Comparison of Suture-Based and Collagen-Based Vascular Closure Devices for Large Bore Arteriotomies—A Meta-Analysis of Bleeding and Vascular Outcomes
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Data Extraction and Outcomes
2.3. Statistical Analysis
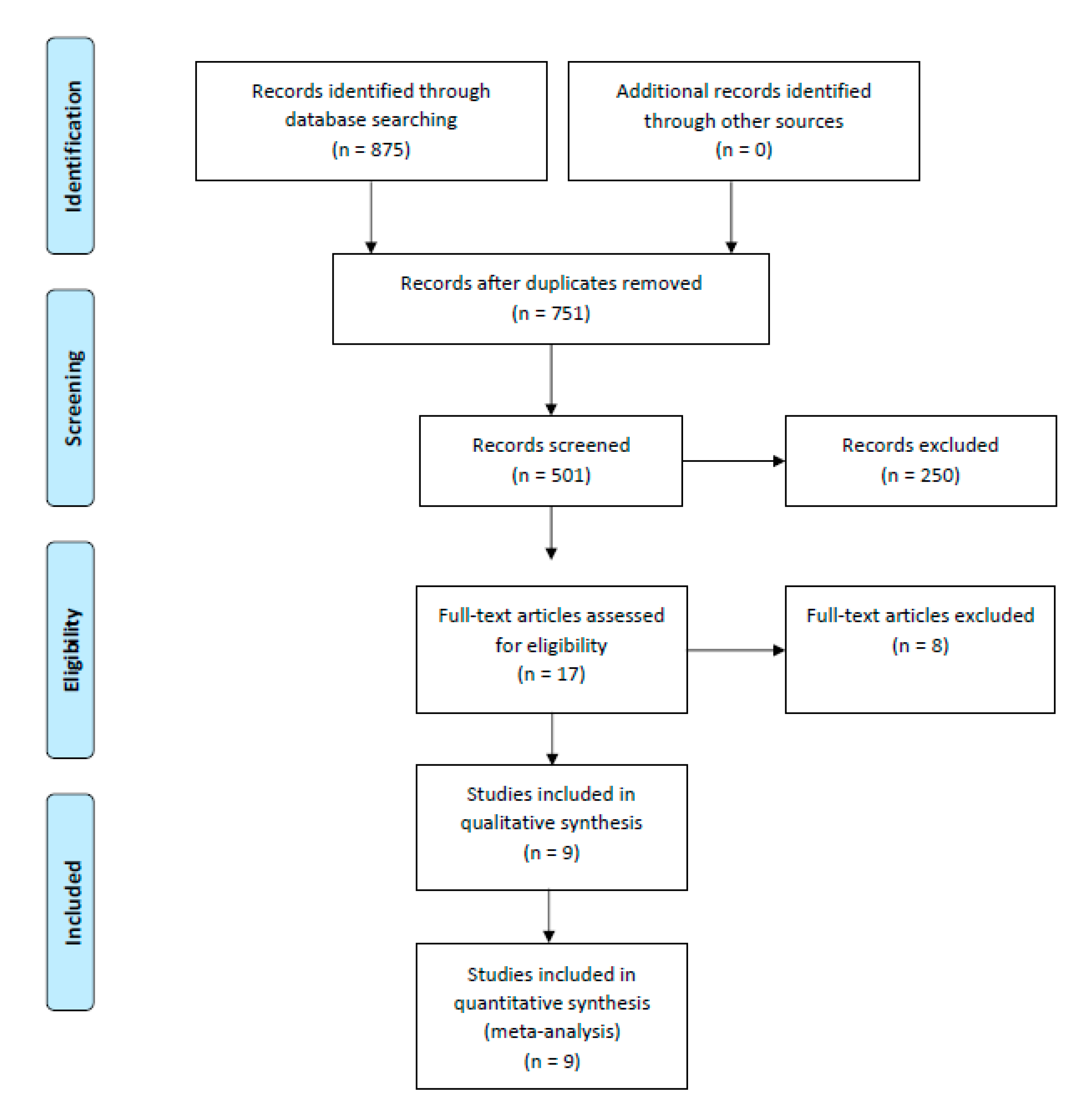
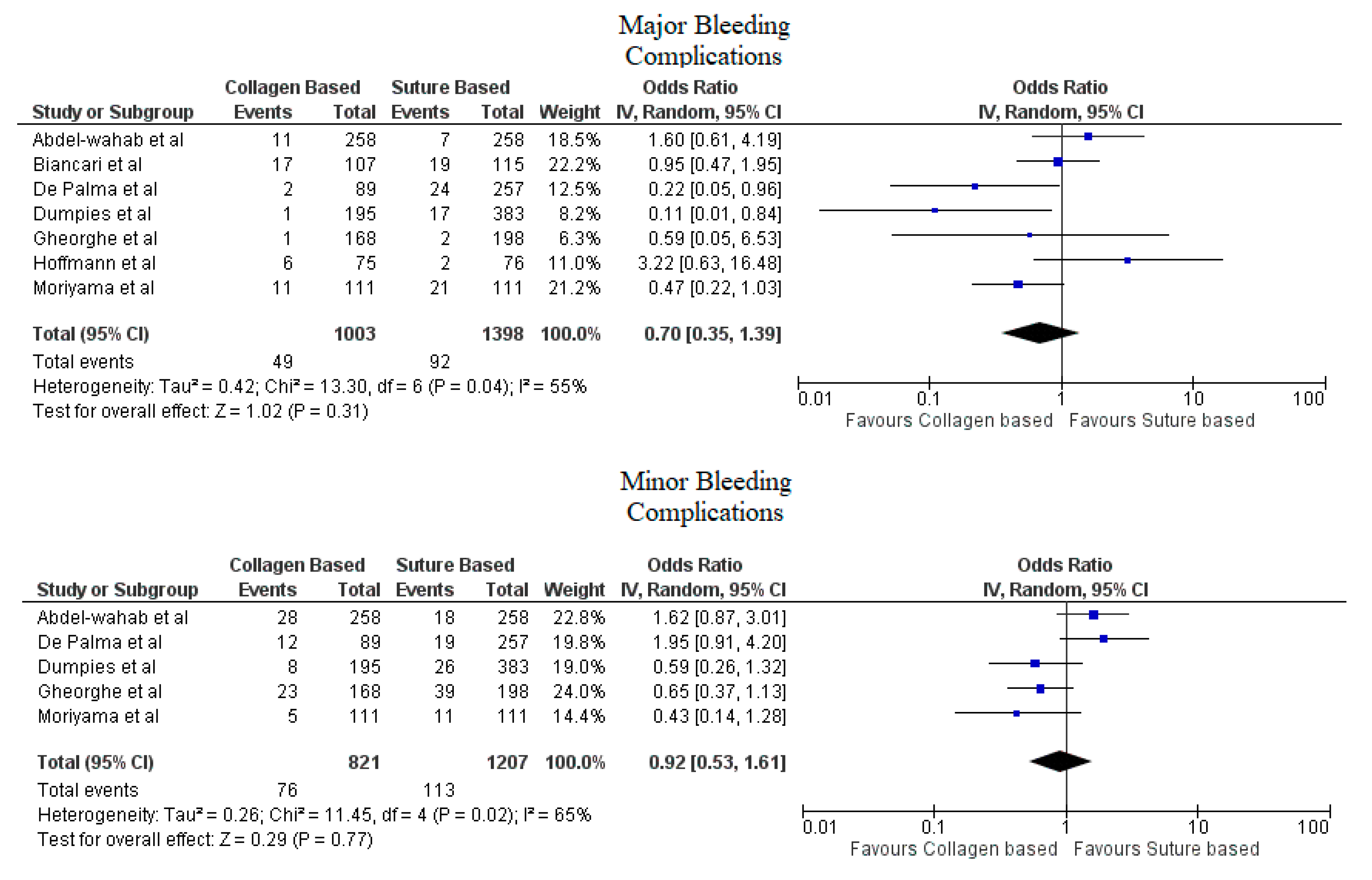
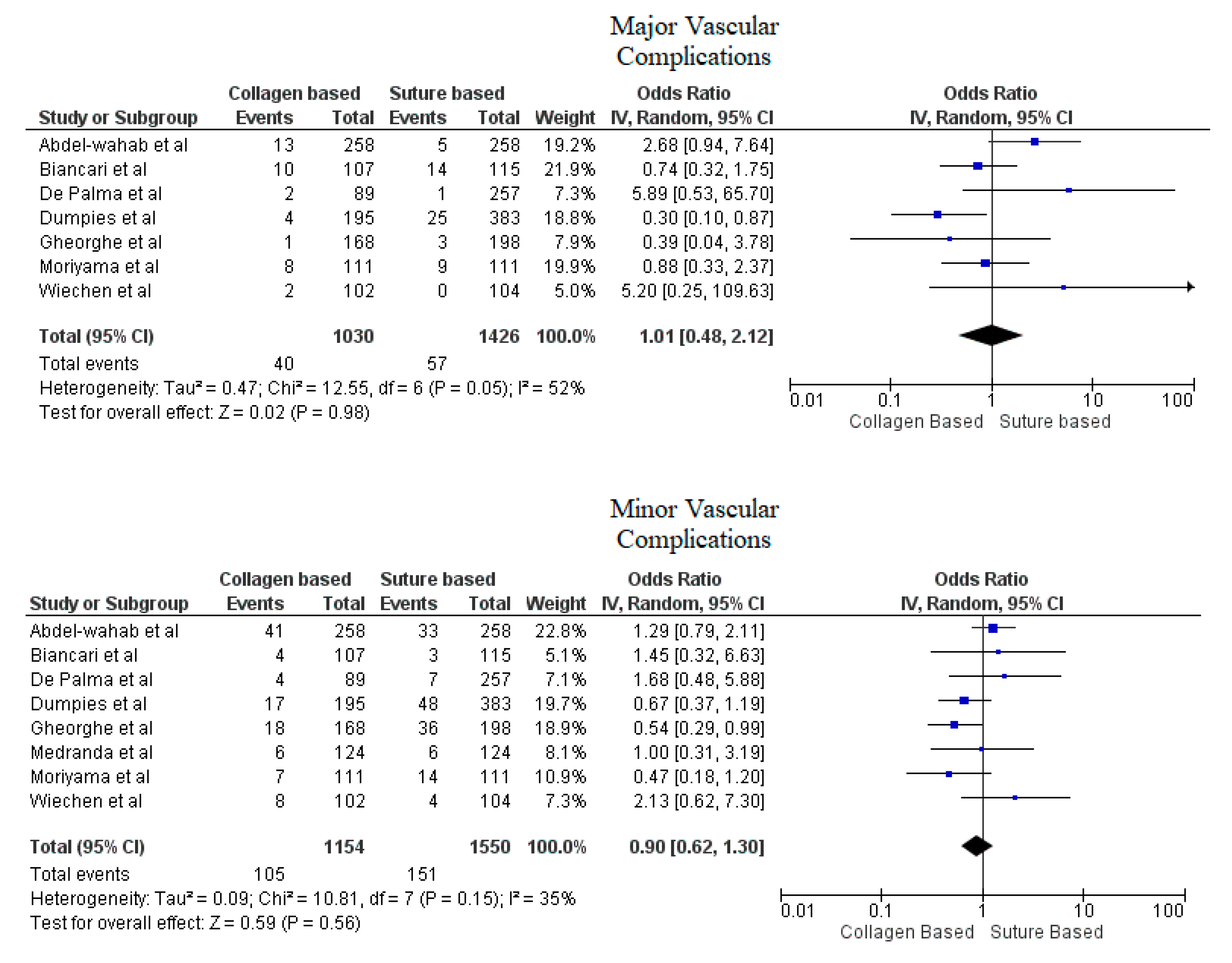
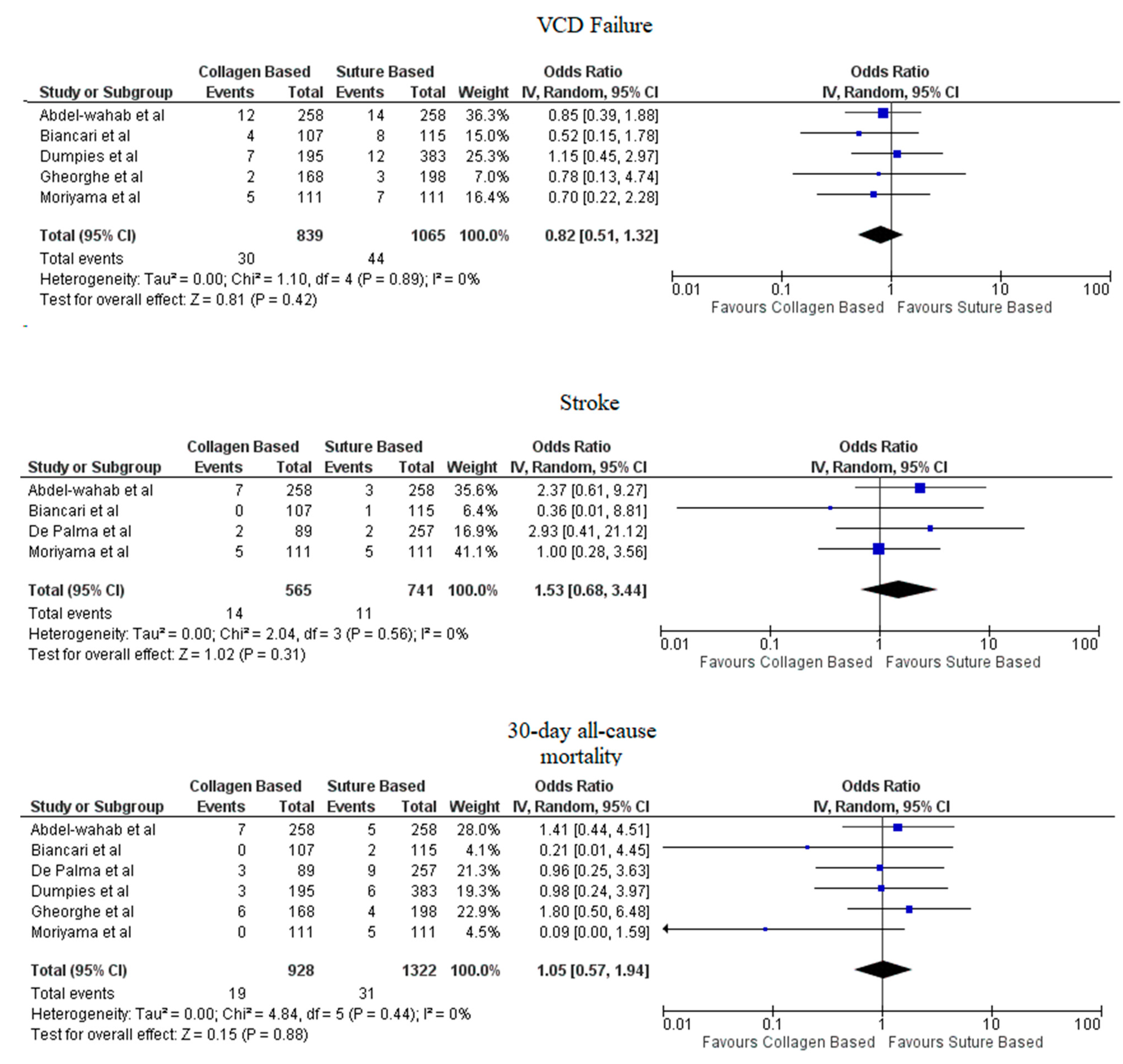
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Wiechen, M.P.; Ligthart, J.M.; Van Mieghem, N.M. Large-bore Vascular Closure: New Devices and Techniques. Interv. Cardiol. Rev. Res. Resour. 2019, 14, 17–21. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.P.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Use of Mechanical Circulatory Support Devices Among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA Netw. Open 2021, 4, e2037748. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Redfors, B.; Watson, B.M.; McAndrew, T.; Palisaitis, E.; Francese, D.P.; Razavi, M.; Safirstein, J.; Mehran, R.; Kirtane, A.J.; Généreux, P. Mortality, Length of Stay, and Cost Implications of Procedural Bleeding After Percutaneous Interventions Using Large-Bore Catheters. JAMA Cardiol. 2017, 2, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Kaki, A.; Blank, N.; Alraies, M.C.; Kajy, M.; Grines, C.L.; Hasan, R.; Htun, W.W.; Glazier, J.; Mohamad, T.; Elder, M.; et al. Access and closure management of large bore femoral arterial access. J. Interv. Cardiol. 2018, 31, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Tchetche, D.; Chieffo, A.; Dumonteil, N.; Messika-Zeitoun, D.; van der Boon, R.M.; Vahdat, O.; Buchanan, G.L.; Marcheix, B.; Himbert, D.; et al. Incidence, Predictors, and Implications of Access Site Complications with Transfemoral Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2012, 110, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.; Tayal, R. Mechanical Circulatory Support Devices: Management and Prevention of Vascular Complications. Interv. Cardiol. Clin. 2021, 10, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G. MANTA Dedicated Large-Bore Vessel Closure Device. Circ. Cardiovasc. Interv. 2019, 12, e008203. [Google Scholar] [CrossRef]
- Chen, I.-M.; Lee, T.-H.; Chen, P.-L.; Shih, C.-C.; Chang, H.-H. Factors in ProGlide® Vascular Closure Failure in Sheath Arteriotomies Greater than 16 French. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 615–622. [Google Scholar] [CrossRef]
- Hu, G.; Chen, B.; Fu, W.; Xu, X.; Guo, D.; Jiang, J.; Yang, J.; Wang, Y. Predictors and treatments of Proglide-related complications in percutaneous endovascular aortic repair. PLoS ONE 2015, 10, e0123739. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.A.; Krajcer, Z.; Sathananthan, J.; Strickman, N.; Metzger, C.; Fearon, W.; Aziz, M.; Satler, L.F.; Waksman, R.; Eng, M.; et al. Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Percutaneous Vascular Closure Device. Circ. Cardiovasc. Interv. 2019, 12, e007258. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Romppanen, H.; Savontaus, M.; Siljander, A.; Mäkikallio, T.; Piira, O.-P.; Piuhola, J.; Vilkki, V.; Ylitalo, A.; Vasankari, T.; et al. MANTA versus ProGlide vascular closure devices in transfemoral transcatheter aortic valve implantation. Int. J. Cardiol. 2018, 263, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Al-Ani, A.; Von Lueder, T.; Hoffmann, J.; Majak, P.; Hagen, O.; Loose, H.; Kløw, N.E.; Opdahl, A. Access site complications after transfemoral aortic valve implantation—A comparison of Manta and ProGlide. CVIR Endovasc. 2018, 1, 20. [Google Scholar] [CrossRef]
- Gheorghe, L.; Brouwer, J.; Mathijssen, H.; Nijenhuis, V.J.; Rensing, B.J.; Swaans, M.J.; Yin, D.R.C.P.; Heijmen, R.H.; De Kroon, T.; Sonker, U.; et al. Early Outcomes After Percutaneous Closure of Access Site in Transfemoral Transcatheter Valve Implantation Using the Novel Vascular Closure Device Collagen Plug-Based MANTA. Am. J. Cardiol. 2019, 124, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.; Settergren, M.; Rück, A.; Linder, R.; Saleh, N. Impact of percutaneous femoral arteriotomy closure using the MANTATM device on vascular and bleeding complications after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2018, 92, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, N.; Lindström, L.; Laine, M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve re-placement using MANTA versus ProGlide. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2019, 14, e1558–e1565. [Google Scholar] [CrossRef]
- Dumpies, O.; Kitamura, M.; Majunke, N.; Hartung, P.; Haag, A.; Wilde, J.; Desch, S.; Sandri, M.; Crusius, L.; Noack, T.; et al. Manta versus Perclose ProGlide vascular closure device after transcatheter aortic valve implantation: Initial experience from a large European center. Cardiovasc. Revascularization Med. 2021, 37, 34–40. [Google Scholar] [CrossRef]
- Medranda, G.A.; Case, B.C.; Zhang, C.; Rappaport, H.; Weissman, G.; Bernardo, N.L.; Satler, L.F.; Ben-Dor, I.; Rogers, T.; Waksman, R. Propensity-matched comparison of large-bore access closure in transcatheter aortic valve replacement using MANTA versus Perclose: A real-world experience. Catheter. Cardiovasc. Interv. 2021, 98, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Van Wiechen, M.P.; Tchétché, D.; Ooms, J.F.; Hokken, T.W.; Kroon, H.; Ziviello, F.; Ghattas, A.; Siddiqui, S.; Laperche, C.; Spitzer, E.; et al. Suture- or Plug-Based Large-Bore Arteriotomy Closure. JACC Cardiovasc. Interv. 2020, 14, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Hartung, P.; Dumpies, O.; Obradovic, D.; Wilde, J.; Majunke, N.; Boekstegers, P.; Müller, R.; Seyfarth, M.; Vorpahl, M.; et al. Comparison of a Pure Plug-Based Versus a Primary Suture-Based Vascular Closure Device Strategy for Transfemoral Transcatheter Aortic Valve Replacement: The CHOICE-CLOSURE Randomized Clinical Trial. Circulation 2022, 145, 170–183. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; Van Es, G.-A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardio-Thoracic Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sherwood, M.; Xiang, K.; Matsouaka, R.; Li, Z.; Vemulapalli, S.; Vora, A.N.; Fanaroff, A.; Harrison, J.K.; Thourani, V.H.; Holmes, D.; et al. Incidence, Temporal Trends, and Associated Outcomes of Vascular and Bleeding Complications in Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2020, 13, e008227. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Qamar, A.; Kolte, D.; Pandey, A.; Paladugu, M.B.; Borhade, M.B.; Ramm, C.J.; Bhatt, D.L.; Vavalle, J.P. Trends in Inpatient Complications After Transcatheter and Surgical Aortic Valve Replacement in the Transcatheter Aortic Valve Replacement Era. Circ. Cardiovasc. Interv. 2018, 11, e007517. [Google Scholar] [CrossRef]
- Sardar, M.R.; Goldsweig, A.M.; Abbott, J.D.; Sharaf, B.L.; Gordon, P.C.; Ehsan, A.; Aronow, H.D. Vascular complications associated with transcatheter aortic valve replacement. Vasc. Med. 2017, 22, 234–244. [Google Scholar] [CrossRef]
- Nuis, R.-J.; Wood, D.; Kroon, H.; Van Wiechen, M.; Bigelow, D.; Buller, C.; Daemen, J.; de Jaegere, P.; Krajcer, Z.; Webb, J.; et al. Frequency, Impact, and Predictors of Access Complications with Plug-Based Large-Bore Arteriotomy Closure—A Patient-Level Meta-Analysis. Cardiovasc. Revascularization Med. 2022, 34, 69–74. [Google Scholar] [CrossRef]
- Généreux, P.; Webb, J.G.; Svensson, L.G.; Kodali, S.K.; Satler, L.F.; Fearon, W.F.; Davidson, C.J.; Eisenhauer, A.C.; Makkar, R.R.; Bergman, G.W.; et al. Vascular Complications After Transcatheter Aortic Valve Replacement: Insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J. Am. Coll. Cardiol. 2012, 60, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, S.; Bedogni, F.; Giordano, A.; Petronio, A.S.; Iadanza, A.; Bartorelli, A.L.; Reimers, B.; Spaccarotella, C.; Trani, C.; Attisano, T.; et al. Efficacy and Safety of ProGlide Versus Prostar XL Vascular Closure Devices in Transcatheter Aortic Valve Replacement: The RISPEVA Registry. J. Am. Heart Assoc. 2020, 9, e018042. [Google Scholar] [CrossRef]
- Maniotis, C.; Andreou, C.; Karalis, I.; Koutouzi, G.; Agelaki, M.; Koutouzis, M. A systematic review on the safety of Prostar XL versus ProGlide after TAVR and EVAR. Cardiovasc. Revascularization Med. 2017, 18, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Barbash, I.M.; Barbanti, M.; Webb, J.; De Nicolas, J.M.-M.; Abramowitz, Y.; Latib, A.; Nguyen, C.; Deuschl, F.; Segev, A.; Sideris, K.; et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur. Heart J. 2015, 36, 3370–3379. [Google Scholar] [CrossRef]
- Bangalore, S.; Arora, N.; Resnic, F.S. Vascular Closure Device Failure: Frequency and Implications. Circ. Cardiovasc. Interv. 2009, 2, 549–556. [Google Scholar] [CrossRef]
- Masiero, G.; D’Angelo, L.; Fovino, L.N.; Fabris, T.; Cardaioli, F.; Rodinò, G.; Benedetti, A.; Boiago, M.; Continisio, S.; Montonati, C.; et al. Real-World Experience with a Large Bore Vascular Closure Device during TAVI Procedure: Features and Predictors of Access-Site Vascular Complications. Front. Cardiovasc. Med. 2022, 9, 832242. [Google Scholar] [CrossRef]
- Kroon, H.G.; Tonino, P.A.; Savontaus, M.; Amoroso, G.; Laine, M.; Christiansen, E.H.; Toggweiler, S.; Berg, J.; Sathananthan, J.; Daemen, J.; et al. Dedicated plug based closure for large bore access—The MARVEL prospective registry. Catheter. Cardiovasc. Interv. 2020, 97, 1270–1278. [Google Scholar] [CrossRef]
- Fonseca, P.; Almeida, J.; Bettencourt, N.; Ferreira, N.; Carvalho, M.; Ferreira, W.; Caeiro, D.; Gonçalves, H.; Ribeiro, J.; Rodrigues, A.; et al. Incidence and predictors of vascular access site complications following transfemoral transcatheter aortic valve implantation. Rev. Port. Cardiol. 2017, 36, 747–753. [Google Scholar] [CrossRef]
- Langouet, Q.; Martinez, R.; Saint-Etienne, C.; Soulami, R.B.; Harmouche, M.; Aupart, M.; Le Breton, H.; Verhoye, J.-P.; Bourguignon, T. Incidence, predictors, impact, and treatment of vascular complications after transcatheter aortic valve implantation in a modern prospective cohort under real conditions. J. Vasc. Surg. 2020, 72, 2120–2129.e2. [Google Scholar] [CrossRef]
- Noori, V.J.; Eldrup-Jørgensen, J. A systematic review of vascular closure devices for femoral artery puncture sites. J. Vasc. Surg. 2018, 68, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Giniyani, L.L.; Rana, Y.P.; Hanumanthu, B.K.J.; Chan, D.; Kwan, T.W. Perclose ProGlide embolization as a complication: Case report and review of literature. Futur. Cardiol. 2021, 17, 1193–1197. [Google Scholar] [CrossRef]
- Wong, Y.-H.; De Backer, O.; Søndergaard, L.; Bieliauskas, G. Percutaneous management of an embolised MANTA large bore arteriotomy closure device. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2019, 15, 74–75. [Google Scholar] [CrossRef]
- Nardi, G.; De Backer, O.; Ristalli, F.; Meucci, F.; Stolcova, M.; Wang, X.I.; Sondergaard, L.; Palmerini, T.; Bruno, A.G.; Al Jabri, A.G.; et al. Peripheral intravascular lithotripsy to facilitate transfemoral TAVR: A multicentric prospective registry. Eur. Heart J. 2021, 42, ehab724.2131. [Google Scholar] [CrossRef]
- Tayal, R.; Sohal, S.; Okoh, A.; Wasty, N.; Waxman, S.; Salemi, A. Intravascular Lithotripsy Enabled Transfemoral Transcatheter Aortic Valve Implantation via Percutaneous Axillary Access Approach. Cardiovasc. Revascularization Med. 2020, 28, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Atreya, A.R. Mechanical Circulatory Support in Advanced Heart Failure. Indian J. Clin. Cardiol. 2021, 2, 158–170. [Google Scholar] [CrossRef]
- Halim, J.; Missault, L.; Lycke, M.; Van Der Heyden, J. Assessment of the MANTA closure device in transfemoral transcatheter aortic valve replacement: A single-centre observational study. Neth. Heart J. 2020, 28, 639–644. [Google Scholar] [CrossRef]
| S. No | Study | Type of Study | Year | Comparison | Primary Outcome |
|---|---|---|---|---|---|
| 1. | De Palma et al. [15]. | Observational | 2018 | MANTA vs. Prostar XL | Closure success and time to hemostasis |
| 2. | Biancari et al. [12]. | Observational | 2018 | MANTA vs. ProGlide | Invasive treatment of bleeding, life-threatening/disabling bleeding and major vascular complications |
| 3. | Hoffman et al. [13]. | Observational | 2018 | MANTA vs. ProGlide | Vascular complications or non-planned vascular surgery |
| 4. | Gheorghe et al. [14]. | Observational | 2019 | MANTA vs. Prostar XL | Acute closure success and occurrence of any access site related vascular injury as well as major and life threatening/disabling bleeding |
| 6. | Moriyama et al. [16]. | Observational | 2019 | MANTA vs. ProGlide | Bleeding and vascular complications |
| 7. | Wiechen et al. [19]. | Randomized Controlled Trial | 2021 | MANTA vs. ProGlide | Composite end point of access site related major and minor vascular complications |
| 8. | Medranda et al. [18]. | Observational | 2021 | MANTA vs. ProGlide | Vascular closure device success |
| 9. | Dumpies et al. [17]. | Observational | 2021 | MANTA vs. ProGlide | In-hospital vascular and access-site-related complications |
| 10. | Abdel-Wahab et al. [20]. | Randomized controlled trial | 2021 | MANTA vs. ProGlide | In-hospital access-site or access related major and minor vascular complications |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohal, S.; Mathai, S.V.; Nagraj, S.; Kurpad, K.; Suthar, K.; Mehta, H.; Kaur, K.; Wasty, N.; Waxman, S.; Cohen, M.; et al. Comparison of Suture-Based and Collagen-Based Vascular Closure Devices for Large Bore Arteriotomies—A Meta-Analysis of Bleeding and Vascular Outcomes. J. Cardiovasc. Dev. Dis. 2022, 9, 331. https://doi.org/10.3390/jcdd9100331
Sohal S, Mathai SV, Nagraj S, Kurpad K, Suthar K, Mehta H, Kaur K, Wasty N, Waxman S, Cohen M, et al. Comparison of Suture-Based and Collagen-Based Vascular Closure Devices for Large Bore Arteriotomies—A Meta-Analysis of Bleeding and Vascular Outcomes. Journal of Cardiovascular Development and Disease. 2022; 9(10):331. https://doi.org/10.3390/jcdd9100331
Chicago/Turabian StyleSohal, Sumit, Sheetal Vasundara Mathai, Sanjana Nagraj, Krishna Kurpad, Kandarp Suthar, Harsh Mehta, Komaldeep Kaur, Najam Wasty, Sergio Waxman, Marc Cohen, and et al. 2022. "Comparison of Suture-Based and Collagen-Based Vascular Closure Devices for Large Bore Arteriotomies—A Meta-Analysis of Bleeding and Vascular Outcomes" Journal of Cardiovascular Development and Disease 9, no. 10: 331. https://doi.org/10.3390/jcdd9100331
APA StyleSohal, S., Mathai, S. V., Nagraj, S., Kurpad, K., Suthar, K., Mehta, H., Kaur, K., Wasty, N., Waxman, S., Cohen, M., Visveswaran, G. K., & Tayal, R. (2022). Comparison of Suture-Based and Collagen-Based Vascular Closure Devices for Large Bore Arteriotomies—A Meta-Analysis of Bleeding and Vascular Outcomes. Journal of Cardiovascular Development and Disease, 9(10), 331. https://doi.org/10.3390/jcdd9100331






