Neurofibromin Encoded by the Neurofibromatosis Type 1 (NF1) Gene Promotes the Membrane Translocation of SPRED2, Thereby Inhibiting the ERK Pathway in Breast Cancer Cells
Abstract
1. Introduction
2. Results
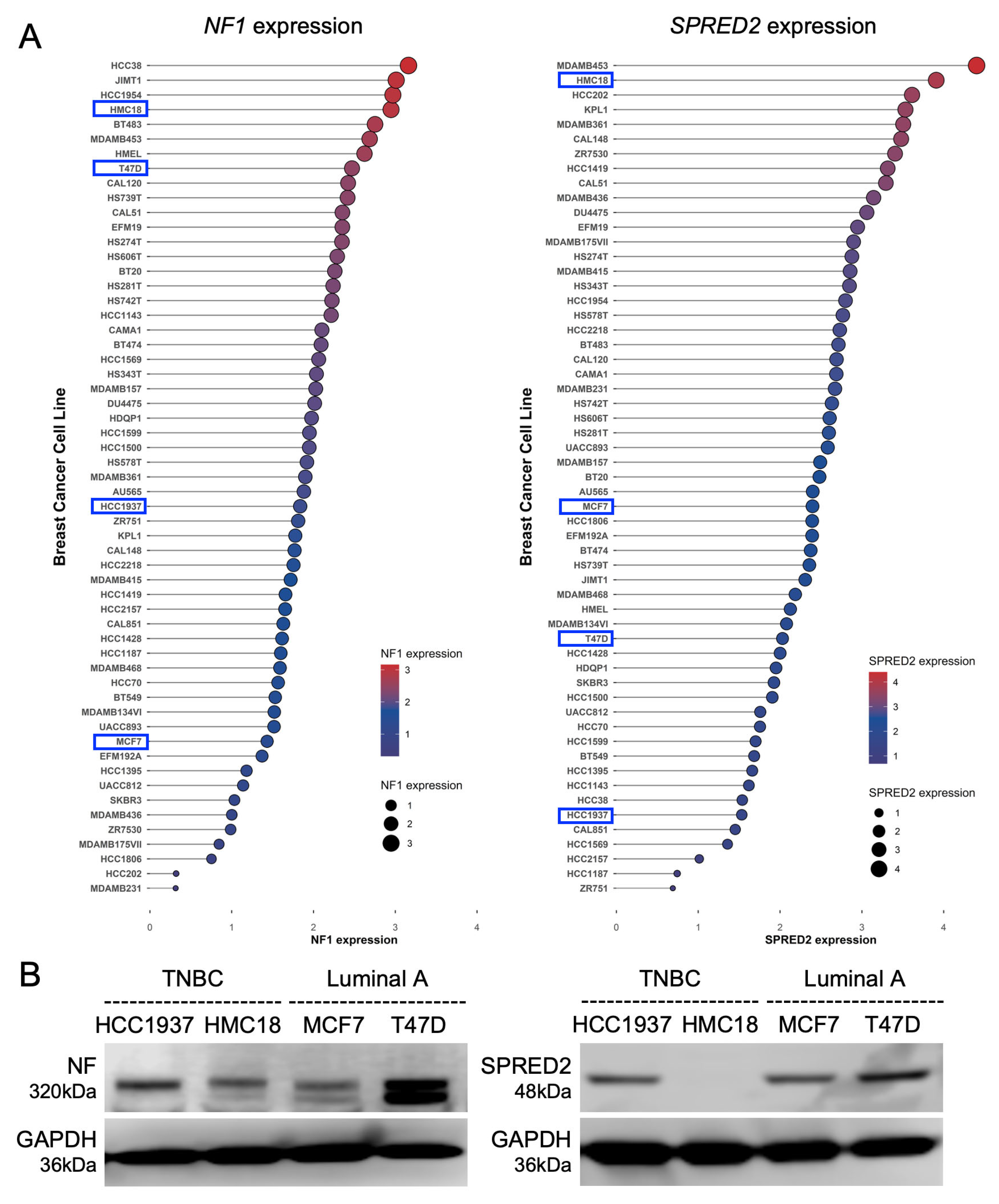
2.1. Expression of NF1 and SPRED2 in Human BC Cell Lines
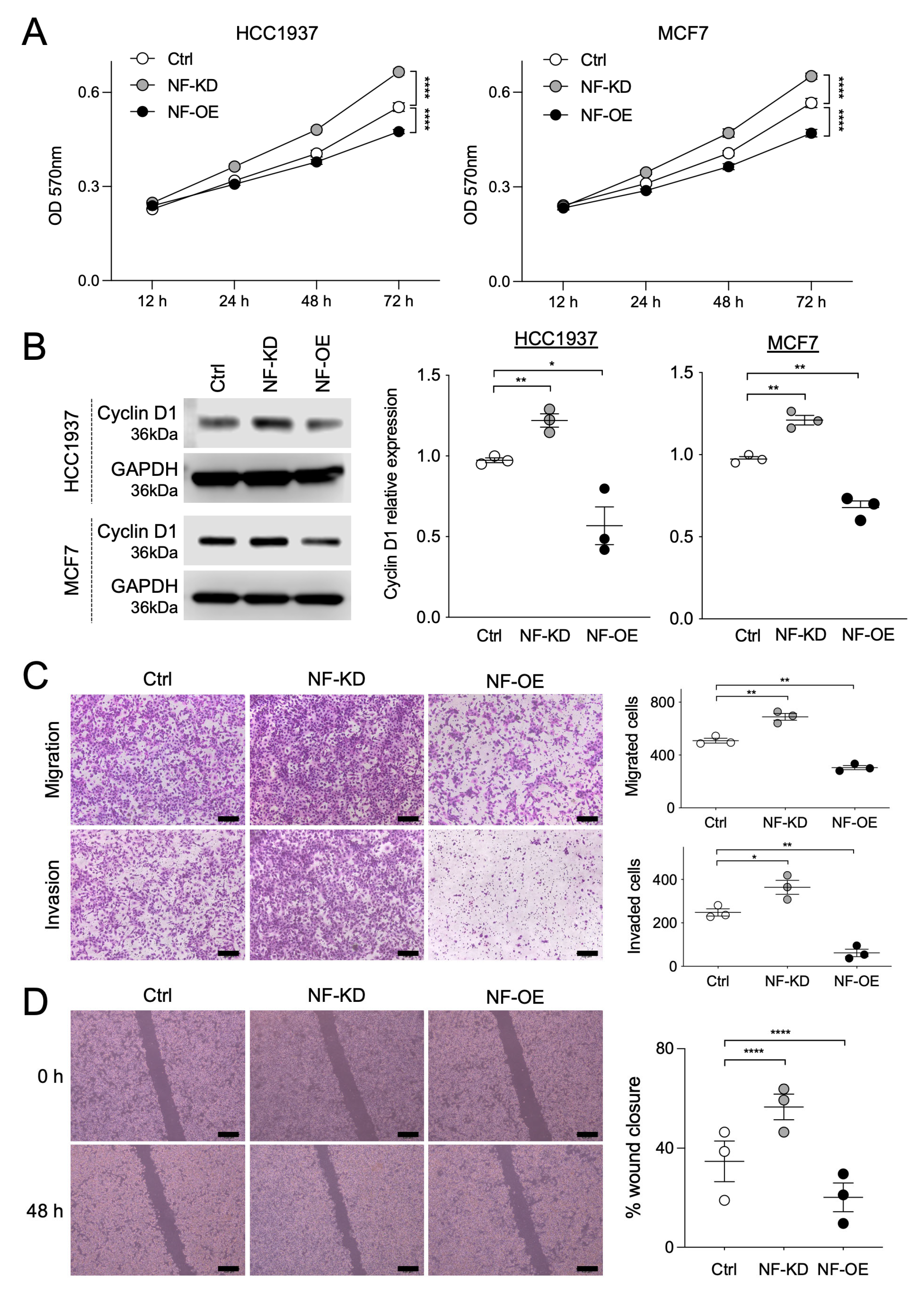
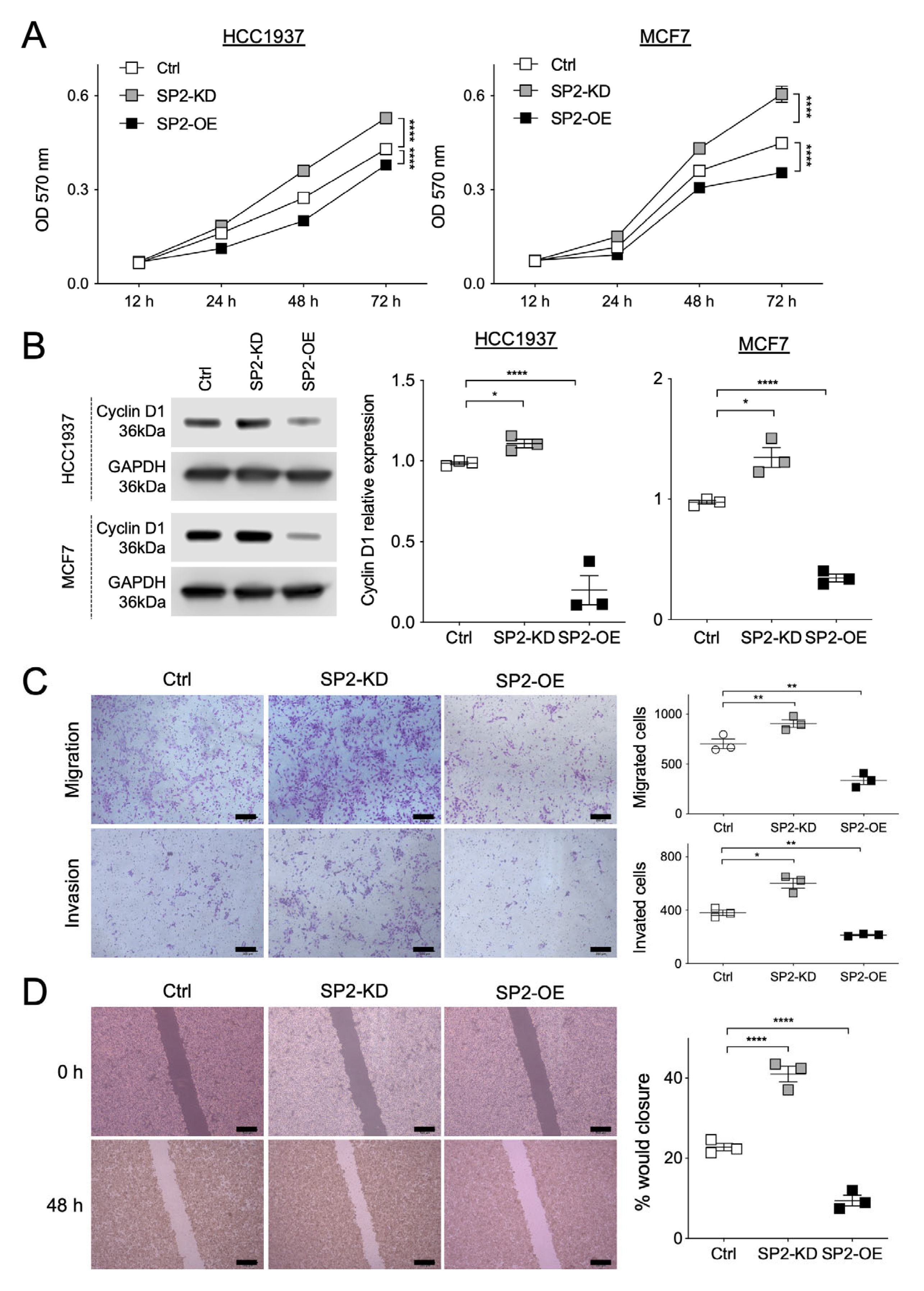
2.2. NF and SPRED2 Downregulates BC Cell Proliferation, Migration and Invasiveness
2.3. NF and SPRED2 Downregulate BC Cell Proliferation, Migration and Invasiveness via the RAF/ERK Pathway
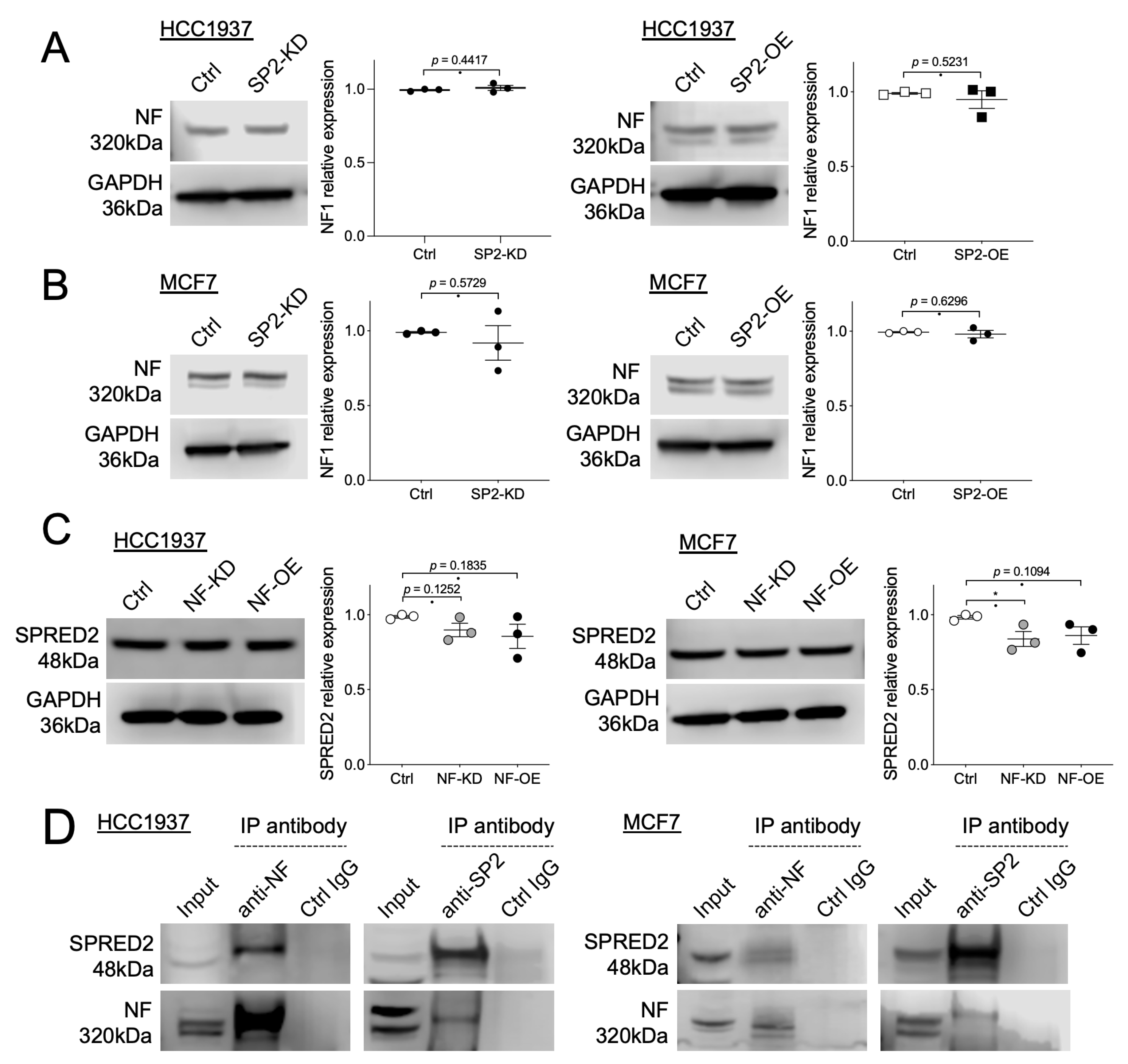
2.4. Interaction of NF and SPRED2 in BC Cells
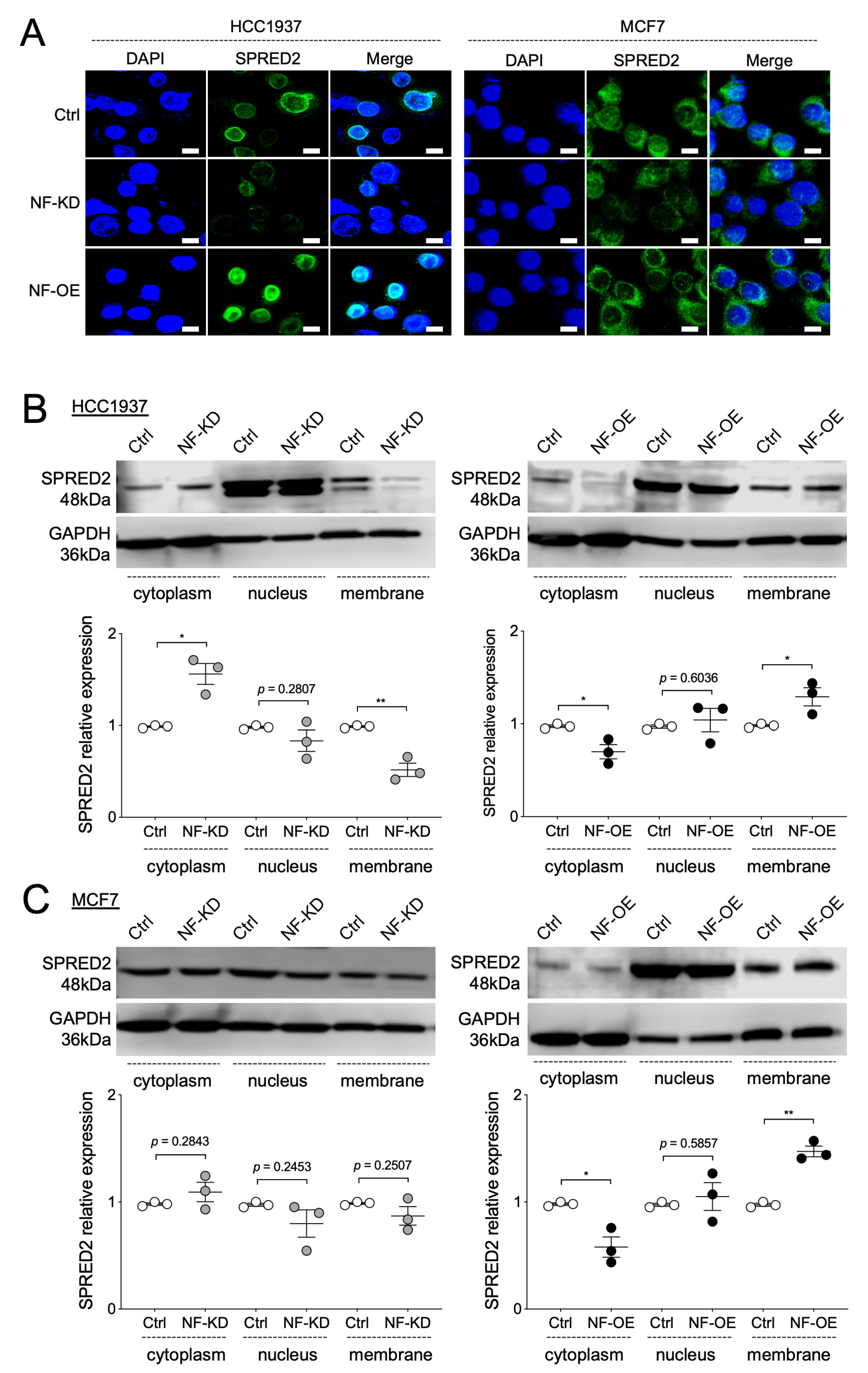
2.5. NF Promotes the Membrane Translocation of SPRED2
2.6. NF and SPRED2 Expression in Clinical Specimens
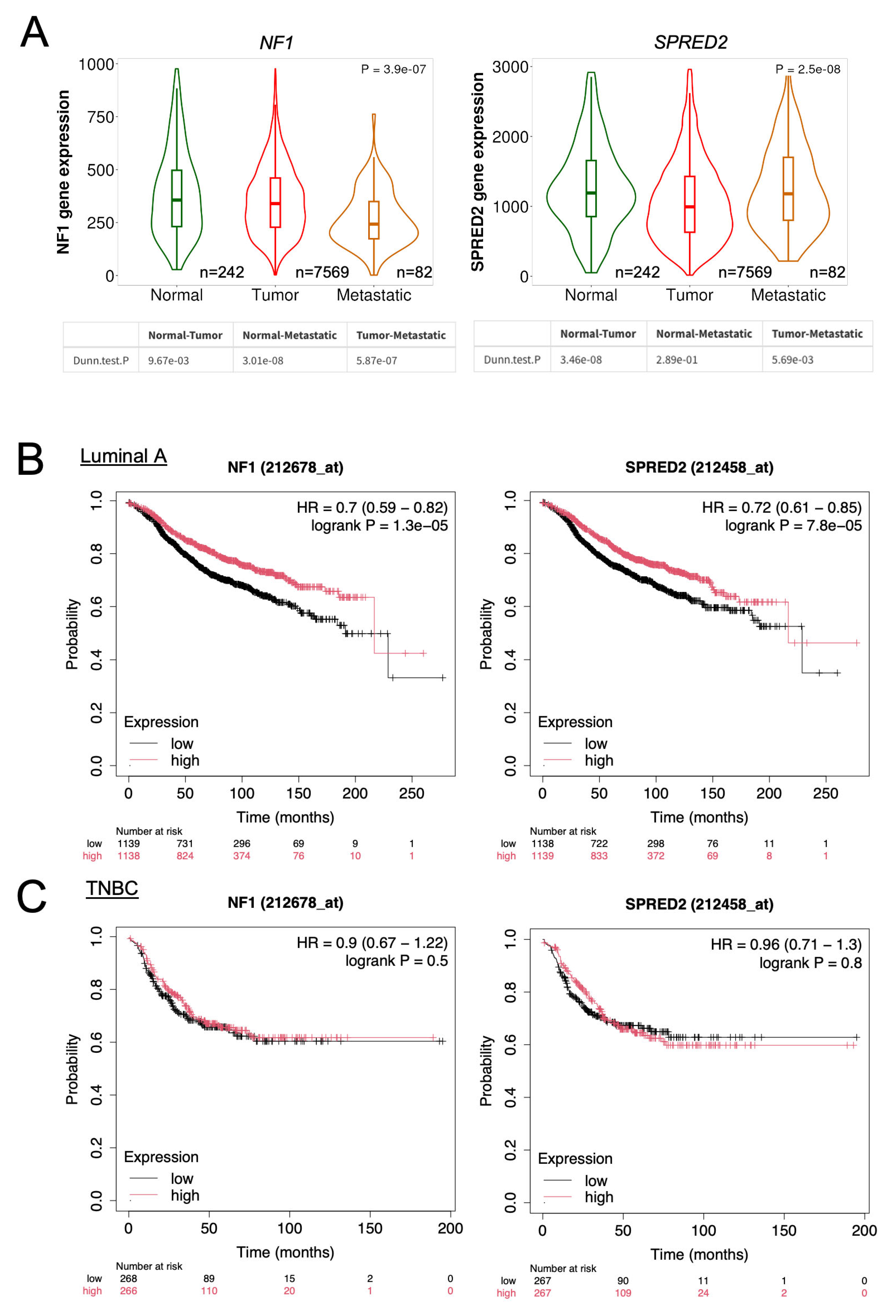
2.7. Database Analysis of NF1 and SPRED2 mRNA Expression and Their Prognostic Significance in BC
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. Cell Culture
4.3. Transfection
4.4. Cell Proliferation Assay
4.5. Transwell Migration and Invasion Assay
4.6. Scratch Assay
4.7. RT-qPCR
4.8. Western Blotting
4.9. Immunocytochemistry (ICC) and Immunofluorescence (IF)
4.10. Immunohistochemistry (IHC)
4.11. Co-Immunoprecipitation Assay (Co-IP)
4.12. Clinical Samples
4.13. Database Analyses
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NF1 | neurofibromatosis type 1 |
| NF | Neurofibromin |
| SPRED | Sprouty-related EVH1 domain-containing protein |
| BC | breast cancer |
| IHC | immunohistochemistry |
| TNBC | triple-negative breast cancer |
| ER | estrogen receptor |
| PR | progesterone receptor |
| HER2 | human epidermal growth factor receptor 2 |
| HCC | hepatocellular carcinoma |
| siRNA | small interfering RNA |
| KD | knockdown |
| OE | overexpression |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Peduto, C.; Zanobio, M.; Nigro, V.; Perrotta, S.; Piluso, G.; Santoro, C. Neurofibromatosis Type 1: Pediatric Aspects and Review of Genotype-Phenotype Correlations. Cancers 2023, 15, 1217. [Google Scholar] [CrossRef]
- Maani, N.; Westergard, S.; Yang, J.; Scaranelo, A.M.; Telesca, S.; Thain, E.; Schachter, N.F.; McCuaig, J.M.; Kim, R.H. NF1 Patients Receiving Breast Cancer Screening: Insights from The Ontario High Risk Breast Screening Program. Cancers 2019, 11, 707. [Google Scholar] [CrossRef]
- Uusitalo, E.; Kallionpää, R.A.; Kurki, S.; Rantanen, M.; Pitkäniemi, J.; Kronqvist, P.; Härkönen, P.; Huovinen, R.; Carpen, O.; Pöyhönen, M.; et al. Breast cancer in neurofibromatosis type 1: Overrepresentation of unfavourable prognostic factors. Br. J. Cancer. 2017, 116, 211–217. [Google Scholar] [CrossRef]
- Pearson, A.; Proszek, P.; Pascual, J.; Fribbens, C.; Shamsher, M.K.; Kingston, B.; O’Leary, B.; Herrera-Abreu, M.T.; Cutts, R.J.; Garcia-Murillas, I.; et al. Inactivating NF1 Mutations Are Enriched in Advanced Breast Cancer and Contribute to Endocrine Therapy Resistance. Clin. Cancer Res. 2020, 26, 608–622. [Google Scholar] [CrossRef] [PubMed]
- House, R.R.J.; Tovar, E.A.; Redlon, L.N.; Essenburg, C.J.; Dischinger, P.S.; Ellis, A.E.; Beddows, I.; Sheldon, R.D.; Lien, E.C.; Graveel, C.R.; et al. NF1 deficiency drives metabolic reprogramming in ER+ breast cancer. Mol. Metab. 2024, 80, 101876. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.; Tovell, H.; Frayling, I.M.; Cooper, D.N.; Upadhyaya, M. The NF1 somatic mutational landscape in sporadic human cancers. Hum. Genom. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Safonov, A.; Nomakuchi, T.T.; Chao, E.; Horton, C.; Dolinsky, J.S.; Yussuf, A.; Richardson, M.; Speare, V.; Li, S.; Bogus, Z.C.; et al. A genotype-first approach identifies high incidence of NF1 pathogenic variants with distinct disease associations. Nat. Commun. 2025, 16, 3121. [Google Scholar] [CrossRef]
- Báez-Flores, J.; Rodríguez-Martín, M.; Lacal, J. The therapeutic potential of neurofibromin signaling pathways and binding partners. Commun. Biol. 2023, 6, 436. [Google Scholar] [CrossRef] [PubMed]
- Bergoug, M.; Doudeau, M.; Godin, F.; Mosrin, C.; Vallée, B.; Bénédetti, H. Neurofibromin Structure, Functions and Regulation. Cells 2020, 9, 2365. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Moye, S.L.; McKay, R.M.; Le, L.Q. Neurofibromin and suppression of tumorigenesis: Beyond the GAP. Oncogene 2022, 41, 1235–1251. [Google Scholar] [CrossRef]
- Acosta-Casique, A.; Montes-Alvarado, J.B.; Barragán, M.; Larrauri-Rodríguez, K.A.; Perez-Gonzalez, A.; Delgado-Magallón, A.; Millán-Perez-Peña, L.; Rosas-Murrieta, N.H.; Maycotte, P. ERK activation modulates invasiveness and Reactive Oxygen Species (ROS) production in triple negative breast cancer cell lines. Cell Signal 2023, 101, 110487. [Google Scholar] [CrossRef]
- Wakioka, T.; Sasaki, A.; Kato, R.; Shouda, T.; Matsumoto, A.; Miyoshi, K.; Tsuneoka, M.; Komiya, S.; Baron, R.; Yoshimura, A. Spred is a Sprouty-related suppressor of Ras signalling. Nature 2001, 412, 647–651. [Google Scholar] [CrossRef]
- Dunzendorfer-Matt, T.; Mercado, E.L.; Maly, K.; McCormick, F.; Scheffzek, K. The neurofibromin recruitment factor Spred1 binds to the GAP related domain without affecting Ras inactivation. Proc. Natl. Acad. Sci. USA 2016, 113, 7497–7502. [Google Scholar] [CrossRef]
- Yan, W.; Markegard, E.; Dharmaiah, S.; Urisman, A.; Drew, M.; Esposito, D.; Scheffzek, K.; Nissley, D.V.; McCormick, F.; Simanshu, D.K. Structural Insights into the SPRED1-Neurofibromin-KRAS Complex and Disruption of SPRED1-Neurofibromin Interaction by Oncogenic EGFR. Cell Rep. 2020, 32, 107909. [Google Scholar] [CrossRef]
- Kachroo, N.; Valencia, T.; Warren, A.Y.; Gnanapragasam, V.J. Evidence for downregulation of the negative regulator SPRED2 in clinical prostate cancer. Br. J. Cancer. 2013, 108, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Kong, F.; Xiao, F.; Li, Y.; Wang, H.; Zhang, S.; Huang, D.; Wang, L.; Yang, Y. Spred2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by impairing ERK signaling. Oncol. Rep. 2020, 44, 174–184. [Google Scholar] [CrossRef]
- Oda, S.; Fujisawa, M.; Chunning, L.; Ito, T.; Yamaguchi, T.; Yoshimura, T.; Matsukawa, A. Expression of Spred2 in the urothelial tumorigenesis of the urinary bladder. PLoS ONE 2021, 16, e0254289. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yang, X.; Fujisawa, M.; Ohara, T.; Wang, T.; Tomonobu, N.; Sakaguchi, M.; Yoshimura, T.; Matsukawa, A. SPRED2: A Novel Regulator of Epithelial-Mesenchymal Transition and Stemness in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2023, 24, 4996. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Gao, T.; Fujisawa, M.; Sumardika, I.W.; Sakaguchi, M.; Toyooka, S.; Yoshimura, T.; Matsukawa, A. Expression of SPRED2 in the lung adenocarcinoma. Pathol. Res. Pract. 2025, 265, 155721. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, V.; Hany, D.; Picard, D. Hyperactivation of MAPK Induces Tamoxifen Resistance in SPRED2-Deficient ERα-Positive Breast Cancer. Cancers 2022, 14, 954. [Google Scholar] [CrossRef]
- Wang, T.; Gao, T.; Fujisawa, M.; Ohara, T.; Sakaguchi, M.; Yoshimura, T.; Matsukawa, A. SPRED2 Is a Novel Regulator of Autophagy in Hepatocellular Carcinoma Cells and Normal Hepatocytes. Int. J. Mol. Sci. 2024, 25, 6269. [Google Scholar] [CrossRef]
- Lorenzo, C.; McCormick, F. SPRED proteins and their roles in signal transduction, development, and malignancy. Genes. Dev. 2020, 34, 1410–1421. [Google Scholar] [CrossRef]
- Nusinow, D.P.; Szpyt, J.; Ghandi, M.; Rose, C.M.; McDonald, E.R.; Kalocsay, M.; Jané-Valbuena, J.; Gelfand, E.; Schweppe, D.K.; Jedrychowski, M.; et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell 2020, 180, 387–402.e16. [Google Scholar] [CrossRef]
- Lopez, J.; Bonsor, D.A.; Sale, M.J.; Urisman, A.; Mehalko, J.L.; Cabanski-Dunning, M.; Castel, P.; Simanshu, D.K.; McCormick, F. The ribosomal S6 kinase 2 (RSK2)-SPRED2 complex regulates the phosphorylation of RSK substrates and MAPK signaling. J. Biol. Chem. 2023, 299, 104789. [Google Scholar] [CrossRef]
- Hirata, Y.; Brems, H.; Van der Auweraer, S.; Ohyagi, M.; Iizuka, M.; Mise-Omata, S.; Ito, M.; Messiaen, L.; Mizuno, S.; Takahashi, S.; et al. Legius syndrome mutations in the Ras-regulator SPRED1 abolish its membrane localization and potentially cause neurodegeneration. J. Biol. Chem. 2024, 300, 107969. [Google Scholar] [CrossRef]
- Dischinger, P.S.; Tovar, E.A.; Essenburg, C.J.; Madaj, Z.B.; Gardner, E.E.; Callaghan, M.E.; Turner, A.N.; Challa, A.K.; Kempston, T.; Eagleson, B.; et al. NF1 deficiency correlates with estrogen receptor signaling and diminished survival in breast cancer. NPJ Breast Cancer 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shen, H.; Huang, W.; He, S.; Chen, J.; Zhang, D.; Shen, Y.; Sun, Y. Genome-scale CRISPR-Cas9 knockout screening in hepatocellular carcinoma with lenvatinib resistance. Cell Death Discov. 2021, 7, 359, Correction in Cell Death Discov. 2022, 8, 1. [Google Scholar] [CrossRef]
- Qiao, G.; Jia, X.; Zhang, Y.; Chen, B. Neurofibromin 1 expression is negatively correlated with malignancy and prognosis of epithelial ovarian cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 1702–1712. [Google Scholar] [PubMed]
- Arai, H.; Elliott, A.; Millstein, J.; Xiu, J.; Ou, F.S.; Innocenti, F.; Wang, J.; Battaglin, F.; Jayachandran, P.; Kawanishi, N.; et al. Molecular characteristics and clinical outcomes of patients with Neurofibromin 1-altered metastatic colorectal cancer. Oncogene 2022, 41, 260–267. [Google Scholar] [CrossRef]
- Barron, V.A.; Lou, H. Alternative splicing of the neurofibromatosis type I pre-mRNA. Biosci. Rep. 2012, 32, 131–138. [Google Scholar] [CrossRef]
- Rossi, S.; Gasparotto, D.; Cacciatore, M.; Sbaraglia, M.; Mondello, A.; Polano, M.; Mandolesi, A.; Gronchi, A.; Reuss, D.E.; von Deimling, A.; et al. Neurofibromin C terminus-specific antibody (clone NFC) is a valuable tool for the identification of NF1-inactivated GISTs. Mod. Pathol. 2018, 31, 160–168. [Google Scholar] [CrossRef]
- Führer, S.; Tollinger, M.; Dunzendorfer-Matt, T. Pathogenic Mutations Associated with Legius Syndrome Modify the Spred1 Surface and Are Involved in Direct Binding to the Ras Inactivator Neurofibromin. J. Mol. Biol. 2019, 431, 3889–3899. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, M.; Lin, G.; Mao, B.; Cheng, W.; Liu, H.; Gal, J.; Zhu, H.; Yuan, Z.; Deng, W.; et al. Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death. Oncotarget 2016, 7, 25652–25667. [Google Scholar] [CrossRef]
- King, J.A.; Straffon, A.F.; D’Abaco, G.M.; Poon, C.L.; I, S.T.; Smith, C.M.; Buchert, M.; Corcoran, N.M.; Hall, N.E.; Callus, B.A.; et al. Distinct requirements for the Sprouty domain for functional activity of Spred proteins. Biochem. J. 2005, 388, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Mardakheh, F.K.; Yekezare, M.; Machesky, L.M.; Heath, J.K. Spred2 interaction with the late endosomal protein NBR1 down-regulates fibroblast growth factor receptor signaling. J. Cell Biol. 2009, 187, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment landscape of triple-negative breast cancer—Expanded options, evolving needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Akter, S.; Ramproshad, S.; Mondal, B.; Riaz, T.A.; Islam, M.T.; Khan, I.N.; Docea, A.O.; Calina, D.; Sharifi-Rad, J.; et al. Targeting Ras-ERK cascade by bioactive natural products for potential treatment of cancer: An updated overview. Cancer Cell Int. 2022, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Rashwan, S.; Albrahim, Y.; Wadan, A.S.; Radwan, F.; Alqosaibi, A.I.; Abdel-Ghany, S.; Arneth, B. Targeting Resistance Pathways in Breast Cancer Through Precision Oncology: Nanotechnology and Immune Modulation Approaches. Biomedicines 2025, 13, 1691. [Google Scholar] [CrossRef] [PubMed]


| Total Cases | Negative | Low | High | p | |
|---|---|---|---|---|---|
| TNBC | 49 | 21 (42.9%) | 20 (40.8%) | 8 (16.3%) | 0.0213 |
| Luminal A | 45 | 10 (22.2%) | 21 (46.7%) | 14 (31.1%) |
| Total Cases | C−M− | C+M− | C+M+ | p | |
|---|---|---|---|---|---|
| TNBC | 49 | 11 (22.4%) | 38 (77.6%) | 0 (0%) | <0.0001 |
| Luminal A | 45 | 6 (13.3%) | 21 (46.7%) | 18 (40.0%) |
| NF-Negative | NF-Positive | ||||
|---|---|---|---|---|---|
| C+M− | C+M+ | C+M− | C+M+ | p | |
| TNBC | 21 | 0 | 28 | 0 | 0.0213 |
| Luminal A | 10 | 0 | 17 | 18 | |
| Antigen | Company (Cat. Number) |
|---|---|
| Neurofibromin (NF) | Proteintech (27249-1-AP) 1,2 |
| Santa Cruz Biotechnology (sc-376886) 1,2 | |
| SPRED2 | Proteintech (24091-1-AP) 1,2 |
| Santa Cruz Biotechnology (sc-517018) 1,2 | |
| p44/42 MAPK (ERK1/2) | Cell Signaling Technology (4695) 1 |
| Phospho-p44/42 MAPK (pERK1/2) | Cell Signaling Technology (4370) 1 |
| c-RAF (D4B3J) | Cell Signaling Technology (53745) 1 |
| Phospho-c-RAF (Ser338) (56A6) | Cell Signaling Technology (9427) 1 |
| CyclinD1 | Cell Signaling Technology (92G2) 1 |
| GAPDH | Cell Signaling Technology (5174) 1 |
| Estrogen Receptor a (D6R2W) | Cell Signaling Technology (13258) 1 |
| Progesterone Receptor A/B (D8Q2J) | Cell Signaling Technology (8757) 1 |
| HER2/ErbB2 (29D8) | Cell Signaling Technology (2165) 1 |
| Mouse IgG Isotype control | Cell Signaling Technology (14269S) 1 |
| Normal Rabbit IgG | Cell Signaling Technology (5415S) 1 |
| HRP-goat anti-rabbit IgG | Cell Signaling Technology (7074) 1,2 |
| HRP-anti-mouse IgG | Cell Signaling Technology (7076) 1,2 |
| Goat anti-Rabbit IgG, Alexa Fluor 488 conjugated | Thermo Fisher Scientific (A-11008) 1 |
| Characteristics | n | (%) | |
|---|---|---|---|
| Age | ≤49 | 29 | 30.85 |
| ≥50 | 65 | 69.15 | |
| Tumor size | pT1 (≤5 mm) | 6 | 6.38 |
| pT1b (6–10 mm) | 14 | 14.89 | |
| pT1c (10–20 mm) | 46 | 48.94 | |
| pT2 (20–50 mm) | 24 | 25.53 | |
| pT3 (>50 mm) | 4 | 4.25 | |
| Lymph node metastasis | pN0 | 69 | 73.40 |
| pN1 | 15 | 15.96 | |
| pN2 | 3 | 3.19 | |
| pN3 | 5 | 5.32 | |
| not accessible | 2 | 2.13 | |
| Histologic grade | Grade 1 and 2 | 52 | 55.32 |
| Grade 3 | 42 | 44.68 | |
| Estrogen receptor | Negative | 49 | 52.13 |
| Positive | 45 | 47.87 | |
| Progesterone receptor | Negative | 49 | 52.13 |
| Positive | 45 | 47.87 | |
| Her2 overexpression | Negative | 94 | 100 |
| Positive | 0 | 0 | |
| Ki67 index | ≤20% | 51 | 54.26 |
| >20% | 43 | 45.74 | |
| Intrinsic subtypes | Luminal A | 45 | 47.87 |
| Triple negative | 49 | 52.13 | |
| Histologic type | No special type (NST) | 94 | 100 |
| Invasive lobular carcinoma | 0 | 0 | |
| Mucinous carcinoma | 0 | 0 | |
| Invasive micropapillary carcinoma | 0 | 0 | |
| Other special types | 0 | 0 | |
| Outcome | Disease free survival 1 | 70 | 74.47 |
| Overall survival 2 | 74 | 78.72 | |
| Death | 19 | 15.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su Pwint, N.T.; Li, C.; Gao, T.; Wang, Y.; Fujisawa, M.; Ohara, T.; Sakaguchi, M.; Yoshimura, T.; Matsukawa, A. Neurofibromin Encoded by the Neurofibromatosis Type 1 (NF1) Gene Promotes the Membrane Translocation of SPRED2, Thereby Inhibiting the ERK Pathway in Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 10072. https://doi.org/10.3390/ijms262010072
Su Pwint NT, Li C, Gao T, Wang Y, Fujisawa M, Ohara T, Sakaguchi M, Yoshimura T, Matsukawa A. Neurofibromin Encoded by the Neurofibromatosis Type 1 (NF1) Gene Promotes the Membrane Translocation of SPRED2, Thereby Inhibiting the ERK Pathway in Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(20):10072. https://doi.org/10.3390/ijms262010072
Chicago/Turabian StyleSu Pwint, Nang Thee, Chunning Li, Tong Gao, Yuze Wang, Masayoshi Fujisawa, Toshiaki Ohara, Masakiyo Sakaguchi, Teizo Yoshimura, and Akihiro Matsukawa. 2025. "Neurofibromin Encoded by the Neurofibromatosis Type 1 (NF1) Gene Promotes the Membrane Translocation of SPRED2, Thereby Inhibiting the ERK Pathway in Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 20: 10072. https://doi.org/10.3390/ijms262010072
APA StyleSu Pwint, N. T., Li, C., Gao, T., Wang, Y., Fujisawa, M., Ohara, T., Sakaguchi, M., Yoshimura, T., & Matsukawa, A. (2025). Neurofibromin Encoded by the Neurofibromatosis Type 1 (NF1) Gene Promotes the Membrane Translocation of SPRED2, Thereby Inhibiting the ERK Pathway in Breast Cancer Cells. International Journal of Molecular Sciences, 26(20), 10072. https://doi.org/10.3390/ijms262010072









