Evaluation of the Efficacy and Effects of Common Hepatic Artery Reconstruction in Pancreas Transplantation: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
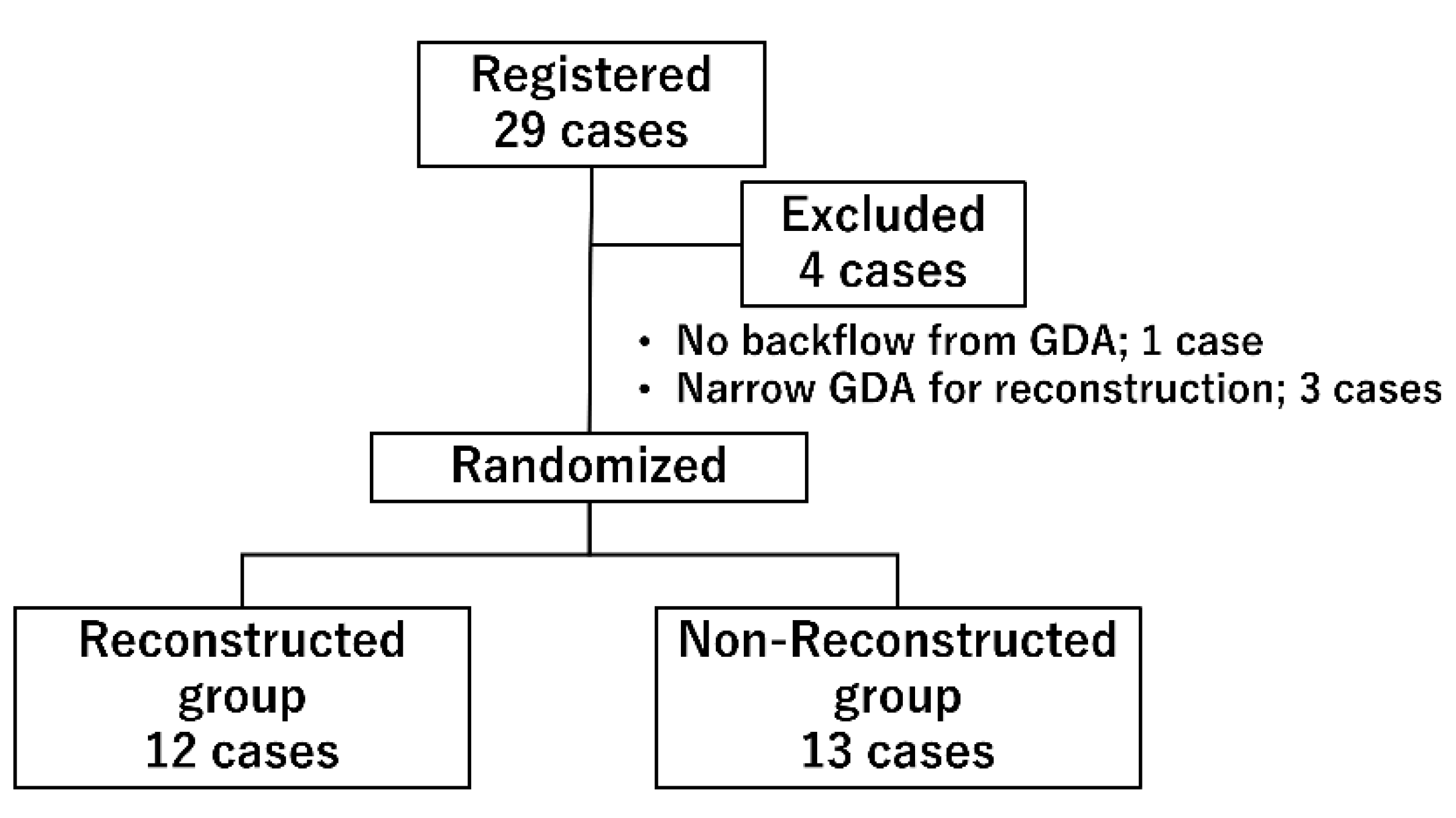
2.2. Study Population
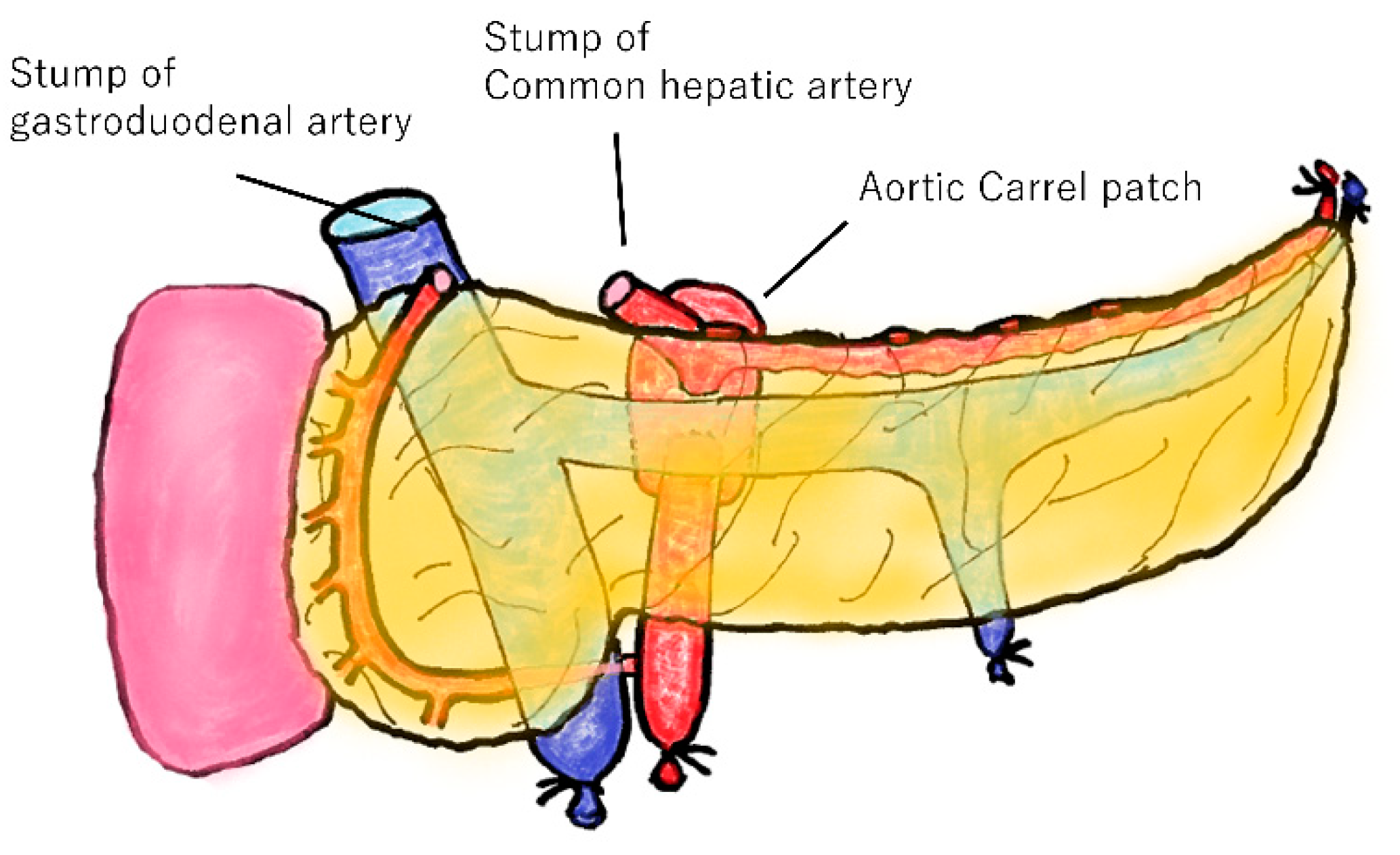
2.3. Transplantation Procedure
2.4. Immunosuppression Protocol
2.5. Evaluation Items
2.5.1. Primary Endpoints and Secondary Endpoints
2.5.2. Assessment of Graft Function
2.5.3. Assessment of Tissue Perfusion
2.6. Statistical Analysis
3. Results
3.1. Backgrounds of the Participents
3.2. Graft Survival and Complications
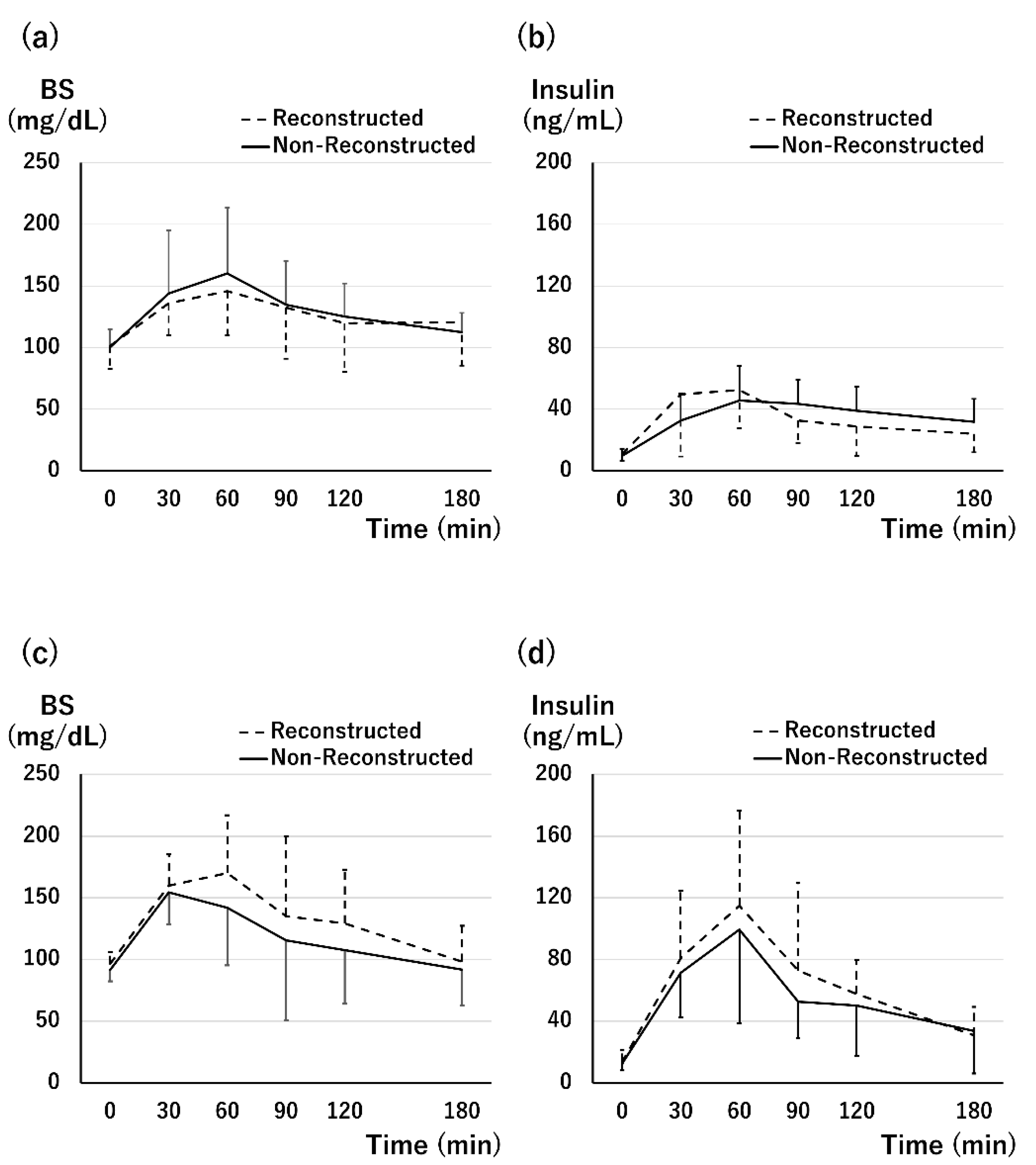
3.3. Graft Function at 1 Month after Tx
3.4. Graft Function at 1 Year after Tx
3.5. Tissue Perfusion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Tomimaru, Y.; Ito, T. Impact of the Revision of the Law on Pancreatic Transplants in Japan-An Analysis of the Japanese Pancreas Transplants Registry. J. Hepatobiliary-Pancreat. Sci. 2021, 28, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, C.; Perrone, V.G.; Amorese, G.; Vistoli, F.; Baronti, W.; Marchetti, P.; Boggi, U. Update on Pancreatic Transplantation in the Management of Diabetes. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc. Copyright © 2000-2022; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278943/ (accessed on 15 December 2021).
- Blundell, J.; Shahrestani, S.; Lendzion, R.; Pleass, H.J.; Hawthorne, W.J. Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620942589. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, V.N.; Goldaracena, N.; Marquez, M.A.; Singh, S.K.; Norgate, A.; McGilvray, I.D.; Schiff, J.; Greig, P.D.; Cattral, M.S.; Selzner, M. Duodenal Leaks After Pancreas Transplantation With Enteric Drainage-Characteristics and Risk Factors. Transpl. Int. 2015, 28, 720–728. [Google Scholar] [CrossRef] [PubMed]
- White, S.A.; Shaw, J.A.; Sutherland, D.E. Pancreas Transplantation. Lancet 2009, 373, 1808–1817. [Google Scholar] [CrossRef] [Green Version]
- Prudhomme, T.; Mulvey, J.F.; Young, L.A.J.; Mesnard, B.; Lo Faro, M.L.; Ogbemudia, A.E.; Dengu, F.; Friend, P.J.; Ploeg, R.; Hunter, J.P.; et al. Ischemia-Reperfusion Injuries Assessment During Pancreas Preservation. Int. J. Mol. Sci. 2021, 22, 5172. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; Bergt, S.; Obermaier, R.; Wiessner, R.; Pfeffer, F.; Schareck, W.; Hopt, U.T. Impairment of Microcirculation in the Early Reperfusion Period Predicts the Degree of Graft Pancreatitis in Clinical Pancreas Transplantation. Transplantation 2001, 71, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Ito, T.; Sugitani, A.; Furukawa, H.; Sekiguchi, S.; Gotoh, M.; Teraoka, S.; Sato, Y.; Matsuno, N.; Kenmochi, S.; et al. Present Status of Pancreas Transplantation in Japan-Donation Predominantly From Marginal Donors and Modified Surgical Technique: Report of Japan Pancreas Transplantation Registry. Transplant. Proc. 2008, 40, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Tomimaru, Y.; Eguchi, H.; Doki, Y.; Ito, T.; Kenmochi, T. Current State of Pancreas Transplantation in Japan Based on the Nationwide Registry. Ann. Gastroenterol. Surg. 2021, 5, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.G.; Lam, H.D.; Braat, A.E.; Schaapherder, A.F. The Dorsal Pancreatic Artery in Pancreas Procurement and Transplantation: Anatomical Considerations and Potential Implications. Clin. Transplant. 2016, 30, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kenmochi, T.; Nishikawa, T.; Maruyama, M.; Kusaka, M.; Sasaki, H.; Asano, T.; Matsubara, H.; Hoshinaga, K. A Novel Screening Test for Detecting Graft Thrombosis after Pancreatic Transplantation Using Contrast-Enhanced Ultrasonography with Sonazoid. Transplant. Proc. 2014, 46, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Aida, N.; Kenmochi, T.; Ito, T.; Nishikawa, T.; Hiratsuka, I.; Shibata, M.; Suzuki, A.; Hasegawa, M.; Kawai, A.; Kusaka, M.; et al. Prediction of Insulin Secretion Ability With Microcirculation Evaluated by Contrast-Enhanced Ultrasonography in Pancreas Transplantation. Pancreas 2018, 47, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briceño, J.; Sánchez-Hidalgo, J.M.; Arjona-Sanchez, A. Back-Table Surgery Pancreas Allograft for Transplantation: Implications in Complications. World J. Transplant. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Adamson, D.; Holzner, M.L.; Wadhera, V.; Shapiro, R. Reconstruction of a Pancreatic Allograft with Variant Arterial Anatomy for Transplantation. Transplant. Direct. 2019, 5, e425. [Google Scholar] [CrossRef]
- Nghiem, D.D. Revascularization of the Gastroepiploic Artery in Pancreas Transplant. Transpl. Int. 2008, 21, 774–777. [Google Scholar] [CrossRef]
- Pinchuk, A.V.; Dmitriev, I.V.; Anisimov, Y.A.; Storozhev, R.V.; Balkarov, A.G.; Kondrashkin, A.S.; Khodilina, I.V.; Muslimov, R.S. Pancreas Transplantation With Isolated Splenic Artery Blood Supply-Single Center Experience. Asian J. Surg. 2020, 43, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Kawai, A.; Suzuki, A.; Shibata, M.; Hiratsuka, I.; Hasegawa, M. The Outcomes of Pancreatic Transplantation from Pediatric Donors-A Single Institution Experience. J. Clin. Med. 2019, 8, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Fàbrega, J.; Cano-Vargas, B.; Ventura-Aguiar, P.; Cárdenas, G.; García-Criado, Á.; López-Boado, M.A.; Rull, R.; García, R.; Cuatrecasas, M.; Esmatjes, E.; et al. Early intestinal complications following pancreas transplantation: Lessons learned from over 300 case—A retrospective single-center study. Transpl. Int. 2021, 34, 139–152. [Google Scholar] [CrossRef]
- Orsenigo, E.; Fiorina, P.; Dell’Antonio, G.; Cristallo, M.; Socci, C.; Invernizzi, L.; Maffi, P.; Secchi, A.; Di Carlo, V. Gastrointestinal Bleeding From Enterically Drained Transplanted Pancreas. Transpl. Int. 2005, 18, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.; Gruessner, R.W.; Dunn, D.L.; Matas, A.J.; Humar, A.; Kandaswamy, R.; Mauer, S.M.; Kennedy, W.R.; Goetz, F.C.; Robertson, R.P.; et al. Lessons Learned From More Than 1,000 Pancreas Transplant at a Single Institution. Ann. Surg. 2001, 233, 463–501. [Google Scholar] [CrossRef] [PubMed]



| Non-Reconstructed Group (n = 13) | Reconstructed Group (n = 12) | p-Value | ||
|---|---|---|---|---|
| Recipient factor | ||||
| Age (years) | 48.2 ± 9.5 | 47.9 ± 8.3 | 0.93 | |
| Sex (male, %) | 5 (38.5) | 5 (41.7) | 1 | |
| Duration of DM (yr) | 30.6 ± 10.0 | 26.8 ± 7.7 | 0.29 | |
| Duration of HD (yr) | 6.3 ± 5.8 | 4.9 ± 3.6 | 0.52 | |
| Donor factor | ||||
| Age (years) | 35.5 ± 17.8 | 42.2 ± 12.6 | 0.30 | |
| Sex (male, %) | 5 (38.5) | 8 (66.7) | 0.24 | |
| BMI (kg/m2) | 20.7 ± 4.3 | 23.7 ± 3.5 | 0.067 | |
| Cause of death (%) | 0.69 | |||
| non-CVD | 7 (53.8) | 8 (66.7) | ||
| CVD | 6 (46.2) | 4 (33.3) | ||
| Episode of CPA (%) | 10 (76.9) | 6 (50.0) | 0.23 | |
| Serum creatinine (mg/dL) | 0.80 ± 0.70 | 0.79 ± 0.31 | 0.97 | |
| HbA1c (%) | 5.58 ± 0.19 | 5.67 ± 0.29 | 0.36 | |
| Operative procedure (%) | 1 | |||
| PAK | 1 (7.7) | 1 (9.1) | ||
| SPK | 12 (92.3) | 11 (90.9) | ||
| Cold ischemia time (hr) | 13.2 ± 4.1 | 12.7 ± 1.9 | 0.69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aida, N.; Ito, T.; Kurihara, K.; Hiratsuka, I.; Shibata, M.; Suzuki, A.; Kenmochi, T. Evaluation of the Efficacy and Effects of Common Hepatic Artery Reconstruction in Pancreas Transplantation: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 2258. https://doi.org/10.3390/jcm11082258
Aida N, Ito T, Kurihara K, Hiratsuka I, Shibata M, Suzuki A, Kenmochi T. Evaluation of the Efficacy and Effects of Common Hepatic Artery Reconstruction in Pancreas Transplantation: A Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(8):2258. https://doi.org/10.3390/jcm11082258
Chicago/Turabian StyleAida, Naohiro, Taihei Ito, Kei Kurihara, Izumi Hiratsuka, Megumi Shibata, Atsushi Suzuki, and Takashi Kenmochi. 2022. "Evaluation of the Efficacy and Effects of Common Hepatic Artery Reconstruction in Pancreas Transplantation: A Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 8: 2258. https://doi.org/10.3390/jcm11082258






