Infrared Thermal Imaging as a Novel Non-Invasive Point-of-Care Tool to Assess Filarial Lymphoedema
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Participants
2.2. Lymphoedema Stage
- (i)
- No lymphoedema (Stage 0);
- (ii)
- Mild lymphoedema (Stage 1), variable swelling may reduce on elevation or overnight, no skin changes;
- (iii)
- Moderate lymphoedema (Stage 2), permanent shallow skin folds, irreversible limb enlargement, presence of minor skin changes such as small knobs;
- (iv)
- Severe lymphoedema (Stage 3), permanent deep skin folds, pathological skin changes including large knobs, sclerosis, discolouration and/or mossy lesions (papillomatosis).
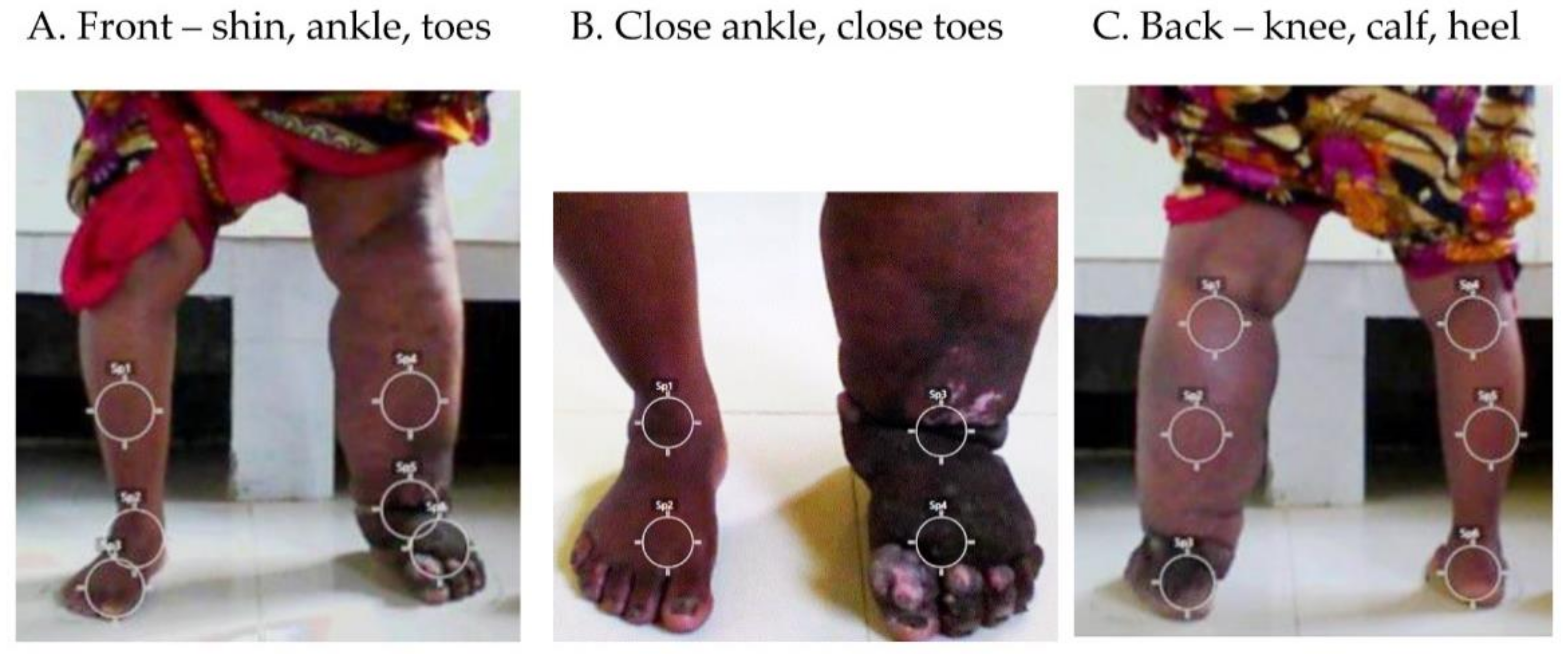
2.3. Temperature and Regions of Interest (ROIs)
2.4. Objective Limb Measurements
2.5. Room Measures
2.6. Data Collection, Visualisation and Analysis
2.7. Ethics Statement
2.8. COVID-19 Precautions
3. Results
3.1. Summary
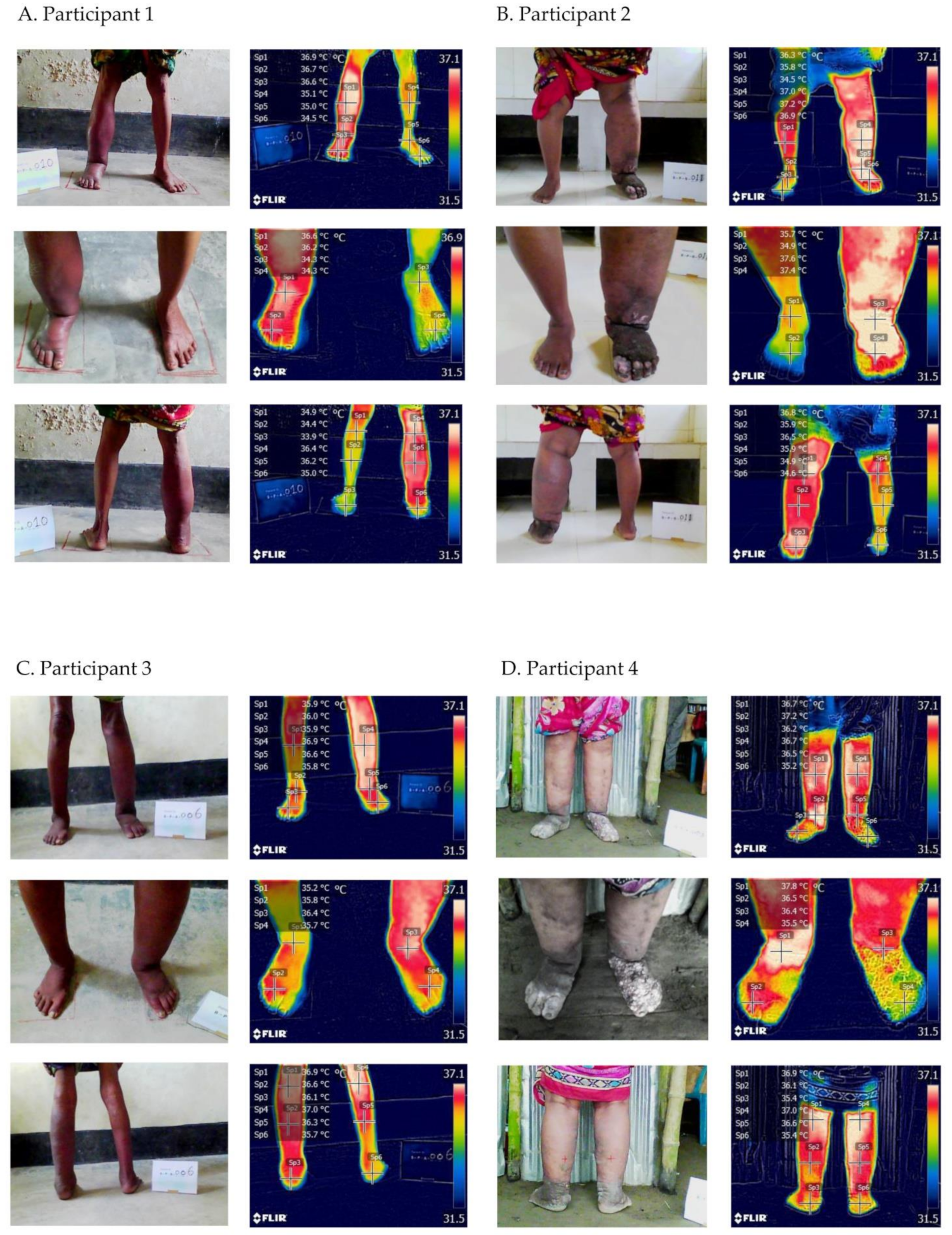
3.2. Temperature Visualisation
3.2.1. Participant 1
- Right leg—moderate lymphoedema (Stage 2) with shallow skin folds
- ⚬
- Temperatures—shin 36.9 °C, ankle 36.7 °C, foot 36.6 °C, close ankle 36.6 °C, close foot 36.2 °C, back knee 36.4 °C, calf 36.2 °C, heel 35.0 °C
- ⚬
- Circumference 33.0 cm and compressibility 2.96
- Left leg—no lymphoedema (Stage 0)
- ⚬
- Temperatures—shin 35.1 °C, ankle 35.0 °C, foot 34.5 °C, close ankle 34.3 °C, close foot 34.3 °C, back knee 34.9 °C, calf 34.4 °C, heel 33.9 °C
- ⚬
- Circumference 23 cm and compressibility 3.995
- ADLA presence, one in last month, three in last six months
- Room temperature 33.7 °C; room humidity 55%
3.2.2. Participant 2
- Right leg—no lymphoedema (Stage 0)
- ⚬
- Temperatures—shin 36.3 °C. ankle 35.7 °C, foot 34.3 °C, close ankle 35.7 °C, close toes 33.9 °C, back knee 35.9 °C, calf 34.9 °C, heel 34.6 °C
- ⚬
- Circumference 28.5 cm and compressibility 3.315
- Left leg—severe lymphoedema (Stage 3) with deep skin folds, skin knobs and mossy lesions
- ⚬
- Temperatures—shin 37.0 °C, ankle 37.2 °C, foot 36.6 °C, close ankle 37.6 °C, close toes 36.4 °C, back knee 36.8 °C, calf 35.9 °C, heel 36.5 °C
- ⚬
- Circumference 46.0 cm and compressibility 3.68
- ADLA presence, one in last month, three in last six months
- Room temp 30.5 °C, room humidity 80%
3.2.3. Participant 3
- Right leg—mild lymphoedema (Stage 1)
- ⚬
- Temperatures—shin 35.9 °C; ankle 36.0 °C; foot 35.9 °C, close ankle 35.2 °C; close foot 35.8 °C; back knee 37.0 °C, calf 36.3 °C, heel 35.7 °C
- ⚬
- Circumference 25.7 cm and Compressibility 2.81
- Left leg—moderate lymphoedema (Stage 2) with shallow skin folds
- ⚬
- Temperatures—shin 36.9 °C; ankle 36.6 °C, foot 35.8 °C, close ankle 36.4 °C, close foot 35.7 °C, back knee 36.9 °C, calf 36.6 °C, heel 36.1 °C
- ⚬
- Circumference 35.5 cm and Compressibility 2.025
- ADLA presence, one in last month, eight in last six months
- Room temp 32.3 °C, room humidity 70%
3.2.4. Participant 4
- Right leg—severe lymphoedema (Stage 3)
- ⚬
- Temperatures—shin 36.7 °C; ankle 37.2 °C; foot 36.2 °C, close ankle 37.8 °C, close foot 36.5 °C, back knee 37.0 °C, calf 36.6 °C, heel 35.4 °C
- ⚬
- Circumference 41.0 cm and Compressibility 2.470
- Left leg—severe lymphoedema (Stage 3) with deep ankle folds, skin knobs and mossy lesions
- ⚬
- Temperatures—shin 36.7 °C; ankle 36.5 °C, foot 35.2 °C, close ankle 36.4 °C; close foot 35.5 °C, back knee 36.9 °C, calf 36.1 °C, heel 35.4 °C
- ⚬
- Circumference 40.0 cm and Compressibility 2.475
- ADLA presence, one in last month, one in last six months
- Room temp 29.1 °C, room humidity 84%
3.3. Temperature Differences at Each Region of Interest (ROI) by Lymphoedema Stage
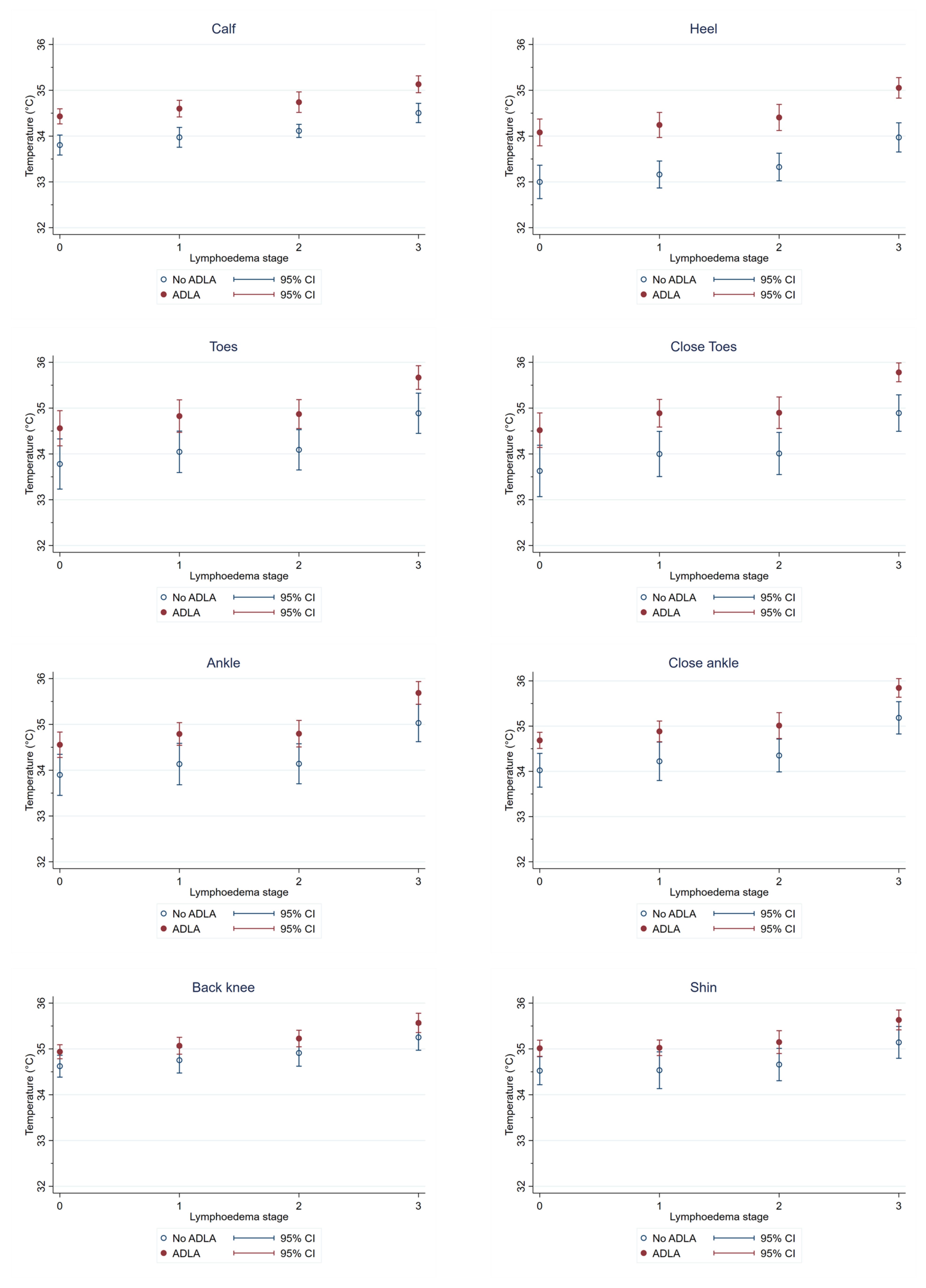
3.4. Temperature Differences at Each Region of Interest (ROI) by ADLA History
3.5. Correlation between Temperature Measures at Different Regions of Interest (ROIs)
3.6. Correlation between Objective Outcome Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Furlong-Silva, J.; Cross, S.D.; Marriott, A.E.; Pionnier, N.; Archer, J.; Steven, A.; Merker, S.S.; Mack, M.; Hong, Y.-K.; Taylor, M.J.; et al. Tetracyclines Improve Experimental Lymphatic Filariasis Pathology by Disrupting Interleukin-4 Receptor-Mediated Lymphangiogenesis. J. Clin. Invest. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Lymphatic Filariasis-Managing Morbidity and Preventing Disability: An Aide-Memoire for National Programme Managers, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. Lymphatic Filariasis. Available online: https://www.who.int/lymphatic_filariasis/en/ (accessed on 4 July 2019).
- Ton, T.G.; Mackenzie, C.; Molyneux, D. The Burden of Mental Health in Lymphatic Filariasis. Infect. Dis. Poverty 2015, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.C.; Yamauchi, M.; Mkwanda, S.Z.; Ndhlovu, P.; Matipula, D.E.; Mackenzie, C.; Kelly-Hope, L.A. Measuring the Physical and Economic Impact of Filarial Lymphoedema in Chikwawa District, Malawi: A Case-Control Study. Infect. Dis. Poverty 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Mensah, E.O.; Aikins, M.K.; Gyapong, M.; Anto, F.; Bockarie, M.J.; Gyapong, J.O. Extent of Integration of Priority Interventions into General Health Systems: A Case Study of Neglected Tropical Diseases Programme in the Western Region of Ghana. PLoS Negl. Trop. Dis. 2016, 10, e0004725. [Google Scholar] [CrossRef] [PubMed]
- Semrau, M.; Ali, O.; Deribe, K.; Mengiste, A.; Tesfaye, A.; Kinfe, M.; Bremner, S.A.; Hounsome, N.; Kelly-Hope, L.A.; MacGregor, H.; et al. EnDPoINT: Protocol for an Implementation Research Study to Integrate a Holistic Package of Physical Health, Mental Health and Psychosocial Care for Podoconiosis, Lymphatic Filariasis and Leprosy into Routine Health Services in Ethiopia. BMJ Open 2020, 10, e037675. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Graves, P.; Gordon, S. Intrarater Reliability of Tonometry and Bioimpedance Spectroscopy to Measure Tissue Compressibility and Extracellular Fluid in the Legs of Healthy Young People in Australia and Myanmar. Lymphat. Res. Biol. 2017, 15, 57–63. [Google Scholar] [CrossRef]
- Stocks, M.E.; Freeman, M.C.; Addiss, D.G. The Effect of Hygiene-Based Lymphedema Management in Lymphatic Filariasis-Endemic Areas: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2015, 9, e0004171. [Google Scholar] [CrossRef]
- Douglass, J.; Kelly-Hope, L. Comparison of Staging Systems to Assess Lymphedema Caused by Cancer Therapies, Lymphatic Filariasis, and Podoconiosis. Lymphat. Res. Biol. 2019, 17, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Addiss, D.; Dreyer, P.; Noroes, J. Basic Lymphoedema Management: Treatment and Prevention of Problems Associated with Lymphatic Filariasis; Hollis Publishing Company: Hollis, NY, USA, 2002. [Google Scholar]
- Pani, S.P.; Vanamail, P.; Yuvaraj, J. Limb Circumference Measurement for Recording Edema Volume in Patients with Filarial Lymphedema. Lymphology 1995, 28, 57–63. [Google Scholar]
- International Society of Lymphology. The Diagnosis and Treatment of Peripheral Lymphoedema; 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Douglass, J.; Mableson, H.; Martindale, S.; Karim, J.; Mahmood, A.S.M.S.; Hailekiros, F.; Kelly-Hope, L. Intra-Rater Reliability and Agreement of the Indurometer When Used to Assess Mid-Calf Tissue Compressibility Among People Affected by Moderate to Severe lymphedema in Bangladesh and Ethiopia. Lymphat. Res. Biol. 2019, 18, 374–380. [Google Scholar] [CrossRef]
- Yahathugoda, C.; Weiler, M.J.; Rao, R.; De Silva, L.; Dixon, J.B.; Weerasooriya, M.V.; Weil, G.J.; Budge, P.J. Use of a Novel Portable Three-Dimensional Imaging System to Measure Limb Volume and Circumference in Patients with Filarial Lymphedema. Am. J. Trop. Med. Hyg. 2017, 97, 836–1842. [Google Scholar] [CrossRef]
- Zhou, C.; Yahathugoda, C.; De Silva, L.; Rathnapala, U.; Owen, G.; Weerasooriya, M.; Rao, R.U.; Weil, G.J.; Budge, P.J. Portable Infrared Imaging for Longitudinal Limb Volume Monitoring in Patients with Lymphatic Filariasis. PLoS Negl. Trop. Dis. 2019, 13, e0007762. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.F. A Reappraisal of the Use of Infrared Thermal Image Analysis in Medicine. IEEE Trans. Med. Imaging 1998, 17, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Ring, E.F.J.; Ammer, K. Infrared Thermal Imaging in Medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Bagavathiappan, S.; Jayakumar, T.; Philip, J. Medical Applications of Infrared Thermography: A Review. Infrared Phys. Technol. 2012, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.G.; Costello, J.T.; Brito, C.J.; Adamczyk, J.G.; Ammer, K.; Bach, A.J.E.; Costa, C.M.A.; Eglin, C.; Fernandes, A.A.; Fernández-Cuevas, I.; et al. Thermographic Imaging in Sports and Exercise Medicine: A Delphi Study and Consensus Statement on the Measurement of Human Skin Temperature. J. Therm. Biol. 2017, 69, 155–162. [Google Scholar] [CrossRef]
- Ko, L.N.; Raff, A.B.; Garza-Mayers, A.C.; Dobry, A.S.; Ortega-Martinez, A.; Anderson, R.R.; Kroshinsky, D. Skin Surface Temperatures Measured by Thermal Imaging Aid in the Diagnosis of Cellulitis. J. Investig. Dermatol. 2018, 138, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; Kwon, S.R.; Jung, K.-H.; Joo, K.; Park, S.-G.; Park, W. Digital Thermography of the Fingers and Toes in Raynaud′s Phenomenon. J. Korean Med. Sci. 2014, 29, 502–506. [Google Scholar] [CrossRef]
- Chojnowski, M. Infrared Thermal Imaging in Connective Tissue Diseases. Reumatologia 2017, 55, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.; Thomson, R.; Bamford, A.; Moiemen, N. A Pilot Evaluation Study of High Resolution Digital Thermal Imaging in the Assessment of Burn Depth. Burns 2013, 39, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Nakagami, G.; Goto, T.; Noguchi, H.; Oe, M.; Miyagaki, T.; Hayashi, A.; Sasaki, S.; Sanada, H. Use of Smartphone Attached Mobile Thermography Assessing Subclinical Inflammation: A Pilot Study. J. Wound Care 2016, 25, 177–180, 182. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, P.; Bammigatti, C.; Deepanjali, S.; Suryanarayana, B.S.; Kadhiravan, T. Point-of-Care Infrared Thermal Imaging for Differentiating Venomous Snakebites from Non-Venomous and Dry Bites. PLoS Negl. Trop. Dis. 2021, 15, e0008580. [Google Scholar] [CrossRef]
- Karim, M.J.; Haq, R.; Mableson, H.E.; Mahmood, A.S.M.S.; Rahman, M.; Chowdhury, S.M.; Rahman, A.K.M.F.; Hafiz, I.; Betts, H.; Mackenzie, C.; et al. Developing the First National Database and Map of Lymphatic Filariasis Clinical Cases in Bangladesh: Another Step Closer to the Elimination Goals. PLoS Negl. Trop. Dis. 2019, 13, e0007542. [Google Scholar] [CrossRef]
- Skouroliakou, A.; Seferis, I.; Sianoudis, I.; Valais, I.; Fragopoulou, A.; Margaritis, L. Infrared Thermography Imaging: Evaluating Surface Emissivity and Skin Thermal Response to IR Heating. E J. Sci. Technol. 2014, 3, 9–14. [Google Scholar]
- Costa, C.M.A.; Moreira, D.G.; Sillero-Quintana, M.; Brito, C.J.; de Azambuja Pussieldi, G.; de Andrade Fernandes, A.; Cano, S.P.; Bouzas Marins, J.C. Daily Rhythm of Skin Temperature of Women Evaluated by Infrared Thermal Imaging. J. Therm. Biol. 2018, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Mableson, H.E.; Martindale, S.; Kelly-Hope, L.A. An Enhanced Self-Care Protocol for People Affected by Moderate to Severe Lymphedema. Methods Protoc. 2019, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Hailekiros, F.; Martindale, S.; Mableson, H.; Seife, F.; Bishaw, T.; Nigussie, M.; Meribo, K.; Tamiru, M.; Agidew, G.; et al. Addition of Lymphatic Stimulating Self-Care Practices Reduces Acute Attacks among People Affected by Moderate and Severe Lower-Limb Lymphedema in Ethiopia, a Cluster Randomized Controlled Trial. J. Clin. Med. 2020, 9, 4077. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Mableson, H.; Martindale, S.; Jhara, S.T.; Karim, M.J.; Rahman, M.M.; Al Kawsar, A.; Khair, A.; Mahmood, A.S.; Rahman, A.F.; et al. Effect of an Enhanced Self-Care Protocol on Lymphedema Status among People Affected by Moderate to Severe Lower-Limb Lymphedema in Bangladesh, a Cluster Randomized Controlled Trial. J. Clin. Med. 2020, 9, 2444. [Google Scholar] [CrossRef]
- FLIR. Available online: https://www.flir.com/ (accessed on 1 April 2021).
- Vanderstelt, S.; Pallotta, O.J.; McEwen, M.; Ullah, S.; Burrow, L.; Piller, N. Indurometer vs. Tonometer: Is the Indurometer Currently Able to Replace and Improve Upon the Tonometer? Lymphat. Res. Biol. 2015, 13, 131–136. [Google Scholar] [CrossRef]
- Ministry of Health and Family Welfare Government of Bangladesh Environment and Social Management Framework for COVID-19 Emergency Response and Pandemic Preparedness Project. Available online: https://dghs.gov.bd/index.php/en/home/5454-environment-and-social-management-framework-covid-19-emergency-response-and-pandemic-preparedness-project (accessed on 1 April 2021).
- Carlson, J.A. Lymphedema and Subclinical Lymphostasis (Microlymphedema) Facilitate Cutaneous Infection, Inflammatory Dermatoses, and Neoplasia: A Locus Minoris Resistentiae. Clin. Dermatol. 2014, 32, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.; Graves, P.; Lindsay, D.; Becker, L.; Roineau, M.; Masson, J.; Aye, N.; Win, S.; Wai, T.; Win, Y.; et al. Lymphatic Filariasis Increases Tissue Compressibility and Extracellular Fluid in Lower Limbs of Asymptomatic Young People in Central Myanmar. Trop. Med. Infect. Dis. 2017, 2, 50. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Philip, J.; Jayakumar, T.; Raj, B.; Rao, P.N.S.; Varalakshmi, M.; Mohan, V. Correlation between Plantar Foot Temperature and Diabetic Neuropathy: A Case Study by Using an Infrared Thermal Imaging Technique. J. Diabetes Sci. Technol. 2010, 4, 1386–1392. [Google Scholar] [CrossRef]
- Mues, K.E.; Deming, M.; Kleinbaum, D.G.; Budge, P.J.; Klein, M.; Leon, J.S.; Prakash, A.; Rout, J.; Fox, L.M. Impact of a Community-Based Lymphedema Management Program on Episodes of Adenolymphangitis (ADLA) and Lymphedema Progression-Odisha State, India. PLoS Negl. Trop. Dis. 2014, 8, e3140. [Google Scholar] [CrossRef] [PubMed]
- Budge, P.J.; Little, K.M.; Mues, K.E.; Kennedy, E.D.; Prakash, A.; Rout, J.; Fox, L.M. Impact of Community-Based Lymphedema Management on Perceived Disability among Patients with Lymphatic Filariasis in Orissa State, India. PLoS Negl. Trop. Dis. 2013, 7, e2100. [Google Scholar] [CrossRef] [PubMed]
- McPherson, T.; Fay, M.P.; Singh, S.; Penzer, R.; Hay, R. Health Workers′ Agreement in Clinical Description of Filarial Lymphedema. Am. J. Trop. Med. Hyg. 2006, 74, 500–504. [Google Scholar] [CrossRef]
- Douglass, J.; Graves, P.; Gordon, S. Self-Care for Management of Secondary Lymphedema: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004740. [Google Scholar] [CrossRef]
- Nahm, F.S. Infrared Thermography in Pain Medicine. Korean J. Pain 2013, 26, 219–222. [Google Scholar] [CrossRef]
- Martindale, S.; Mkwanda, S.Z.; Smith, E.; Molyneux, D.; Stanton, M.C.; Kelly-Hope, L.A. Quantifying the Physical and Socio-Economic Burden of Filarial Lymphoedema in Chikwawa District, Malawi. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 759–767. [Google Scholar] [CrossRef]
- Patrick Brooks, J.; Brooks, J.M.; Seals, T. Smartphone Thermal Imaging in the Detection of Testicular Ischemia. Urology 2021. [Google Scholar] [CrossRef]
- Deribe, K.; Beng, A.A.; Cano, J.; Njouendo, A.J.; Fru-Cho, J.; Awah, A.R.; Eyong, M.E.; Chounna Ndongmo, P.W.; Giorgi, E.; Pigott, D.M.; et al. Mapping the Geographical Distribution of Podoconiosis in Cameroon Using Parasitological, Serological, and Clinical Evidence to Exclude Other Causes of Lymphedema. PLoS Negl. Trop. Dis. 2018, 12, e0006126. [Google Scholar] [CrossRef] [PubMed]
- Deribe, K.; Florence, L.; Kelemework, A.; Getaneh, T.; Tsegay, G.; Cano, J.; Giorgi, E.; Newport, M.J.; Davey, G. Developing and Validating a Clinical Algorithm for the Diagnosis of Podoconiosis. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Kebede, B.; Martindale, S.; Mengistu, B.; Kebede, B.; Mengiste, A.; Kiros, F.H.; Tamiru, A.; Davey, G.; Kelly-hope, L.A.; Mackenzie, D. Integrated Morbidity Mapping of Lymphatic Filariasis and Podoconiosis Cases in 20 Co-Endemic Districts of Ethiopia. PLoS Negl. Trop. Dis. 2018, 12, e0006491. [Google Scholar] [CrossRef] [PubMed]
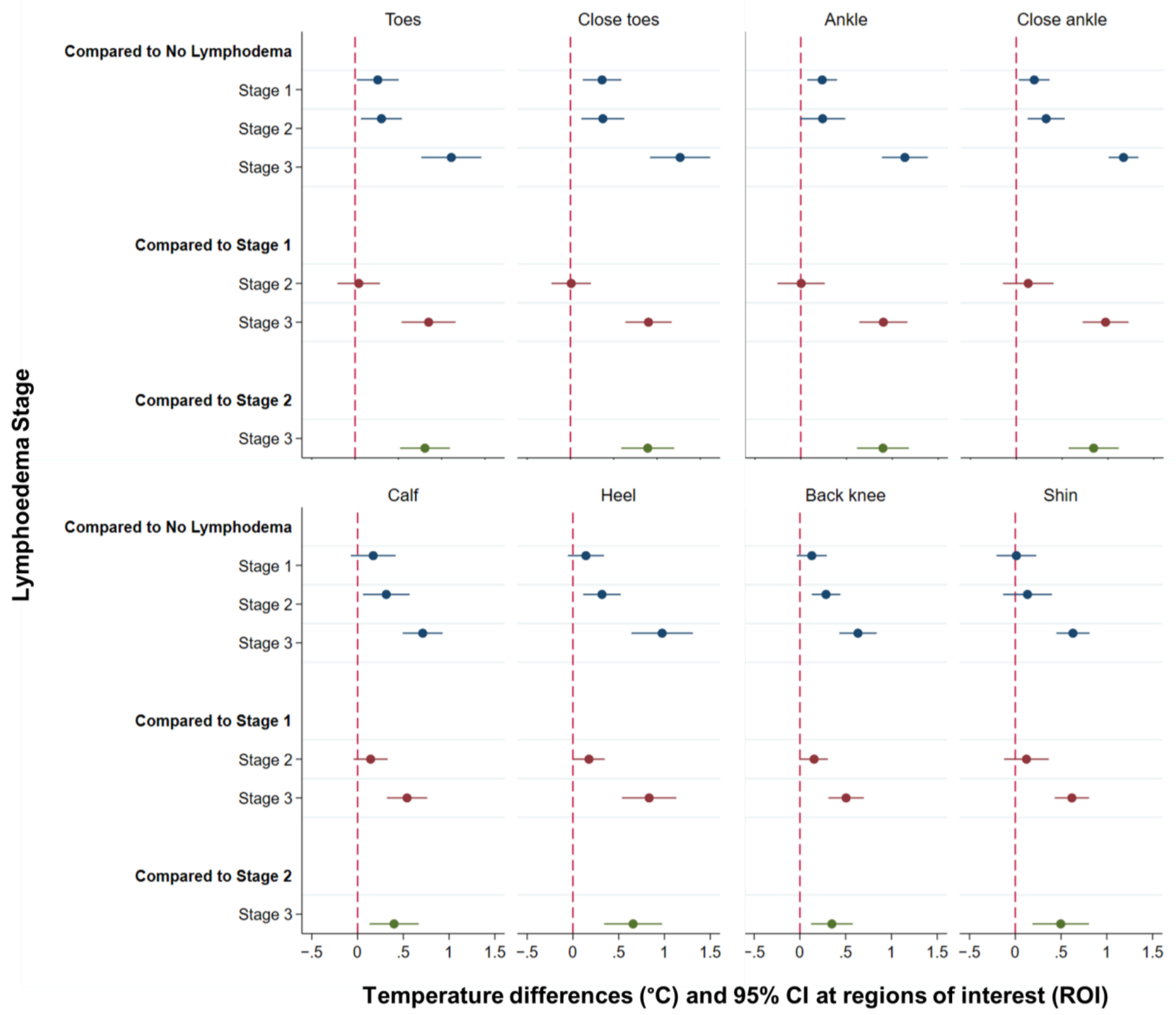
| (A) Compared to No Lymphoedema (Stage 0) | |||
| Temperature difference °C | 95% CI | p-value | |
| Mild (Stage 1) | |||
| Toes | 0.26 | 0.02–0.50 | 0.033 * |
| Close toes | 0.37 | 0.14–0.59 | 0.001 * |
| Ankle | 0.23 | 0.07–0.40 | 0.005 * |
| Close ankle | 0.20 | 0.03–0.36 | 0.022 * |
| Calf | 0.17 | −0.07–0.42 | 0.17 |
| Heel | 0.14 | −0.06–0.34 | 0.159 |
| Back knee | 0.13 | −0.03–0.29 | 0.119 |
| Shin | 0.01 | −0.21–0.23 | 0.916 |
| Moderate (Stage 2) | |||
| Toes | 0.30 | 0.07–0.54 | 0.012 * |
| Close toes | 0.37 | 0.13–0.62 | 0.003 * |
| Ankle | 0.24 | −0.01–0.49 | 0.058 |
| Close ankle | 0.33 | 0.12–0.53 | 0.002 * |
| Calf | 0.31 | 0.06–0.57 | 0.016 * |
| Heel | 0.32 | 0.11–0.52 | 0.002 * |
| Back knee | 0.29 | 0.13–0.44 | <0.0001 * |
| Shin | 0.13 | −0.13–0.40 | 0.327 |
| Severe (Stage 3) | |||
| Toes | 1.11 | 0.76–1.46 | <0.0001 * |
| Close toes | 1.27 | 0.92–1.61 | <0.0001 * |
| Ankle | 1.14 | 0.89–1.39 | <0.0001 * |
| Close ankle | 1.17 | 1.01–1.33 | <0.0001 * |
| Calf | 0.71 | 0.49–0.93 | <0.0001 * |
| Heel | 0.97 | 0.64–1.31 | <0.0001 * |
| Back knee | 0.63 | 0.43–0.84 | <0.0001 * |
| Shin | 0.63 | 0.45–0.81 | <0.0001 * |
| (B) Compared to Mild Lymphoedema (Stage 1) | |||
| Moderate (Stage 2) | |||
| Toes | 0.04 | −0.20–0.29 | 0.735 |
| Close toes | 0.01 | −0.22–0.24 | 0.94 |
| Ankle | 0.01 | −0.25–0.26 | 0.971 |
| Close ankle | 0.13 | −0.15–0.41 | 0.357 |
| Calf | 0.14 | −0.04–0.33 | 0.135 |
| Heel | 0.18 | 0.004–0.35 | 0.045 * |
| Back knee | 0.16 | 0.005–0.31 | 0.043 * |
| Shin | 0.12 | −0.12–0.37 | 0.329 |
| Severe (Stage 3) | |||
| Toes | 0.85 | 0.54–1.16 | <0.0001 * |
| Close toes | 0.90 | 0.63–1.17 | <0.0001 * |
| Ankle | 0.90 | 0.64–1.16 | <0.0001 * |
| Close ankle | 0.97 | 0.72–1.23 | <0.0001 * |
| Calf | 0.54 | 0.32–0.76 | <0.0001 * |
| Heel | 0.83 | 0.54–1.13 | <0.0001 * |
| Back knee | 0.50 | 0.31–0.70 | <0.0001 * |
| Shin | 0.62 | 0.43–0.81 | <0.0001 * |
| (C) Compared to Moderate Lymphoedema (Stage 2) | |||
| Severe (Stage 3) | |||
| Toes | 0.81 | 0.52–1.09 | <0.0001 |
| Close toes | 0.89 | 0.59–1.20 | <0.0001 |
| Ankle | 0.90 | 0.61–1.18 | <0.0001 |
| Close ankle | 0.84 | 0.57–1.12 | <0.0001 |
| Calf | 0.40 | 0.13–0.67 | 0.004 |
| Heel | 0.66 | 0.34–0.97 | <0.0001 |
| Back knee | 0.35 | 0.12–0.58 | 0.003 |
| Shin | 0.50 | 0.19–0.81 | 0.002 |
| ADLA | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Toes | 0.56 | 0.06–1.06 | 0.028 * |
| Close toes | 0.68 | 0.21–1.15 | 0.005 * |
| Ankle | 0.54 | 0.10–0.97 | 0.015 * |
| Close ankle | 0.58 | 0.17–0.99 | 0.006 * |
| Calf | 0.53 | 0.32–0.74 | <0.0001 * |
| Heel | 0.82 | 0.36–1.28 | <0.0001 * |
| Back knee | 0.31 | 0.07–0.54 | 0.012 * |
| Shin | 0.49 | 0.19–0.79 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly-Hope, L.A.; Karim, M.J.; Sultan Mahmood, A.; Al Kawsar, A.; Khair, A.; Betts, H.; Douglass, J.; Forrer, A.; Taylor, M.J. Infrared Thermal Imaging as a Novel Non-Invasive Point-of-Care Tool to Assess Filarial Lymphoedema. J. Clin. Med. 2021, 10, 2301. https://doi.org/10.3390/jcm10112301
Kelly-Hope LA, Karim MJ, Sultan Mahmood A, Al Kawsar A, Khair A, Betts H, Douglass J, Forrer A, Taylor MJ. Infrared Thermal Imaging as a Novel Non-Invasive Point-of-Care Tool to Assess Filarial Lymphoedema. Journal of Clinical Medicine. 2021; 10(11):2301. https://doi.org/10.3390/jcm10112301
Chicago/Turabian StyleKelly-Hope, Louise A., Mohammad Jahirul Karim, ASM Sultan Mahmood, Abdullah Al Kawsar, Abul Khair, Hannah Betts, Janet Douglass, Armelle Forrer, and Mark J. Taylor. 2021. "Infrared Thermal Imaging as a Novel Non-Invasive Point-of-Care Tool to Assess Filarial Lymphoedema" Journal of Clinical Medicine 10, no. 11: 2301. https://doi.org/10.3390/jcm10112301
APA StyleKelly-Hope, L. A., Karim, M. J., Sultan Mahmood, A., Al Kawsar, A., Khair, A., Betts, H., Douglass, J., Forrer, A., & Taylor, M. J. (2021). Infrared Thermal Imaging as a Novel Non-Invasive Point-of-Care Tool to Assess Filarial Lymphoedema. Journal of Clinical Medicine, 10(11), 2301. https://doi.org/10.3390/jcm10112301






