Prognostic Impact of Acute Cardiovascular Events in COVID-19 Hospitalized Patients—Results from the CORONA Germany Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
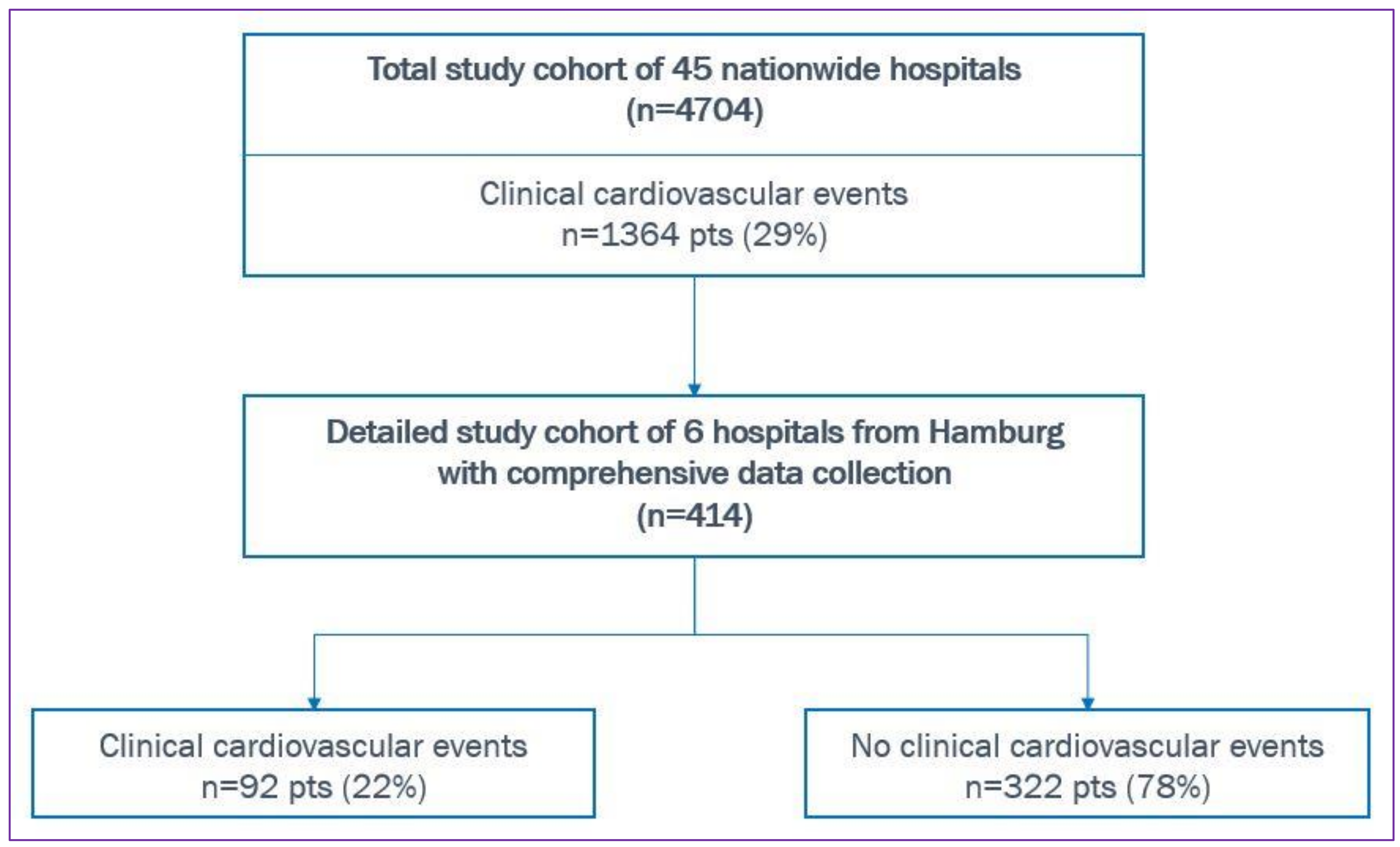
2.2. Study Cohort
2.3. Endpoints
2.4. Statistics
3. Results—Hamburg Cohort
3.1. Baseline Characteristics
3.2. Cardiovascular Comorbidities and Medication
3.3. Clinical Results
3.4. Effects on Cardiovascular Outcome
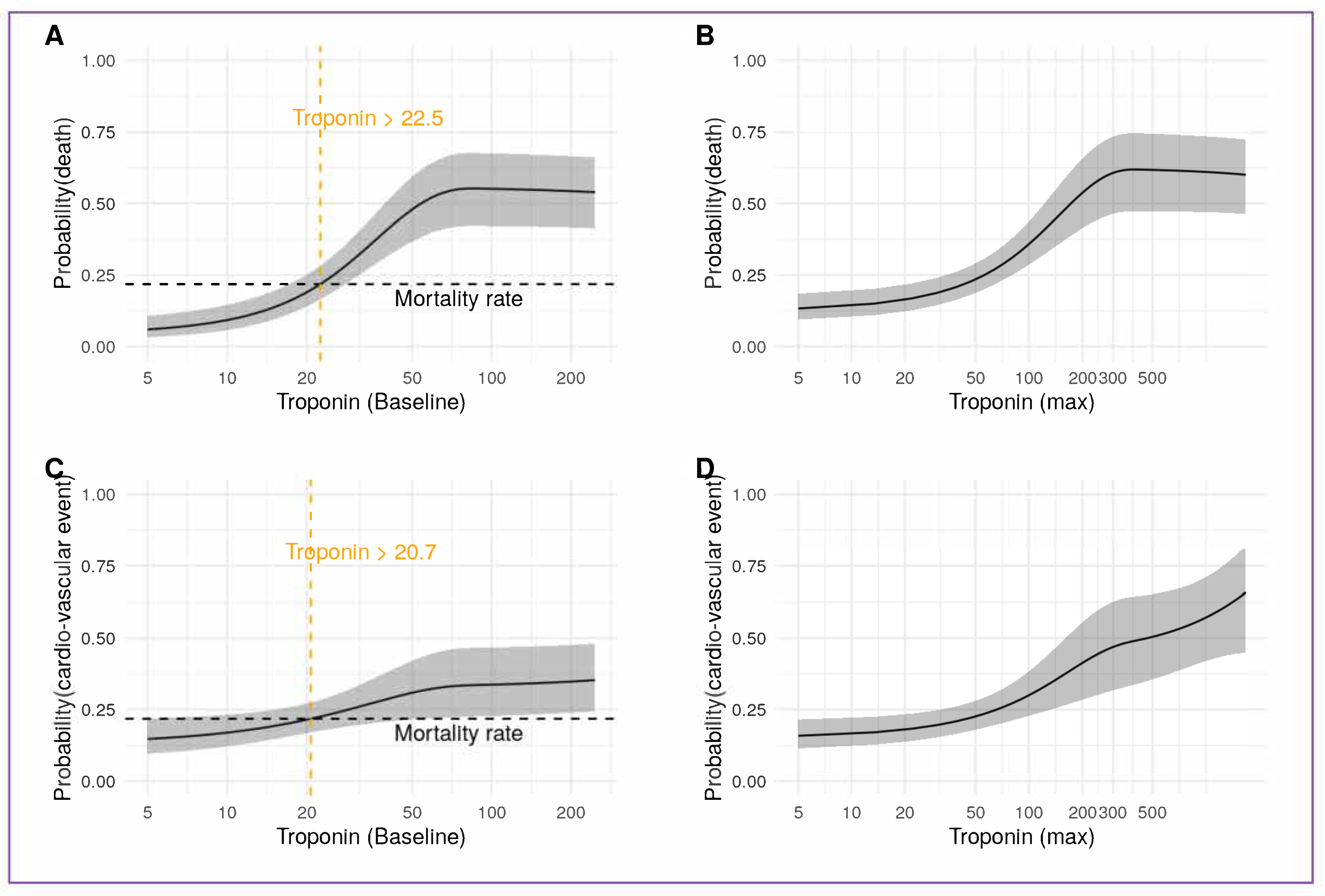
3.5. Acute Myocardial Injury and High Sensitivity Troponin
4. Discussion
4.1. Cardiovascular Events and Troponin
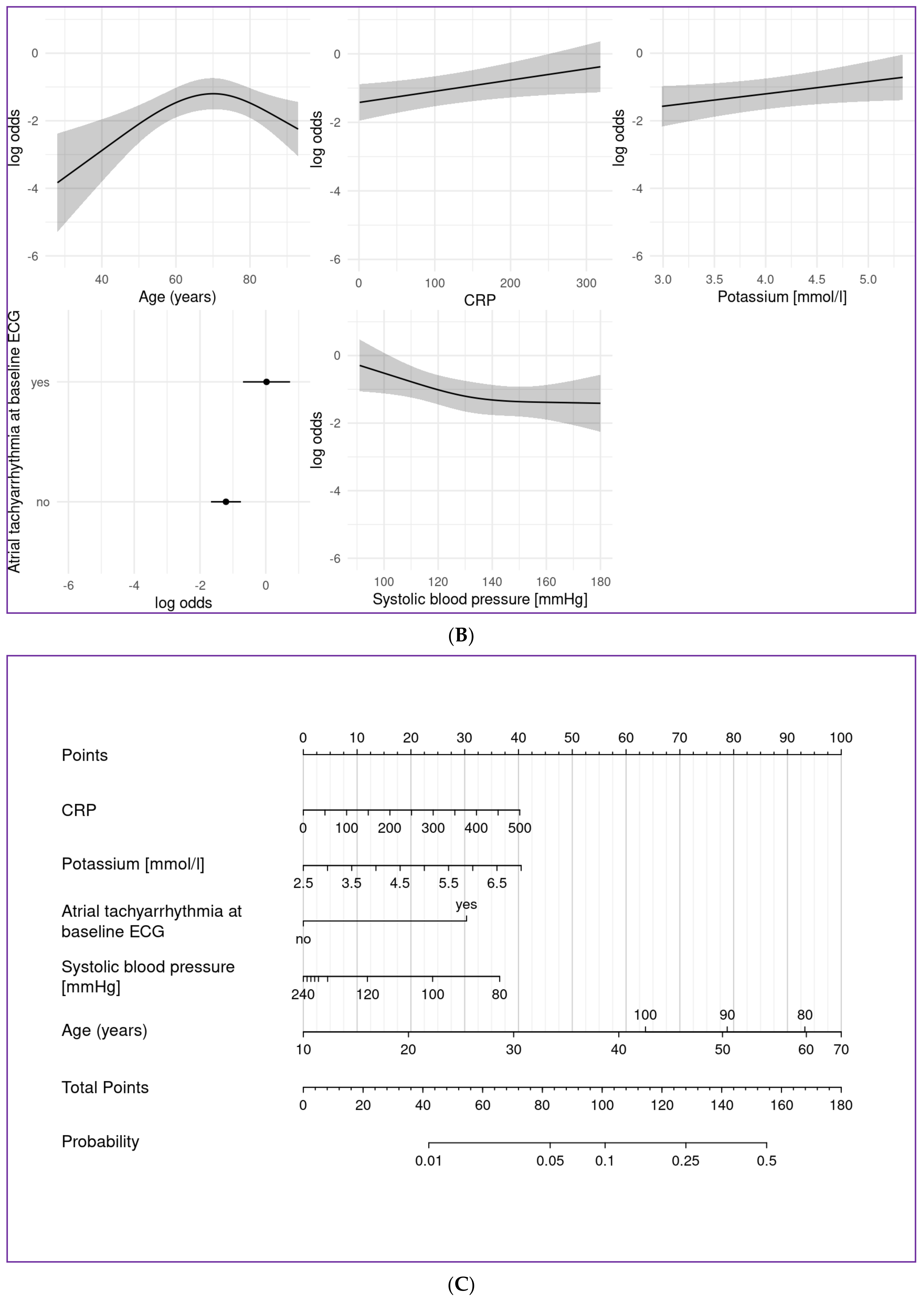
4.2. Prediction Models
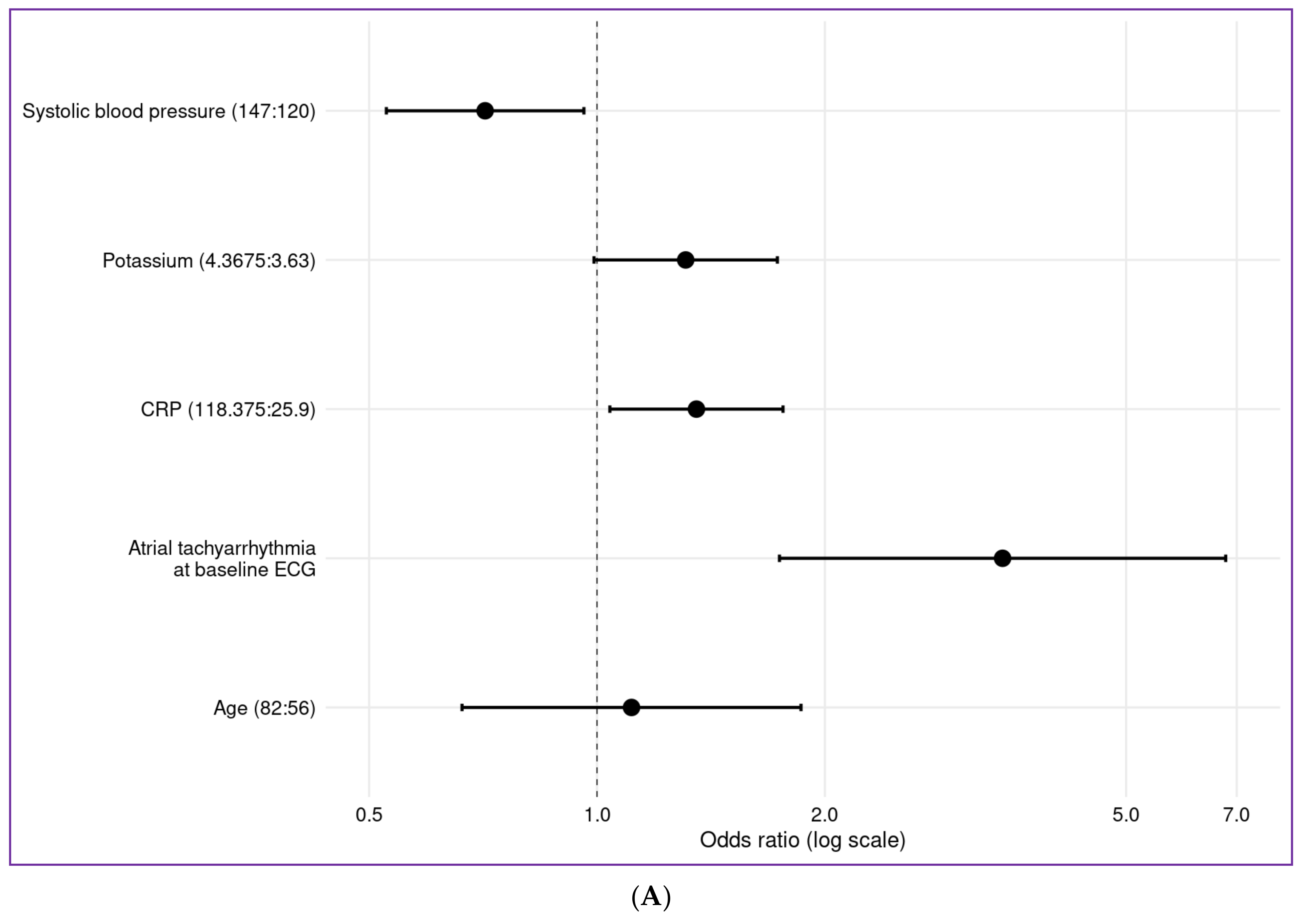
4.3. Impact of Clinical Baseline Parameters—Age
4.4. Atrial Fibrillation
4.5. Blood Pressure
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Corbett, S.; Chettibi, M.; Hayrapetyan, H.; et al. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, 618–651. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Jangaard, N.; Hosbond, S.; Diederichsen, A.C.P.; Thygesen, K.; Mickley, H. Clinical Characteristics and Outcomes of Patients with Myocardial Infarction, Myocardial Injury, and Nonelevated Troponins. Am. J. Med. 2016, 129, 446.e5–446.e21. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Alger, H.M.; Rutan, C.; Williams, J.H.; Walchok, J.G.; Bolles, M.; Hall, J.L.; Bradley, S.M.; Elkind, M.S.V.; Rodriguez, F.; Wang, T.Y.; et al. American Heart Association COVID-19 CVD Registry Powered by Get with the Guidelines. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006967. [Google Scholar] [CrossRef]
- Linschoten And, M.; Asselbergs, F.W. CAPACITY-COVID: A European Registry to determine the role of cardiovascular disease in the COVID-19 pandemic. Eur. Heart J. 2020, 41, 1795–1796. [Google Scholar] [CrossRef]
- Stefanini, G.G.; Chiarito, M.; Ferrante, G.; Cannata, F.; Azzolini, E.; Viggiani, G.; De Marco, A.; Briani, M.; Bocciolone, M.; Bragato, R.; et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart 2020, 106, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Vleck, T.; Van Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. Lancet 2020, 76, 533–546. [Google Scholar]
- Huang, D.; Yang, H.; Yu, H.; Wang, T.; Yao, R.; Liang, Z. A novel risk score to predict cardiovascular complications in patients with coronavirus disease 2019 (COVID-19): A retrospective, multicenter, observational study. Immunity Inflamm. Dis. 2020, 8, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Prebensen, C.; Røysland, R.; Søvik, S.; Sørensen, V.; Røsjø, H.; Svensson, M.; Berdal, J.E.; Myhre, P.L. Established Cardiovascular Biomarkers Provide Limited Prognostic Information in Unselected Patients Hospitalized with COVID-19. Circulation 2020, 142, 1878–1880. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Putot, A.; Bouhey, E.; Tetu, J.; Putot, S.; Ray, P.; Manckoundia, P. Troponin Elevation in Older Patients with Acute Pneumonia: Frequency and Prognostic Value. J. Clin. Med. 2020, 9, 3632. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Iodice, S.; Tersalvi, G.; Rota, I.; Ghidini, S.; Terranova, L.; Valenti, L.; Amati, F.; Aliberti, S.; et al. Prognostic parameters of in-hospital mortality in COVID-19—An Italian experience. Eur J. Clin. Investig. 2021, 51, e13629. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 7, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Dherange, P.; Lang, J.; Qian, P.; Oberfeld, B.; Sauer, W.H.; Koplan, B.; Tedrow, U. Arrhythmias and COVID-19: A Review. JACC Clin. Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, F.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Serbia, C.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the EuropeanAssociation of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Kruse, J.M.; Magomedov, A.; Kurreck, A.; Münch, F.H.; Koerner, R.; Kamhieh-Milz, J.; Kahl, A.; Gotthardt, I.; Piper, S.K.; Eckardt, K.U.; et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gessler, N.; Gunawardene, M.A.; Wohlmuth, P.; Arnold, D.; Behr, J.; Gloeckner, C.; Herrlinger, K.; Hoelting, T.; Pape, F.; Schreiber, R.; et al. Clinical outcome, risk assessment, and seasonal variation in hospitalized COVID-19 patients—Results from the CORONA Germany study. PLoS ONE 2021, 16, e0252867. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 practice guidelines for the management of arterial hypertension of the European society of cardiology and the European society of hypertension ESC/ESH task force for the management of arterial hypertension. J. Hypertens 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imazio, M.; Klingel, K.; Kindermann, I.; Brucato, A.; De Rosa, F.G.; Adler, Y.; De Ferrari, G.M. COVID-19 pandemic and troponin: Indirect myocardial injury, myocardial inflammation or myocarditis? Heart 2020, 106, 1127–1131. [Google Scholar] [CrossRef]
- Castiglione, V.; Chiriacò, M.; Emdin, M.; Taddei, S.; Vergaro, G. Statin therapy in COVID-19 infection. Eur. Heart J. 2020, 6, 258–259. [Google Scholar] [CrossRef]
| N | n = 414 | |
|---|---|---|
| Age (years) | 73 (56–82); 68 ± 18 | |
| Gender (female) | 0.42 (171) | |
| Duration between symptoms onset until hospitalization, prop(n) | 7.0 (3.0–10.0); 7.4 ± 6.4 | |
| Sick contact in patient history, prop(n) | 0.24 (98) | |
| First allocation of patients in the hospital: | ||
| normal ward, prop(n) | 0.86 (349) | |
| intermediate care unit, prop(n) | 0.02 (9) | |
| intensive care unit, prop(n) | 0.12 (47) | |
| Hospitalization for elective procedure, prop(n) | 0.02 (6) | |
| Already hospitalized, prop(n) | 0.00 (1) | |
| Baseline symptoms | ||
| Cough, prop(n) | 0.51 (210) | |
| Fever (>38.5 °C), prop(n) | 0.47 (196) | |
| Dyspnea, prop(n) | 0.53 (219) | |
| Headache, prop(n) | 0.08 (34) | |
| Diarrhea, prop(n) | 0.15 (62) | |
| Expectoration, prop(n) | 0.14 (57) | |
| Sore throat, prop(n) | 0.07 (29) | |
| Loss of smell/taste, prop(n) | 0.04 (18) | |
| Body aches, prop(n) | 0.16 (66) | |
| Nausea, prop(n) | 0.16 (66) | |
| Chills, prop(n) | 0.08 (33) | |
| Body temperature | 37.5 ± 1.2 | |
| Glasgow coma scale < 15 points, prop(n) | 0.03 (12) | |
| Baseline vital parameters | ||
| Heart rate (/min) | 88 ± 19 | |
| Systolic blood pressure (mmHg) | 134 ± 22 | |
| Diastolic blood pressure (mmHg) | 77 ± 12 | |
| Respiratory rate (/min) | 19.6 ± 6.2 | |
| spO2 (%) | 93.2 ± 6.2 | |
| Baseline laboratory findings | ||
| Lymphocytes, /nL | 219 | 1.0 (0.6–1.5); 1.8 ± 3.7 |
| Leukocytes, /nL | 402 | 7.0 (5.2–9.9); 8.1 ± 4.2 |
| Neutrophile, /nL | 240 | 5.0 (3.4–7.6); 7.0 ± 12.4 |
| D-Dimer, mg/L | 214 | 1.04 (0.53–2.13); 4.47 ± 14.60 |
| Creatinine, mg/dL | 398 | 1.1 (0.8–1.4); 1.4 ± 1.7 |
| C-reactive protein, mg/L | 398 | 65 (28–121); 87± 81 |
| Lactate dehydrogenase, U/L | 284 | 329 (256–455); 436 ± 832 |
| Activated partial thromboplastin time, s | 297 | 32 (29–37); 35 ± 15 |
| Potassium, mmol/L | 395 | 3.97 (3.62–4.36); 4.03 ± 0.63 |
| Procalcitonin, µg/L | 252 | 0.10 (0.05–0.32); 1.61 ± 11.68 |
| High sensitivity troponin I, ng/L | 311 | 14 (8–37); 537 ± 7022 |
| Hemoglobin, g/dL | 400 | 13.3 (11.7–14.6); 13.1± 2.5 |
| Thrombocytes, /nL | 395 | 208 (160–280); 226 ± 94 |
| International normalized ratio (INR) | 336 | 1.11 (1.03–1.23); 1.24 ± 0.54 |
| Sodium, mmol/L | 395 | 137.0 (134.0–140.0); 137.4 ± 5.9 |
| Aspartate Aminotransferase, U/L | 95 | 48 (32–82); 98 ± 277 |
| Bilirubin, mg/dL | 86 | 0.60 (0.40–0.80); 0.78 ± 0.90 |
| N/L Ratio | 217 | 5.0 (2.9–9.5) |
| Quick, % | 336 | 84 (71–95); 81 ± 21 |
| Glomerular filtration rate, mL/min | 397 | 65 (43–85); 61 ± 25 |
| Thyroid stimulating hormon, mU/L | 303 | 1.0 (0.6–1.6); 1.6 ± 3.5 |
| Glycated Hemoglobin (HbA1c) (%) | 41 | 6.3 (5.8–8.4); 24.8 ± 98.4 |
| Interleukin 6, pg/mL | 22 | 47 (22–102); 2774 ± 12488 |
| Lactate (mmol/dL) | 282 | 1.4 (1.0–2.0); 3.3 ± 16.1 |
| BE (Base excess), mmol/L | 212 | 1.2 (1.3- 3.2); 1.0 ± 8.3 |
| Creatine Kinase (CK), U/L | 333 | 116 (58–314); 1734 ± 23932 |
| CK-myocardial band (CK-MB), U/L | 209 | 25 (18–41); 40 ± 49 |
| N-terminal prohormone of brain natriuretic peptide (NT-proBNP), ng/L | 26 | 1241 (169–4190); 5052 ± 7735 |
| Troponin, ng/L | 311 | 14 (8–37); 537 ± 7022 |
| Baseline Electrocardiogram | ||
| Rhythm at baseline | ||
| Sinus rhythm, prop(n) | 0.84 (286) | |
| Atrial fibrillation, prop(n) | 0.16 (53) | |
| Heart Rate, /min | 88 (77–103); 91 ± 22 | |
| ST-segment abnormalities * | 0.1 (40) | |
| Intensive care treatment | ||
| Need for ventilation, prop(n) | 414 | 0.21 (86) |
| Hours of ventilation | 86 | 354 (168–582); 479± 621 |
| primary non-invasive ventilation, prop(v) | 86 | 0.26 (22) |
| primary invasive ventilation, prop(n) | 86 | 0.74 (64) |
| progress from non-invasive to invasive ventilation, prop(n) | 22 | 0.68 (15) |
| days of intensive care treatment | 116 | 12 (4–23); 16 ±14 |
| Extracorporeal membrane oxygenation therapy, prop(n) | 414 | 0.02 (10) |
| Invasive coronary angiogram (ICA), prop(n) | 0.05 (19) | |
| -requiring percutaneous coronary intervention of these with (ICA),prop(n) | 0.58 (11) |
| (n = 414) | |
|---|---|
| Comorbidities | |
| Diabetes mellitus, prop(n) | 0.25 (103) |
| Hypertension, prop(n) | 0.55 (226) |
| Dyslipidemia, prop(n) | 0.15 (61) |
| Smoking, prop(n) | 0.07 (28) |
| Cardiomyopathy, prop(n) | 0.05 (20) |
| Coronary artery disease, prop(n) | 0.17 (69) |
| Myocardial infarction, prop(n) | 0.07 (29) |
| CABG *, prop(n) | 0.03 (12) |
| Prior percutaneous coronary intervention, prop(n) | 0.07 (31) |
| Vascular disease †, prop(n) | 0.21 (87) |
| Prior arrhythmias, prop(n) | 0.18 (75) |
| Implanted device ‡, prop(n) | 0.04 (16) |
| Congenital heart disease, prop(n) | 0.01 (5) |
| Chronic kidney disease, prop(n) | 0.19 (79) |
| Chronic liver disease, prop(n) | 0.02 (9) |
| Pulmonary disease, prop(n) | 0.15 (64) |
| Medication | |
| Antiplatelet therapy, prop(n) | 0.23 (96) |
| Oral anticoagulation, prop(n) | 0.15 (63) |
| ACE-Inhibitor/ARB §, prop(n) | 0.37 (155) |
| Aldosterone Antagonist, prop(n) | 0.06 (23) |
| Angiotensin-receptor-neprilysin-inhibito, prop(n) | 0 (2) |
| Antidiabetic medication II, prop(n) | 0.19 (79) |
| Diuretics, prop(n) | 0.25 (103) |
| Statins, prop(n) | 0.21 (88) |
| Betablocker, prop(n) | 0.3 (124) |
| Antiarrhythmic drugs, prop(n) | 0.05 (22) |
| Immuno-suppressive medication #, prop(n) | 0.08 (33) |
| Prednisolone, prop(n) | 0.04 (18) |
| Proton pump inhibitor, prop(n) | 0.28 (115) |
| Hamburg Cohort n = 414 | Total Cohort n = 4704 | |
|---|---|---|
| Primary Endpoints | ||
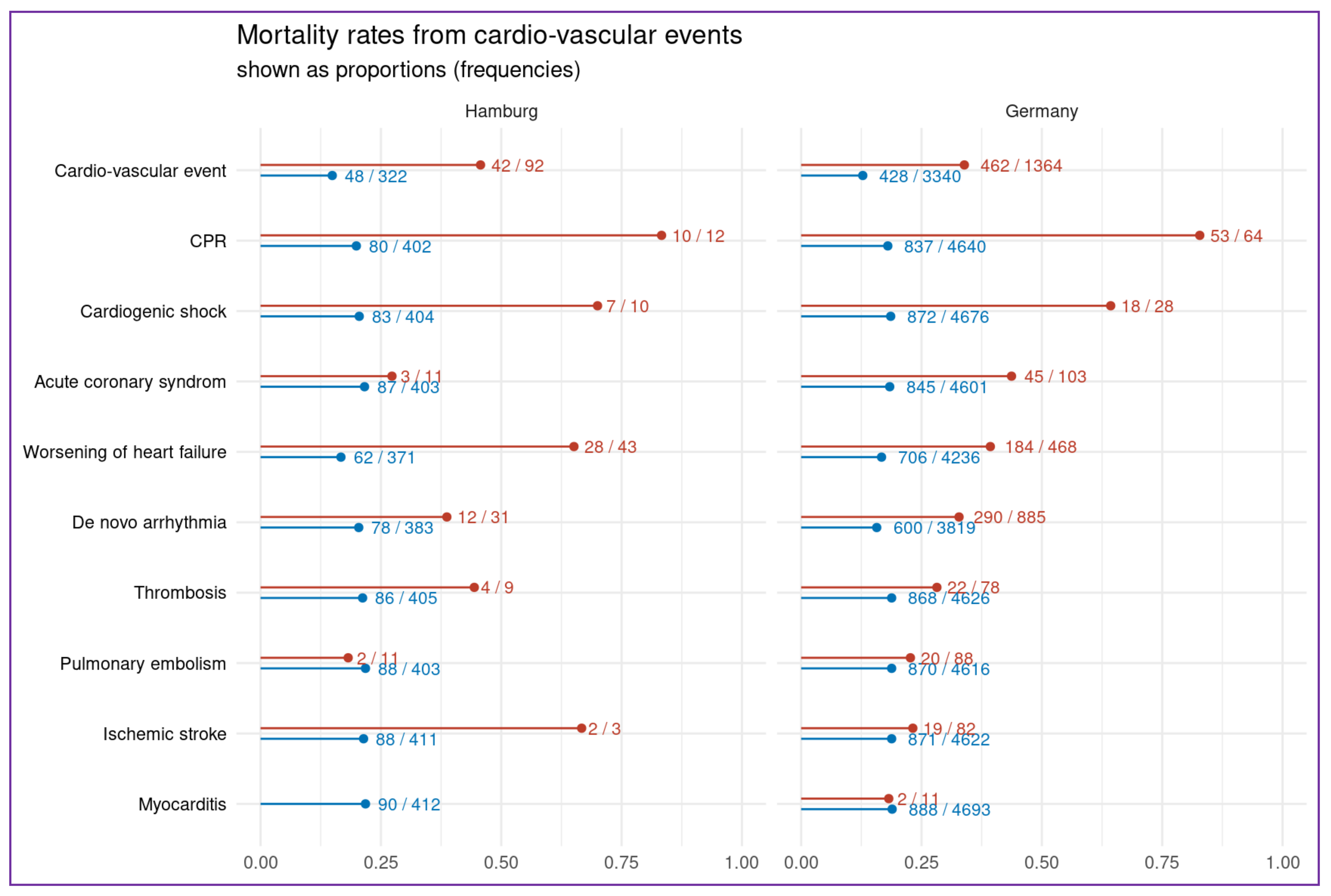
| At least one cardiovascular event per patient, prop(n) | 0.22 (92) | 0.29 (1364) |
| Number of total cardiovascular events, n (per patient, pp) | 132 (1.4 pp) | 1845 (1.4 pp) |
| Cardiopulmonary resuscitation, prop(n) | 0.03 (12) | 0.01 (64) |
| Cardiogenic shock, prop(n) | 0.02 (10) | 0.01 (28) |
| Acute coronary syndrome, prop(n): | 0.03 (11) | 0.02 (103) |
| STEMI * | 0.18 (2) | 0.19 (20) |
| NSTEMI † | 0.82 (9) | 0.81 (83) |
| Worsening or new onset heart failure, prop(n) | 0.1 (43) | 0.1 (464) |
| De novo Arrhythmia (all), prop(n): | 0.07 (31) | 0.20 (927) |
| Atrial tachyarrhythmias ‡, prop(n) | 0.87 (27) | 0.90 (837) |
| Other arrhythmias §, prop(n): | 0.13 (4) II | 0.10 (90) |
| - Supraventricular arrhythmias, prop(n): | 0 | N/A |
| - Ventricular arrhythmias, prop(n): | 0.13 (4) | 0.03 (24) |
| - Ventricular tachycardia, prop(n): | 0.06 (2) | N/A |
| ◦ Ventricular fibrillation, prop(n): | 0.06 (2) | N/A |
| Acute Myocarditis, prop(n) | 0.005 (2) | 0 (11) |
| Pulmonary embolism, prop(n) | 0.03 (11) | 0.02 (88) |
| Thrombosis, prop(n) | 0.02 (9) | 0.02 (78) |
| Ischemic stroke, prop(n) | 0.01 (3) | 0.02 (82) |
| Mortality | ||
| Death, prop(n) | (90) | 0.19 (890) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardene, M.A.; Gessler, N.; Wohlmuth, P.; Heitmann, K.; Anders, P.; Jaquet, K.; Herborn, C.U.; Arnold, D.; Bein, B.; Bergmann, M.W.; et al. Prognostic Impact of Acute Cardiovascular Events in COVID-19 Hospitalized Patients—Results from the CORONA Germany Study. J. Clin. Med. 2021, 10, 3982. https://doi.org/10.3390/jcm10173982
Gunawardene MA, Gessler N, Wohlmuth P, Heitmann K, Anders P, Jaquet K, Herborn CU, Arnold D, Bein B, Bergmann MW, et al. Prognostic Impact of Acute Cardiovascular Events in COVID-19 Hospitalized Patients—Results from the CORONA Germany Study. Journal of Clinical Medicine. 2021; 10(17):3982. https://doi.org/10.3390/jcm10173982
Chicago/Turabian StyleGunawardene, Melanie A., Nele Gessler, Peter Wohlmuth, Kathrin Heitmann, Philipp Anders, Kai Jaquet, Christoph U. Herborn, Dirk Arnold, Berthold Bein, Martin W. Bergmann, and et al. 2021. "Prognostic Impact of Acute Cardiovascular Events in COVID-19 Hospitalized Patients—Results from the CORONA Germany Study" Journal of Clinical Medicine 10, no. 17: 3982. https://doi.org/10.3390/jcm10173982
APA StyleGunawardene, M. A., Gessler, N., Wohlmuth, P., Heitmann, K., Anders, P., Jaquet, K., Herborn, C. U., Arnold, D., Bein, B., Bergmann, M. W., Herrlinger, K. R., Stang, A., Schreiber, R., Wesseler, C., & Willems, S. (2021). Prognostic Impact of Acute Cardiovascular Events in COVID-19 Hospitalized Patients—Results from the CORONA Germany Study. Journal of Clinical Medicine, 10(17), 3982. https://doi.org/10.3390/jcm10173982






