The CCR2+ Monocyte Subsets Increase in Obese Boys but Not Girls with Abnormally High Carotid Intima-Media Thickness: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Measurements
2.3. Biochemical Markers
2.4. Ultrasound Measurements
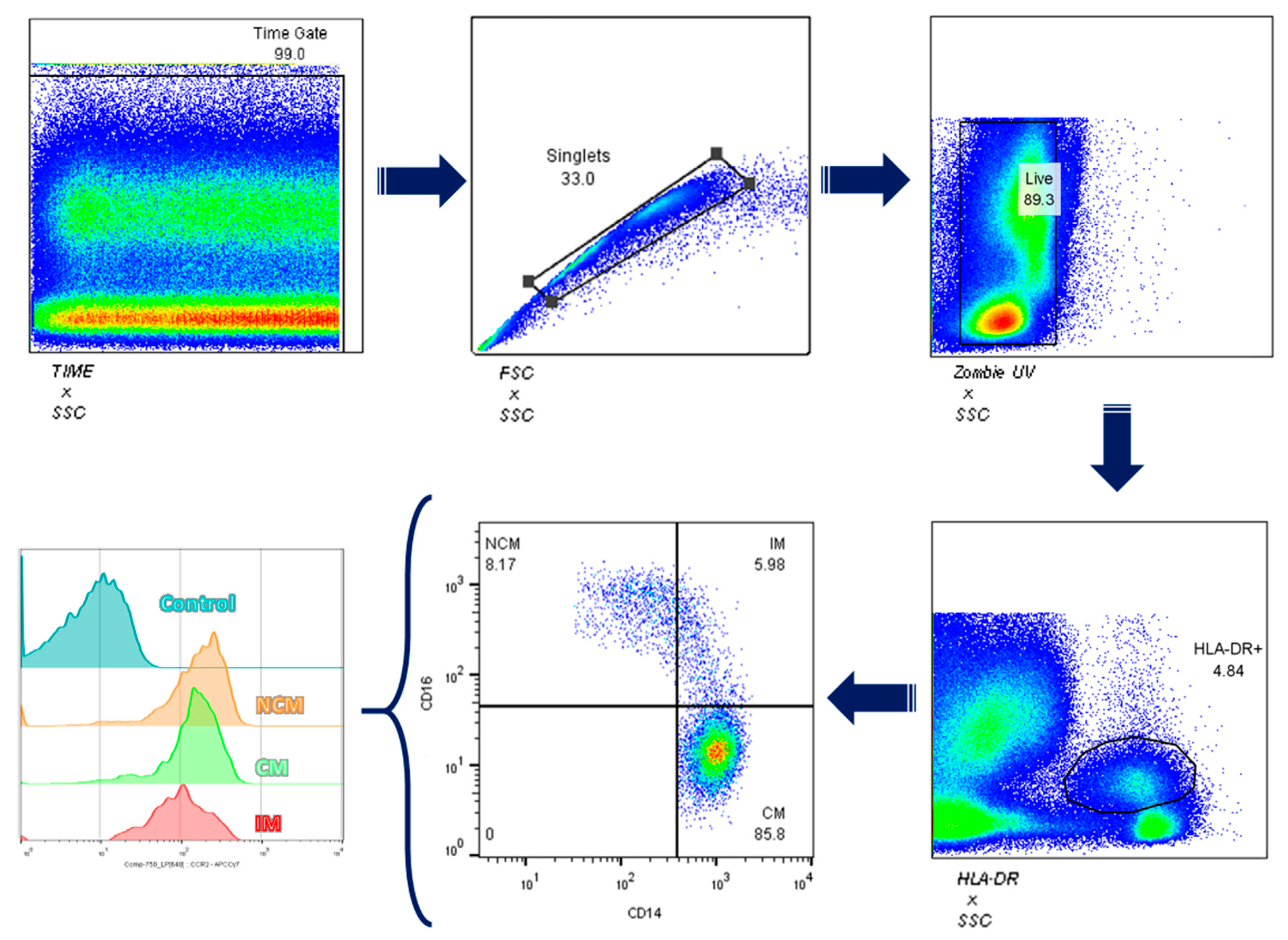
2.5. Total Leukocyte Isolation and Flow Cytometry
2.6. Statistics
3. Results
3.1. Participants Characteristics
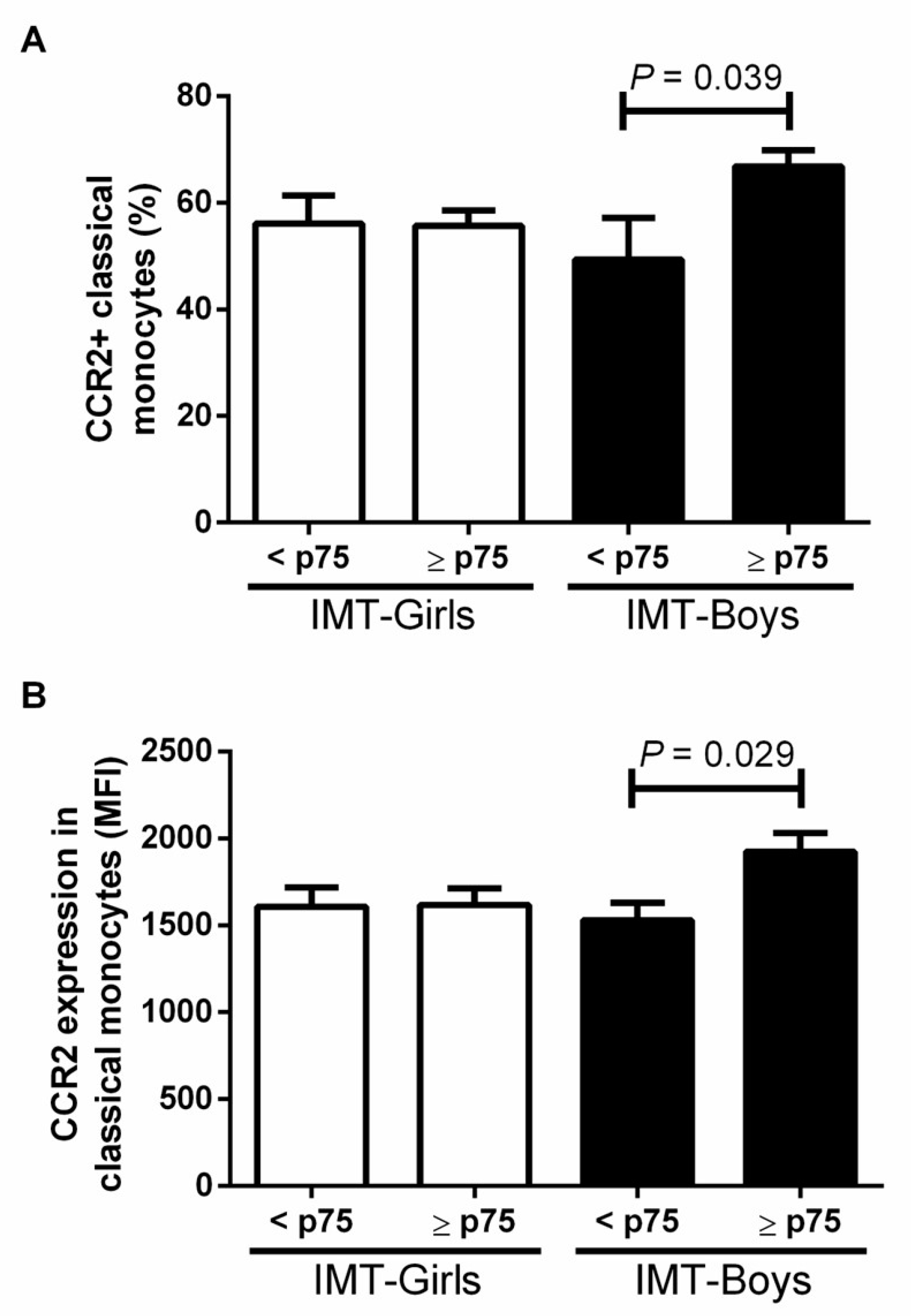
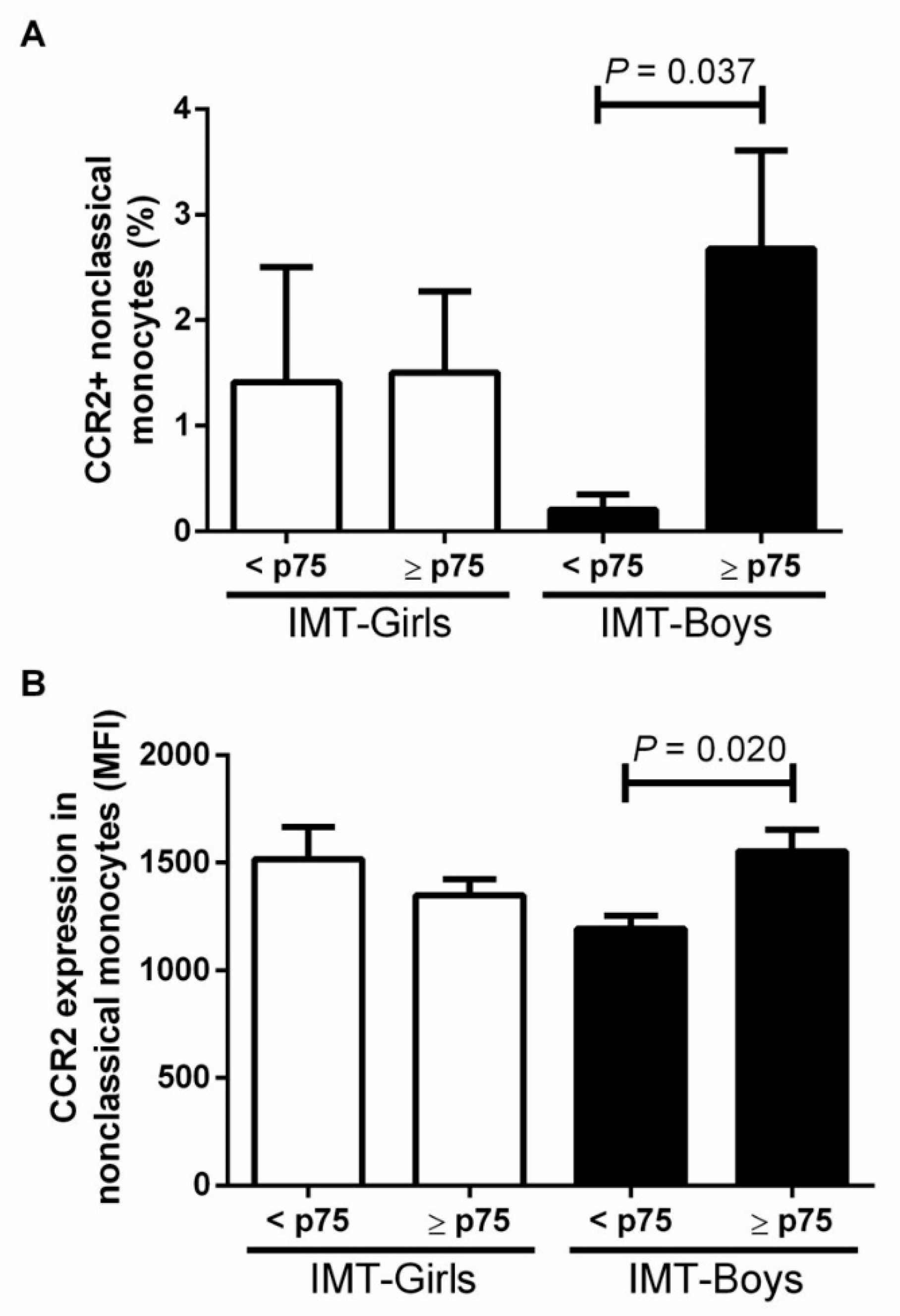
3.2. CCR2+ Monocyte Subsets in Boys and Girls with Obesity and High Risk IMT
3.3. Correlation between CCR2+ Monocyte Subsets and Children with Obesity and High Risk IMT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Medina-leyte, D.J.; Zepeda-garc, O.; Dom, M. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, D.P.; Gotto, A.M. MINI-REVIEW Biological Relevance of In fl ammation and Oxidative Stress in the Pathogenesis of Arterial Diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus–atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, 89–91. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C.; McMahan, C.A.; Herderick, E.E.; Zieske, A.W.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002, 105, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Santoro, N.; Caprio, S.; Kim, G.; Lartaud, D.; Shaw, M.; Pierpont, B.; Weiss, R. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011, 34, 1869–1874. [Google Scholar] [CrossRef]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and cardiometabolic risk factors: From childhood to adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef]
- Di Pino, A.; Defronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447–1467. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Norata, G.D.; Magnoni, M.; Camici, P.G. The role of monocytes and macrophages in human atherosclerosis, plaque neoangiogenesis, and atherothrombosis. Med. Infl. 2019, 2019, 7434376. [Google Scholar] [CrossRef] [PubMed]
- França, C.N.; Izar, M.C.O.; Hortêncio, M.N.S.; do Amaral, J.B.; Ferreira, C.E.S.; Tuleta, I.D.; Fonseca, F.A.H. Monocyte subtypes and the CCR2 chemokine receptor in cardiovascular disease. Clin. Sci. 2017, 131, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, K.; Ludew, D.; Filip, G.; Filip, M.; Sagan, A.; Szczepaniak, P.; Grudzien, G. CD14+CD16++ “nonclassical” monocytes are associated with Endothelial dysfunction in patients with coronary artery disease. Thromb. Haemost. 2017, 117, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef]
- Böhm, B.; Hartmann, K.; Buck, M.; Oberhoffer, R. Sex differences of carotid intima-media thickness in healthy children and adolescents. Atherosclerosis 2009, 206, 458–463. [Google Scholar] [CrossRef]
- Petty, K.H.; Li, K.; Dong, Y.; Fortenberry, J.; Stallmann-Jorgensen, I.; Guo, D.; Zhu, H. Sex dimorphisms in inflammatory markers ad adiposity in African American youth. Int. J. Ped. Obes. 2010, 5, 327–333. [Google Scholar] [CrossRef][Green Version]
- Dhanraj, P.; van Heerden, M.B.; Pepper, M.S.; Ambele, M.A. Sexual dimorphism in changes that occur in tissues, organs and plasma during the early stages of obesity development. Biology 2021, 10, 717. [Google Scholar] [CrossRef]
- Verweij, S.L.; Duivenvoorden, R.; Stiekema, L.C.A.; Nurmohamed, N.S.; Van Der Valk, F.M.; Versloot, M.; Verberne, H.J.; Stroes, E.S.G.; Nahrendorf, M.; Bekkering, S.; et al. CCR2 expression on circulating monocytes is associated with arterial wall inflammation assessed by 18F-FDG PET/CT in patients at risk for cardiovascular disease. Cardiovasc. Res. 2018, 114, 468–475. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 2002, 11, 1–190. [Google Scholar]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity—Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endo. Metabo. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Lentferink, Y.E.; Elst, M.A.J.; Knibbe, C.A.J.; Vorst, M.M.J. Van Der. Predictors of Insulin Resistance in Children versus Adolescents with Obesity. J. Obes. 2017, 2017, 3793868. [Google Scholar] [CrossRef] [PubMed]
- Van Der Aa, M.P.; Farsani, S.F.; Kromwijk, L.A.J.; De Boer, A.; Knibbe, C.A.J.; Van Der Vorst, M.M.J. How to Screen Obese Children at Risk for Type 2 Diabetes Mellitus? Clin. Pediatr. 2014, 53, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Mook-Kanamori, D.O.; Holzhauer, S.; Hollestein, L.M.; Durmus, B.; Manniesing, R.; Koek, M.; Boehm, G.; van der Beek, E.M.; Hofman, A.; Witteman, J.C.M.; et al. Abdominal Fat in Children Measured by Ultrasound and Computed Tomography. Ultrasound Med. Biol. 2009, 35, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Schneitler, S.; Rathmann, W.; Haastert, B.; Schneitler, H.; Winkler, H.; Bredahl, R.; Hahnloser, E.; Martin, S. Low-grade inflammation, obesity, and insulin resistance in adolescents. J. Clin. Endocrinol. Metab. 2007, 92, 4569–4574. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.D.; Shim, J.W.; Lee, S.Y.; Lee, H.; Cho, K.-H.; Yun, J.; Bae, Y.-S. Serum Amyloid A Induces CCL2 Production via Formyl Peptide Receptor-Like 1-Mediated Signaling in Human Monocytes. J. Immunol. 2008, 181, 4332–4339. [Google Scholar] [CrossRef]
- Simoes, E.; Correia-Lima, J.; Sardas, L.; Storti, F.; dos Santos Otani, T.Z.; Vasques, D.A.C.; Otani, V.H.O.; Bertolazzi, P.; Kochi, C.; Seelaender, M.; et al. Sex dimorphism in inflammatory response to obesity in childhood. Int. J. Obes. 2021, 45, 879–887. [Google Scholar] [CrossRef]
- Kang, H.; Li, X.; Xiong, K.; Song, Z.; Tian, J.; Wen, Y.; Sun, A.; Deng, X. The Entry and Egress of Monocytes in Atherosclerosis: A Biochemical and Biomechanical Driven Process. Cardiovas. Therap. 2021, 2021, 6642927. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Y.; Li, W.; Deng, F.; Liu, X.; Wang, X.; Gui, Y.; Qin, L.; Hu, C.; Chen, L. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima-media thickness. Atherosclerosis 2014, 232, 199–203. [Google Scholar] [CrossRef]
- Okumoto, S.; Taniguchi, Y.; Nakashima, A.; Masaki, T.; Ito, T.; Ogawa, T.; Takasugi, N.; Kohno, N.; Yorioka, N. C-C chemokine receptor 2 expression by circulating monocytes influences atherosclerosis in patients on chronic hemodialysis. Therap. Apher. Dial. 2009, 13, 205–212. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.J.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef]
- Cannon, J.G.; Sharma, G.; Sloan, G.; Dimitropoulou, C.; Baker, R.R.; Mazzoli, A.; Kraj, B.; Mulloy, A.; Cortez-Cooper, M. Leptin regulates CD16 expression on human monocytes in a sex-specific manner. Physiol. Rep. 2014, 2, e12177. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, M.J.; Doyle, M.F.; Stein, J.H.; Sitlani, C.M.; Fohner, A.E.; Huber, S.A.; Landay, A.L.; Heckbert, S.R.; Rice, K.; Kronmal, R.A.; et al. Nonclassical Monocytes (CD14dimCD16+) Are Associated with Carotid Intima-Media Thickness Progression for Men but Not Women: The Multi-Ethnic Study of Atherosclerosis—Brief Report. Arter. Thromb Vasc Biol. 2021, 41, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Große-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012, 60, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.E.; Ljungcrantz, I.; Andersson, L.; Bryngelsson, C.; Hedblad, B.; Fredrikson, G.N.; Nilsson, J.; Björkbacka, H. Elevated CD14++CD16-monocytes predict cardiovascular events. Circ. Cardiovasc Gen. 2012, 5, 122–131. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Di Pietrantonio, N.; Palmerini, C.; Pipino, C.; Baldassarre, M.P.A.; Bologna, G.; Mohn, A.; Giannini, C.; Lanuti, P.; Chiarelli, F.; Pandolfi, A.; et al. BBA—Molecular Basis of Disease Plasma from obese children increases monocyte-endothelial adhesion and affects intracellular insulin signaling in cultured endothelial cells: Potential role of mTORC1-S6K1. BBA Mol. Basis Dis. 2021, 1867, 166076. [Google Scholar] [CrossRef]
- Poitou, C.; Dalmas, E.; Renovato, M.; Benhamo, V.; Hajduch, F.; Abdennour, M.; Kahn, J.-F.; Veyrie, N.; Rizkalla, S.; Fridman, W.-H.; et al. CD14dim CD16+ and CD14+CD16+Monocytes in obesity and During Weight Loss Relationships With Fat Mass and Subclinical Atherosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 2322–2330. [Google Scholar] [CrossRef]
- Mine, S.; Okada, Y.; Tanikawa, T.; Kawahara, C.; Tabata, T.; Tanaka, Y. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochem. Biophys. Res. Commun. 2006, 344, 780–785. [Google Scholar] [CrossRef]
- Gállego-Suárez, C.; Bulan, A.; Hirschfeld, E.; Wachowiak, P.; Abrishami, S.; Griffin, C.; Sturza, J.; Tzau, A.; Hayes, T.; Woolford, S.J.; et al. Enhanced Myeloid Leukocytes in Obese Children and Adolescents at Risk for Metabolic Impairment. Front. Endocrinol. 2020, 11, 327. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Bernhagen, J.; Heitman, L.H.; Weber, C.; Dichgans, M. Targeting the CCL2–CCR2 axis for atheroprotection. Eur. Heart J. 2022, 43, 1799–1808. [Google Scholar] [CrossRef]
- McEwan, S.; Kwon, H.; Tahiri, A.; Shanmugarajah, N.; Cai, W.; Ke, J.; Huang, T.; Belton, A.; Singh, B.; Wang, L.; et al. Deconstructing the origins of sexual dimorphism in sensory modulation of pancreatic β cells. Mol. Metab. 2021, 53, 101260. [Google Scholar] [CrossRef] [PubMed]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation—A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diab. Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]

| All Participants (n = 33) | Girls a (n = 19) | Boys b (n = 14) | p a vs. b | |
|---|---|---|---|---|
| Anthropometric variables | ||||
| Age (years) | 10.9 ± 2.1 | 11.0 ± 2.4 | 10.7 ± 1.7 | 0.741 |
| Weight (kg) | 59.1 ± 15.1 | 57.8 ± 14.9 | 60.9 ± 15.8 | 0.559 |
| Height (cm) | 147.6 ± 10.8 | 145.5 ± 11.32 | 150.4 ± 9.8 | 0.210 |
| BMI (kg/m2) | 26.6 ± 3.3 | 26.8 ± 2.9 | 26.4 ± 3.8 | 0.786 |
| Waist (cm) | 86.5 ± 10.3 | 84.2 ± 8.3 | 89.7 ± 12.2 | 0.134 |
| SBP(mmHg) | 103.3 ± 9.8 | 101.5 ± 9.7 | 105.6 ± 9.8 | 0.247 |
| DBP (mmHg) | 68.7 ± 8.7 | 66.6 ± 7.0 | 71.5 ± 10.1 | 0.118 |
| BFP (%) | 31.4 ± 5.0 | 33.3 ± 3.8 | 28.7 ± 5.4 | 0.008 |
| VFA (cm3) | 86.5 ± 34.5 | 71.3 ± 22.9 | 107.1 ± 37.5 | 0.002 |
| PFA (cm2) | 4.6 ± 1.6 | 4.5 ± 1.9 | 4.6 ± 1.1 | 0.860 |
| Puberty Stages | ||||
| Tanner Stage | 43.3% | 33.3% | 58.3% | 0.111 |
| 1 | ||||
| 2 | 20% | 16.7% | 25% | |
| 3 | 10% | 5.6% | 26.7% | |
| 4 | 23.3% | 38.9% | 0% | |
| 5 | 3.3% | 5.6% | 0% | |
| Glucose Homeostasis | ||||
| Glucose (mg/dL) | 88.6 ± 8.4 | 88.9 ± 8.7 | 86.8 ± 8.2 | 0.493 |
| HbA1c | 5.3 ± 0.2 | 5.2 ± 0.1 | 5.3 ± 0.2 | 0.183 |
| Insulin (mg/dL) | 16.4 ± 1.4 | 16.9 ± 7.2 | 15.6 ± 10.2 | 0.687 |
| HOMA | 3.7 ± 1.9 | 4.0 ± 1.7 | 3.4 ± 2.3 | 0.403 |
| Biochemical variables | ||||
| Uric acid (mg/dL) | 5.7 ± 1.0 | 5.8 ± 1.0 | 5.6 ± 1.1 | 0.958 |
| TG (mg/dL) | 138.5 ± 54.5 | 133.4 ± 45.3 | 145.4 ± 66.3 | 0.567 |
| ALT (UI/L) | 24.8 ± 1.8 | 22.8 ± 8.5 | 27.5 ± 13.3 | 0.235 |
| AST (UI/L) | 28.1 ± 7.2 | 26.3 ± 6.8 | 30.5 ± 6.8 | 0.105 |
| GGT (UI/L) | 16.2 ± 0.9 | 14.9 ± 3.7 | 18.0 ± 6.5 | 0.134 |
| Cardiovascular variables | ||||
| Tot Chol (mg/dL) | 163.7 ± 21.0 | 162.0 ± 18.6 | 166.0 ± 24.5 | 0.603 |
| HDL (mg/dL) | 40.0 ± 7.8 | 40.0 ± 8.0 | 39.9 ± 7.8 | 0.965 |
| LDL (mg/dL) | 107.2 ± 17.9 | 107.0 ± 16.5 | 107.5 ± 20.3 | 0.938 |
| LDL/HDL Ratio | 2.7 ± 0.1 | 2.7 ± 0.6 | 2.7 ± 0.7 | 0.924 |
| IMT (mm) | 0.56 ± 0.1 | 0.58 ± 0.10 | 0.55 ± 0.11 | 0.399 |
| CCR2 % | CCR2 MFI | ||||
|---|---|---|---|---|---|
| IMT ≥ p75 | HOMA ≥ 3.4 | IMT ≥ p75 | HOMA ≥ 3.4 | ||
| CM | Boys (n = 14) | r = 0.56 *(0.07–0.85) | r = 0.21 (−0.45–0.72) | r = 0.61 *(0.20–0.86) | r = 0.22 (−0.45–0.70) |
| Girls (n = 19) | r = −0.05 (−0.68–0.58) | r = −0.11 (−0.72–0.60) | r = 0.22 (−0.36–0.72) | r = −0.21 (−0.88–0.42) | |
| IM | Boys (n = 14) | r = 0.62 *(0.17–0.88) | r = 0.41 (−0.12–0.88) | r = 0.62 *(0.19–0.91) | r = 0.52 (−0.00–0.86) |
| Girls (n = 19) | r = 0.27 (−0.27–0.88) | r = 0.03 (−0.62–0.80) | r = −0.04 (−0.60–0.48) | r = −0.07 (−0.68–0.50) | |
| NCM | Boys (n = 14) | r = 0.72 **(0.38–0.91) | r = 0.80 **(0.60–0.93) | r = 0.73 **(0.45–0.91) | r = 0.70 **(0.40–0.90) |
| Girls (n = 19) | r = 0.15 (−0.39–0.74) | r = −0.22 (−0.71–0.40) | r = −0.21 (−0.75–0.33) | r = −0.20 (−0.69–0.37) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés-Hernández, M.J.; Pedraza-Escudero, K.; Garibay-Nieto, N.; Hernández-Ruiz, J.; Prieto-Chávez, J.L.; Arriaga-Pizano, L.A.; Villanueva-Ortega, E.; Escobedo, G.; Manjarrez-Reyna, A.N.; López-Alvarenga, J.C.; et al. The CCR2+ Monocyte Subsets Increase in Obese Boys but Not Girls with Abnormally High Carotid Intima-Media Thickness: A Pilot Study. J. Cardiovasc. Dev. Dis. 2022, 9, 330. https://doi.org/10.3390/jcdd9100330
Garcés-Hernández MJ, Pedraza-Escudero K, Garibay-Nieto N, Hernández-Ruiz J, Prieto-Chávez JL, Arriaga-Pizano LA, Villanueva-Ortega E, Escobedo G, Manjarrez-Reyna AN, López-Alvarenga JC, et al. The CCR2+ Monocyte Subsets Increase in Obese Boys but Not Girls with Abnormally High Carotid Intima-Media Thickness: A Pilot Study. Journal of Cardiovascular Development and Disease. 2022; 9(10):330. https://doi.org/10.3390/jcdd9100330
Chicago/Turabian StyleGarcés-Hernández, María José, Karen Pedraza-Escudero, Nayely Garibay-Nieto, Joselin Hernández-Ruiz, Jessica Lakshmi Prieto-Chávez, Lourdes Andrea Arriaga-Pizano, Eréndira Villanueva-Ortega, Galileo Escobedo, Aaron Noe Manjarrez-Reyna, Juan Carlos López-Alvarenga, and et al. 2022. "The CCR2+ Monocyte Subsets Increase in Obese Boys but Not Girls with Abnormally High Carotid Intima-Media Thickness: A Pilot Study" Journal of Cardiovascular Development and Disease 9, no. 10: 330. https://doi.org/10.3390/jcdd9100330
APA StyleGarcés-Hernández, M. J., Pedraza-Escudero, K., Garibay-Nieto, N., Hernández-Ruiz, J., Prieto-Chávez, J. L., Arriaga-Pizano, L. A., Villanueva-Ortega, E., Escobedo, G., Manjarrez-Reyna, A. N., López-Alvarenga, J. C., Pérez-Hernández, J. L., & Queipo-García, G. (2022). The CCR2+ Monocyte Subsets Increase in Obese Boys but Not Girls with Abnormally High Carotid Intima-Media Thickness: A Pilot Study. Journal of Cardiovascular Development and Disease, 9(10), 330. https://doi.org/10.3390/jcdd9100330








