRef]
- Helmy, Y.; Farouk, N.; Dahy, A.A.; Abu-Elsoud, A.; Khattab, R.F.; Mohammed, S.E.; Gad, L.A.; Altramsy, A.; Hussein, E.; Farahat, A. Objective assessment of platelet-rich plasma (prp) potentiality in the treatment of chronic leg ulcer: Rct on 80 patients with venous ulcer. J. Cosmet. Dermatol. 2021, 20, 3257–3263. [Google Scholar] [CrossRef]
- Hongying, J.; Liang, Z.; Xi, Y.; Jing, H.; Xiaona, X.; Zhengyan, L.; Hongchen, H. Effect of platelet-rich plasma on pressure ulcers after spinal cord injury. Chin. J. Tissue Eng. Res. 2020, 25, 1149–1153. [Google Scholar]
- Karimi, R.; Afshar, M.; Salimian, M.; Sharif, A.; Hidariyan, M. The effect of platelet rich plasma dressing on healing diabetic foot ulcers. Nurs. Midwifery Stud. 2016, 5, e30314. [Google Scholar] [CrossRef]
- Khorvash, F.; Pourahmad, M.; Khoshchingol, N.; Avijgan, M.; Mohammadi, M.; Sahebnazar, K. Comparing the effects of the platelet-rich plasma gel with wound therapeutic methods on the treatment of diabetic foot. J. Isfahan Med. Sch. 2017, 35, 1389–1395. [Google Scholar]
- Kulkarni, S.R.; Chawla, A. Study of efficacy of platelet rich plasma dressing in management of chronic non-healing leg ulcers. J. Evol. Med. Dent. Sci. 2019, 8, 1307–1310. [Google Scholar]
- Li, L.; Chen, D.; Wang, C.; Yuan, N.; Wang, Y.; He, L.; Yang, Y.; Chen, L.; Liu, G.; Li, X.; et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015, 23, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Milek, T.; Nagraba, L.; Mitek, T.; Wozniak, W.; Mlosek, K.; Olszewski, W.; Ciostek, P.; Deszczynski, J.; Kuchar, E.; Stolarczyk, A. Autologous platelet-rich plasma reduces healing time of chronic venous leg ulcers: A prospective observational study. In Advances in Biomedicine; Pokorski, M., Ed.; Springer: Cham, Switzerland, 2019; Volume 1176, pp. 109–117. [Google Scholar]
- Mohammad, A.; Rohangiz, K.; Morteza, S.; Alireza, S.; Abolfazl, A. Comparison of platelet rich plasma and normal saline dressing effectiveness in the improvement of diabetic foot ulcers. J. Diabet. Nurs. 2017, 5, 246–255. [Google Scholar]
- Moneib, H.A.; Youssef, S.S.; Aly, D.G.; Rizk, M.A.; Abdelhakeem, Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Dermatol. 2018, 17, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Obolenskiy, V.N.; Ermolova, D.A.; Laberko, L.A. Clinical and economic effectiveness of the use of platelet-rich plasma in the treatment of chronic wounds. Wound Med. 2017, 19, 27–32. [Google Scholar] [CrossRef]
- Obolenskiy, V.N.; Ermolova, D.A.; Laberko, L.A.; Semenova, T.V. Efficacy of platelet-rich plasma for the treatment of chronic wounds. EWMA J. 2014, 14, 37–41. [Google Scholar]
- Pires, B.; de Oliveira, B.G.R.B.; Bokehi, L.C.; Luiz, R.R.; Carvalho, B.T.F.; Santana, R.F.; de Souza, P.A.; de Paula, G.R.; Teixeira, L.A. Clinical and microbiological outcomes associated with use of platelet-rich plasma in chronic venous leg uclers: A randomized controlled trial. J. Wound Ostomy Cont. Nurs. Off. Publ. Wound Ostomy Cont. Nurses Soc. 2021, 48, 292–299. [Google Scholar] [CrossRef]
- Pu, D.; Lei, X.; Leng, W.; Zheng, Y.; Chen, L.; Liang, Z.; Chen, B.; Wu, Q. Lower limb arterial intervention or autologous platelet-rich gel treatment of diabetic lower extremity arterial disease patients with foot ulcers. Ann. Transl. Med. 2019, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, J. Clinical study of local injection of autologous platelet-rich plasma in treatment of diabetic foot ulcer. Chin. J. Reparative Reconstr. Surg. 2019, 33, 1547–1551. [Google Scholar]
- Rainys, D.; Cepas, A.; Dambrauskaite, K.; Nedzelskiene, I.; Rimdeika, R. Effectiveness of autologous platelet-rich plasma gel in the treatment of hard-to-heal leg ulcers: A randomised control trial. J. Wound Care 2019, 28, 658–667. [Google Scholar] [CrossRef]
- Ramos-Torrecillas, J.; García-Martínez, O.; de Luna-Bertos, E.; Ocaña-Peinado, F.M.; Ruiz, C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol. Res. Nurs. 2015, 17, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Saad Setta, H.; Elshahat, A.; Elsherbiny, K.; Massoud, K.; Safe, I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int. Wound J. 2011, 8, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Patra, A.C.; Gowda, S.P.; Mondal, N.; Rahaman, S.; Ahmed, S.K.S.; Debbarma, S.; Vitthal, K.P.K.; Sarkar, S.; Sil, A.; et al. Effectiveness and safety of autologous platelet-rich plasma therapy with total contact casting versus total contact casting alone in treatment of trophic ulcer in leprosy: An observer-blind, randomized controlled trial. Indian J. Derm. Venereol. Leprol. 2020, 86, 262–271. [Google Scholar]
- Semenič, D.; Cirman, T.; Rožman, P.; Smrke, D.M. Regeneration of chronic wounds with allogeneic platelet gel versus hydrogel treatment: A prospective study. Acta Clin. Croat. 2018, 57, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Borah, D.; Khanna, G.; Jain, S. Efficacy of local autologous platelet-rich plasma in the treatment of pressure ulcer in spinal cord injury patients. Cureus 2021, 13, e18668. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, V.; Pandey, A.; Pandey, P.; Gupta, V.; Verma, R. Role of platelet-rich plasma in healing diabetic foot ulcers: A prospective study. J. Wound Care 2018, 27, 550–556. [Google Scholar] [CrossRef]
- Sokolov, T.; Manukova, A.; Karakoleva, S.; Valentinov, B.; Petrova, N. Analysis of the results of applying the method platelet-rich plasma (prp) for the treatment of problematic skin wounds. J. IMAB 2017, 23, 1460–1465. [Google Scholar] [CrossRef]
- Somani, A.; Rai, R. Comparison of efficacy of autologous platelet-rich fibrin versus saline dressing in chronic venous leg ulcers: A randomised controlled trial. J. Cutan. Aesthetic Surg. 2017, 10, 8–12. [Google Scholar]
- Tsachiridi, M.; Galyfos, G.; Andreou, A.; Sianou, A.; Sigala, F.; Zografos, G.; Filis, K. Autologous platelet-rich plasma for nonhealing ulcers: A comparative study. Vasc. Spec. Int. 2019, 35, 22–27. [Google Scholar] [CrossRef]
- Tsai, H.C.; Lehman, C.W.; Chen, C.M. Use of platelet-rich plasma and platelet-derived patches to treat chronic wounds. J. Wound Care 2019, 28, 15–21. [Google Scholar] [CrossRef]
- Ucar, O.; Celik, S. Comparison of platelet-rich plasma gel in the care of the pressure ulcers with the dressing with serum physiology in terms of healing process and dressing costs. Int. Wound J. 2020, 17, 831–841. [Google Scholar] [CrossRef]
- Yang, L.; Gao, L.; Lv, Y.; Wang, J. Autologous platelet-rich gel for lower-extremity ischemic ulcers in patients with type 2 diabetes. Int. J. Clin. Exp. Med. 2017, 10, 13796–13801. [Google Scholar]
- Yuvasri, G.; Rai, R. Comparison of efficacy of autologous platelet-rich fibrin versus unna’s paste dressing in chronic venous leg ulcers: A comparative study. Indian Dermatol. Online J. 2020, 11, 58–61. [Google Scholar] [PubMed]
- Kakagia, D.D.; Kazakos, K.J.; Xarchas, K.C.; Karanikas, M.; Georgiadis, G.S.; Tripsiannis, G.; Manolas, C. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J. Diabetes Its Complicat. 2007, 21, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Senet, P.; Bon, F.-X.; Benbunan, M.; Bussel, A.; Traineau, R.; Calvo, F.; Dubertret, L.; Dosquet, C. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J. Vasc. Surg. 2003, 38, 1342–1348. [Google Scholar] [CrossRef]
- Baranovskiy, Y.G.; Ilchenko, F.N.; Shapovalova, E.Y.; Kaliberdenko, V.B.; Shanmugaraj, K.; Keerthanaa, B. Influence of autologous platelet concentrates on the dynamics of regenerative processes in treatment of trophic ulcers of lower extremities. Indian J. Public Health Res. Dev. 2019, 10, 1850–1855. [Google Scholar]
- Capoano, R.; Businaro, R.; Tesori, M.C.; Donello, C.; Lombardo, F.; Vasco, V.R.L.; Capriotti, L.; Corsi, M.; Raimo, T.D.; Leopizzi, M.; et al. Wounds difficult to heal: An effective treatment strategy. Curr. Vasc. Pharmacol. 2017, 15, 582–588. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Wang, Y.; He, L.P.; Yang, Y.Z.; Chen, L.H.; Chen, D.W.; Li, X.J.; Ran, X.W. Impact of topical application of autologous platelet-rich gel on medical expenditure and length of stay in hospitals in diabetic patients with refractory cutaneous ulcers. J. Sichuan Univ. (Med. Sci. Ed.) 2012, 43, 762–765. [Google Scholar]
- Liu, G.Y.; Deng, X.L.; Sun, Y.; Wang, M.Z.; Gao, J.; Gou, J. Effect of autologous platelet-rich gel on the treatment of diabetic foot ulcers. J. Xi’an Jiaotong Univ. (Med. Sci.) 2016, 37, 264–267. [Google Scholar]
- Madhumitha, M.; Srinivasan, S. A study of efficacy of autologous platelet rich plasma in the treatment of chronic diabetic foot ulcers. Int. J. Pharm. Res. 2019, 11, 190–196. [Google Scholar]
- Martí, X.; Linares, P.; Bonell, A.; Acosta, M.; Llort, C.; Lapiedra, O. Growth factors used in healing venous ulcers. Chirurgia 2008, 21, 17–20. [Google Scholar]
- Slaninka, I.; Klein, L.; Čáp, R.; Hošek, F.; Guňka, I.; Šedivý, O.; Jiška, S.; Kaška, M. Optimizing the treatment procedure in crural ulcers—A pilot study of the surgical method. Rozhl. Chir. 2015, 94, 69–73. [Google Scholar] [PubMed]
- Smagin, M.A.; Shumkov, O.A.; Soluianov, M.I.; Demura, A.U.; Smagin, A.A.; Lykov, A.P.; Nimaev, V.V. Treatment of torpid trophic ulcers in patients of the older age group. Adv. Gerontol. 2020, 33, 373–378. [Google Scholar] [PubMed]
- Wang, L.; Liu, G.; Li, Z.; Jia, B.C.; Wang, Y. Clinical application of platelet-rich fibrin in chronic wounds combined with subcutaneous stalking sinus. Chin. J. Burn. 2018, 34, 637–642. [Google Scholar]
- Gupta, A.; Channaveera, C.; Sethi, S.; Ranga, S.; Anand, V. Efficacy of intralesional platelet-rich plasma in diabetic foot ulcer. J. Am. Podiatr. Med. Assoc. 2021, 111, 7. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The academia europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
| First Author, Year of Publication | Type of Publication | Study Type | Country | Ulcer Etiology | Outcome |
|---|---|---|---|---|---|
| Abd El-Mabood, 2018 [25] | Journal article | RCT | Egypt | Diabetic | Complete closure, healing rate, infection, and pain |
| Ahmed, 2017 [26] | Journal article | RCT | Egypt | Diabetic | Complete closure, healing rate, and infection |
| Alamdari, 2021 [27] | Journal article | RCT | Iran | Diabetic | Healing time, and amputation |
| Amato, 2020 [28] | Journal article | RCT | Italy | Mixed | Reduction of wound area, complete closure, infection, and pain |
| Anitua, 2008 [29] | Journal article | RCT | Spain | Mixed | Reduction of wound area and infection |
| Burgos-Alonso, 2018 [30] | Journal article | RCT | Spain | Venous | Reduction of wound area, complete closure, infection, pain, adverse events, and quality of life |
| Cardenosa, 2017 [31] | Journal article | RCT | Spain | Venous | Reduction of wound area, pain, and adverse events |
| Chandanwale, 2020 [32] | Journal article | RCT | India | Arterial | Reduction of wound area |
| de Oliveira, 2017 [33] | Journal article | RCT | Brazil | Venous | Reduction of wound area and infection |
| Driver, 2006 [34] | Journal article | RCT | US | Diabetic | Reduction of wound area, healing rate, complete closure, healing time, and adverse events |
| Elbarbary, 2020 [35] | Journal article | RCT | India | Venous | Reduction of wound area, complete closure, healing time, and recurrence |
| Elgarhy, 2020 [36] | Journal article | RCT | India | Venous | Reduction of wound area, complete closure, and healing time |
| Elsaid, 2020 [37] | Journal article | RCT | Egypt | Diabetic | Reduction of wound area, complete closure, and healing time |
| Game, 2018 [38] | Journal article | RCT | UK | Diabetic | Reduction of wound area, complete closure, healing time, infection, pain, amputation, and adverse events |
| Glukhov, 2017 [39] | Journal article | RCT | Russia | Venous | Complete closure, and pain |
| Goda, 2018 1 [41] | Journal article | RCT | Egypt | Diabetic | Healing rate, and complete closure |
| Goda, 2018 2 [40] | Journal article | RCT | Egypt | Venous | Reduction of wound area, and complete closure |
| Gude, 2019 [42] | Journal article | RCT | US | Diabetic | Complete closure, and amputation |
| Helmy, 2021 [43] | Journal article | RCT | Egypt | Venous | Reduction of wound area, complete closure, healing time, pain, adverse events, and recurrence |
| Hongying, 2020 [44] | Journal article | RCT | China | Pressure | Reduction of wound area, and complete closure |
| Kakagia, 2007 [71] | Journal article | RCT | Greece | Diabetic | Reduction of wound area, and complete closure |
| Karimi, 2016 [45] | Journal article | RCT | Iran | Diabetic | Reduction of wound area, complete closure, and amputation |
| Khorvash, 2017 [46] | Journal article | RCT | Iran | Diabetic | Reduction of wound area, infection, pain, and quality of life |
| Kulkarni, 2019 [47] | Journal article | RCT | India | N/A | Reduction of wound area, healing time, and adverse events |
| Li, 2015 [48] | Journal article | RCT | China | Diabetic | Reduction of wound area, complete closure, healing time, infection, amputation, and adverse events |
| Milek, 2019 [49] | Journal article | RCT | Poland | Venous | Reduction of wound area and complete closure |
| Mohammad, 2017 [50] | Journal article | RCT | Iran | Diabetic | Reduction of wound area |
| Moneib, 2018 [51] | Journal article | RCT | Egypt | Venous | Reduction of wound area, complete closure, pain, and adverse events |
| Obolenskiy, 2014 [53] | Journal article | RCT | Russia | Mixed | Complete closure and healing time |
| Obolenskiy, 2017 [52] | Journal article | RCT | Russia | Mixed | Healing rate, complete closure, and healing time |
| Pires, 2021 [54] | Journal article | RCT | Brazil | Venous | Infection |
| Pu, 2019 [55] | Journal article | RCT | China | Arterial | Reduction of wound area, healing rate, and amputation |
| Qin, 2019 [56] | Journal article | RCT | China | Diabetic | Reduction of wound area |
| Rainys, 2019 [57] | Journal article | RCT | Lithuania | N/A | Reduction of wound area, complete closure, infection, and adverse events |
| Ramos-Torrecilla, 2015 [58] | Journal article | RCT | Spain | Pressure | Reduction of wound area, complete closure, and infection |
| Saad Setta, 2011 [59] | Journal article | RCT | Egypt | Diabetic | Complete closure and healing time |
| Saha, 2020 [60] | Journal article | RCT | India | Leprosy | Reduction of wound area, complete closure, and pain |
| Semenic, 2018 [61] | Journal article | RCT | Slovenia | Mixed | Reduction of wound area and adverse events |
| Senet, 2003 [72] | Journal article | RCT | France | Venous | Reduction of wound area, healing rate, complete closure, infection, and adverse events |
| Singh, 2018 [63] | Journal article | RCT | India | Diabetic | Complete closure, healing time, amputation, and adverse events |
| Singh, 2021 [62] | Journal article | RCT | India | Pressure | Reduction of wound area |
| Sokolov, 2017 [64] | Journal article | RCT | Bulgaria | Not defined | Complete closure |
| Somani, 2017 [65] | Journal article | RCT | India | Venous | Reduction of wound area and complete closure |
| Tsachiridi, 2019 [66] | Journal article | RCT | Greece | Pressure | Reduction of wound area and healing rate |
| Tsai, 2019 [67] | Journal article | RCT | US | Mixed | Reduction of wound area |
| Ucar, 2020 [68] | Journal article | RCT | Turkey | Pressure | Reduction of wound area |
| Yang, 2017 [69] | Journal article | RCT | China | Diabetic | Healing rate, healing time, infection, pain, and adverse events |
| Yuvasri, 2020 [70] | Journal article | RCT | India | Venous | Reduction of wound area and complete closure |
| First Author, Year of Publication | Main Conclusion |
|---|---|
| Healing Time | |
| Alamdari, 2021 [27] | Shorter healing time in the PRP group than in the control group |
| Driver, 2006 [34] | Shorter healing time in the PRP group than in the control group |
| Elbarbary, 2020 [35] | Shorter healing time in the PRP group than in the control group * |
| Elgarhy, 2020 [36] | Shorter healing time in the topical and injected PRP groups than in the control group * |
| Elsaid, 2020 [37] | Shorter healing time in the PRP group than in the control group * |
| Game, 2018 [38] | Shorter healing time in the PRP group than in the control group * |
| Helmy, 2021 [43] | Shorter healing time in the PRP group than in the control group * |
| Kulkarni, 2019 [47] | Shorter healing time in the PRP group than in the control group * |
| Li, 2015 [48] | Shorter healing time in the PRP group than in the control group * |
| Obolenskiy, 2014 [53] | Shorter healing time in the PRP group than in the control group |
| Obolenskiy, 2017 [52] | Shorter healing time in the PRP group than in the control group * |
| Saad Setta, 2011 [59] | Shorter healing time in the PRP group than in the control group * |
| Singh, 2018 [63] | Shorter healing time in the PRP group than in the control group * |
| Yang, 2017 [69] | Shorter healing time in the PRP group than in the control group * |
| Infection Rates | |
| Abd El-Mabood, 2018 [25] | More infection in the control group than in the PRP group * |
| Ahmed, 2017 [26] | More infection in the control group than in the PRP group * |
| Amato, 2020 [28] | More infection in the control group than in the PRP group * |
| Anitua, 2008 [29] | No statistically significant difference between the PRP and the control groups |
| Burgos-Alonso, 2018 [30] | No statistically significant difference between the PRP and the control groups |
| de Oliveira, 2017 [33] | No statistically significant difference between the PRP and the control groups |
| Game, 2018 [38] | No statistically significant difference between the PRP and the control groups |
| Khorvash, 2017 [46] | No statistically significant difference between the PRP and the control groups |
| Li, 2015 [48] | No statistically significant difference between the PRP and the control groups |
| Pires, 2021 [54] | No statistically significant differences in antimicrobial resistance between P. aeruginosa and S. aureus in the PRP and control groups. PRP decreased bacteriological growth or the microbial load and resistance profile in the case of P. aeruginosa |
| Rainys, 2019 [57] | No statistically significant difference between the PRP and the control groups |
| Ramos-Torrecilla, 2015 [58] | No signs of infection were recorded during the study |
| Senet, 2003 [72] | No statistically significant difference between the PRP and the control groups |
| Yang, 2017 [69] | More infection in the control group than in the PRP group * |
| Pain | |
| Abd El-Mabood, 2018 [25] | Pain occurred more frequently in the control group * |
| Amato, 2020 [28] | Pain occurred more frequently in the control group * |
| Burgos-Alonso, 2018 [30] | No statistically significant difference in pain reduction between the PRP and the control groups |
| Cardenosa, 2017 [31] | Pain reduction was higher in the PRP group * |
| Game, 2018 [38] | No statistically significant difference in pain reduction between the PRP and the control groups |
| Glukhov, 2017 [39] | All patients subjectively experienced pain reduction in both groups |
| Helmy, 2021 [43] | All patients subjectively experienced pain reduction in the PRP group |
| Khorvash, 2017 [46] | pain reduction was higher in the PRP group * |
| Moneib, 2018 [51] | All patients subjectively experienced pain reduction in both groups |
| Saha, 2020 [60] | Administration-related pain was reported by 10 participants in the PRP group |
| Yang, 2017 [69] | pain reduction was higher in the PRP group * |
| Amputation Rates | |
| Alamdari, 2021 [27] | No statistically significant difference between the PRP and the control groups |
| Game, 2018 [38] | No statistically significant difference between the PRP and the control group |
| Gude, 2019 [42] | Two amputations in the control group and no amputation in the PRP group |
| Karimi, 2016 [45] | No statistically significant difference between the PRP and the control groups |
| Li, 2015 [48] | Four amputations in the control group one amputation in the PRP group |
| Pu, 2019 [55] | No statistically significant difference between the PRP and the control groups |
| Singh, 2018 [63] | Two amputations in the control group, and no amputation in the PRP group |
| Adverse Events | |
| Burgos-Alonso, 2018 [30] | No statistically significant difference between the PRP and the control groups |
| Cardenosa, 2017 [31] | No adverse events recorded |
| Chandanwale, 2020 [32] | No adverse event in the PRP group |
| Driver, 2006 [34] | No administration related serious adverse event was recorded in either group; one case of Contact dermatitis in the PRP group and one case of maceration in the control group |
| Game, 2018 [38] | No statistically significant difference between the PRP and the control groups |
| Helmy, 2021 [43] | No adverse events recorded |
| Kulkarni, 2019 [47] | No adverse event in the PRP group |
| Li, 2015 [48] | No adverse events were recorded in the PRP group |
| Moneib, 2018 [51] | No adverse events recorded |
| Rainys, 2019 [57] | No statistically significant difference between the PRP and the control groups, and no serious adverse event was recorded |
| Semenic, 2018 [61] | No adverse events recorded |
| Senet, 2003 [72] | No statistically significant difference between the PRP and the control groups |
| Singh, 2018 [63] | No adverse events recorded |
| Yang, 2017 [69] | No statistically significant difference between the PRP and the control groups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meznerics, F.A.; Fehérvári, P.; Dembrovszky, F.; Kovács, K.D.; Kemény, L.V.; Csupor, D.; Hegyi, P.; Bánvölgyi, A. Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2022, 11, 7532. https://doi.org/10.3390/jcm11247532
Meznerics FA, Fehérvári P, Dembrovszky F, Kovács KD, Kemény LV, Csupor D, Hegyi P, Bánvölgyi A. Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Medicine. 2022; 11(24):7532. https://doi.org/10.3390/jcm11247532
Chicago/Turabian StyleMeznerics, Fanni Adél, Péter Fehérvári, Fanni Dembrovszky, Kata Dorottya Kovács, Lajos Vince Kemény, Dezső Csupor, Péter Hegyi, and András Bánvölgyi. 2022. "Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Journal of Clinical Medicine 11, no. 24: 7532. https://doi.org/10.3390/jcm11247532
APA StyleMeznerics, F. A., Fehérvári, P., Dembrovszky, F., Kovács, K. D., Kemény, L. V., Csupor, D., Hegyi, P., & Bánvölgyi, A. (2022). Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Clinical Medicine, 11(24), 7532. https://doi.org/10.3390/jcm11247532






