Advances in Vitreoretinal Surgery
Abstract
:1. Introduction
2. Development
2.1. Advances in Management of Rhegmatogenous Retinal Detachments
2.1.1. Primary Vitrectomy
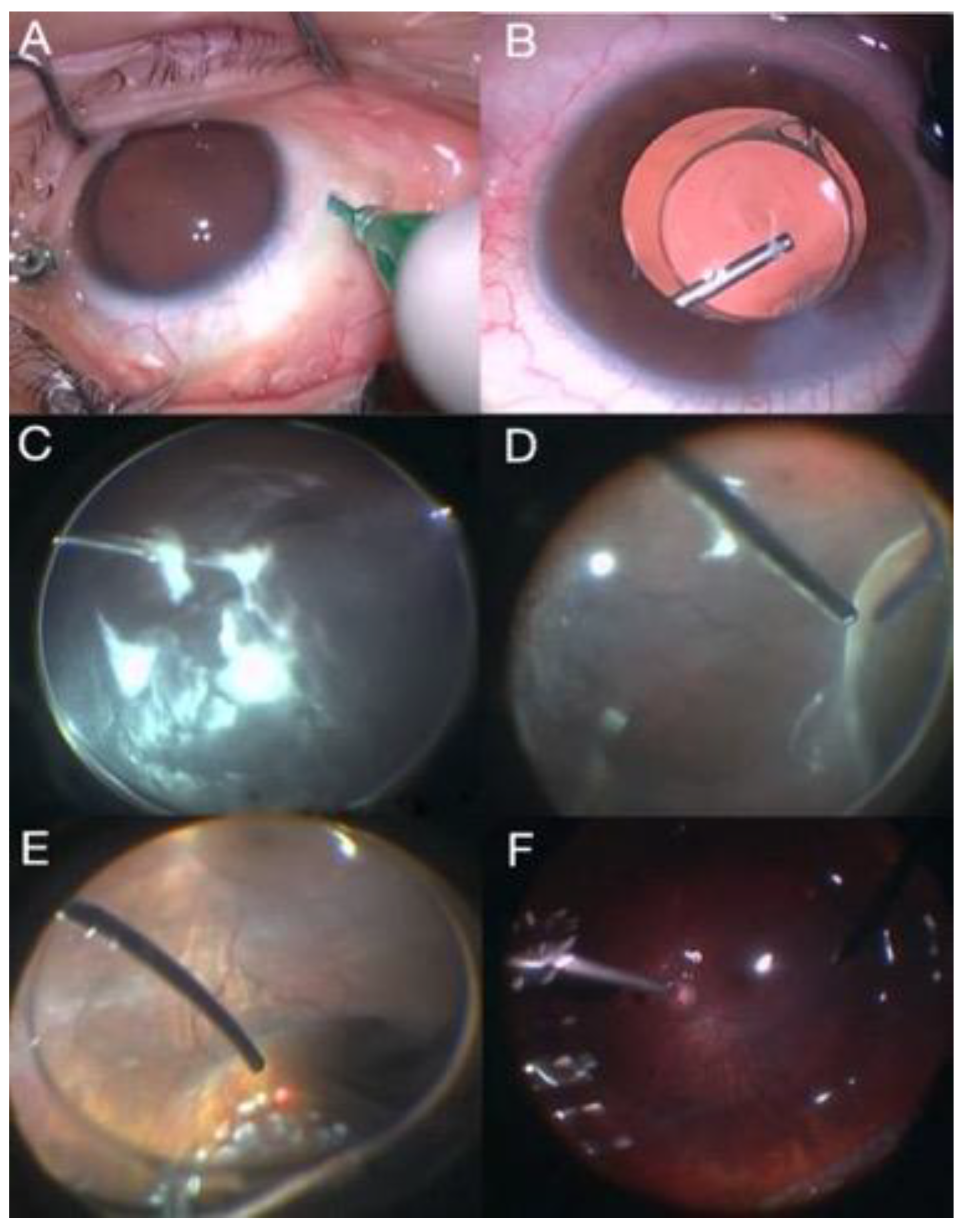
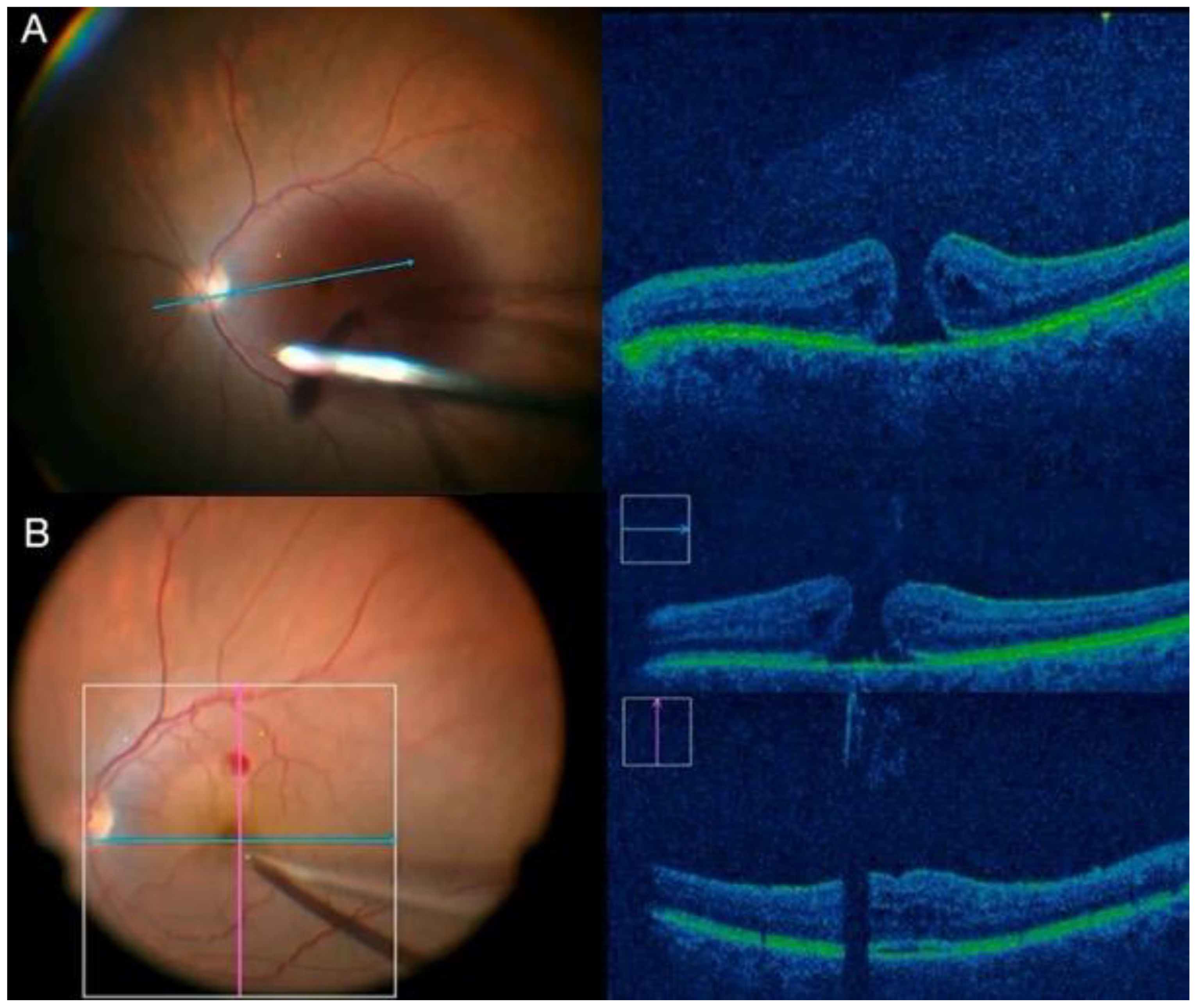
2.1.2. Scleral Buckling Using the Chandelier Illumination Guided by Non-Contact Visualization Systems
2.2. Sclerotomy/Valved Trocar Diameters
2.3. Posterior Vitrectomy Systems and Ergonomic Vitrectomy Probes
2.4. Chromovitrectomy
2.5. Vitreous Substitutes
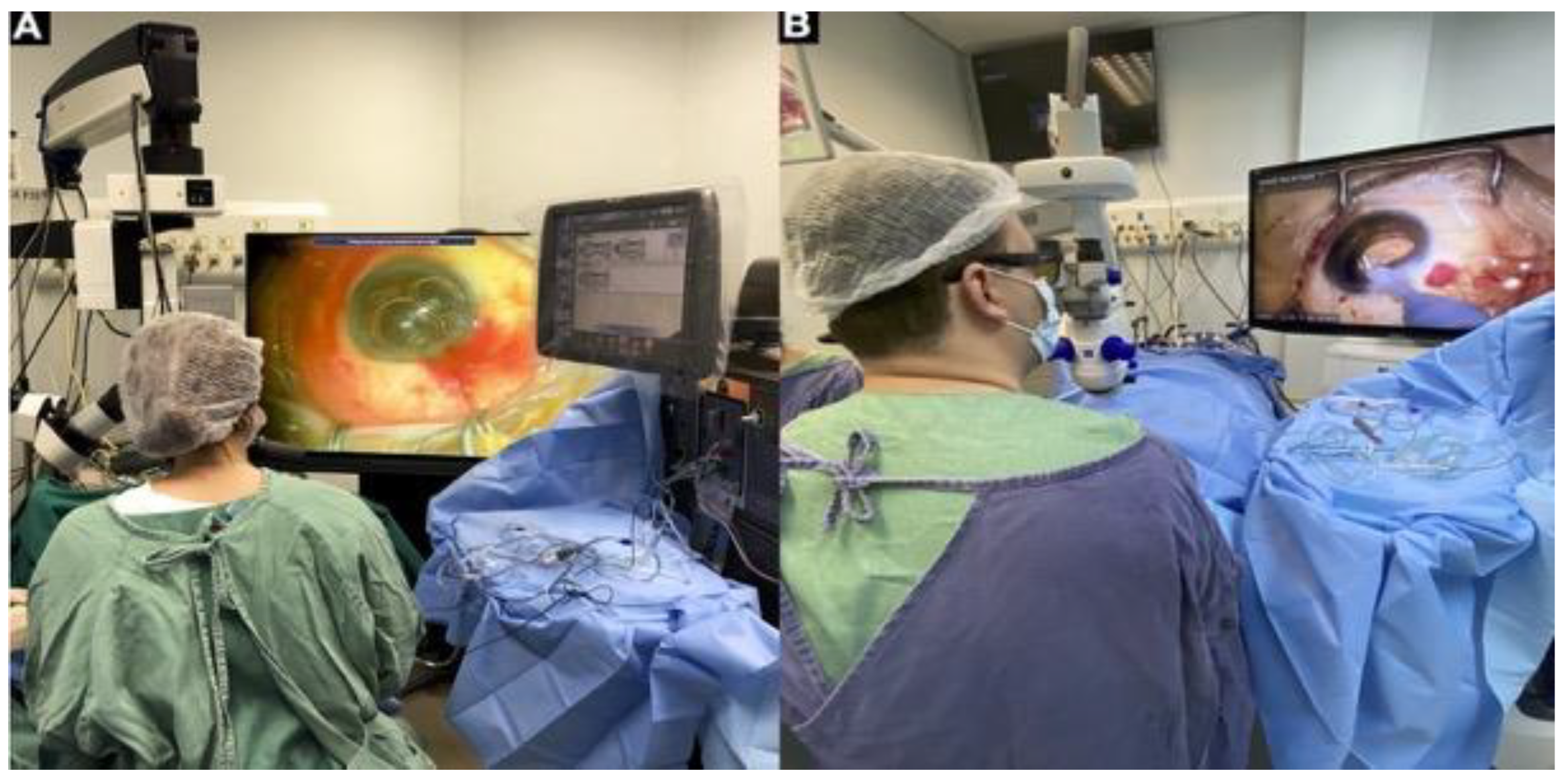
2.6. Intraoperative Visualization Systems including 3D Technology
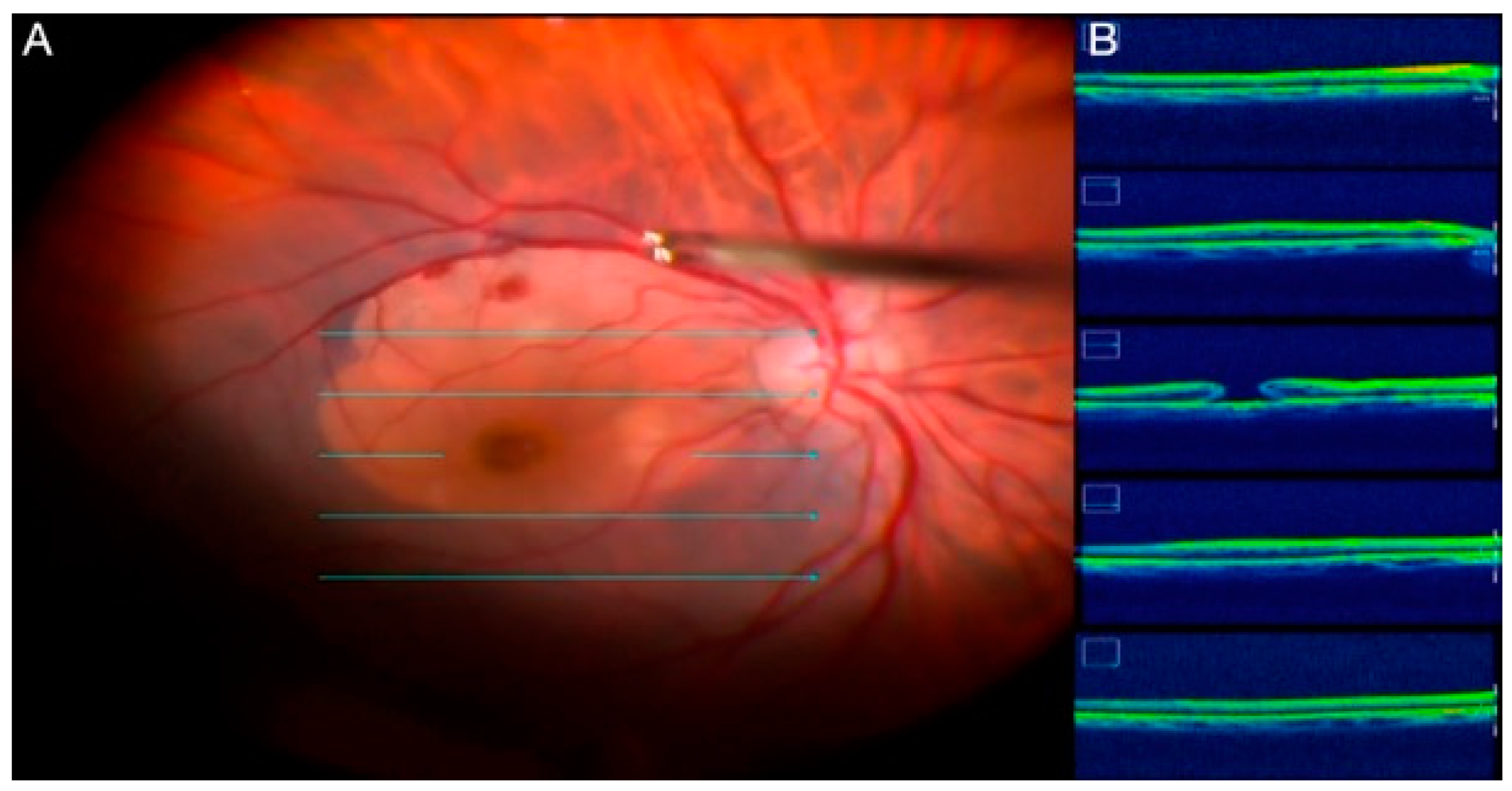
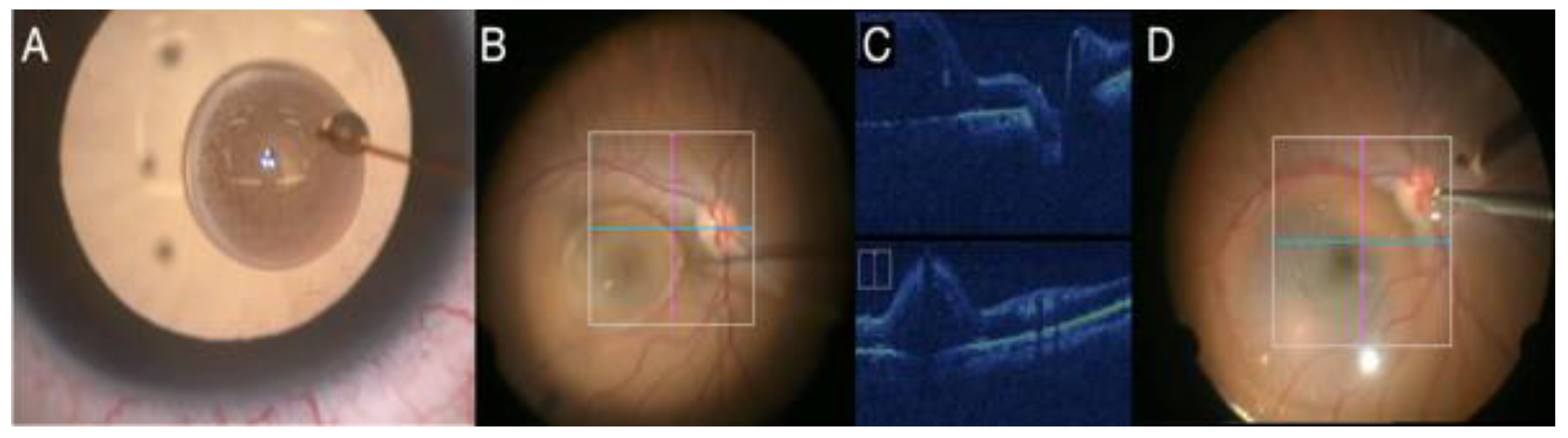
2.7. Intraoperative OCT
2.8. New Instrumentation in Vitreoretinal Surgery
2.9. Anti-VEGF Injection before PPV in Eyes with PDR and New Surgical Techniques
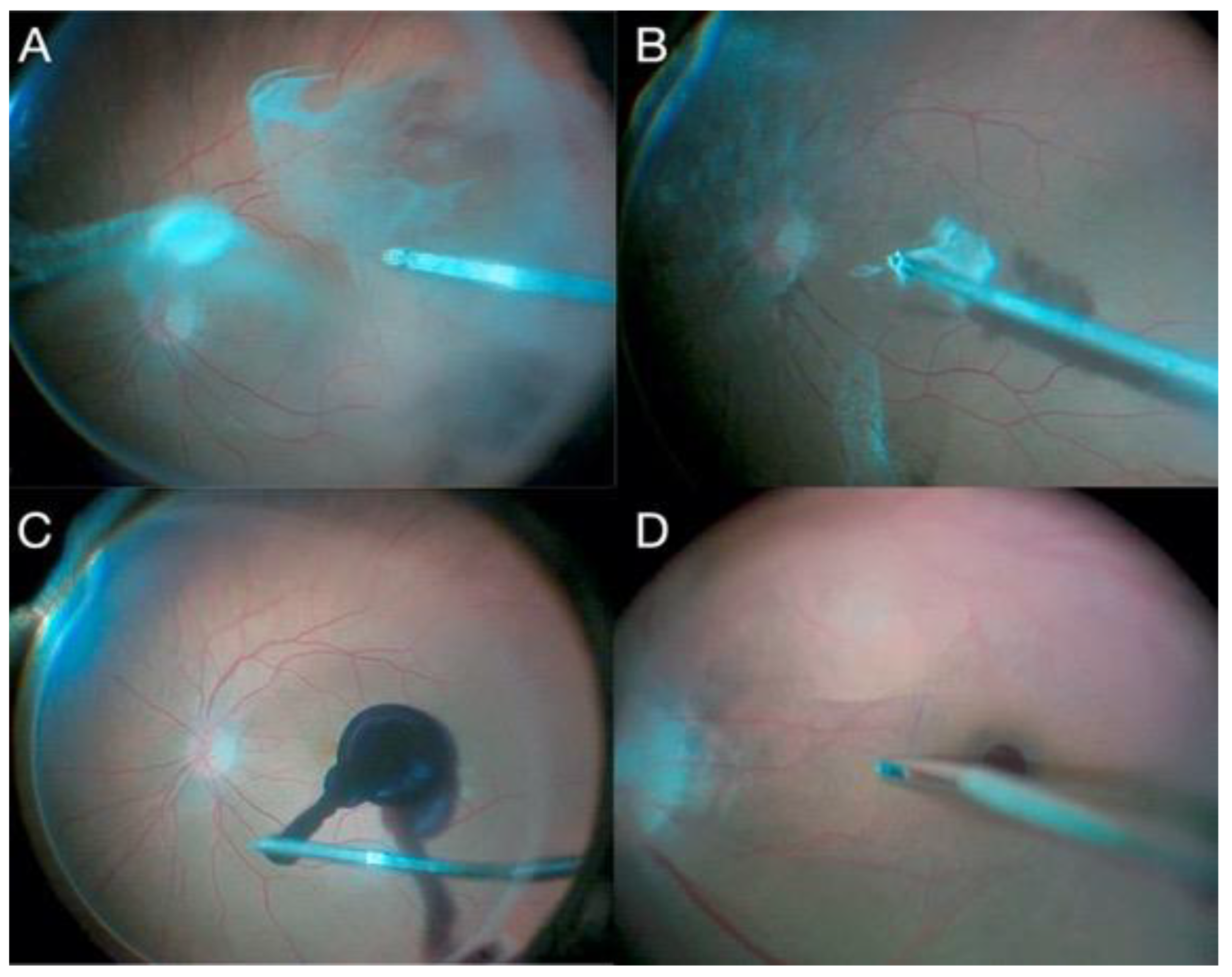
2.10. Endoscopic Surgery
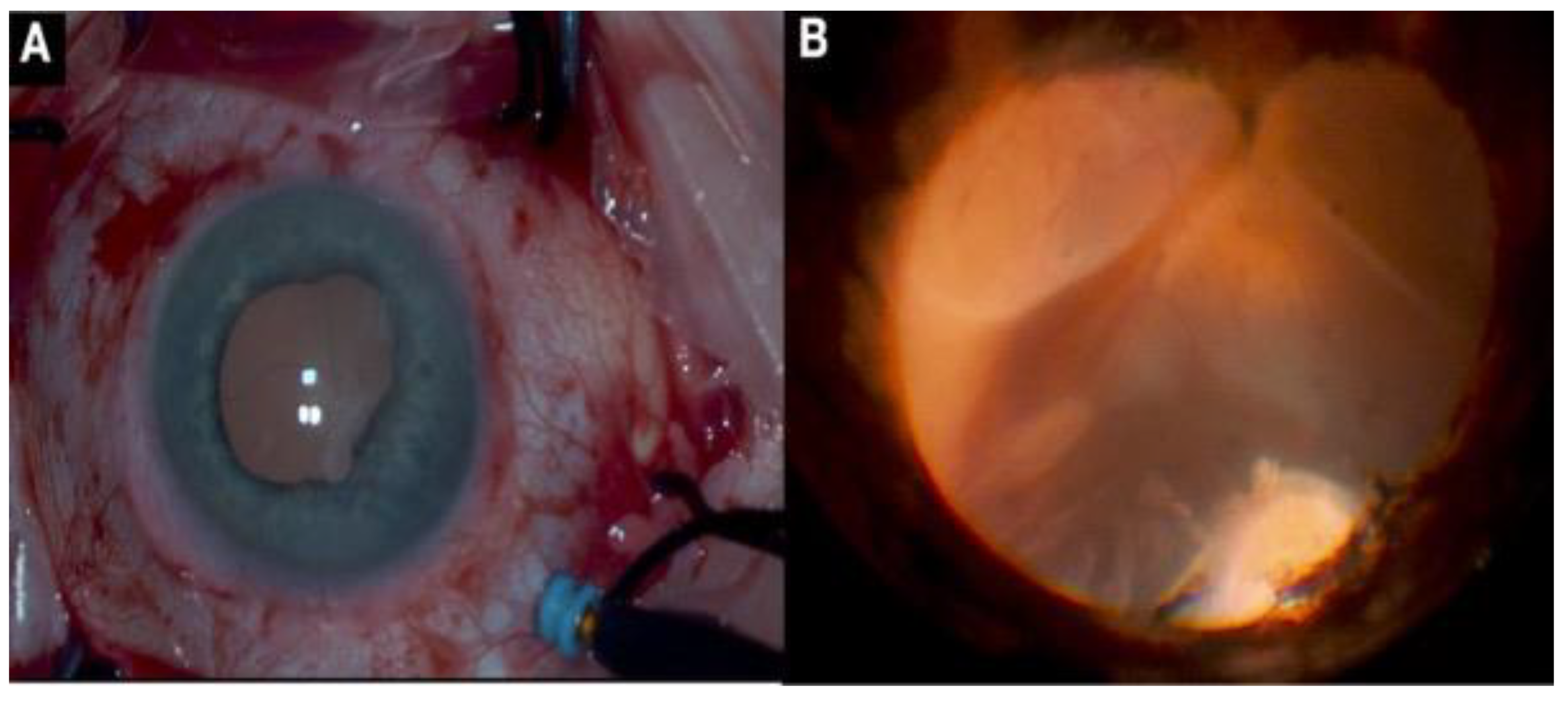
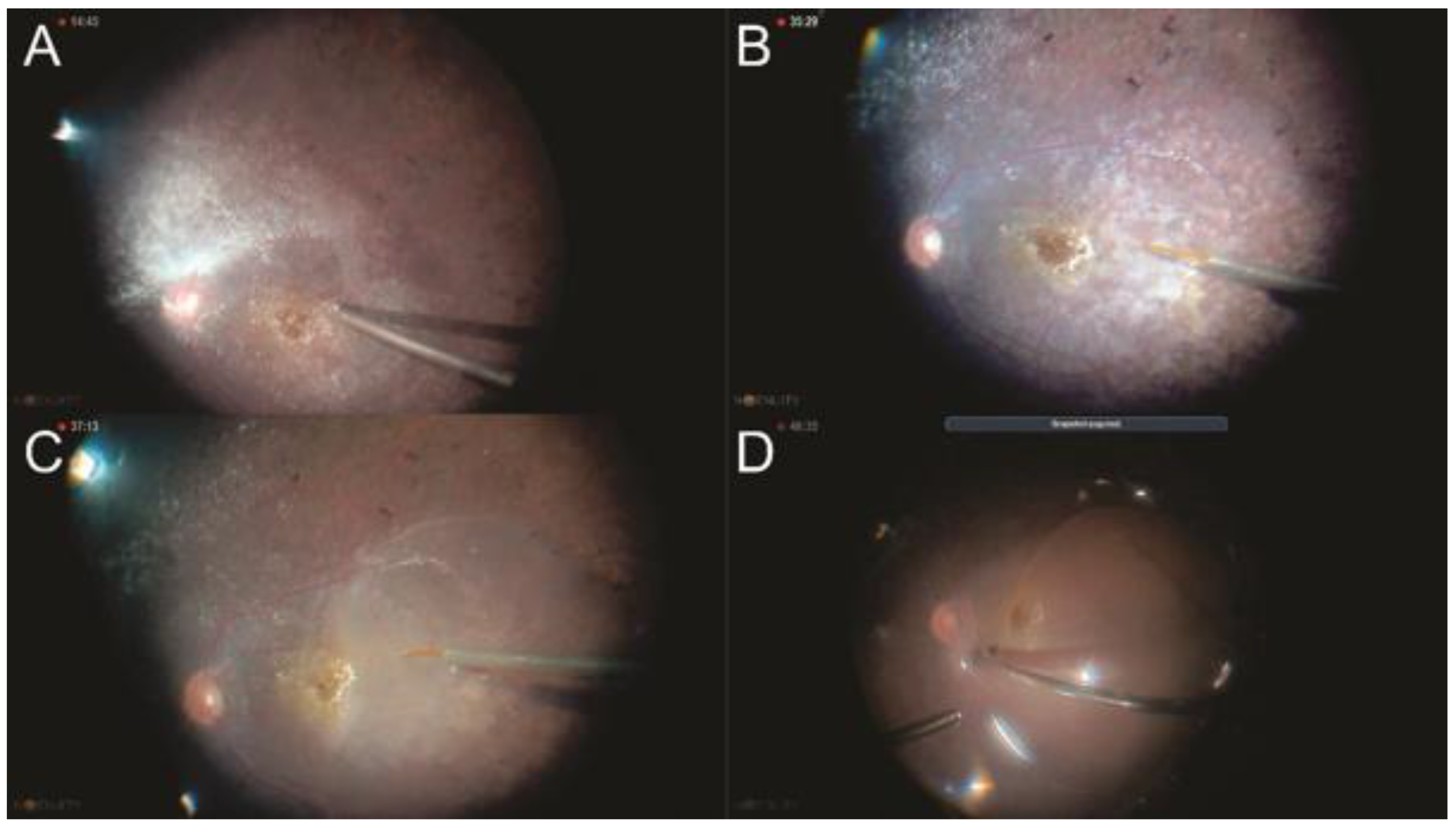
2.11. Management of Subretinal Hemorrhages
2.12. Gene Therapy Using Viral Vectors
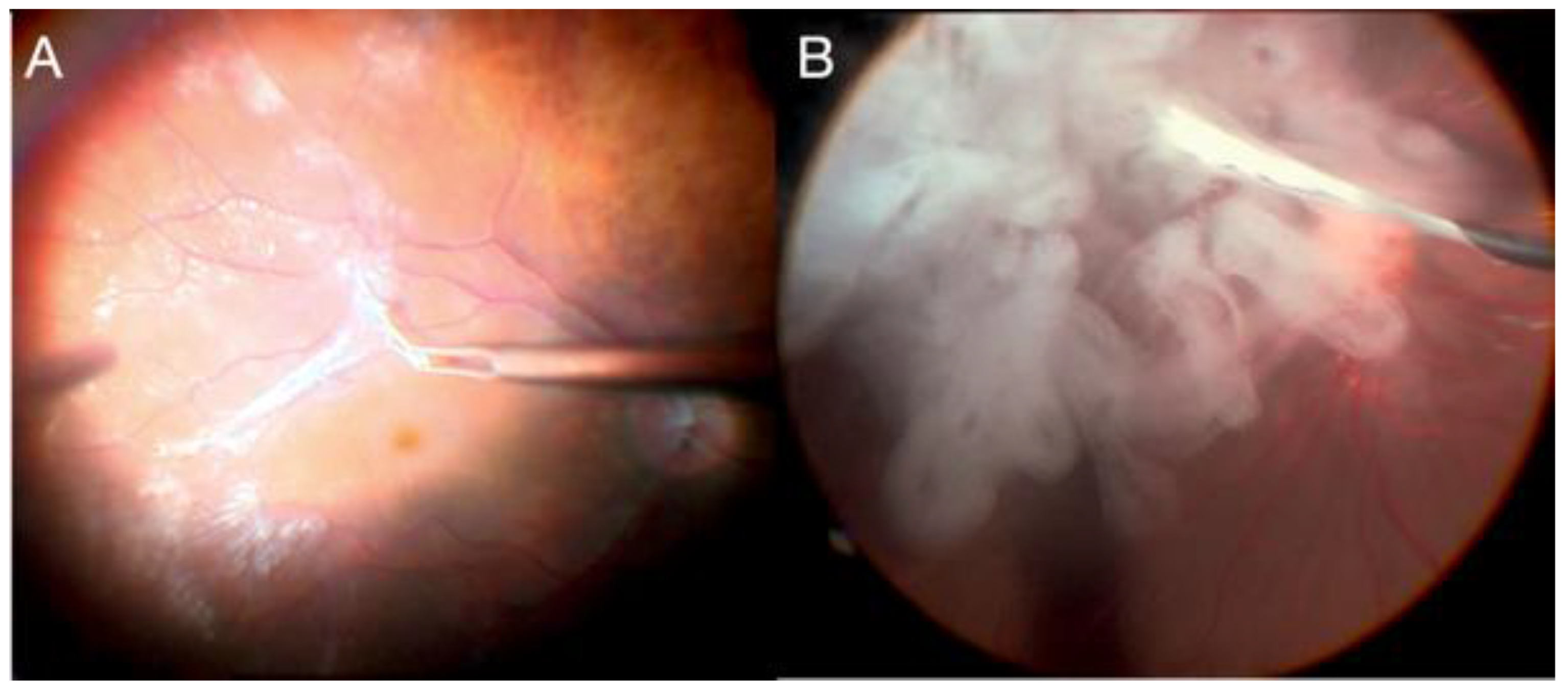
2.13. Alternative Techniques for Refractory Macular Holes
2.14. Perspectives for Stem Cell Therapy, RPE Transplantation, and Prevention of PVR
2.15. Port Delivery System
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, G.W.; Topping, T. An Improved Method for Practice Vitrectomy. Arch. Ophthalmol. 1978, 96, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Abulon, D. Vitreous flow rates of 27 gauge dual-cutting 20,000 cpm vitrectomy probes. In Proceedings of the 22th Ever Congress, Nice, France, 17–19 October 2019. [Google Scholar]
- Süsskind, D.; Neuhann, I.; Hilgers, R.D.; Hagemann, U.; Szurman, P.; Bartz-Schmidt, K.U.; Aisenbrey, S. Primary Vitrectomy for Rhegmatogenous Retinal Detachment in Pseudophakic Eyes: 20-Gauge Versus 25-Gauge Vitrectomy. Acta Ophthalmol. 2016, 94, 824–828. [Google Scholar] [CrossRef]
- Heimann, H.; Bartz-Schmidt, K.U. Scleral Buckling Versus Primary Vitrectomy in Rhegmatogenous Retinal Detachment: A Prospective Randomized Multicenter Clinical Study. Ophthalmology 2007, 114, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.X.; Du, Y.; Liu, W.; Huang, S.Y.; Zhang, S.C. Using surgical microscope for sclera buckling and transscleral cryopexy: An alternative procedure of treatment for rhegmatogenous retinal detachment. Biomed. Res. Int. 2014, 2014, 364961. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liang, X.; Sun, D.; Peng, S. The scleral buckling of primary rhegmatogenous retinal detachment under the surgical microscope. Ophthalmic Surg. Lasers Imaging 2011, 42, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Kim, W.J. Scleral Buckling Technique Using A 25-Gauge Chandelier Endoilluminator. Retina 2013, 33, 880–882. [Google Scholar] [CrossRef]
- Caporossi, T.; Finocchio, L. Scleral Buckling for Primary Rhegmatogenous Retinal Detachment Using a Noncontact Wide-angle Viewing System With a Cannula-Based 27-G Chandelier Endoilluminator. Retina 2019, 39 (Suppl. 1), S144–S150. [Google Scholar] [CrossRef]
- Nam, K.Y.; Lim, H.B. Twenty-seven-gauge endoilluminator-assisted scleral buckling using a wide-field viewing system: Case report. Medicine 2021, 100, e27206. [Google Scholar] [CrossRef]
- Aras, C.; Ucar, D. Scleral Buckling with A NonContact WideAngle Viewing System. Ophthalmologica 2012, 227, 107110. [Google Scholar] [CrossRef]
- Seider, M.I.; Nomides, R.E. Scleral Buckling with Chandelier Illumination. J. Ophthalmic Vis. Res. 2016, 11, 304–309. [Google Scholar] [CrossRef]
- Gogia, V.; Venkatesh, P. Endoilluminator-Assisted Scleral Buckling: Our Results. Indian J. Ophthalmol. 2014, 62, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Fujii, Y. Scleral Buckling with A Noncontact WideAngle Viewing System in the Management of Retinal Detachment with Undetected Retinal Break: A Case Report. Clin. Ophthalmol. 2013, 7, 587589. [Google Scholar]
- Oshima, Y.; Wakabayashi, T. A 27-Gauge Instrument System for Transconjunctival Sutureless Microincision Vitrectomy Surgery. Ophthalmology 2010, 117, 93–102.e2. [Google Scholar] [CrossRef] [PubMed]
- Juan, E.D.; Machemer, R. Surgery for Stage 5 Retinopathy of Prematurity. Arch. Ophthalmol. 1987, 105, 21. [Google Scholar]
- Stalmans, P. A Comparative Study Of 23-Gauge And 27-Gauge Vitrectomy for Puckers or Floaters, Including Evaluation of the Effect of Combined Phaco-Vitrectomy Surgery on Postoperative Outcome. Ophthalmologica 2021, 244, 245–249. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.M. Outcomes of Transconjunctival Sutureless 27-Gauge Vitrectomy For Vitreoretinal Diseases. Int. J. Ophthalmol. 2018, 11, 408–415. [Google Scholar]
- Abulon, D. The impact of vitrectomy probe design on pulsatile motion during aspiration. In Proceedings of the 19th Euretina Congress, Paris, France, 5–8 September 2019. [Google Scholar]
- Abulon, D.; Gariepy, H. 27 Gauge vitreous traction comparison: Dual-cutting vs. single-cutting vitrectomy probes. In Proceedings of the 34th Congress of Asia-Pacific Academy of Ophthalmology, Bangkok, Thailand, 6–9 March 2019. [Google Scholar]
- Bergamo, V.C.; Caiado, R.R. Role of Vital Dyes in Chromovitrectomy. Asia Pac. J. Ophthalmol. 2021, 10, 26–38. [Google Scholar] [CrossRef]
- Caiado, R.R.; de Moraes-Filho, M.N. State of the Art in Chromovitrectomy. Ver. Bras. Oftalmol. 2014, 73, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Burk, S.E.; Mata, A.P.D. Indocyanine Green-Assisted Peeling of the Retinal Internal Limiting Membrane. Ophthalmology 2000, 107, 2010–2014. [Google Scholar] [CrossRef]
- Rodrigues, E.B.; Meyer, C.H. Intravitreal Staining of the Internal Limiting Membrane Using Indocyanine Green in the Treatment of Macular Holes. Ophthalmologica 2005, 219, 251–262. [Google Scholar] [CrossRef]
- Penha, F.M.; Maia, M. Morphologic and Clinical Effects of Subretinal Injection of Indocyanine Green and Infracyanine Green in Rabbits. J. Ocul. Pharmacol. Ther. 2008, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nakashizuka, H. Double Staining with Brilliant Blue G and Double Peeling for Epiretinal Membranes. Ophthalmology 2009, 116, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Enaida, H.; Hisatomi, T. Preclinical Investigation of Internal Limiting Membrane Staining and Peeling Using Intravitreal Brilliant Blue G. Retina 2006, 26, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.B.; Costa, E.F. The Use of Vital Dyes in Ocular Surgery. Surv. Ophthalmol. 2009, 54, 576–617. [Google Scholar]
- Rodrigues, E.B.; Maia, M. Vital Dyes for Chromovitrectomy. Curr. Opin. Ophthalmol. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Soiberman, U.; Shai, D. Macular Hole Surgery with Internal Limiting Membrane Peeling Facilitated by Membrane-Blue® versus Membrane-Blue-Dual®: A Retrospective Comparative Study. J. Ophthalmol. 2016, 2016, 1292735. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Kreissig, I. Intravitreal Triamcinolone Acetonide for Treatment of Intraocular Proliferative, Exudative, and Neovascular Diseases. Prog. Retin. Eye Res. 2005, 24, 587–611. [Google Scholar]
- Landers, M.B., III; Stefánsson, E.; Wolbarsht, M.L. The Optics of Vitreous Surgery. Am. J. Ophthalmol. 1981, 91, 611–614. [Google Scholar] [CrossRef]
- Maia, M.; Furlani, B.A. Lutein: A New Dye for Chromovitrectomy. Retina 2014, 34, 262–272. [Google Scholar]
- Caiado, R.R.; Peris, C.S. Retinal Toxicity of Açaí Fruit (Euterpe Oleracea) Dye Concentrations in Rabbits: Basic Principles of a New Dye for Chromovitrectomy in Humans. Curr. Eye Res. 2017, 42, 1185–1193. [Google Scholar] [CrossRef]
- Alovisi, C.; Panico, C.; de Sanctis, U.; Eandi, C.M. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery. J. Ophthalmol. 2017, 2017, 3172138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.S.; Oberstein, S.Y.; Mura, M.; Bijl, H.M. Air versus gas tamponade in retinal detachment surgery. Br. J. Ophthalmol. 2013, 97, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Towards an ideal biomaterial for vitreous replacement: Historical overview and future trends. Acta Biomater. 2011, 7, 921–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati, S.; Caprani, S.M.; Airaghi, G.; Vinciguerra, R.; Bartalena, L.; Testa, F.; Mariotti, C.; Porta, G.; Simonelli, F.; Azzolini, C. Vitreous substitutes: The present and the future. Biomed Res Int. 2014, 2014, 351804. [Google Scholar] [CrossRef]
- Kleinberg, T.T.; Tzekov, R.T.; Stein, L.; Ravi, N.; Kaushal, S. Vitreous substitutes: A comprehensive review. Surv. Ophthalmol. 2011, 56, 300–323. [Google Scholar] [CrossRef]
- Inoue, M.; Iriyama, A.; Kadonosono, K.; Tamaki, Y.; Yanagi, Y. Effects of perfluorocarbon liquids and silicone oil on human retinal pigment epithelial cells and retinal ganglion cells. Retina 2009, 29, 677–681. [Google Scholar] [CrossRef]
- Spitznas, M. A binocular indirect ophthalmomicroscope (BIOM) for non-contact wide-angle vitreous surgery. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 13–15. [Google Scholar] [CrossRef]
- Eckardt, C.; Paulo, E.B. Heads-up Surgery for Vitreoretinal Procedures: An Experimental and Clinical Study. Retina 2016, 36, 137–147. [Google Scholar] [CrossRef]
- Coppola, M.; Spina, C.L. Heads-Up 3D Vision System for Retinal Detachment Surgery. Int. J. Retin. Vitreous 2017, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Tang, W. Comparative Analysis of Three-Dimensional Heads-Up Vitrectomy and Traditional Microscopic Vitrectomy for Vitreoretinal Diseases. Curr. Eye Res. 2019, 44, 1080–1086. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerling, G.; Müller, M. Intraoperative 2-Dimensional Optical Coherence Tomography as a New Tool for Anterior Segment Surgery. Arch. Ophthalmol. 2005, 123, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Dayani, P.N.; Maldonado, R. Intraoperative Use of Handheld Spectral Domain Optical Coherence Tomography Imaging in Macular Surgery. Retina 2009, 29, 1457–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, J.P.; Dupps, W.J. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am. J. Ophthalmol. 2014, 158, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlers, J.P.; Goshe, J. Determination of Feasibility and Utility of Microscope-Integrated Optical Coherence Tomography During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015, 133, 1124–1132. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Uchida, A. Intraoperative Optical Coherence Tomography-Compatible Surgical Instruments for Real-Time Image-Guided Ophthalmic Surgery. Br. J. Ophthalmol. 2017, 101, 1306–1308. [Google Scholar] [CrossRef]
- Stalmans, P. Enhancing Visual Acuity. Dev. Ophthalmol. 2014, 54, 23–30. [Google Scholar] [CrossRef]
- Francisconi, C.L.M.; Chow, D.R.C. What’s New in vitreoretinal Instrumentation? New Designs, Cut Rates, and Port Migration Aim to Increase Efficiency. Retin. Phys. 2018, 15, 34–37. [Google Scholar]
- Stalmans, P.; Mijnsbrugge, J.V. Investigation of the Effectiveness of a New Portable ‘Cryopen’ Probe for Cryotherapy (CryoTreq®). Int. J. Retin. Vitreous 2021, 7, 28. [Google Scholar] [CrossRef]
- Oyakawa, R.T.; Schachat, A.P. Complications of Vitreous Surgery for Diabetic Retinopathy. (I. Postoperative Complications). Ophthalmology 1983, 90, 517e521. [Google Scholar] [CrossRef]
- Velazquez, J.C.; Aleman, I. Bevacizumab before Diabetic Vitrectomy: A Clinical Trial Assessing 3 Dosing Amounts. Ophthalmol. Retin. 2018, 2, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Iwata, E.; Ueno, S. Focal Macular Electroretinograms after Intravitreal Injections of Bevacizumab for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4185–4190. [Google Scholar] [CrossRef] [Green Version]
- Farahvash, M.S.; Majidi, A.R. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011, 31, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Park, C.H. Use of Intravitreal Bevacizumab as a Preoperative Adjunct for Tractional Retinal Detachment Repair in Severe Proliferative Diabetic Retinopathy. Retina 2006, 26, 699–700. [Google Scholar] [CrossRef] [PubMed]
- El-Sabagh, H.A.; Abdelghaffar, W. Preoperative Intravitreal Bevacizumab Use as an Adjuvant to Diabetic Vitrectomy: Histopathologic Findings and Clinical Implications. Ophthalmology 2011, 118, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Fukutomi, A. Anti-VEGF crunch syndrome in proliferative diabetic retinopathy: A review. Surv. Ophthalmol. 2021, 66, 926–932. [Google Scholar] [CrossRef]
- di Lauro, R.; Ruggiero, P.D. Intravitreal Bevacizumab for Surgical Treatment of Severe Proliferative Diabetic Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 785–791. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X. Comparative Analysis of the Effects of the Anti-VEGF Drug and Glucocorticoid by Injection before the End of Vitrectomy for Proliferative Diabetic Retinopathy. Evid. Based Complement. Altern. Med. 2021, 2021, 1285372. [Google Scholar] [CrossRef]
- Sabti, K.A.; Raizada, S. Endoscope-Assisted Pars Plana Vitrectomy in Severe Ocular Trauma. Br. J. Ophthalmol. 2012, 96, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Marra, K.V.; Yonekawa, Y. Indications and Techniques of Endoscope Assisted Vitrectomy. J. Ophthalmic Vis. Res. 2013, 8, 282–290. [Google Scholar]
- Ben-nun, J. Cornea Sparing by Endoscopically Guided Vitreoretinal Surgery. Ophthalmology 2001, 108, 1465–1470. [Google Scholar] [CrossRef]
- Ayyildiz, O.; Durukan, A.H. Comparison of Endoscopic-Assisted and Temporary Keratoprosthesis-Assisted Vitrectomy in Combat Ocular Trauma: Experience at a Tertiary Eye Center in Turkey. J. Int. Med. Res. 2018, 46, 2708–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, D.C.M.; Nagiel, A.; Yang, U.; Lee, T.C.; Wong, S.C. Endoscopy for pediatric retinal disease. Asia Pac. J. Ophthalmol. 2018, 7, 200–207. [Google Scholar]
- Bhisitku, R.B.; Winn, B.J. Neuroprotective Effect of Intravitreal Triamcinolone Acetonide Against Photoreceptor Apoptosis in a Rabbit Model of Subretinal Hemorrhage. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Hillenkamp, J.; Surguch, V. Management of Submacular Hemorrhage with Intravitreal Versus Subretinal Injection of Recombinant Tissue Plasminogen Activator. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kumar, J.B. Pneumatic Displacement of Submacular Hemorrhage with Subretinal Air and Tissue Plasminogen Activator: Initial United States Experience. Ophthalmol. Retin. 2018, 2, 180–186. [Google Scholar] [CrossRef]
- Garafalo, A.V.; Cideciyan, A.V. Progress in Treating Inherited Retinal Diseases: Early Subretinal Gene Therapy Clinical Trials and Candidates for Future Initiatives. Prog. Retin. Eye Res. 2020, 77, 100827. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J. Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Xue, K.; Jolly, J.K. Beneficial Effects on Vision in Patients Undergoing Retinal Gene Therapy for Choroideremia. Nat. Med. 2018, 24, 1507–1512. [Google Scholar] [CrossRef]
- McCannel, C.A.; Ensminger, J.L. Population Based Incidence of Macular Holes. Ophthalmology 2009, 116, 1366–3669. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Enkh-Amgalan, I. Results of Macular Hole Surgery: Evaluation Based on the International Vitreomacular Traction Study Classification. Retina 2018, 38, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Karahan, E. Comparative Analysis of Gliosis Induced by Covering of Macular Holes with a Patch of Retinal Autograft vs. Human Amniotic Membrane. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4392. [Google Scholar]
- Grewal, D.S.; Mahmoud, T.H. Autologous Neurosensory Retinal Free Flap for Closure of Refractory Myopic Macular Holes. JAMA Ophthalmol. 2016, 134, 229–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.N.; Mahmoud, T.H. Autologous neurosensory retinal transplantation: Bridging the gap. Retina 2021, 41, 2417–2423. [Google Scholar] [CrossRef]
- Frisina, R.; Gius, I. Refractory Full Thickness Macular Hole: Current Surgical Management. Eye 2022, 36, 1344–1354. [Google Scholar] [CrossRef]
- Dhamodaran, K.; Subramani, M. Ocular Stem Cells: A Status Update! Stem Cell Res. Ther. 2014, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Mead, B.; Berry, M. Stem Cell Treatment of Degenerative Eye Disease. Stem Cell Res. 2015, 14, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khateb, S.; Jha, S. Cell-Based Therapies for Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2021, 1256, 265–293. [Google Scholar]
- Binder, S.; Stanzel, B.V.; Krebs, I.; Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 2007, 26, 516–554. [Google Scholar] [CrossRef]
- Wongpichedchai, S.; Weiter, J.J. Comparison of External and Internal Approaches for Transplantation of Autologous Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3341–3352. [Google Scholar]
- Joussen, A.M.; Heussen, F.M.; Joeres, S.; Llacer, H.; Prinz, B.; Rohrschneider, K.; Maaijwee, K.J.M.; van Meurs, J.; Kirchhof, B. Autologous Translocation of the Choroid and Retinal Pigment Epithelium in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2006, 142, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Zandi, S.; Pfister, I.B.; Traine, P.G.; Tappeiner, C.; Despont, A.; Rieben, R.; Skowronska, M.; Garweg, J.G. Biomarkers for PVR in Rhegmatogenous Retinal Detachment. PLoS ONE 2019, 14, e0214674. [Google Scholar] [CrossRef]
- Charteris, D.G.; Sethi, C.S. Proliferative Vitreoretinopathy-Developments in Adjunctive Treatment and Retinal Pathology. Eye 2002, 16, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Longo, A.; Avitabile, T.; Bonfiglio, V.; Toro, M.D.; Russo, A.; Viti, F.; Nicolai, M.; Saitta, A.; Giovannini, A.; et al. Transconjunctival Nonvitrectomizing Vitreous Surgery Versus 25-Gauge Vitrectomy in Patients with Epiretinal Membrane: A Prospective Randomized Study. Retina 2015, 35, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Amarnani, D.; Machuca-Parra, A.I. Effect of Methotrexate on an In Vitro Patient-Derived Model of Proliferative Vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3940–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benner, J.D.; Dao, D. Intravitreal Methotrexate for the Treatment of Proliferative Vitreoretinopathy. BMJ Open Ophthalmol. 2019, 4, e000293. [Google Scholar] [CrossRef]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307. [Google Scholar] [CrossRef]
- Khanani, A.M.; Aziz, A.A.; Weng, C.Y.; Lin, W.V.; Vannavong, J.; Chhablani, J.; Danzig, C.J.; Kaiser, P.K. Port delivery system: A novel drug delivery platform to treat retinal diseases. Expert Opin. Drug Deliv. 2021, 18, 1571–1576. [Google Scholar] [CrossRef]
- Ghoraba, H.H.; Do, D.V.; Nguyen, Q.D. Port Delivery System With Ranibizumab: As Always-Risks Versus Benefits for the Recipients. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 416–417. [Google Scholar] [CrossRef]
- Khanani, A.M.; Callanan, D.; Dreyer, R.; Chen, S.; Howard, J.G.; Horpkins, J.J.; Lin, C.-Y.; Lorenz-Candlin, M.; Makadia, S.; Patel, S.; et al. End-of-Study Results for the Ladder Phase 2 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2021, 5, 775–787. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.; Oliveira, J.; Kuroiwa, D.; Kolko, M.; Fernandes, R.; Junior, O.; Moraes, N.; Vasconcelos, H.; Oliveira, T.; Maia, M. Advances in Vitreoretinal Surgery. J. Clin. Med. 2022, 11, 6428. https://doi.org/10.3390/jcm11216428
Ribeiro L, Oliveira J, Kuroiwa D, Kolko M, Fernandes R, Junior O, Moraes N, Vasconcelos H, Oliveira T, Maia M. Advances in Vitreoretinal Surgery. Journal of Clinical Medicine. 2022; 11(21):6428. https://doi.org/10.3390/jcm11216428
Chicago/Turabian StyleRibeiro, Lucas, Juliana Oliveira, Dante Kuroiwa, Mohamed Kolko, Rodrigo Fernandes, Octaviano Junior, Nilva Moraes, Huber Vasconcelos, Talita Oliveira, and Mauricio Maia. 2022. "Advances in Vitreoretinal Surgery" Journal of Clinical Medicine 11, no. 21: 6428. https://doi.org/10.3390/jcm11216428






