Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women
Abstract
:1. Introduction
2. Materials and Methods
3. Search Strategy
4. Eligibility Criteria
5. Study Selection
6. Data Extraction and Management
7. Data Synthesis and Statistical Analyses
8. Results
| Author’s Name, Country, Year | Sample Size | Age (Years) | Study Design | No. of Incident Breast Cancer/Case | Follow Up (Mean, Year) | Data Presented | Adjusted | Results | Study Score |
|---|---|---|---|---|---|---|---|---|---|
| Lars J. Vatten et al. Norway (1990) [14] | 24,329 | 31–51 | Cohort | 242 | 14 | TG, TC | Age, BMI | There were no statistically significant associations of lipid measures with breast cancer risk. | 5 |
| Annette Pernille Hoyer et al. Denmark (1992) [15] | 5207 | 30–80 | Cohort | 51 | 26 | HDL, LDL, TG, TC | Age, Smoking, Menopause Age, Alcohol Intake, BMI, Socioeconomic Status | There was a significant association of HDL with breast cancer risk. | 5 |
| Maria Gaard et al. Norway (1994) [16] | 30,666 | 20–54 | Cohort | 302 | 10.4 | HDL, LDL, TG, TC | Age, Smoking, Menopause Age, BMI, Lipid Baseline | There were no statistically significant associations of lipid measures with breast cancer risk. | 6 |
| Kyle Steenland et al. USA (1995) [17] | 14,407 | 25–74 | Cohort | 163 | 17 | TC | Age, Smoking, Menopause Age, Alcohol Intake, BMI, Socioeconomic Status, Physical Activity, Parity | There was no statistically significant association of TC with breast cancer risk. | 7 |
| Patricia. G Moorman et al. USA (1998) [33] | 400 (control), 196 (case) | 41.3 | Nested case-control | Case | _ | HDL | Age, Menopause Age, Education, BMI, Alcohol Intake, Family History Cancer, Hormone Use, History of Hysterectomy, Parity, Smoking | There was no statistically significant association of HDL with breast cancer risk. | 6 |
| J Manjer et al. Sweden (2001) [18] | 9738 | 49.6 ± 7.8 | Cohort | 269 | 13.1 | TG, TC | Age, Smoking, Alcohol Intake, BMI, Parity | There were no statistically significant associations of lipid measures with breast cancer risk. | 8 |
| Anne-Sofie Furberg et al. Norway (2004) [9] | 38,823 | 43 | cohort | 708 | 17.2 | HDL | Age, Menopause Age, Smoking Socioeconomic Status, BMI, Parity, Lipid Baseline | There was no statistically significant association of HDL with breast cancer risk. | 9 |
| A. Heather Eliassen et al. USA (2005) [19] | 71,921 | 66 (mean) | Cohort | 2468 | 10 | TC | Age, Menopause Age, Alcohol intake, BMI, Physical Activity, Parity, Family History Cancer, Hormone Use | There was no statistically significant association of TC with breast cancer risk. | 8 |
| Manami Inouea et al. Japan (2008) [22] | 18,176 | 55.5 ± 8.1 | Cohort | 120 | 10 | HDL, TG | Age, Smoking, Alcohol Intake, Lipid Baseline | There were no statistically significant associations of lipid measures with breast cancer risk. | 6 |
| Anna M. Kuchareska-Newton et al. USA (2008) [20] | 7575 | 53.7 ± 5.7 | Cohort | 359 | 13 | HDL | Age, Menopause Age, BMI, Race, Smoking Hormone Use | There was no statistically significant association of HDL with breast cancer risk. | 8 |
| Guy Fagherazzi et al. France (2009) [25] | 69,088 | 40–65 | Cohort | 2932 | 12 | TC | Menopause Age, Alcohol Intake, BMI, Family History Cancer, Hormone Use | There was no statistically significant association of TC with breast cancer risk. | 7 |
| Hiroyasu Iso et al. Japan (2009) [23] | 21,685 | 54.2 (mean) | Cohort | 178 | 12.4 | TC | Age, Smoking, Alcohol Intake, BMI | There was a significant association of TC with breast cancer risk. | 8 |
| H Ulmer et al. Austria (2009) [24] | 84,460 | 41.8 ± 15.1 | Cohort | 1204 | 10.6 | TG | Smoking, BMI, Socioeconomic status, Lipid Baseline | There was no statistically significant association of TG with breast cancer risk. | 9 |
| Mina Ha et al. Korean (2009) [21] | 170,374 | 40–64 | Cohort | 714 | 10 | TC | Age, Age at Menarche, Age at First Childbirth, Nulliparity, Hormone Replacement Therapy, Duration of Breast Feeding, Smoking Habit, Alcohol Consumption | There was a positive association between cholesterol level and breast cancer risk. | 6 |
| Immacolata Capasso et al. Italy (2010) [35] | 777 [control), 210 [case) | 57.5 | Nested case-control | Case | _ | HDL | Age, Menopause Age, BMI, Alcohol Intake, Family History cancer, Hormone Use, Parity, Smoking, Socioeconomic status | There was a significant association of HDL with breast cancer risk. | 7 |
| C. Agnoli et al. Italy (2010) [34] | 1089 (control), 163 (case) | 58 ± 5.6 | Nested case-control | Case | _ | HDL, TG | Age, Smoking, Menopause Age, Education, Alcohol Intake, Family History Cancer, Hormone Use | There were significant associations of HDL and TG with breast cancer risk. | 6 |
| Wegene Borena et al. Norway, Austria, and Swede (2011) [26] | 256,512 | 44.2 | Cohort | 5006 | 11.9 | TG | Age, Smoking, BMI | There was no statistically significant association of TG with breast cancer risk. | 8 |
| Jennifer C. Melvin et al. Sweden (2012) [28] | 234,494 | 25< | Cohort | 6105 | 8.25 | HDL, LDL, TG, TC, APO A, APO B, TC/HDL, LDL/HDL, TG/HD, APO B/APO A | Age, Socioeconomic Status, Lipid Baseline, Parity | There was a significant association of TG with breast cancer risk. | 7 |
| Jaclyn L. F. Bosco et al. USA (2012) [27] | 49,172 | 21–69 | Cohort | 1228 | 10.5 | TC | Age, Race, Education, BMI, Physical Activity | There was no statistically significant association of TC with breast cancer risk. | 7 |
| Susanne Strohmaier et al. Norway, Austria, and Sweden (2013) [29] | 288,057 | 33–48 | Cohort | 5228 | 11.7 | TC | Age, Smoking, BMI | There was a significant association of TC with breast cancer risk. | 8 |
| Signe Borgquist et al. Sweden (2016) [30] | 17,035 | 57.9 | Cohort | 1024 | 14.3 | APO A, APO B, APO B/APO A | Age, Menopause Age, Socioeconomic status, BMI, Hormone Use, Parity | There were significant associations of APO B/APO A with breast cancer risk. | 7 |
| Mathilde His et al. France (2017) [36] | 1043 (control), 583 [case) | 50–63 | Nested case-control | Case | _ | HDL, LDL, TG, TC, TC/HDL, LDL/HDL | Age, Menopause Age, Smoking, BMI, Family History Cancer, Education, Alcohol Intake, Hormone Use | There were no statistically significant associations of lipid measures with breast cancer risk. | 7 |
| Daniel T. Dibaba et al. USA (2018) [31] | 94,555 | 50–71 | Cohort | 5380 | 14 | TC | Age, Education, BMI, Physical Activity, Family History Cancer, Hormone Use, History of Hysterectomy, Parity, Smoking | There was a significant association of TC with breast cancer risk. | 8 |
| Kasper Mønsted Pedersen et al. Denmark (2020) [8] | 56,790 | 57.5 | Cohort | 1641 | 7.4 | HDL, APO A | Age, Smoking, BMI, Physical Activity, Education, Alcohol Intake, Socioeconomic status, Lipid Baseline | There was a significant association of Apo A with breast cancer risk. | 7 |
| Catherine Schairer et al. USA (2020) [37] | 2470 (control), 247 (case) | 60.7 | Nested case-control | Case | _ | HDL, TG, TG/HDL | Age, Race | There were significant associations of HDL and TG/HDL with breast cancer risk. | 6 |
| Rhonda S. Arthur et al. UK (2021) [32] | 58,629 | 60 (56–64) | Cohort | 1268 | 7 | HDL, TG | Age, BMI, Physical activity, Family History Cancer, Alcohol intake, Hormone Use, Smoking, Socioeconomic status | There were no statistically significant associations of lipid measures with breast cancer risk. | 7 |
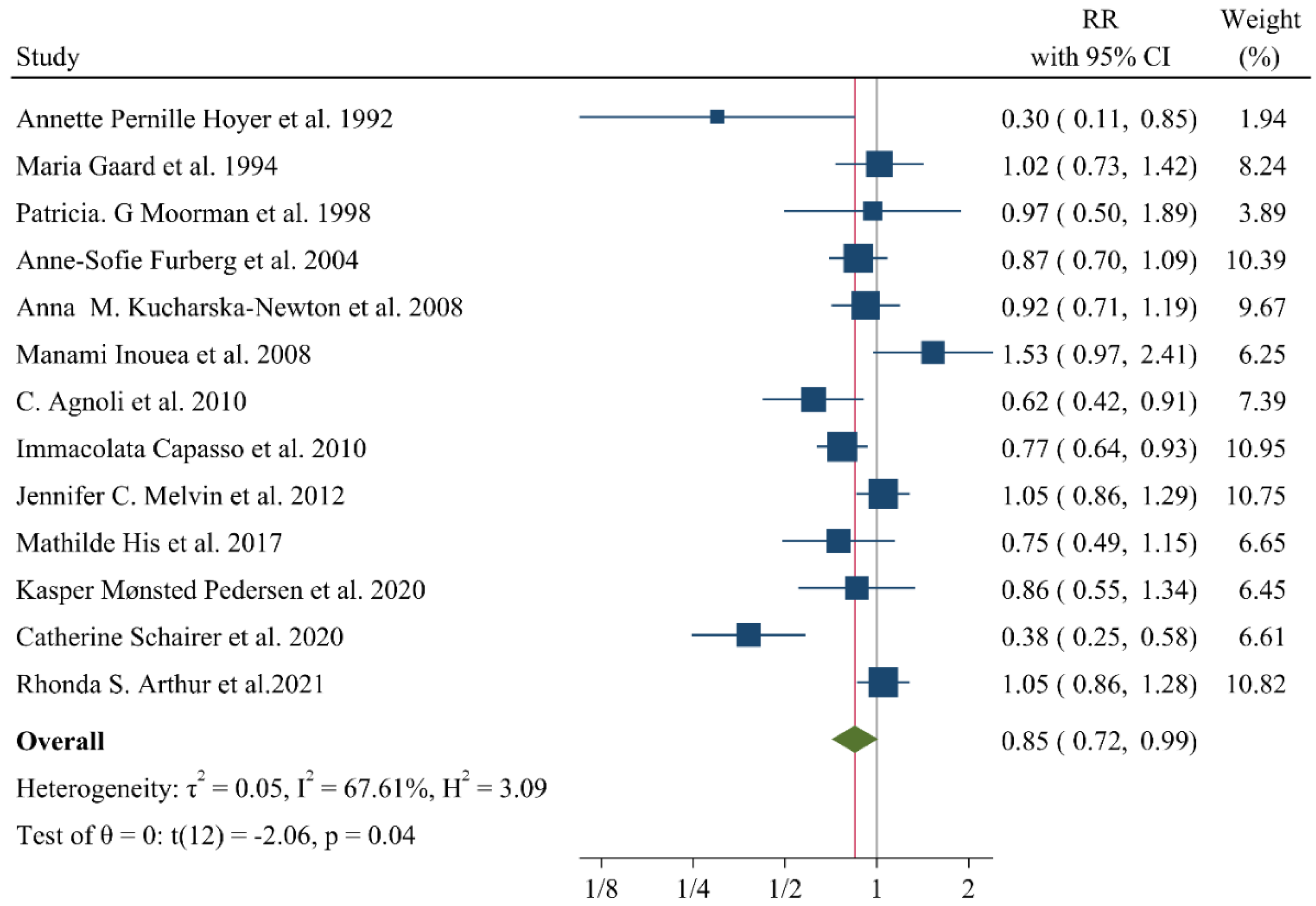
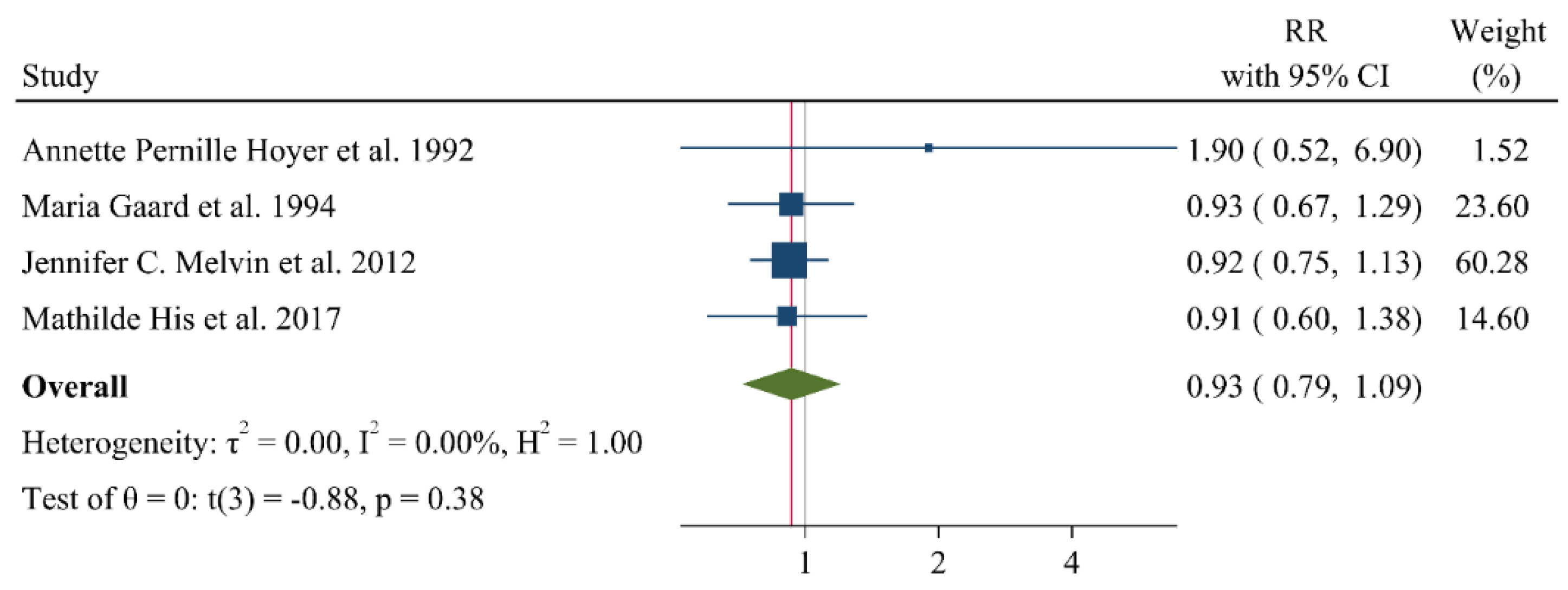
9. Associations of Lipid Profile with Risk of Breast Cancer
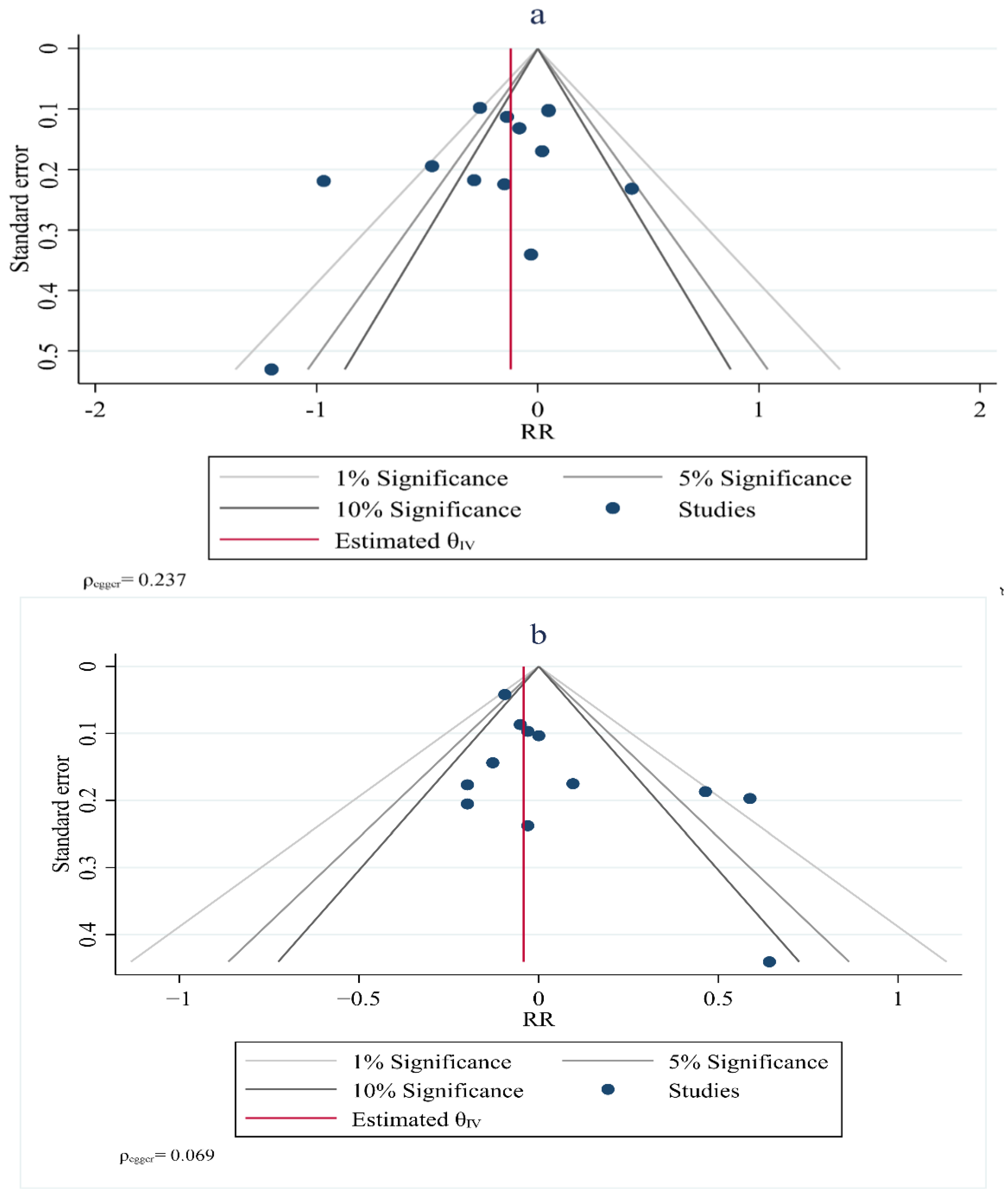
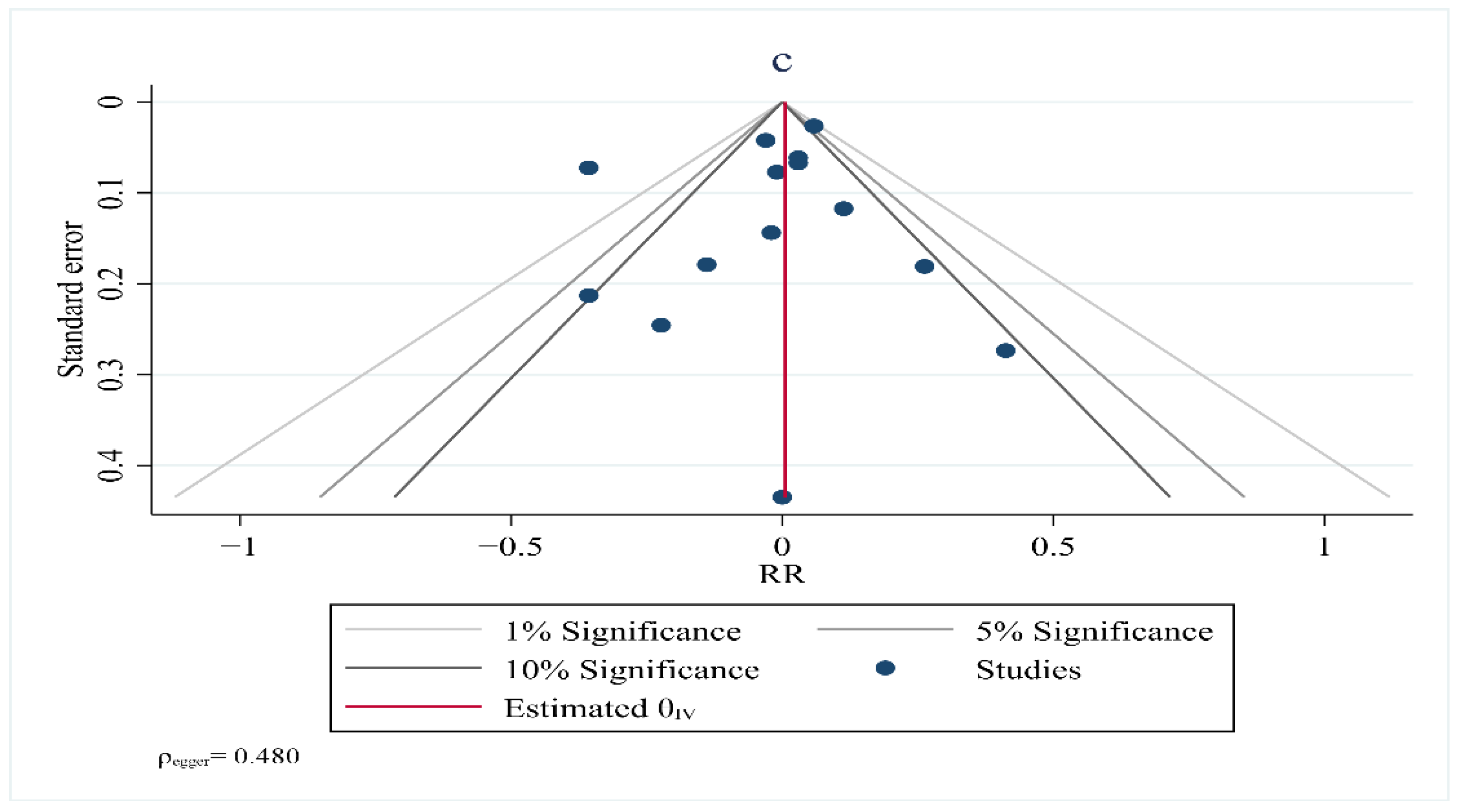
10. Publication Bias
11. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudet, M.M.; Gierach, G.L.; Carter, B.D.; Luo, J.; Milne, R.L.; Weiderpass, E.; Giles, G.G.; Tamimi, R.M.; Eliassen, A.H.; Rosner, B. Pooled analysis of nine cohorts reveals breast cancer risk factors by tumor molecular subtype. Cancer Res. 2018, 78, 6011–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laisupasin, P.; Thompat, W.; Sukarayodhin, S.; Sornprom, A.; Sudjaroen, Y. Comparison of serum lipid profiles between normal controls and breast cancer patients. J. Lab. Physicians 2013, 5, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Kapil, U.; Bhadoria, A.S.; Sareen, N.; Singh, P.; Dwivedi, S.N. Total cholesterol and triglyceride levels in patients with breast cancer. J. Breast Cancer 2013, 16, 129–130. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.-Q.; Cui, L.-H.; Li, C.-C.; Yu, Z.; Piao, J.-M. Effects of serum triglycerides on prostate cancer and breast cancer risk: A meta-analysis of prospective studies. Nutr. Cancer 2016, 68, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.-L.; Wu, Y.-T.; Wu, H.; Dai, W.; Arshad, B.; Xu, Z.; Li, H.; Wu, K.-N.; Kong, L.-Q. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis. 2018, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Owiredu, W.; Donkor, S.; Addai, B.W.; Amidu, N. Serum lipid profile of breast cancer patients. Pak. J. Biol. Sci. 2009, 12, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.M.; Çolak, Y.; Bojesen, S.E.; Nordestgaard, B.G. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J. Hematol. Oncol. 2020, 13, 129. [Google Scholar] [CrossRef]
- Furberg, A.-S.; Veierød, M.B.; Wilsgaard, T.; Bernstein, L.; Thune, I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J. Natl. Cancer Inst. 2004, 96, 1152–1160. [Google Scholar] [CrossRef]
- Samadi, S.; Ghayour-Mobarhan, M.; Mohammadpour, A.; Farjami, Z.; Tabadkani, M.; Hosseinnia, M.; Miri, M.; Heydari-Majd, M.; Mehramiz, M.; Rezayi, M. High-density lipoprotein functionality and breast cancer: A potential therapeutic target. J. Cell. Biochem. 2019, 120, 5756–5765. [Google Scholar] [CrossRef]
- Johnson, K.E.; Siewert, K.M.; Klarin, D.; Damrauer, S.M.; Program, V.M.V.; Chang, K.-M.; Tsao, P.S.; Assimes, T.L.; Maxwell, K.N.; Voight, B.F. The relationship between circulating lipids and breast cancer risk: A Mendelian randomization study. PLoS Med. 2020, 17, e1003302. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; Reddy, S.T.; Mato, E.; Blanco-Vaca, F. HDL and LDL: Potential new players in breast cancer development. J. Clin. Med. 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fichtali, K.; Bititi, A.; Elghanmi, A.; Ghazi, B. Serum Lipidomic Profiling in Breast Cancer to Identify Screening, Diagnostic, and Prognostic Biomarkers. BioResearch Open Access 2020, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatten, L.J.; Foss, O.P. Total serum cholesterol and triglycerides and risk of breast cancer: A prospective study of 24,329 Norwegian women. Cancer Res. 1990, 50, 2341–2346. [Google Scholar] [PubMed]
- Høyer, A.P.; Engholm, G. Serum lipids and breast cancer risk: A cohort study of 5,207 Danish women. Cancer Causes Control 1992, 3, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gaard, M.; Tretli, S.; Urdal, P. Risk of breast cancer in relation to blood lipids: A prospective study of 31,209 Norwegian women. Cancer Causes Control 1994, 5, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Nowlin, S.; Palu, S. Cancer incidence in the National Health and Nutrition Survey, I. Follow-up data: Diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol. Prev. Biomark. 1995, 4, 807–811. [Google Scholar]
- Manjer, J.; Kaaks, R.; Riboli, E.; Berglund, G. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: A prospective study within the Malmö Preventive Project. Eur. J. Cancer Prev. 2001, 10, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch. Intern. Med. 2005, 165, 2264–2271. [Google Scholar] [CrossRef]
- Kucharska-Newton, A.M.; Rosamond, W.D.; Mink, P.J.; Alberg, A.J.; Shahar, E.; Folsom, A.R. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann. Epidemiol. 2008, 18, 671–677. [Google Scholar] [CrossRef]
- Ha, M.; Sung, J.; Song, Y.-M. Serum total cholesterol and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control 2009, 20, 1055–1060. [Google Scholar] [CrossRef]
- Inoue, M.; Noda, M.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Iso, H.; Tsugane, S. Impact of metabolic factors on subsequent cancer risk: Results from a large-scale population-based cohort study in Japan. Eur. J. Cancer Prev. 2009, 18, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Ikeda, A.; Inoue, M.; Sato, S.; Tsugane, S. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int. J. Cancer 2009, 125, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, H.; Borena, W.; Rapp, K.; Klenk, J.; Strasak, A.; Diem, G.; Concin, H.; Nagel, G. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br. J. Cancer 2009, 101, 1202–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagherazzi, G.; Fabre, A.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: Results from the prospective E3N cohort. Eur. J. Cancer Prev. 2010, 19, 120–125. [Google Scholar] [CrossRef]
- Borena, W.; Stocks, T.; Jonsson, H.; Strohmaier, S.; Nagel, G.; Bjørge, T.; Manjer, J.; Hallmans, G.; Selmer, R.; Almquist, M. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Causes Control 2011, 22, 291–299. [Google Scholar] [CrossRef]
- Bosco, J.L.; Palmer, J.R.; Boggs, D.A.; Hatch, E.E.; Rosenberg, L. Cardiometabolic factors and breast cancer risk in US black women. Breast Cancer Res. Treat. 2012, 134, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Melvin, J.C.; Seth, D.; Holmberg, L.; Garmo, H.; Hammar, N.; Jungner, I.; Walldius, G.; Lambe, M.; Wigertz, A.; Van Hemelrijck, M. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol. Prev. Biomark. 2012, 21, 1381–1384. [Google Scholar] [CrossRef] [Green Version]
- Strohmaier, S.; Edlinger, M.; Manjer, J.; Stocks, T.; Bjørge, T.; Borena, W.; Häggström, C.; Engeland, A.; Nagel, G.; Almquist, M. Total serum cholesterol and cancer incidence in the Metabolic syndrome and Cancer Project (Me-Can). PLoS ONE 2013, 8, e54242. [Google Scholar] [CrossRef] [Green Version]
- Borgquist, S.; Butt, T.; Almgren, P.; Shiffman, D.; Stocks, T.; Orho-Melander, M.; Manjer, J.; Melander, O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer 2016, 138, 2648–2656. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and the risk of breast cancer and subtypes by race, menopause and BMI. Cancers 2018, 10, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, R.S.; Dannenberg, A.J.; Rohan, T.E. The association of prediagnostic circulating levels of cardiometabolic markers, testosterone and sex hormone-binding globulin with risk of breast cancer among normal weight postmenopausal women in the UK Biobank. Int. J. Cancer 2021, 149, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Hulka, B.S.; Hiatt, R.A.; Krieger, N.; Newman, B.; Vogelman, J.H.; Orentreich, N. Association between high-density lipoprotein cholesterol and breast cancer varies by menopausal status. Cancer Epidemiol. Prev. Biomark. 1998, 7, 483–488. [Google Scholar]
- Agnoli, C.; Berrino, F.; Abagnato, C.A.; Muti, P.; Panico, S.; Crosignani, P.; Krogh, V. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: A nested case–control study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capasso, I.; Esposito, E.; Pentimalli, F.; Crispo, A.; Montella, M.; Grimaldi, M.; De Marco, M.R.; Cavalcanti, E.; D’Aiuto, M.; Fucito, A. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol. Ther. 2010, 10, 1240–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- His, M.; Dartois, L.; Fagherazzi, G.; Boutten, A.; Dupré, T.; Mesrine, S.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Dossus, L. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes Control 2017, 28, 77–88. [Google Scholar] [CrossRef]
- Schairer, C.; Laurent, C.A.; Moy, L.M.; Gierach, G.L.; Caporaso, N.E.; Pfeiffer, R.M.; Kushi, L.H. Obesity and related conditions and risk of inflammatory breast cancer: A nested case–control study. Breast Cancer Res. Treat. 2020, 183, 467–478. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Fassier, P.; His, M.; Norat, T.; Chan, D.S.; Blacher, J.; Hercberg, S.; Galan, P.; Druesne-Pecollo, N.; Latino-Martel, P. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 2015, 114, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J.; Chen, J. The significances and clinical implications of cholesterol components in human breast cancer. Sci. Prog. 2021, 104, 368504211028395. [Google Scholar] [CrossRef] [PubMed]
- Van Wyhe, R.D.; Rahal, O.M.; Woodward, W.A. Effect of statins on breast cancer recurrence and mortality: A review. Breast Cancer 2017, 9, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Borgquist, S.; Broberg, P.; Tojjar, J.; Olsson, H. Statin use and breast cancer survival—A Swedish nationwide study. BMC Cancer 2019, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018, 20, 144. [Google Scholar] [CrossRef]
- Lv, H.; Shi, D.; Fei, M.; Chen, Y.; Xie, F.; Wang, Z.; Wang, Y.; Hu, P. Association Between Statin Use and Prognosis of Breast Cancer: A Meta-Analysis of Cohort Studies. Front. Oncol. 2020, 10, 556243. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Yuan, Q.; Lin, Q.; Shi, W.Q.; Zhu, P.W.; Min, Y.L.; Ge, Q.M.; Shao, Y. Apolipoprotein A1 and Low-Density Lipoprotein as Risk Factors for Intraocular Metastases in Postmenopausal Breast Cancer. Technol. Cancer Res. Treat. 2021, 20, 1533033820984180. [Google Scholar] [CrossRef]
- Liu, J.X.; Yuan, Q.; Min, Y.L.; He, Y.; Xu, Q.H.; Li, B.; Shi, W.Q.; Lin, Q.; Li, Q.H.; Zhu, P.W.; et al. Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag. Res. 2019, 11, 2881–2888. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouri, M.; Mohsenpour, M.A.; Katsiki, N.; Ghobadi, S.; Jafari, A.; Faghih, S.; Banach, M.; Mazidi, M. Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women. J. Clin. Med. 2022, 11, 4503. https://doi.org/10.3390/jcm11154503
Nouri M, Mohsenpour MA, Katsiki N, Ghobadi S, Jafari A, Faghih S, Banach M, Mazidi M. Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women. Journal of Clinical Medicine. 2022; 11(15):4503. https://doi.org/10.3390/jcm11154503
Chicago/Turabian StyleNouri, Mehran, Mohammad Ali Mohsenpour, Niki Katsiki, Saeed Ghobadi, Alireza Jafari, Shiva Faghih, Maciej Banach, and Mohsen Mazidi. 2022. "Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women" Journal of Clinical Medicine 11, no. 15: 4503. https://doi.org/10.3390/jcm11154503







