Dose Optimization of Meropenem in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Cardiac Patients: Pharmacokinetic/Pharmacodynamic Modeling
Abstract
1. Introduction
2. Methods
Ethical Aspects
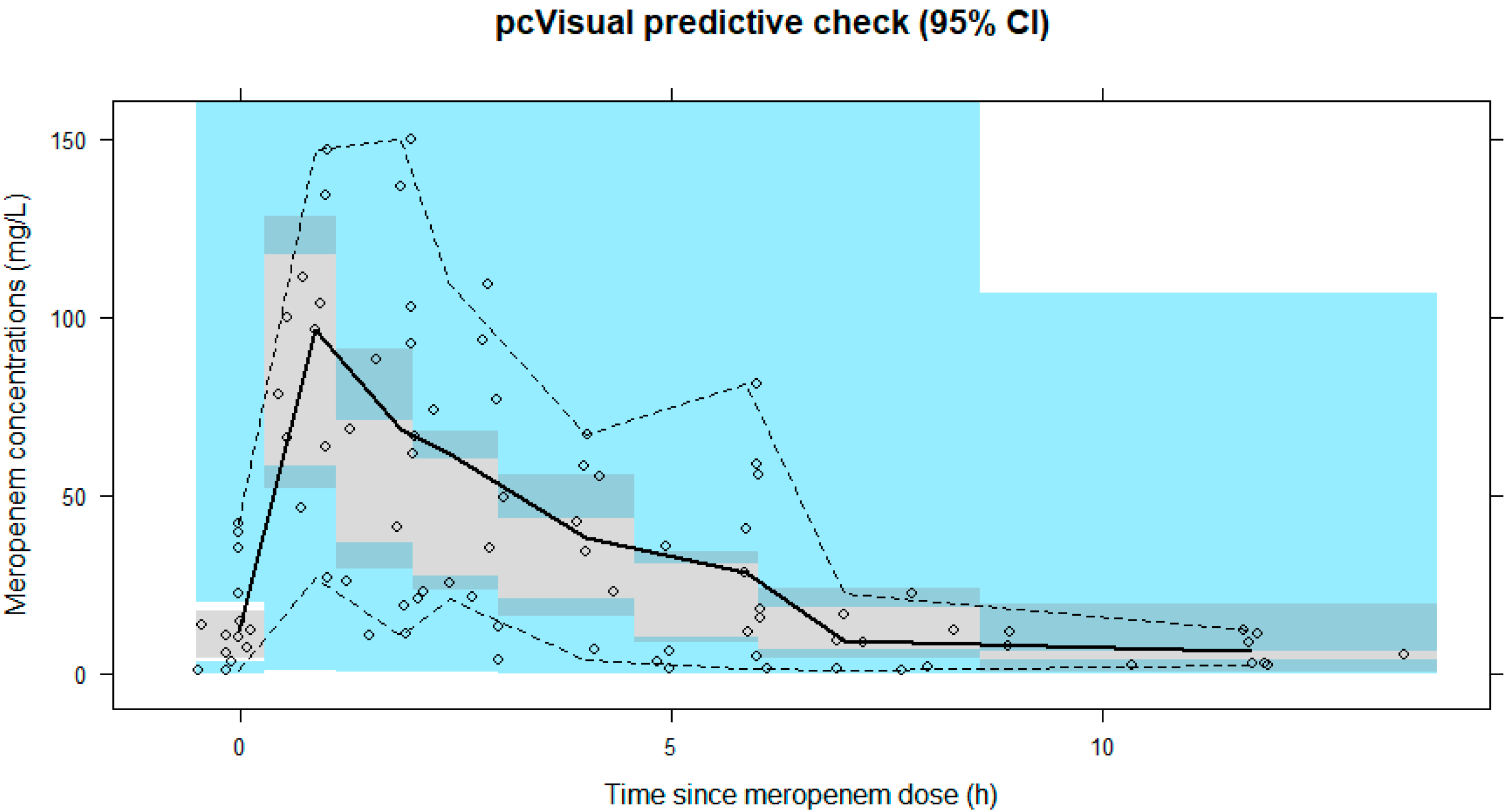
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engström, A.E.; Lagrand, W.K.; Cherpanath, T.G.V.; Driessen, A.H.G.; de Mol, B.; Henriques, J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Loforte, A.; Marinelli, G.; Musumeci, F.; Folesani, G.; Pilato, E.; Martin Suarez, S.; Montalto, A.; Lilla Della Monica, P.; Grigioni, F.; Frascaroli, G.; et al. Extracorporeal membrane oxygenation support in refractory cardiogenic shock: Treatment strategies and analysis of risk factors. Artif. Organs 2014, 38, E129–E141. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, S.; Ko, H.H.; Ha, S.O.; Lee, S.H.; Kim, Y.K. Different characteristics of bloodstream infection during venoarterial and venovenous extracorporeal membrane oxygenation in adult patients. Sci. Rep. 2021, 11, 9498. [Google Scholar] [CrossRef] [PubMed]
- Aubron, C.; Cheng, A.C.; Pilcher, D.; Leong, T.; Magrin, G.; Cooper, D.J.; Scheinkestel, C.; Pellegrino, V. Infections acquired by adults who receive extracorporeal membrane oxygenation: Risk factors and outcome. Infect. Control. Hosp. Epidemiol. 2013, 34, 24–30. [Google Scholar] [CrossRef]
- Sherwin, J.; Heath, T.; Watt, K. Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin. Ther. 2016, 38, 1976–1994. [Google Scholar] [CrossRef]
- Hahn, J.; Choi, J.H.; Chang, M.J. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J. Clin. Pharm. Ther. 2017, 42, 661–671. [Google Scholar] [CrossRef]
- Ha, M.A.; Sieg, A.C. Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation. Pharmacotherapy 2017, 37, 221–235. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; McDonald, C.I.; Ghassabian, S.; Anstey, C.; Wallis, S.C.; Mullany, D.V.; Fung, Y.L.; Fraser, J.F. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: Results from an ex vivo study. Crit. Care 2015, 19, 164. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; McDonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T.; et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194. [Google Scholar] [CrossRef]
- Mousavi, S.; Levcovich, B.; Mojtahedzadeh, M. A systematic review on pharmacokinetic changes in critically ill patients: Role of extracorporeal membrane oxygenation. DARU 2011, 19, 312–321. [Google Scholar]
- Thiele, H.; Ohman, E.M.; de Waha-Thiele, S.; Zeymer, U.; Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 2019, 40, 2671–2683. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.H.; Roberts, J.A.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S629–S641. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Roberts, J.A. Antibiotic dosing during extracorporeal membrane oxygenation: Does the system matter? Curr. Opin. Anaesthesiol. 2020, 33, 71–82. [Google Scholar] [CrossRef]
- Hahn, J.; Min, K.L.; Kang, S.; Yang, S.; Park, M.S.; Wi, J.; Chang, M.J. Population Pharmacokinetics and Dosing Optimization of Piperacillin-Tazobactam in Critically Ill Patients on Extracorporeal Membrane Oxygenation and the Influence of Concomitant Renal Replacement Therapy. Microbiol. Spectr. 2021, 9, e0063321. [Google Scholar] [CrossRef]
- Hanberg, P.; Öbrink-Hansen, K.; Thorsted, A.; Bue, M.; Tøttrup, M.; Friberg, L.E.; Hardlei, T.F.; Søballe, K.; Gjedsted, J. Population Pharmacokinetics of Meropenem in Plasma and Subcutis from Patients on Extracorporeal Membrane Oxygenation Treatment. Antimicrob. Agents Chemother. 2018, 62, e02390-17. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Taccone, F.S.; Welch, S.; Wallis, S.C.; Mullany, D.V.; Lipman, J.; Roberts, J.A. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: A matched cohort study. Crit. Care 2014, 18, 565. [Google Scholar] [CrossRef]
- Post, T.M.; Freijer, J.I.; Ploeger, B.A.; Danhof, M. Extensions to the visual predictive check to facilitate model performance evaluation. J. Pharmacokinet. Pharmacodyn. 2008, 35, 185–202. [Google Scholar] [CrossRef]
- Dosne, A.G.; Bergstrand, M.; Karlsson, M.O. An automated sampling importance resampling procedure for estimating parameter uncertainty. J. Pharmacokinet. Pharmacodyn. 2017, 44, 509–520. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741.e9–741.e18. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Antonucci, E.; Cristallini, S.; Roberts, J.A.; Beumier, M.; Scolletta, S.; Jacobs, F.; Rondelet, B.; de Backer, D.; Vincent, J.L.; et al. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int. J. Antimicrob. Agents 2015, 45, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kois, A.K.; Gluck, J.A.; Nicolau, D.P.; Kuti, J.L. Pharmacokinetics and Time above the MIC Exposure of Cefepime in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation (ECMO). Int. J. Antimicrob. Agents 2022, 60, 106603. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jang, J.Y.; Hahn, J.; Kim, D.; Lee, J.Y.; Min, K.L.; Yang, S.; Wi, J.; Chang, M.J. Dose Optimization of Cefpirome Based on Population Pharmacokinetics and Target Attainment during Extracorporeal Membrane Oxygenation. Antimicrob. Agents Chemother. 2020, 64, e00249-20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Abdul-Aziz, M.H.; Burrows, F.; Buscher, H.; Cho, Y.J.; Corley, A.; Gilder, E.; Kim, H.S.; Lim, S.Y.; McGuinness, S.; et al. Population Pharmacokinetics and Dosing Simulations of Ceftriaxone in Critically Ill Patients Receiving Extracorporeal Membrane Oxygenation (An ASAP ECMO Study). Clin. Pharmacokinet. 2022, 61, 847–856. [Google Scholar] [CrossRef]
- Craig, W.A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 1995, 22, 89–96. [Google Scholar] [CrossRef]
- Nielsen, E.I.; Cars, O.; Friberg, L.E. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: A step toward model-based dose optimization. Antimicrob. Agents Chemother. 2011, 55, 4619–4630. [Google Scholar] [CrossRef]
- Onufrak, N.J.; Forrest, A.; Gonzalez, D. Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing. Clin. Ther. 2016, 38, 1930–1947. [Google Scholar] [CrossRef]
- Kristoffersson, A.N.; David-Pierson, P.; Parrott, N.J.; Kuhlmann, O.; Lave, T.; Friberg, L.E.; Nielsen, E.I. Simulation-Based Evaluation of PK/PD Indices for Meropenem Across Patient Groups and Experimental Designs. Pharm. Res. 2016, 33, 1115–1125. [Google Scholar] [CrossRef]
- Drusano, G.L. Prevention of resistance: A goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 2003, 36, S42–S50. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Huttner, A.; Harbarth, S.; Hope, W.W.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of the β-lactam antibiotics: What is the evidence and which patients should we be using it for? J. Antimicrob. Chemother. 2015, 70, 3178–3183. [Google Scholar] [CrossRef]
- Roberts, J.A.; Ulldemolins, M.; Roberts, M.S.; McWhinney, B.; Ungerer, J.; Paterson, D.L.; Lipman, J. Therapeutic drug monitoring of beta-lactams in critically ill patients: Proof of concept. Int. J. Antimicrob. Agents 2010, 36, 332–339. [Google Scholar] [CrossRef]
- Mouton, J.W.; Vinks, A.A. Continuous infusion of beta-lactams. Curr. Opin. Crit. Care 2007, 13, 598–606. [Google Scholar] [CrossRef]
- Mohd Hafiz, A.A.; Staatz, C.E.; Kirkpatrick, C.M.; Lipman, J.; Roberts, J.A. Continuous infusion vs. bolus dosing: Implications for beta-lactam antibiotics. Minerva Anestesiol. 2012, 78, 94–104. [Google Scholar]
- Bauer, K.A.; West, J.E.; O’Brien, J.M.; Goff, D.A. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob. Agents Chemother. 2013, 57, 2907–2912. [Google Scholar] [CrossRef]
- Kuti, J.L.; Nightingale, C.H.; Knauft, R.F.; Nicolau, D.P. Pharmacokinetic properties and stability of continuous-infusion meropenem in adults with cystic fibrosis. Clin. Ther. 2004, 26, 493–501. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kirkpatrick, C.M.; Roberts, M.S.; Robertson, T.A.; Dalley, A.J.; Lipman, J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: Intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J. Antimicrob. Chemother. 2009, 64, 142–150. [Google Scholar] [CrossRef]
- Berthoin, K.; Le Duff, C.S.; Marchand-Brynaert, J.; Carryn, S.; Tulkens, P.M. Stability of meropenem and doripenem solutions for administration by continuous infusion. J. Antimicrob. Chemother. 2010, 65, 1073–1075. [Google Scholar] [CrossRef]
- Patel, P.R.; Cook, S.E. Stability of meropenem in intravenous solutions. Am. J. Health Syst. Pharm. 1997, 54, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Viaene, E.; Chanteux, H.; Servais, H.; Mingeot-Leclercq, M.P.; Tulkens, P.M. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 2002, 46, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Manigaba, K.; Borgert, S.J.; Cope, J.; Peloquin, C.A.; Klinker, K.P. Training a Drug to Do New Tricks: Insights on Stability of Meropenem Administered as a Continuous Infusion. Microbiol. Insights 2018, 11, 1178636118804549. [Google Scholar] [CrossRef] [PubMed]
| ECMO | Patient No. * | Age Range (yr) | Sex | Wt (kg) | Ht (m) | Diagnosis | SCr (mg/dL) | CRRT | eGFR (mL/min/1.73 m2) | APACHE II Score | Length of Hospital Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| On | 1 | 45–49 | M | 74.6 | 1.73 | Acute MI | na # | yes | na # | 34 | 15 |
| 2 | 50–54 | M | 74.6 | 1.70 | Acute MI, | na # | yes | na # | 32 | 27 | |
| 3 | 50–54 | M | 82.9 | 1.68 | Acute MI | na # | yes | na # | 44 | 40 | |
| 4 | 55–59 | F | 69.9 | 1.64 | Acute MI | na # | yes | na # | 30 | 200 | |
| 5 | 70–74 | M | 93.3 | 1.70 | Acute MI | na # | yes | na # | 36 | 21 | |
| 6 | 50–59 | M | 53.1 | 1.68 | Acute MI | 1.06 | no | 76.5 | 29 | 36 | |
| Off | 4 * | 55–59 | F | 67.4 | 1.64 | 1.2 | no | 49.6 | 30 | 200 | |
| 6 * | 55–59 | M | 53.1 | 1.68 | 0.88 | no | 94.9 | 29 | 36 | ||
| 7 | 50–54 | F | 48.2 | 1.46 | Acute MI | na # | yes | na # | 37 | 75 | |
| 8 | 75–79 | M | 53.9 | 1.60 | Acute MI | na # | yes | na # | 40 | 75 | |
| 9 | 45–49 | M | 61.1 | 1.72 | Acute MI | 1.3 | no | 64.3 | 22 | 21 | |
| 10 | 55–59 | F | 60.0 | 1.62 | VF arrest | 0.5 | no | 127.3 | 30 | 29 | |
| 11 | 55–59 | M | 77.5 | 1.68 | Acute MI, VF arrest | 2.0 | no | 36.5 | 28 | 37 | |
| 12 | 50–54 | M | 63.0 | 1.62 | VF arrest | 0.7 | no | 120.4 | 26 | 36 | |
| 13 | 65–69 | M | 67.4 | 1.68 § | Acute MI | 1.3 | no | 60.3 | 14 | 23 | |
| 55 (53–58) | 67.4 (57–74.6) | 1.68 (1.63–1.70) | 1.2 (0.7–1.56) | 70.4 (57.6–101.3) | 30 (28.5–35) | 36 (25–57.5) |
| Parameter | Base Model | Final Model | |
|---|---|---|---|
| Population Estimate (RSE) | Population Estimate (RSE) | SIR Median (2.5th–97.5th Percentile) | |
| Fixed effects (θ) | |||
| CL (L/h) | 2.65 (32%) | 3.79 (26%) | 3.77 (2.69–5.37) |
| Central volume of distribution, V1 (L) | 2.53 (21%) | 2.4 (38%) | 2.76 (0.59–4.84) |
| Peripheral volume of distribution, V2 (L) | 9.61 (38%) | 8.56 (22%) | 8.36 (5.59–12.93) |
| Intercompartmental clearance, Q (L/h) | 20.8 (9%) | 21.3 (17%) | 19.94 (9.37–33.41) |
| θCRRT on CL | - | 0.44 (30%) | 0.45 (0.29–0.62) |
| Random effects (% CV) | |||
| Interindividual variability (ω2) | |||
| CL | 69.4 (36%) | 47.1 (49%) | 49.2 (32.2–74.2) |
| V2 | 61 (103%) | 44 (154%) | 51.1 (7.7–108) |
| Residual unexplained variability (σ2) | 49.7 (18%) | 47.3 (21%) | 49.0 (40.9–60.2) |
| Target | Normal Therapy (40% fT > MIC) | More Aggressive Therapy (100% fT > MIC) | ||
|---|---|---|---|---|
| For Susceptible Pathogens (MIC = 2 mg/L) | For Resistant Pathogens (MIC = 8 mg/L) | For Susceptible Pathogens (MIC = 2 mg/L) | For resistant Pathogens (MIC = 8 mg/L) | |
| without CRRT | 1–2 g q8h II 0.5 g q8h EIs or CI | 1–2 g q8h II 0.5 g q8h EIs or CI | 1–2 g q8h EIs or CI | 2 g q8h EI over 6 h or CI |
| with CRRT | 1 g q12h II 0.5 g q8h II 0.5 g q8h EIs or CI | 1 g q12h II 0.5 g q8h II 0.5 g q8h EIs or CI | 1 g q12h II 0.5 g q8h II 0.5 g q8h EIs or CI | 1 g q8h EIs 0.5–1 g q8h CI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Yang, S.; Hahn, J.; Jang, J.Y.; Min, K.L.; Wi, J.; Chang, M.J. Dose Optimization of Meropenem in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Cardiac Patients: Pharmacokinetic/Pharmacodynamic Modeling. J. Clin. Med. 2022, 11, 6621. https://doi.org/10.3390/jcm11226621
Kang S, Yang S, Hahn J, Jang JY, Min KL, Wi J, Chang MJ. Dose Optimization of Meropenem in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Cardiac Patients: Pharmacokinetic/Pharmacodynamic Modeling. Journal of Clinical Medicine. 2022; 11(22):6621. https://doi.org/10.3390/jcm11226621
Chicago/Turabian StyleKang, Soyoung, Seungwon Yang, Jongsung Hahn, June Young Jang, Kyoung Lok Min, Jin Wi, and Min Jung Chang. 2022. "Dose Optimization of Meropenem in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Cardiac Patients: Pharmacokinetic/Pharmacodynamic Modeling" Journal of Clinical Medicine 11, no. 22: 6621. https://doi.org/10.3390/jcm11226621
APA StyleKang, S., Yang, S., Hahn, J., Jang, J. Y., Min, K. L., Wi, J., & Chang, M. J. (2022). Dose Optimization of Meropenem in Patients on Veno-Arterial Extracorporeal Membrane Oxygenation in Critically Ill Cardiac Patients: Pharmacokinetic/Pharmacodynamic Modeling. Journal of Clinical Medicine, 11(22), 6621. https://doi.org/10.3390/jcm11226621






