A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization
Abstract
1. Introduction
2. Results
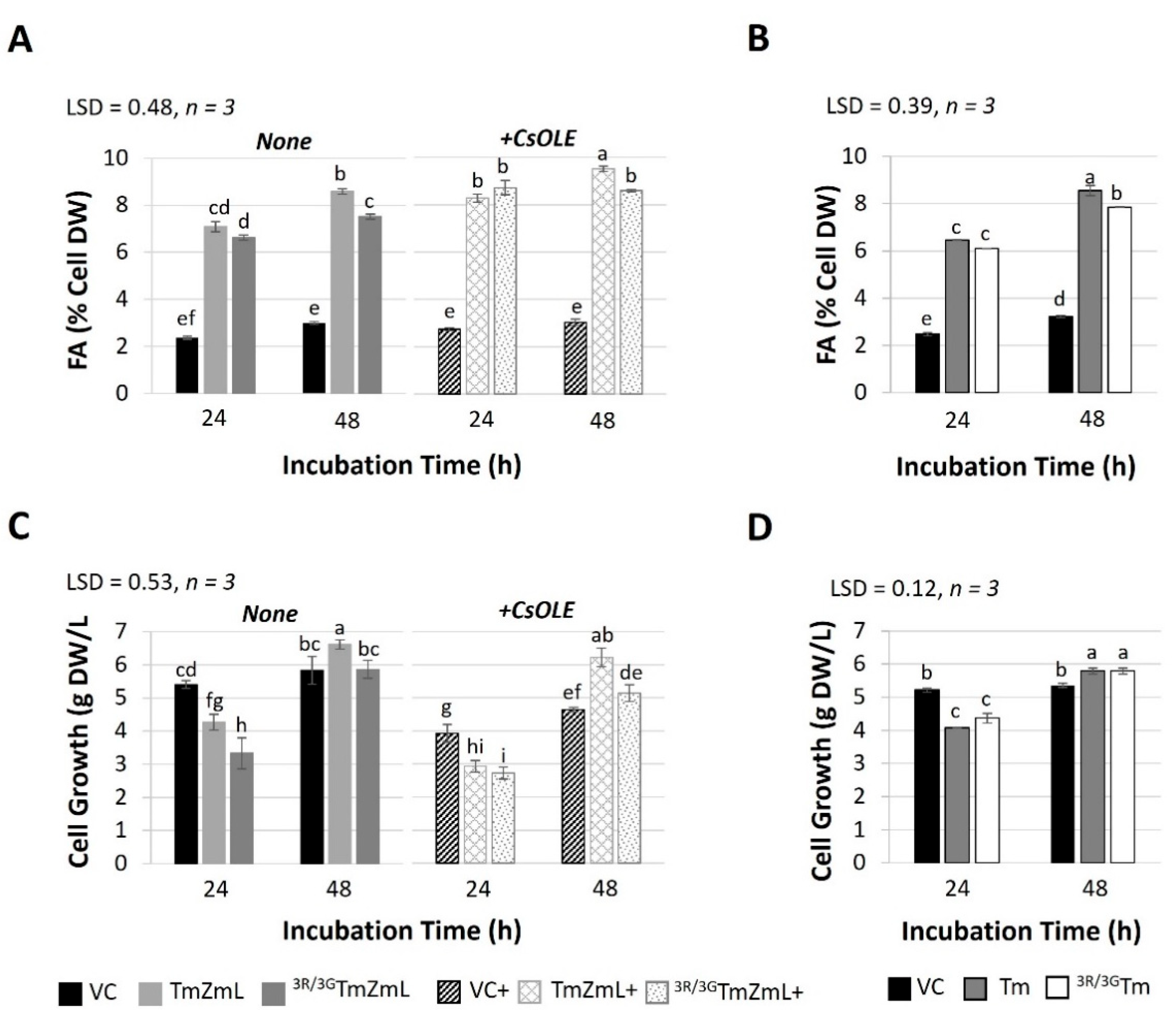
2.1. A Modified N-Terminal di-R Motif of DGAT1 Influences Lipid Content in C. sativa Seeds
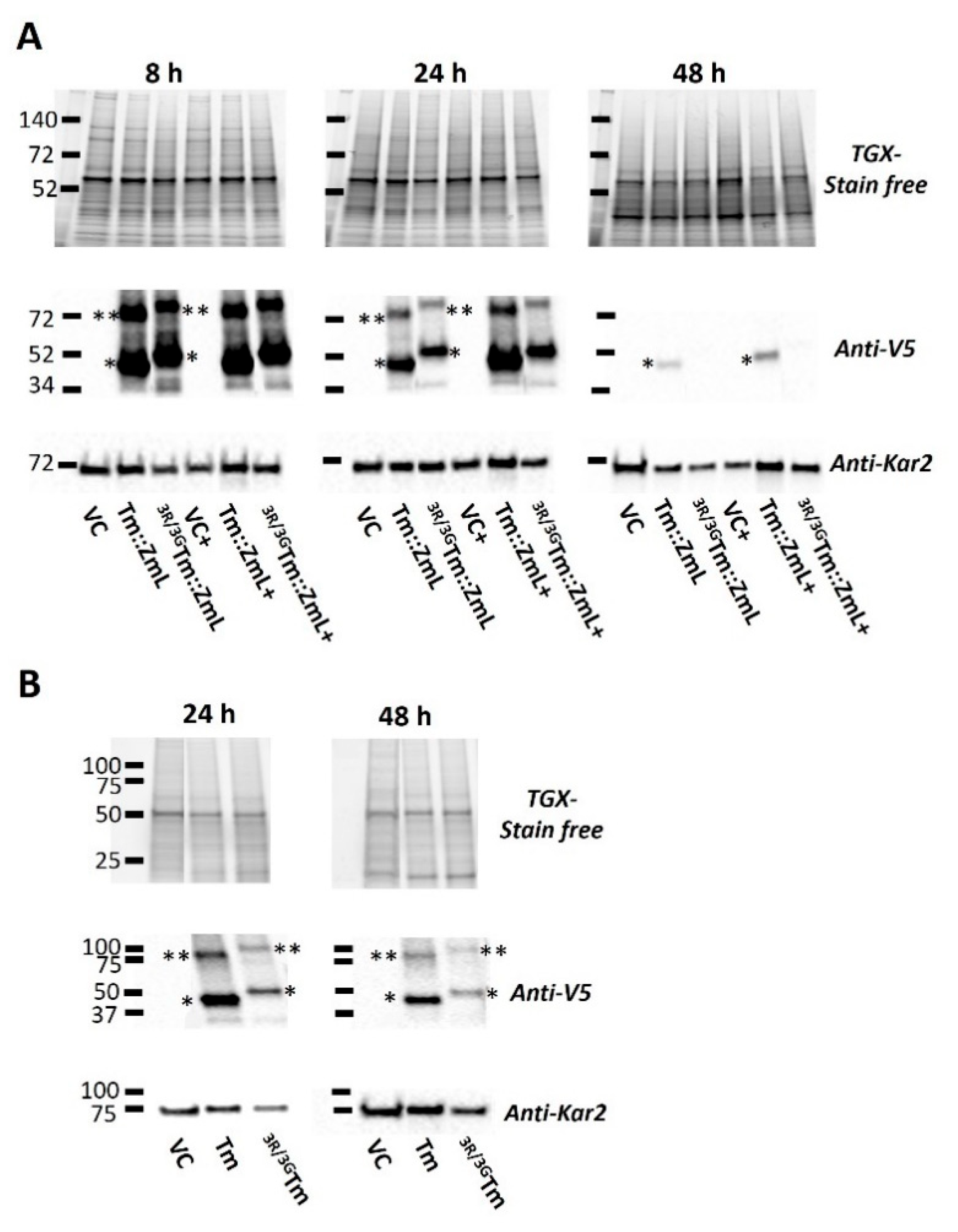
2.2. The Impact of the N-Terminal di-R Motif on the Accumulation of DGAT1 in Yeast
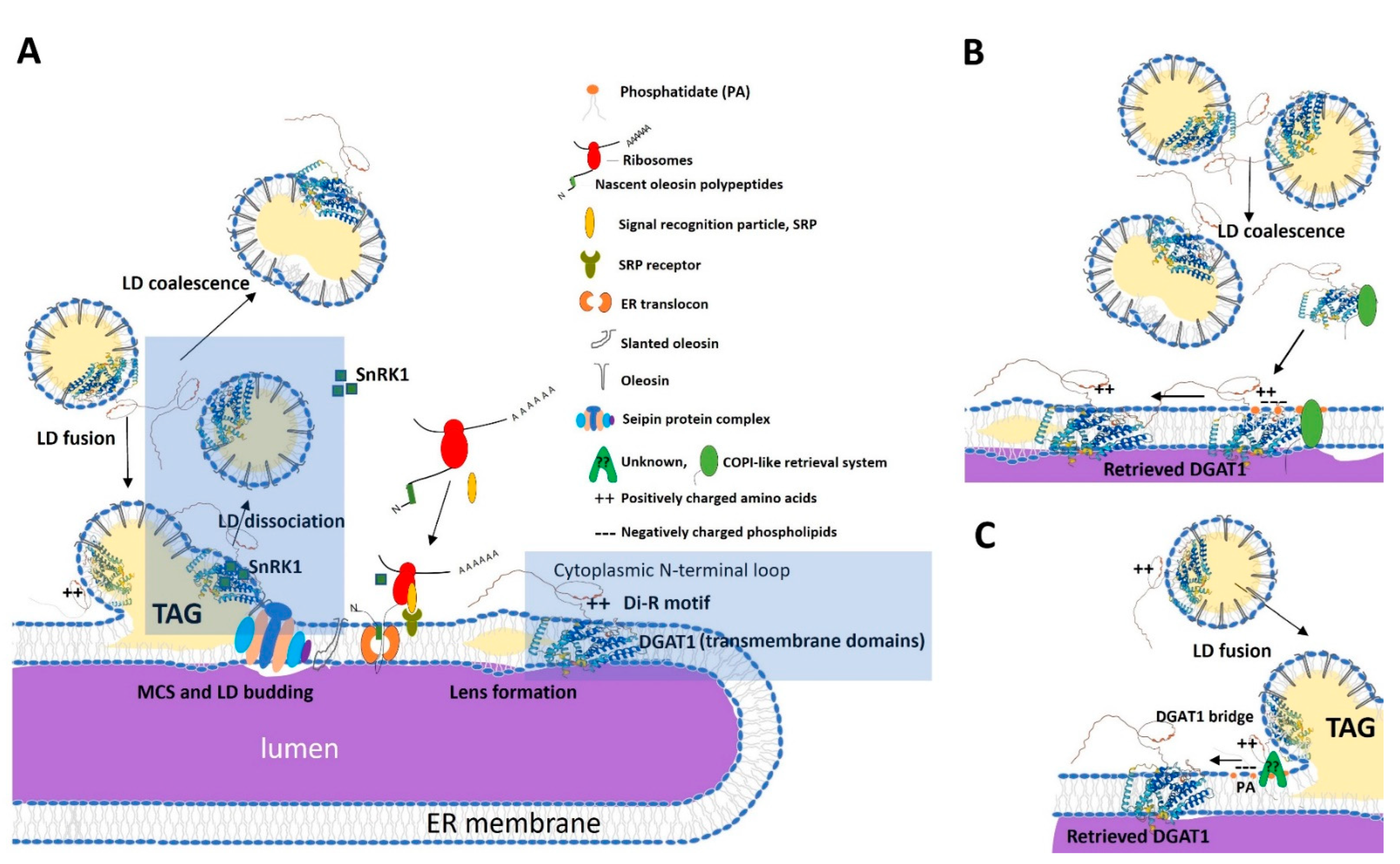
2.3. Peptide Analysis of the N-Terminal DGAT1
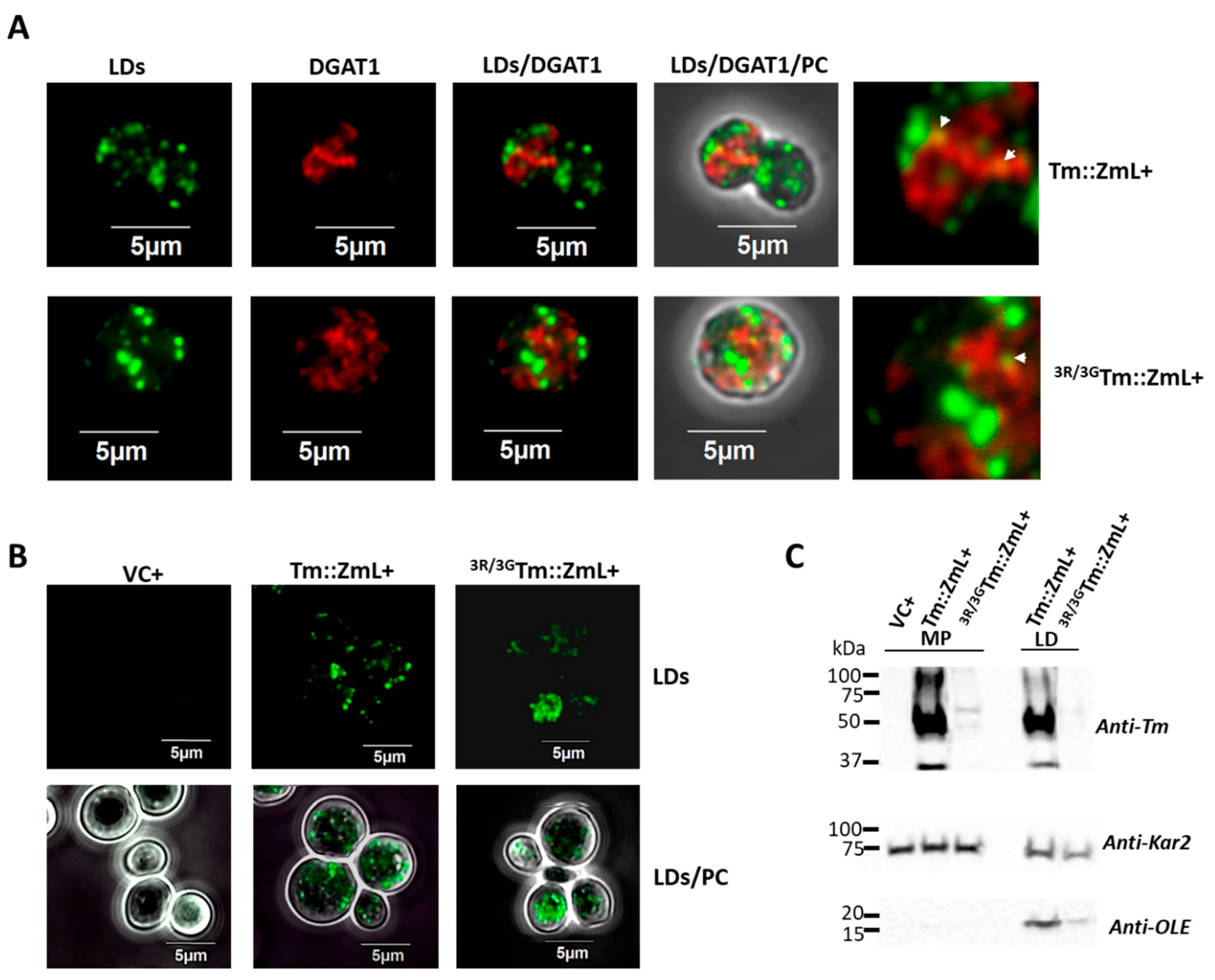
2.4. Disruption of the N-Terminal di-R Motif Within DGAT1 Reduces the Association of Oleosin with LDs
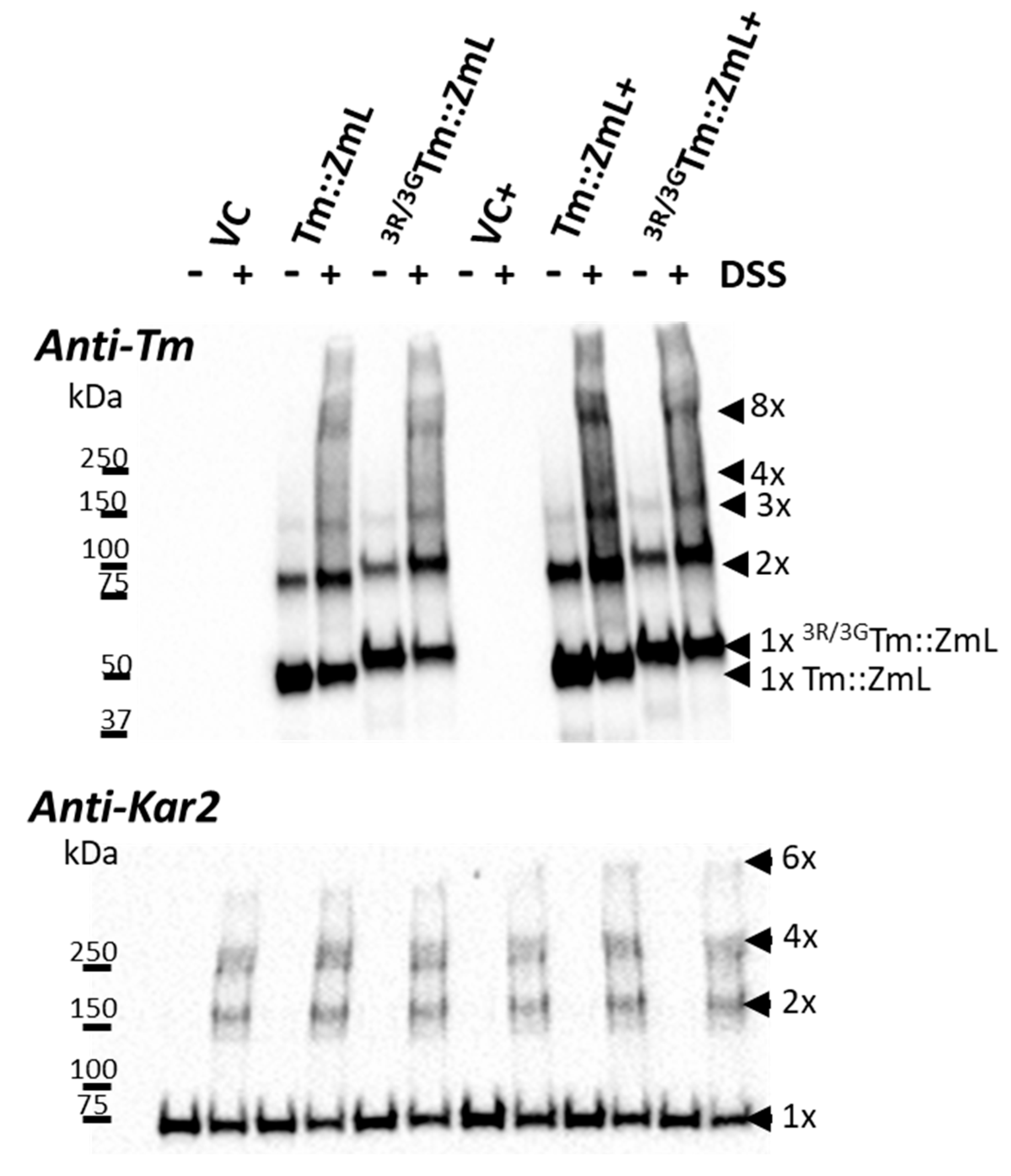
2.5. The 25,26,27R Residues in T. majus DGAT1 Do Not Appear to Be Critical for Oligomerization
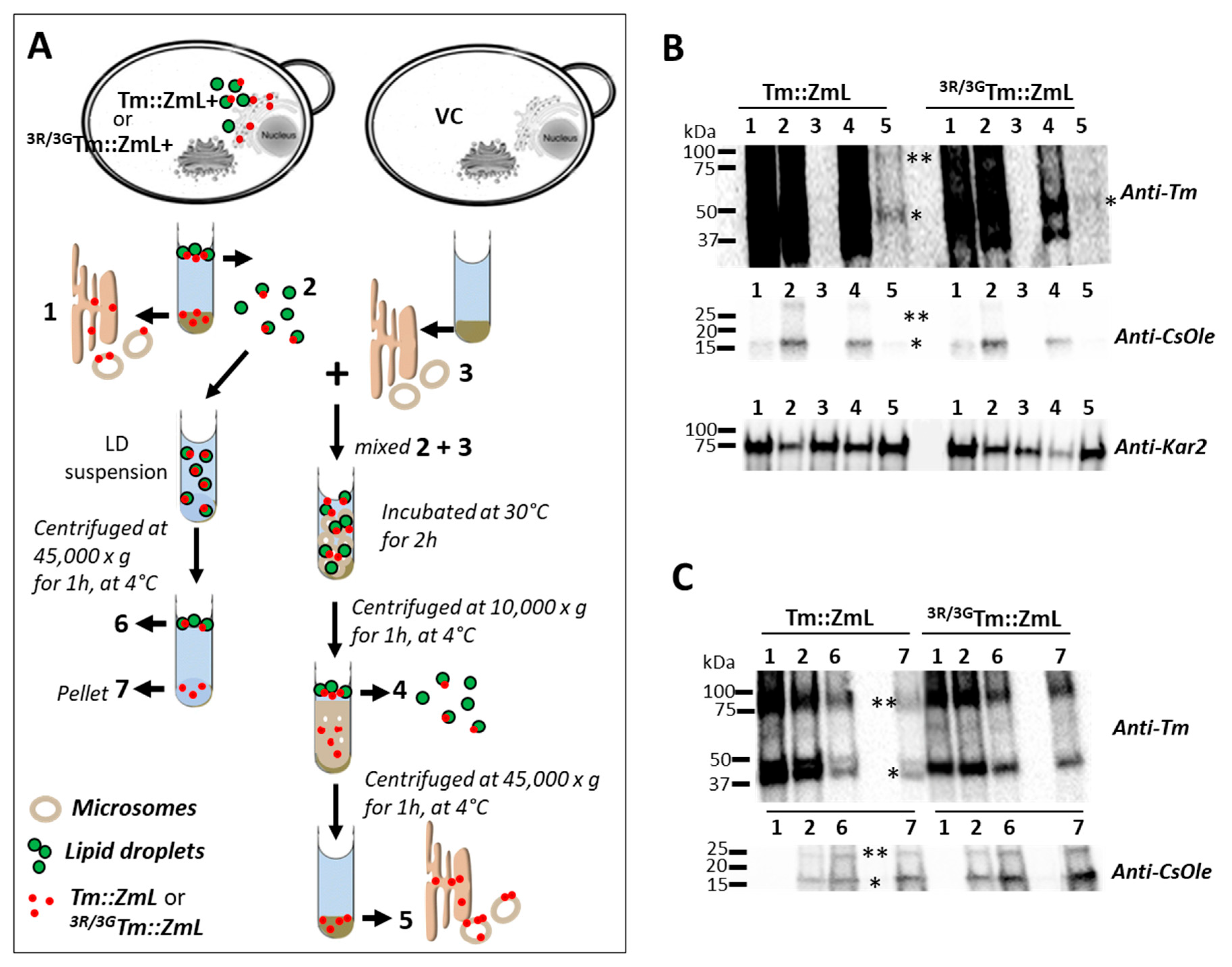
2.6. The T. majus DGAT1 25,26,27R Residues May Serve as an ER Retrieval Signal and/or Aid in Protein Retention in the ER
3. Discussion
4. Materials and Methods
4.1. The di-R Mutated DGAT1 Expression Vectors for C. sativa
4.2. Camelina Experiments
4.3. The di-R Mutated DGAT1 Expression Vectors for S. cerevisiae
4.4. Yeast Experiments
4.5. Lipid Droplet Staining
4.6. In Vitro Study of ER Retrieval of DGAT1s from LDs
4.7. In Vitro Study of LD Retention of DGAT1 and Oleosin
4.8. Immunoblot Analyses
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, D. Biotechnology and the improvement of oil crops—Genes, dreams and realities. Phytochem. Rev. 2022, 1, 67–77. [Google Scholar] [CrossRef]
- Msanne, J.; Kim, H.; Cahoon, E.B. Biotechnology tools and applications for development of oilseeds with healthy vegetable oils. Biochimie 2020, 178, 4–14. [Google Scholar] [CrossRef]
- Van Montagu, M. The future of plant biotechnology in a globalized and environmentally endangered world. Genet. Mol. Biol. 2020, 43, e20190040. [Google Scholar] [CrossRef] [PubMed]
- Fortman, J.L.; Chhabra, S.; Mukhopadhyay, A.; Chou, H.; Lee, T.S.; Steen, E.; Keasling, J.D. Biofuel alternatives to ethanol: Pumping the microbial well. Trend Biotechnol. 2008, 26, 375–381. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Allen, D.; Berguson, B.; Dellapenna, D.; Shachar-Hill, Y.; Stymne, S. Driving on biomass: Burning biomass to produce electricity for battery-driven vehicles can power more travel and displace more petroleum than converting it to ethanol or other fermentation products. Science 2009, 324, 1019–1020. [Google Scholar] [CrossRef]
- Winichayakul, S.; Scott, W.R.; Roldan, M.; Hatier, J.H.B.; Livingston, S.; Cookson, R.; Curran, A.C.; Roberts, N.J. In vivo packaging of triacylglycerols enhances Arabidopsis leaf biomass and energy density. Plant Physiol. 2013, 162, 626–639. [Google Scholar] [CrossRef]
- Mu, J.; Tan, H.; Zheng, Q.; Fu, F.; Liang, Y.; Zhang, J.; Yang, X.; Wang, T.; Chong, K.; Wang, X.-J.; et al. LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 2008, 148, 1042–1054. [Google Scholar] [CrossRef]
- Andrianov, V.; Borisjuk, N.; Pogrebnyak, N.; Brinker, A.; Dixon, J.; Spitsin, S.; Flynn, J.; Matyszczuk, P.; Andryszak, K.; Laurelli, M.; et al. Tobacco as a production platform for biofuel: Overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol. J. 2010, 8, 277–287. [Google Scholar] [CrossRef]
- Sanjaya, M.; Durrett, T.P.; Weise, S.E.; Benning, C. Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol. J. 2011, 9, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Vanhercke, T.; Shrestha, P.; Luo, J.; Akbar, S.; Konik-Rose, C.; Venugoban, L.; Hussain, D.; Tian, L.; Singh, S.; et al. Upregulated lipid biosynthesis at the expense of starch production in potato (Solanum tuberosum) vegetative tissue via simultaneous downregulation of ADP-glucose pyrophosphorylase and sugar dependent 1 expressions. Front. Plant Sci. 2019, 10, 01444. [Google Scholar] [CrossRef] [PubMed]
- James, C.N.; Horn, P.J.; Case, C.R.; Gidda, S.K.; Zhang, D.; Mullen, R.T.; Dyer, J.M.; Anderson, R.G.W.; Chapman, K.D. Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. Proc. Natl. Acad. Sci. USA 2010, 107, 17833–17838. [Google Scholar] [CrossRef] [PubMed]
- Bouvier-Navé, P.; Benveniste, P.; Oelkers, P.; Stephen, L.; Sturley, S.L.; Schaller, H. Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur. J. Biochem. 2000, 267, 85–96. [Google Scholar] [CrossRef]
- Durrett, T.P.; Benning, C.; Ohlrogge, J. Plant triacylglycerol as feedstocks for the production of biofuels. Plant J. 2008, 54, 593–607. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.-M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef]
- Yang, Z.; Ohlrogge, J.B. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis β-oxidation mutants. Plant Physiol. 2009, 150, 1981–1989. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, X.-H.; Anaokar, S.; Shi, H.; Dahl, W.B.; Cai, Y.; Luo, G.; Chai, J.; Cai, Y.; Mollá-Morales, A.; et al. Engineering triacylglycerol accumulation in duckweed (Lemna japonica). Plant Biotechnol. J. 2002, 21, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Lung, S.-C.; Weselake, R.J. Diacylglycerol acyltransferase: A key mediator of plant triacylglycerol synthesis. Lipids 2006, 41, 1073–1088. [Google Scholar] [CrossRef]
- Cases, S.; Smith, S.J.; Zheng, Y.-W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Cases, S.; Stone, S.J.; Zhou, P.; Yen, E.; Tow, B.; Lardizabal, K.D.; Voelker, T.; Farese, R.V., Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001, 276, 38870–38876. [Google Scholar] [CrossRef]
- Qi, J.; Lang, W.; Geisler, J.; Wang, P.; Petrounia, I.; Mai, S.; Smith, C.; Askari, H.; Struble, G.T.; Williams, R.; et al. The use of stable isotope-labelled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J. Lipid Res. 2012, 53, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Wurie, H.R.; Buckett, L.; Zammit, V.A. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilized nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS J. 2012, 279, 3033–3047. [Google Scholar] [CrossRef]
- Zammit, V.A. Hepatic triacylglycerol synthesis and secretion: DGAT2 as the link between glycemia and triglyceridaemia. Biochem. J. 2013, 451, 1–12. [Google Scholar] [CrossRef]
- Irshad, Z.; Dimitri, F.; Christain, M.; Zammit, V.A. Diacylglycerol acyltransferase 2 links glucose utilization to fatty acid oxidation in the brown adipocytes. J. Lipid Res. 2017, 58, 15–30. [Google Scholar] [CrossRef]
- Aymé, L.; Arragain, S.; Canonge, M.; Baud, S.; Touati, N.; Bimai, O.; Jagic, F.; Louis-Mondésir, C.; Briozzo, P.; Fontecave, M.; et al. Arabidopsis thaliana DGAT3 is a [2Fe-2S] protein involved in TAG biosynthesis. Sci. Rep. 2018, 8, 17254. [Google Scholar] [CrossRef]
- Han, L.; Zhai, Y.; Wang, Y.; Shi, X.; Xu, Y.; Gao, S.; Zhang, M.; Luo, J.; Zhang, Q. Diacylglycerol acyltransferase 3 (DGAT3) is responsible for the biosynthesis of unsaturated fatty acids in vegetative organs of Paeonia rockii. Int. J. Mol. Sci. 2022, 23, 14390. [Google Scholar] [CrossRef]
- Kaup, M.T.; Froese, C.D.; Thompson, J.E. A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol. 2002, 129, 1616–1626. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Shockey, J.M.; Dietrich, C.R.; Gidda, S.K.; Mullen, R.T.; Dyer, J.M. Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: Solving bottlenecks in fatty acid flux. Curr. Opin. Plant. Biol. 2007, 10, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, K.; Hildebrand, D.F. DGAT1, DGAT2 and PDAT expression in seeds and other tissues of epoxy and hydroxy fatty acid accumulating plants. Lipids 2010, 45, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Nykiforuk, C.L.; Furukawa-Stoffer, T.L.; Huff, P.W.; Sarna, M.; Laroche, A.; Moloney, M.M.; Weselake, R.J. Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2002, 1580, 95–109. [Google Scholar] [CrossRef]
- McFie, P.J.; Stone, S.L.; Banman, S.L.; Stone, S.J. Topological orientation of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the N terminus in dimer/tetramer formation. J. Biol. Chem. 2010, 285, 37377–37387. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Francis, T.; Mietkiewska, E.; Giblin, E.M.; Barton, D.L.; Zhang, Y.; Zhang, M.; Taylor, D.C. Cloning and characterization of an acyl-CoA-dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site-directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol. J. 2008, 6, 799–818. [Google Scholar] [CrossRef]
- Halford, N.G.; Hardie, D.G. SNF1-related protein kinases: Global regulators of carbon metabolism in plants? Plant Mol. Biol. 1998, 37, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J.; Curran, A.C.; Winichayakul, S.; Marissa, R.; Scott, R.W. Enhanced Acyltransferase Polynucleotides, Polypeptides, and Methods of Use. WO/2014/068437, 26 June 2014. [Google Scholar]
- Roberts, N.J.; Curran, A.C.; Winichayakul, S.; Marissa, R.; Scott, R.W.; Taylor, D.C.; Marillia, E.-F. Improved Acyltransferase Polynucleotides, Polypeptides, and Methods of Use. WO/2014/068439, 8 May 2014. [Google Scholar]
- Winichayakul, S.; Curran, A.; Moraga, R.; Cookson, R.; Xue, H.; Crowther, T.; Roldan, M.; Bryan, G.; Roberts, N. An alternative angiosperm DGAT1 topology and potential motifs in the N-terminus. Front. Plant Sci. 2022, 13, 951389. [Google Scholar] [CrossRef] [PubMed]
- Boulaflous, A.; Saint-Jore-Dupas, C.; Herranz-Gordo, M.-C.; Pagny-Salehabadi, S.; Plasson, C.; Garidou, F.; Kiefer-Meyer, M.-C.; Riitzenthaler, C.; Faye, L.; Gomord, V. Cytosolic N-terminal arginine-based signals together with a luminal signal target a type II membrane protein to the plant ER. BMC Plant Biol. 2009, 9, 144–161. [Google Scholar] [CrossRef]
- Parks, G.D.; Lamb, R.A. Role of NH2-terminal positively charged residues in establishing membrane protein topology. J. Biol. Chem. 1993, 268, 19101–19109. [Google Scholar] [CrossRef]
- Teasdale, R.D.; Jackson, M.R. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Annu. Rev. Cell Dev. Biol. 1996, 12, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Shikano, S.; Li, M. Membrane receptor trafficking: Evidence of proximal and distal zones conferred by two independent endoplasmic reticulum localization signals. Proc. Natl. Acad. Sci. USA 2003, 100, 5783–5788. [Google Scholar] [CrossRef]
- Michelsen, K.; Yuan, H.; Schwappach, B. Hide and run: Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 2005, 6, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Sandager, L.; Gustavsson, M.H.; Ståhl, U.; Dahlqvist, A.; Wiberg, E.; Banas, A.; Lenman, M.; Ronne, H.; Stymne, S. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 2002, 277, 6478–6482. [Google Scholar] [CrossRef]
- West, R.W.; Yocum, R.R.; Ptashne, M. Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: Location and function of the upstream activating sequence UASG. Mol. Cell. Biol. 1984, 4, 2467–2478. [Google Scholar] [PubMed]
- Shen, M.W.Y.; Fang, F.; Sandmeyer, S.; Da Silva, N.A. Development and characterization of a vector set with regulated promoters for systematic metabolic engineering in Saccharomyces cerevisiae. Yeast 2012, 29, 495–503. [Google Scholar] [CrossRef]
- Rose, M.D.; Misra, L.M.; Vogel, J.P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 1989, 57, 1211–1221. [Google Scholar] [CrossRef]
- Rath, A.; Glibowicka, M.; Nadeau, V.G.; Chen, G.; Deber, C.M. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 1760–1765. [Google Scholar] [CrossRef]
- Shirai, A.; Matsuyama, A.; Yashiroda, Y.; Hashimoto, A.; Kawamura, Y.; Arai, R.; Komatsu, Y.; Horinouchi, S.; Yoshida, M. Global analysis of gel mobility of proteins and its use in target identification. J. Biol. Chem. 2008, 283, 10745–10752. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Geiler-Samerotte, K.A.; Dion, M.F.; Budnik, B.A.; Wang, S.M.; Hartl, D.L.; Drummond, D.A. Misfolded proteins impose a dosage-dependent fitness cost and trigger a cytosolic unfolded protein response in yeast. Proc. Natl. Acad. Sci. USA 2010, 108, 680–685. [Google Scholar] [CrossRef]
- Von Heijne, G. Signal sequences: The limits of variation. J. Mol. Biol. 1985, 184, 99–105. [Google Scholar] [CrossRef]
- Martoglio, B.; Dobberstein, B. Signal sequences: More than just greasy peptides. Trends Cell Biol. 1998, 8, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Von Heijne, G. Membrane proteins: From sequences to structure. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Travers, K.J.; Patil, C.K.; Wodicka, L.; Lockhart, D.J.; Weissman, J.S.; Walter, P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 2000, 101, 249–258. [Google Scholar] [CrossRef]
- Liao, P.-C.; Yang, E.J.; Borgman, T.; Boldogh, I.R.; Sing, C.N.; Swayne, T.C.; Pon, L.A. Tough and go: Membrane contact sites between lipid droplets and other organelles. Front. Cell Dev. Biol. 2022, 10, 852021. [Google Scholar] [CrossRef]
- Lajoie, P.; Moir, R.D.; Willis, I.M.; Snapp, E.L. Kar2 availability defines distinct forms of endoplasmic reticulum stress in living cells. Mol. Biol. Cell 2012, 23, 955–964. [Google Scholar] [CrossRef]
- Garcia, E.J.; Liao, P.-C.; Tan, G.; Vevea, J.D.; Sing, C.N.; Tsang, C.A.; McCaffery, J.M.; Boldogh, I.R.; Pon, L.A. Membrane dynamics and protein targets of lipid droplet microautophagy during ER stress-induced proteostasis in the budding yeast, Saccharomyces cerevisiae. Autophagy 2021, 17, 2363–2383. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, X.; Liu, P. Lipid droplet proteins and metabolic diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 1968–1983. [Google Scholar] [CrossRef] [PubMed]
- Guzha, A.; Gautam, B.; Marchiafava, D.; Ver Sagun, J.; Garcia, T.; Javis, B.A.; Barbaglia-Hurlock, A.M.; Johnson, C.; Grotewold, E.; Sedbrook, J.C.; et al. Targeted modulation of pennycress lipid droplet proteins impacts droplet morphology and seed oil content. Plant J. 2024, 120, 2151–2171. [Google Scholar] [CrossRef]
- Jacquier, N.; Mishra, S.; Choudhary, V.; Schneiter, R. Expression of oleosin and perilipins in yeast promotes formation of lipid droplets from the endoplasmic reticulum. J Cell Sci. 2013, 15, 5198–5209. [Google Scholar]
- Wilfling, F.; Wang, H.; Haas, J.T.; Krahmer, N.; Gould, T.J.; Uchida, A.; Cheng, J.-X.; Graham, M.; Christiano, R.; Fröhlich, F.; et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 2013, 24, 384–399. [Google Scholar] [CrossRef]
- Siloto, R.M.P.; Findlay, K.; Lopez-Villalobos, A.; Yeung, E.C.; Nykiforuk, C.L.; Moloney, M.M. The accumulation of oleosins determines the size of seed oilbodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
- Springer, S.; Malkus, P.; Borchert, B.; Wellbrock, U.; Duden, R.; Schekman, R. Regulated oligomerization induces uptake of a membrane protein into COPII vesicles independent of its cytosolic tail. Traffic 2014, 15, 531–545. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Nie, J.; Cao, J.; Zhai, Y.; Tong, D.; Shi, Y. Monoacylglycerol acyltransferase-2 is a tetrameric enzyme that selectively heterodimerizes with diacylglycerol acyltransferase-1. J. Biol. Chem. 2014, 289, 10909–10918. [Google Scholar] [CrossRef] [PubMed]
- Caldo, K.M.P.; Acedo, J.Z.; Panigrahi, R.; Vederas, J.C.; Weselake, R.J.; Lemieux, M.J. Diacylglycerol acyltransferase 1 is regulated by its N-terminal domain in response to allosteric effectors. Plant Physiol. 2017, 175, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qian, H.; Nian, Y.; Han, Y.; Ren, Z.; Zhang, H.; Hu, L.; Prasad, B.V.V.; Laganowsky, A.; Yan, N.; et al. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature 2020, 581, 329–332. [Google Scholar] [CrossRef]
- Scurtu, F.; Zolog, O.; Iacob, B.; Silaghi-Dumitrescu, R. Hemoglobin-albumin cross-linking with disuccinimidyl suberate (DSS) and/or glutaraldehyde for blood substitutes. Artif. Cells Nanomed. Biotechnol. 2014, 42, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakai, K. Tools for the recognition of sorting signals and the prediction of subcellular localization of proteins from their amino acid sequences. Front. Genet. 2020, 11, 103389. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T.; Warren, G. Retention and retrieval in the endoplasmic reticulum and the Golgi apparatus. Curr. Opin. Cell Biol. 1994, 6, 517–521. [Google Scholar] [CrossRef]
- Abell, B.M.; High, S.; Moloney, M.M. Membrane protein topology of oleosin is constrained by its long hydrophobic domain. J. Biol. Chem. 2002, 277, 8602–8610. [Google Scholar] [CrossRef]
- Kellogg, M.K.; Miller, S.C.; Tikhonova, E.B.; Karamyshev, A.L. SRPassing co-translational targeting: The role of the signal recognition particle in protein targeting and mRNA protection. Int. J. Mol. Sci. 2021, 22, 6284. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, Z.; Liu, Z.; Li, B.; Cui, Y.; Gao, C.; Shen, J.; Wang, X.; Zhao, Q.; Zhuang, X.; et al. Recent advances in plant endomembrane research and new microscopical techniques. New Phytol. 2023, 240, 41–60. [Google Scholar] [CrossRef]
- Salo, V.T.; Ikonen, E. Moving out but keeping in touch: Contacts between endoplasmic reticulum and lipid droplets. Curr. Opin. Cell Biol. 2019, 57, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.E.; Liebsch, D.; Diaz, M.G.; Strand, A.; Whelan, J. Dual and dynamic intracellular localization of Arabidopsis thaliana SnRK1.1. J. Exp. Bot. 2019, 70, 2225–2338. [Google Scholar] [CrossRef]
- Salo, V.T.; Belvich, I.; Li, S.; Karhinen, L.; Vihinen, H.; Vigoutoux, C.; Magŕe, J.; Thiele, C.; Hölttä-Vuori, M.; Jokitalo, E.; et al. Seipin regulates ER-lipid droplet contacts and cargo delivery. EMBO J. 2016, 35, 2699–2716. [Google Scholar] [CrossRef]
- Kumari, S.; Tagnik, G.; Varadaraj, S.; Varadaraj, K. Positively charges amino acid residues in the extracellular loops A and C of lens Aquaporin 0 interact with the negative charges in the plasma membrane to facilitate cell-to-cell adhesion. Exp. Eye Res. 2019, 185, 107682. [Google Scholar] [CrossRef]
- Vitrac, H.; MacLean, D.M.; Dowhan, W. Dynamic membrane protein topological switching upon changes in phospholipid environment. Proc. Natl. Acad. Sci. USA 2015, 112, 13874–13879. [Google Scholar] [CrossRef]
- Vitrac, H.; MacLean, D.M.; Karlstaedt, A.; Taegtmeyer, H.; Jayaraman, V.; Bogdanov, M.; Dowhan, W. Dynamic lipid-dependent modulation of protein topology by post-translational phosphorylation. J. Biol. Chem. 2017, 292, 1613–1624. [Google Scholar] [CrossRef]
- Caldo, K.M.P.; Wei, S.; Xu, Y.; Hanley-Bowdoin, L.; Chen, G.; Weselake, R.J.; Lemieux, M.J. Diacylglycerol acyltransferase 1 is activated by phosphatidate and inhibited by SnRK1-catalyzed phosphorylation. Plant J. 2018, 96, 287–299. [Google Scholar] [CrossRef]
- Tzen, J.T.C.; Lie, G.C.; Huang, A.H.C. Characterization of the charged components and their topology on the surface of plant seed oil bodies. J. Biol. Chem. 1992, 267, 15626–15634. [Google Scholar] [CrossRef]
- Josefsson, L.G.; Lenman, M.; Ericson, M.L.; Rask, L. Structure of a gene encoding the 1.7 S storage protein, napin, from Brassica napus. J. Biol. Chem. 1987, 262, 12196–12201. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Plants n = 6 | HOM Seed Size (mg/Seed) | ±SE | Null Seed Size (mg/Seed) | ±SE | HOM Seed Lipid (%) | ±SE | Null Seed Lipid (%) | ±SE | HOM Seed Lipid (mg/Seed) | ±SE | Null Seed Lipid (mg/Seed) | ±SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 1.17 | 0.024 | 27.30 | 0.67 | 0.29 | 0.009 | ||||||
| VC | 1.17 | 0.026 | 25.96 | 0.63 | 0.28 | 0.007 | ||||||
| Tm#2 | 1.15 | 0.045 | 1.15 | 0.036 | 29.58 * | 0.58 | 26.21 | 0.92 | 0.32 * | 0.009 | 0.27 27 | 0.008 |
| SSQ/3RTm#8 | 1.32 **# | 0.034 | 1.16 | 0.032 | 28.97 * | 1.29 | 24.39 | 0.88 | 0.38 **## | 0.018 | 0.28 | 0.014 |
| SSQ/3RTm#13 | 1.37 **## | 0.048 | 1.18 | 0.034 | 27.78 | 1.09 | 27.45 | 0.59 | 0.37 **### | 0.008 | 0.32 | 0.014 |
| SSQ/3RTm#30 | 1.24 | 0.027 | 1.18 | 0.032 | 28.91 * | 0.43 | 27.94 | 0.60 | 0.35 *## | 0.011 | 0.32 | 0.010 |
| SSQ/3RTm#38 | 1.27 | 0.029 | 1.17 | 0.018 | 30.56 * | 1.18 | 26.52 | 0.40 | 0.38 *## | 0.020 | 0.31 | 0.008 |
| Tm::ZmL#13 | 1.29 ** | 0.022 | 1.12 | 0.029 | 31.66 *** | 0.25 | 25.43 | 1.00 | 0.41 *** | 0.010 | 0.29 | 0.017 |
| Δ3RTm::ZmL#1 | 1.16 ## | 0.039 | 1.21 | 0.036 | 25.52 ## | 1.26 | 25.78 | 0.82 | 0.30 ## | 0.021 | 0.31 | 0.016 |
| Δ3RTm::ZmL#15 | 1.18 ## | 0.011 | 1.16 | 0.030 | 28.10 # | 1.17 | 26.87 | 0.48 | 0.33 ## | 0.013 | 0.31 | 0.004 |
| Δ3RTm::ZmL #21 | 1.24 # | 0.054 | 1.19 | 0.044 | 26.70 ## | 1.15 | 25.30 | 1.29 | 0.33 ## | 0.013 | 0.30 | 0.004 |
| 3G/3RTm::ZmL #1 | 1.30 *** | 0.020 | 1.20 | 0.023 | 30.60 *** | 1.34 | 23.36 | 1.41 | 0.40 *** | 0.023 | 0.29 | 0.017 |
| 3G/3RTm::ZmL #20 | 1.15 # | 0.017 | 1.16 | 0.044 | 31.55 *** | 0.74 | 27.49 | 0.36 | 0.36 **# | 0.006 | 0.32 | 0.013 |
| 3G/3RTm::ZmL #21 | 1.14 # | 0.024 | 1.18 | 0.013 | 31.41 *** | 1.39 | 27.30 | 0.89 | 0.36 **# | 0.021 | 0.32 | 0.009 |
| Construct | Length (aa) | MW (kDa) | Isoelectric Point | Charge at pH 7 | % Basic | % Charged | % Hydrophobic |
|---|---|---|---|---|---|---|---|
| Tm and Tm::ZmL | 121 | 12.90 | 6.37 | −0.78 | 13.2 | 24.8 | 43.8 |
| Camelina sativa | |||||||
| SSQ/3RTm | 121 | 13.06 | 9.96 | 2.22 | 15.7 | 27.3 | 43.8 |
| ∆3RTm::ZmL | 118 | 12.43 | 5.01 | −3.78 | 11.0 | 22.9 | 44.9 |
| 3G/3RTm::ZmL | 121 | 13.19 | 9.96 | 2.22 | 15.7 | 27.3 | 41.3 |
| Saccharomyces cerevisiae | |||||||
| 3R/3GTm and 3R/3GTm::ZmL | 121 | 12.60 | 5.01 | −3.78 | 10.7 | 22.3 | 46.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winichayakul, S.; Xue, H.; Roberts, N. A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization. Int. J. Mol. Sci. 2025, 26, 7406. https://doi.org/10.3390/ijms26157406
Winichayakul S, Xue H, Roberts N. A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization. International Journal of Molecular Sciences. 2025; 26(15):7406. https://doi.org/10.3390/ijms26157406
Chicago/Turabian StyleWinichayakul, Somrutai, Hong Xue, and Nick Roberts. 2025. "A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization" International Journal of Molecular Sciences 26, no. 15: 7406. https://doi.org/10.3390/ijms26157406
APA StyleWinichayakul, S., Xue, H., & Roberts, N. (2025). A Conserved N-Terminal Di-Arginine Motif Stabilizes Plant DGAT1 and Modulates Lipid Droplet Organization. International Journal of Molecular Sciences, 26(15), 7406. https://doi.org/10.3390/ijms26157406








