Smell as a Disease Marker in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
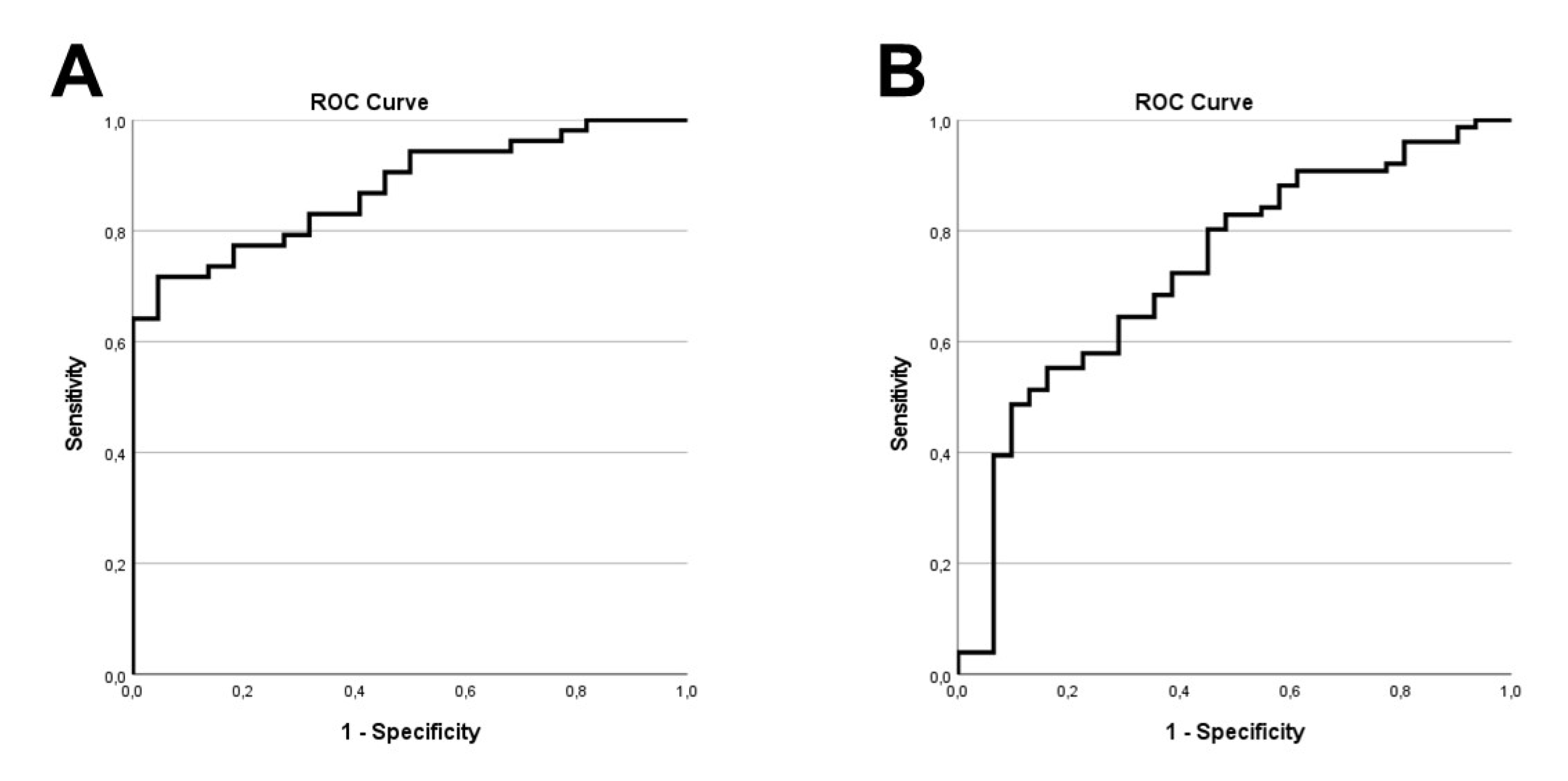
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucassen, E.B.; Turel, A.; Knehans, A.; Huang, X.; Eslinger, P. Olfactory dysfunction in Multiple Sclerosis: A scoping review of the literature. Mult. Scler. Relat. Disord. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Hegen, H.; Ladstätter, F.; Berek, K.; Amprosi, M.; Wurth, S.; Auer, M.; Pauli, F.D.; Deisenhammer, F.; Reindl, M.; et al. Change of olfactory function as a marker of inflammatory activity and disability progression in MS. Mult. Scler. 2019, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.; Li, C.; Mannon, L.; Yousem, D. Olfactory dysfunction in multiple sclerosis. N. Engl. J. Med. 1997, 336, 1918–1919. [Google Scholar] [CrossRef]
- Goektas, O.; Schmidt, F.; Bohner, G.; Erb, K.; Ludemann, L.; Dahlslett, B.; Harms, L.; Fleiner, F. Olfactory bulb volume and olfactory function in patients with multiple sclerosis. Rhinology 2011, 49, 221–226. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.; Pauli, E.; Kobal, G. Sniffin’ Sticks: Olfactory performance assessed by the combined testing of odour identification, odour discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.; Shephard, B.; Kobal, G. Assessment of olfaction in multiple sclerosis: Evidence of dysfunction by olfactory evoked response and identification tests. J. Neurol. Neurosurg. Psychiatry 1997, 63, 145–151. [Google Scholar] [CrossRef][Green Version]
- Silva, A.; Santos, E.; Moreira, I.; Bettencourt, A.; Coutinho, M.E.; Gonçalves, A.; Pinto, C.; Montalban, X.; Cavaco, S. Olfactory dysfunction in multiple sclerosis: Association with secondary progression. Mult. Scler. J. 2012, 18, 616–621. [Google Scholar] [CrossRef]
- Stewart, M.; Witsell, D.; Smith, T.; Weaver, E.; Yueh, B.; Hannley, M. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol. Head Neck Surg. 2004, 130, 157–163. [Google Scholar] [CrossRef]
- Lachanas, V.; Tsiouvaka, S.; Tsea, M.; Hajiioannou, J.; Skoulakis, C. Validation of the nasal obstruction symptom evaluation (NOSE) scale for Greek patients. Otolaryngol. Head Neck Surg. 2014, 151, 819–823. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.; Browne, J. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef]
- Lachanas, V.; Tsea, M.; Tsiouvaka, S.; Hajiioannou, J.; Skoulakis, C.; Bizakis, J. The sino-nasal outcome test (SNOT)-22: Validation for Greek patients. Eur. Arch. Otorhinolaryngol. 2014, 271, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, J.; Hummel, T. Olfactory dysfunction and daily life. Eur. Arch. Otorhinolaryngol. 2005, 262, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, E.; Katotomichelakis, M.; Gouveris, H.; Tripsianis, G.; Livaditis, M.; Danielides, V. Olfaction-associated quality of life in chronic rhinosinusitis: Adaptation and validation of an olfaction-specific questionnaire. Laryngoscope 2012, 122, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Kobal, G.; Hummel, T.; Sekinger, B.; Barz, S.; Roscher, S.; Wolf, S. Sniffin’ Stick: Screening of olfactory performance. Rhinology 1996, 34, 222–226. [Google Scholar] [PubMed]
- Konstantinidis, I.; Printza, A.; Genetzaki, S.; Mamali, K.; Kekes, G.; Constantinidis, J. Cultural adaptation of an olfactory identification test: The Greek version of Sniffin’ Sticks. Rhinology 2008, 46, 292–296. [Google Scholar]
- Oleszkiewicz, A.; Schriever, V.; Croy, I.; Hahner, A.; Hummel, T. Updated Sniffin’ sticks normative data based on an extended sampleof 9139 subjects. Eur. Arch. Otorhinolaryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Langdon, D.; Amato, M.; Boringa, J.; Brochet, B.; Foley, F.; Fredrikson, S.; Hämäläinen, P.; Hartung, H.-P.; Krupp, L.; Penner, I.K.; et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult. Scler. 2012, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Polychroniadou, E.; Bakirtzis, C.; Langdon, D.; Lagoudaki, R.; Kesidou, E.; Theotokis, P.; Tsalikakis, D.; Poulatsidou, K.; Kyriazis, O.; Boziki, M.; et al. Validation of the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) in Greek population with multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 9, 68–72. [Google Scholar] [CrossRef]
- Artemiadis, A.; Bakirtzis, C.; Chatzittofis, A.; Christodoulides, C.; Nikolaou, G.; Boziki, M.K.; Grigoriadis, N. Brief international cognitive assessment for multiple sclerosis (BICAMS) cut-off scores for detecting cognitive impairment in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102751. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test: Manual; Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Messinis, L.; Bakirtzis, C.; Kosmidis, M.H.; Economou, A.; Nasios, G.; Anyfantis, E.; Konitsiotis, S.; Ntoskou, A.; Peristeri, E.; Dardiotis, E.; et al. Symbol Digit Modalities Test: Greek Normative Data for the Oral and Written Version and Discriminative Validity in Patients with Multiple Sclerosis. Arch. Clin. Neuropsychol. 2021, 36, 117–125. [Google Scholar] [CrossRef]
- Vlahou, C.; Kosmidis, M.; Dardagani, A.; Tsotsi, S.; Giannakou, M.; Giazkoulidou, A.; Zervoudakis, E.; Pontikakis, N. Development of the Greek Verbal Learning Test: Reliability, construct validity, and normative standards. Arch. Clin. Neuropsychol. 2013, 28, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R. Brief Visuospatial Memory Test-Revised: Professional Manual; Psychological Assessment Resources: Odessa, FL, USA, 1997. [Google Scholar]
- Bakalidou, D.; Voumvourakis, K.; Tsourti, Z.; Papageorgiou, E.; Poulios, A.; Giannopoulos, S. Validity and reliability of the Greek version of the Modified Fatigue Impact Scale in multiple sclerosis patients. Int. J. Rehabil. Res. 2014, 37, 271–276. [Google Scholar]
- Beck, A.; Steer, R.; Brown, G. Manual for the Beck Depression Inventory-Fast Screen for Medical Patients; Antonio, S., Ed.; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Hobart, J.; Lamping, D.; Fitzpatrick, R.; Riazi, A.A.T. The Multiple Sclerosis Impact Scale (MSIS-29): A new patient-based outcome measure. Brain J. Neurol. 2001, 124, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzis, C.; Nikolaidis, I.; Boziki, M.-K.; Artemiadis, A.; Andravizou, A.; Messinis, L.; Ioannidis, P.; Grigoriadis, N. Cognitive Fatigability is Independent of Subjective Cognitive Fatigue and Mood in Multiple Sclerosis. Cogn. Behav. Neurol. 2020, 33, 113–121. [Google Scholar] [CrossRef]
- Benedict, R.; Fishman, I.; McClellan, M.; Bakshi, R.; Weinstock-Guttman, B. Validity of the Beck Depression Inventory-Fast Screen in multiple sclerosis. Mult. Scler. 2003, 9, 393–396. [Google Scholar] [CrossRef]
- Mirmosayyeb, O.; Ebrahimi, N.; Barzegar, M.; Afshari-Safavi, A.; Bagherieh, S.; Shaygannejad, V. Olfactory dysfunction in patients with multiple sclerosis; A systematic review and meta-analysis. PLoS ONE 2022, 17, e0266492. [Google Scholar] [CrossRef]
- Valsamidis, K.; Printza, A.; Constantinidis, J.; Triaridis, S. The Impact of Olfactory Dysfunction on the Psychological Status and Quality of Life of Patients with Nasal Obstruction and Septal Deviation. Int. Arch. Otorhinolaryngol. 2020, 24, e237–e246. [Google Scholar] [CrossRef]
- Bsteh, G.; Steiger, R.; Tuovinen, N.; Hegen, H.; Berek, K.; Wurth, S.; Auer, M.; Di Pauli, F.; Gizewski, E.R.; Deisenhammer, F.; et al. Impairment of odor discrimination and identification is associated with disability progression and gray matter atrophy of the olfactory system in MS. Mult. Scler. 2020, 26, 706–771. [Google Scholar] [CrossRef]
- Valsamidis, K.; Printza, A.; Titelis, K.; Constantinidis, J.; Triaridis, S. Olfaction and quality of life in patients with nasal septal deviation treated with septoplasty. Am. J. Otolaryngol. 2019, 40, 747–754. [Google Scholar] [CrossRef]
- Printza, A. Patient-Reported Outcome Measures in Diseases of the Head and Neck. J. Clin. Med. 2022, 11, 3358. [Google Scholar] [CrossRef]
- Printza, A.; Triaridis, S. Is the ability of the Eating Assessment Tool (EAT-10) to screen for aspiration in patients with dysphagia depending on the patients’ disease? Eur. Arch. Otorhinolaryngol. 2022, 279, 3745–3746. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Majde, J.A. Neuroinflammation resulting from covert brain invasion by common viruses—A potential role in local and global neurodegeneration. Med. Hypotheses 2010, 75, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; Brenner, M.J.; De Luca, P.; Albanese, M.; D’Ascanio, L.; Ralli, M.; Roccamatisi, D.; Cingolani, C.; Vitelli, F.; Camaioni, A.; et al. Olfactory Dysfunction, Headache, and Mental Clouding in Adults with Long-COVID-19: What Is the Link between Cognition and Olfaction? A Cross-Sectional Study. Brain Sci. 2022, 12, 154. [Google Scholar] [CrossRef]
- Boziki, M.; Bakirtzis, C.; Giantzi, V.; Sintila, S.-A.; Kallivoulos, S.; Afrantou, T.; Nikolaidis, I.; Ioannidis, P.; Karapanayiotides, T.; Koutroulou, I.; et al. Long-Term Efficacy Outcomes of Natalizumab vs. Fingolimod in Patients with Highly Active Relapsing-Remitting Multiple Sclerosis: Real-World Data from a Multiple Sclerosis Reference Center. Front. Neurol. 2021, 12, 699844. [Google Scholar] [CrossRef]
- Walsh, M.C.; Trentham-Dietz, A.; Newcomb, P.A.; Gangnon, R.; Palta, M. Using propensity scores to reduce case-control selection bias. Epidemiology 2012, 23, 772–773. [Google Scholar] [CrossRef]
| MS Patients | Controls | p | |
|---|---|---|---|
| age | 38.69 ± 12.19 | 37.29 ± 12.01 | 0.527 * |
| disease duration (years) | 10.08 ± 8.47 | N/A | N/A |
| disease duration since diagnosis (years) | 7.74 ± 7.80 | N/A | N/A |
| EDSS | 3.7 ± 2.02 | N/A | N/A |
| SDMT | 45.07 ± 14.24 | N/A | N/A |
| GVLT | 55.41 ± 11.9 | N/A | N/A |
| BVMT-R | 22.21 ± 8.76 | N/A | N/A |
| MFIS | 31.06 ± 21.76 | N/A | N/A |
| BDI-FS | 3.85 ± 3.78 | N/A | N/A |
| MSIS | 61.25 ± 22.64 | N/A | N/A |
| SNOT-22 | 21 ± 17.05 | 10.36 ± 12.76 | <0.001 * |
| NOSE | 13.25 ± 17.81 | 8.96 ± 12.63 | 0.532 * |
| QoD | 17.49 ± 9.12 | 17.79 ± 9.13 | 0.967 * |
| MS Patients | Controls | p | |
|---|---|---|---|
| OD | 12.19 ± 0.23 | 13.3 ± 0.3 | 0.005 * |
| OI | 14.08 ± 0.16 | 14.66 ± 0.15 | 0.06 * |
| OT | 6.72 ± 0.26 | 6.96 ± 0.35 | 0.581 ** |
| TDI | 32.98 ± 0.48 | 34.92 ± 0.57 | 0.015 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Printza, A.; Boziki, M.; Valsamidis, C.; Bakirtzis, C.; Constantinidis, J.; Grigoriadis, N.; Triaridis, S. Smell as a Disease Marker in Multiple Sclerosis. J. Clin. Med. 2022, 11, 5215. https://doi.org/10.3390/jcm11175215
Printza A, Boziki M, Valsamidis C, Bakirtzis C, Constantinidis J, Grigoriadis N, Triaridis S. Smell as a Disease Marker in Multiple Sclerosis. Journal of Clinical Medicine. 2022; 11(17):5215. https://doi.org/10.3390/jcm11175215
Chicago/Turabian StylePrintza, Athanasia, Marina Boziki, Constantinos Valsamidis, Christos Bakirtzis, Jannis Constantinidis, Nikolaos Grigoriadis, and Stefanos Triaridis. 2022. "Smell as a Disease Marker in Multiple Sclerosis" Journal of Clinical Medicine 11, no. 17: 5215. https://doi.org/10.3390/jcm11175215
APA StylePrintza, A., Boziki, M., Valsamidis, C., Bakirtzis, C., Constantinidis, J., Grigoriadis, N., & Triaridis, S. (2022). Smell as a Disease Marker in Multiple Sclerosis. Journal of Clinical Medicine, 11(17), 5215. https://doi.org/10.3390/jcm11175215








