Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model
Abstract
1. Introduction
2. Results
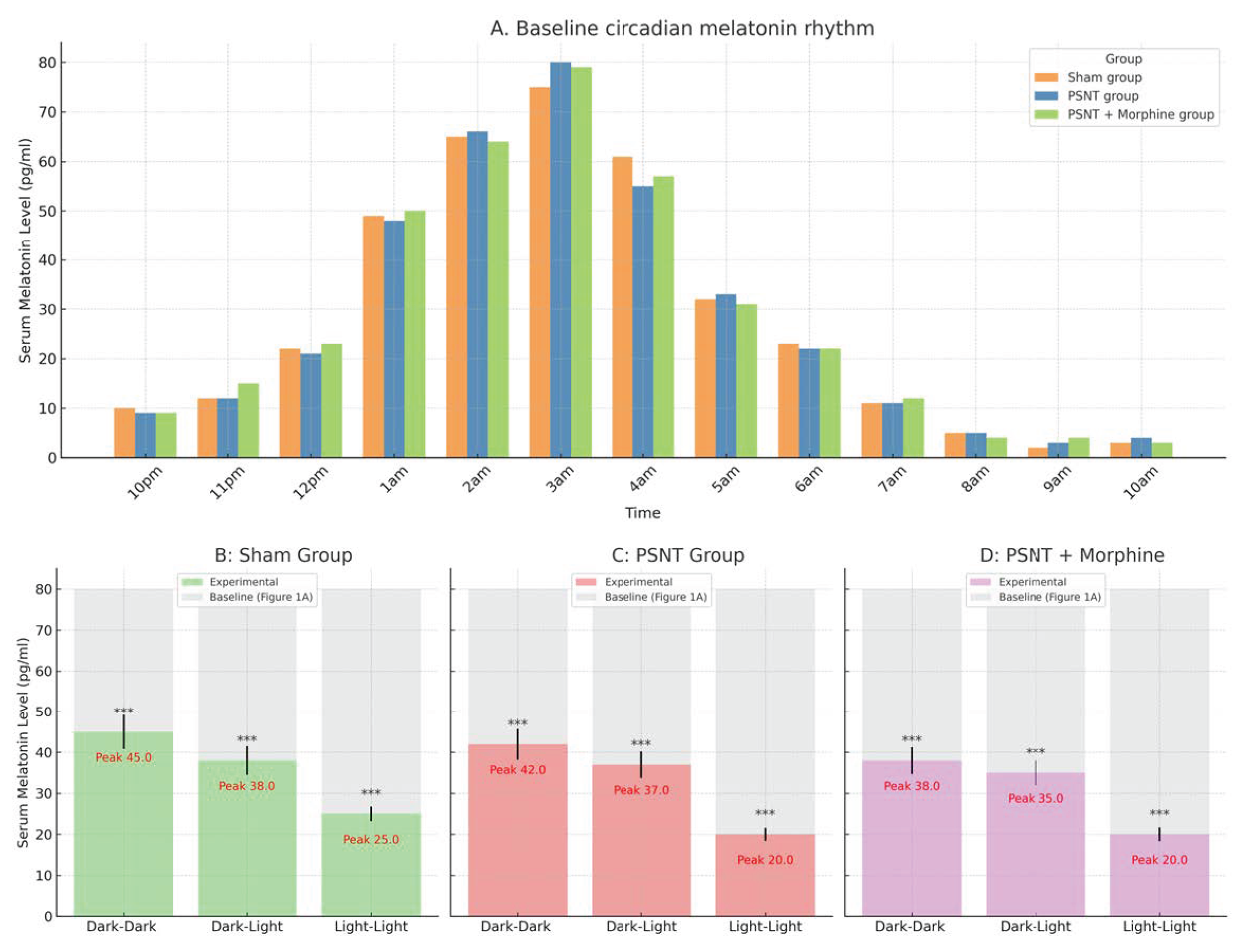
2.1. Circadian Regulation of Serum Melatonin Under Light Exposure and Treatment Conditions
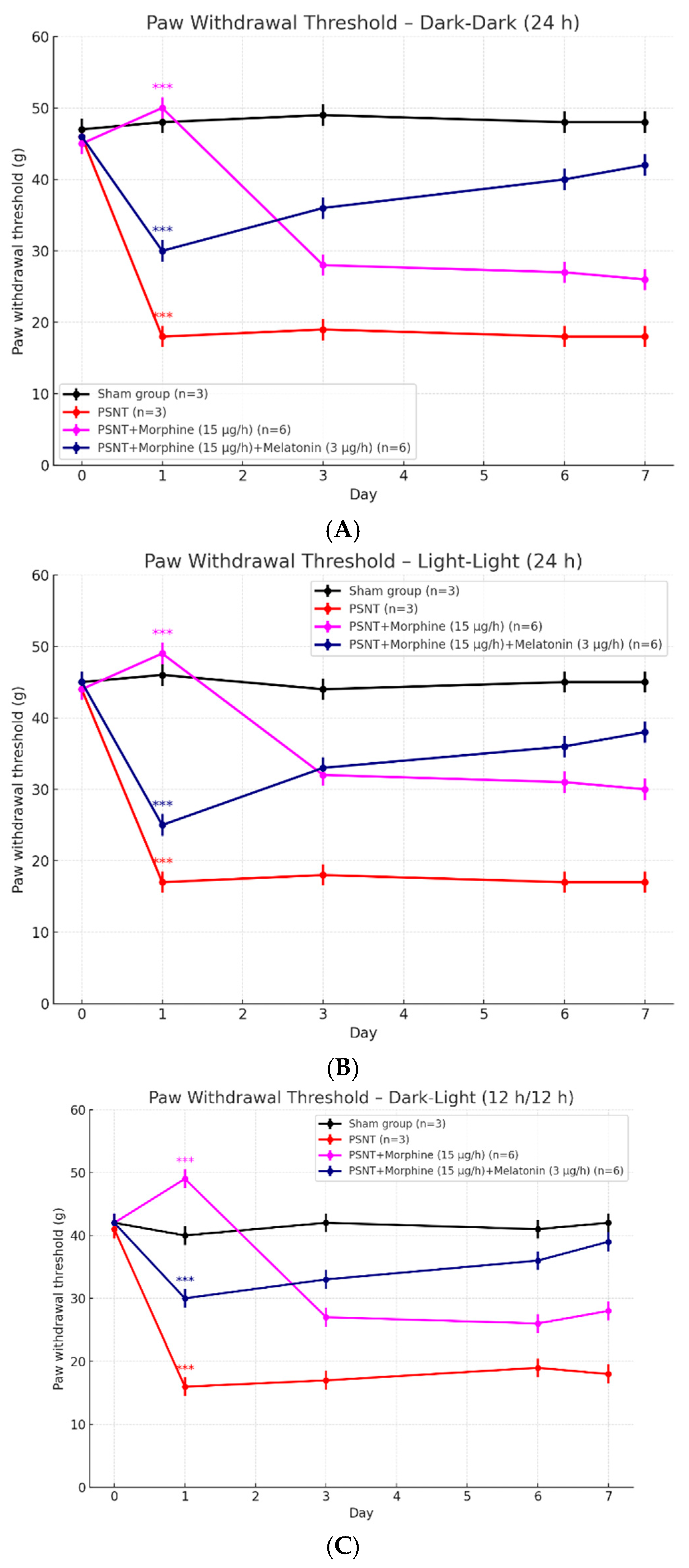
2.2. Effects of Light Exposure on Paw Withdrawal Threshold in PSNT Rats Treated with Morphine and Melatonin
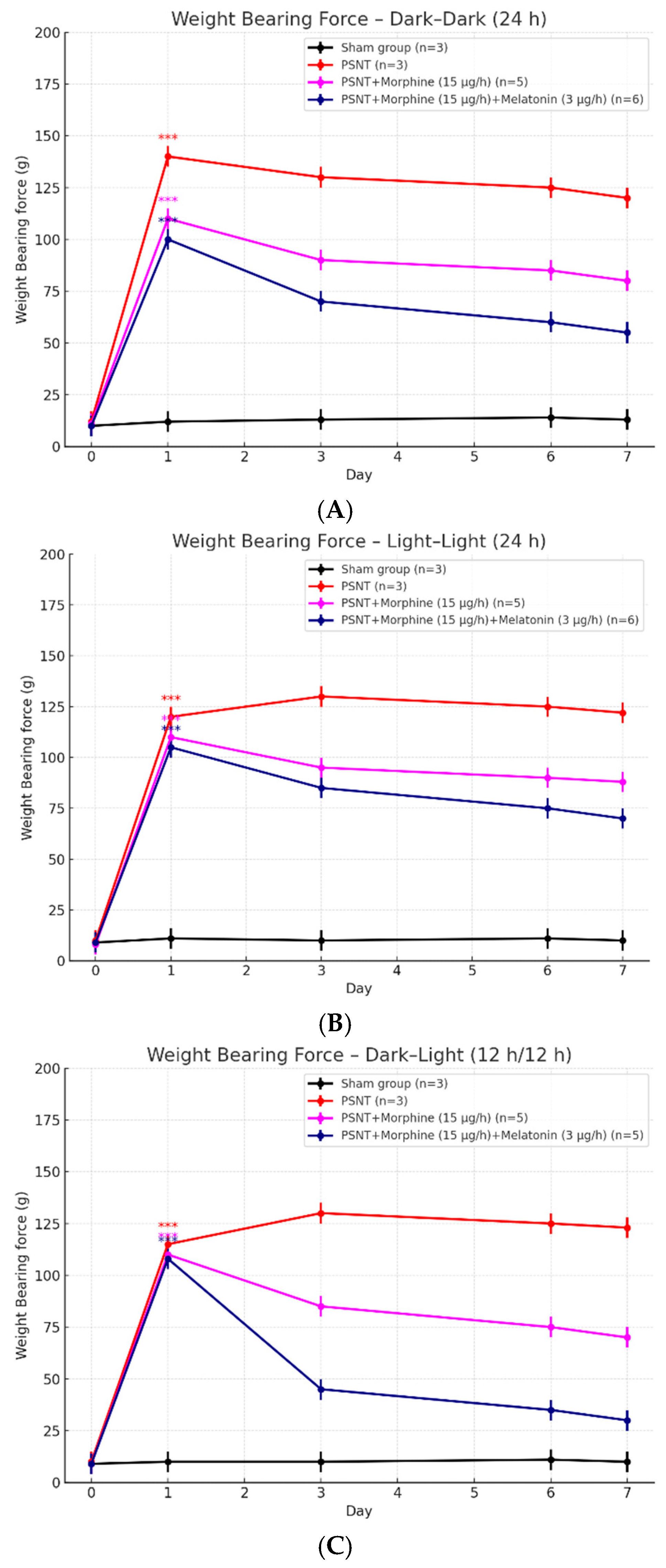
2.3. Influence of Light Conditions on Weight-Bearing Test to PSNT and Morphine Treatments
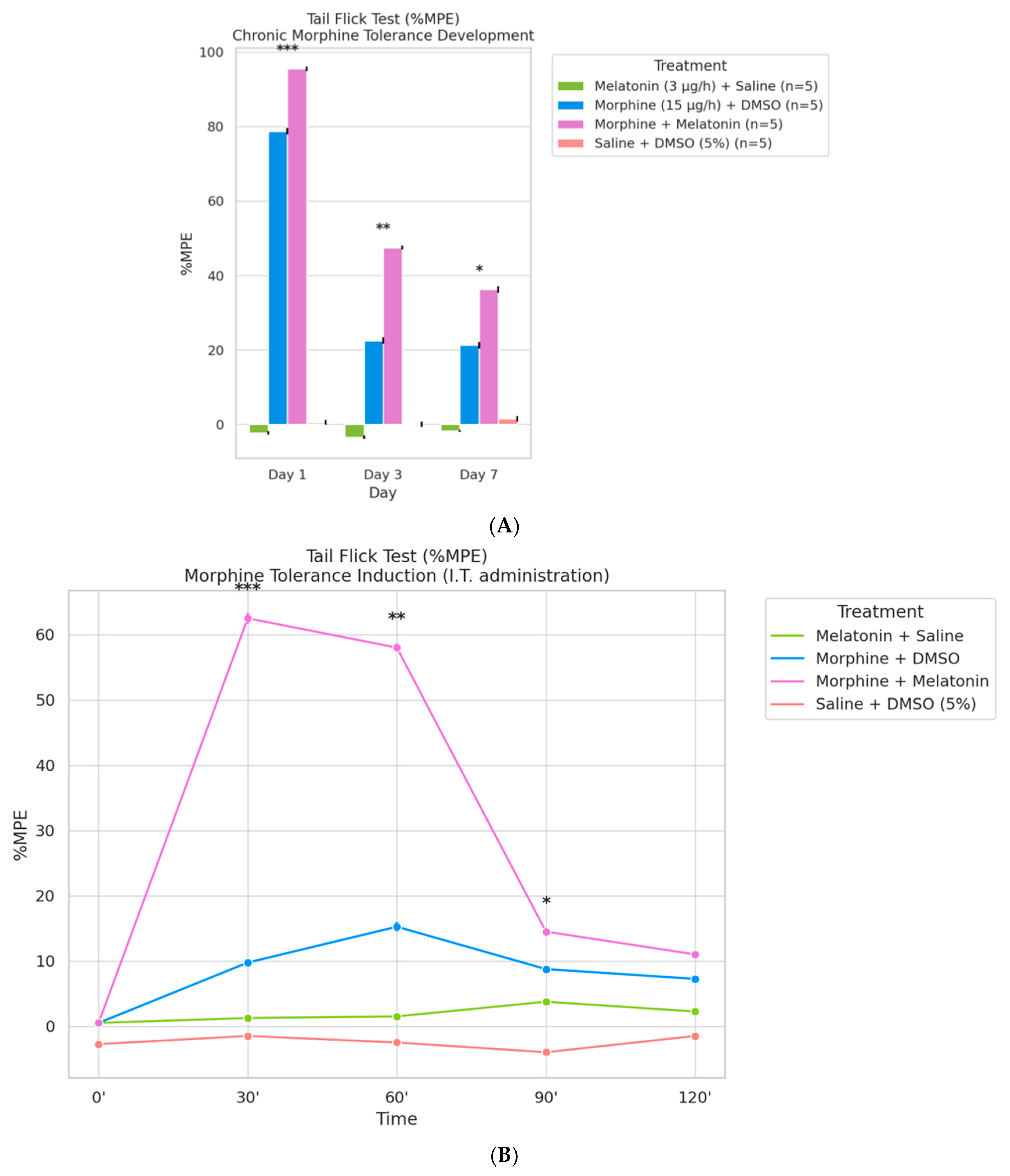
2.4. Co-Infusion of Melatonin Attenuates Morphine Tolerance Development
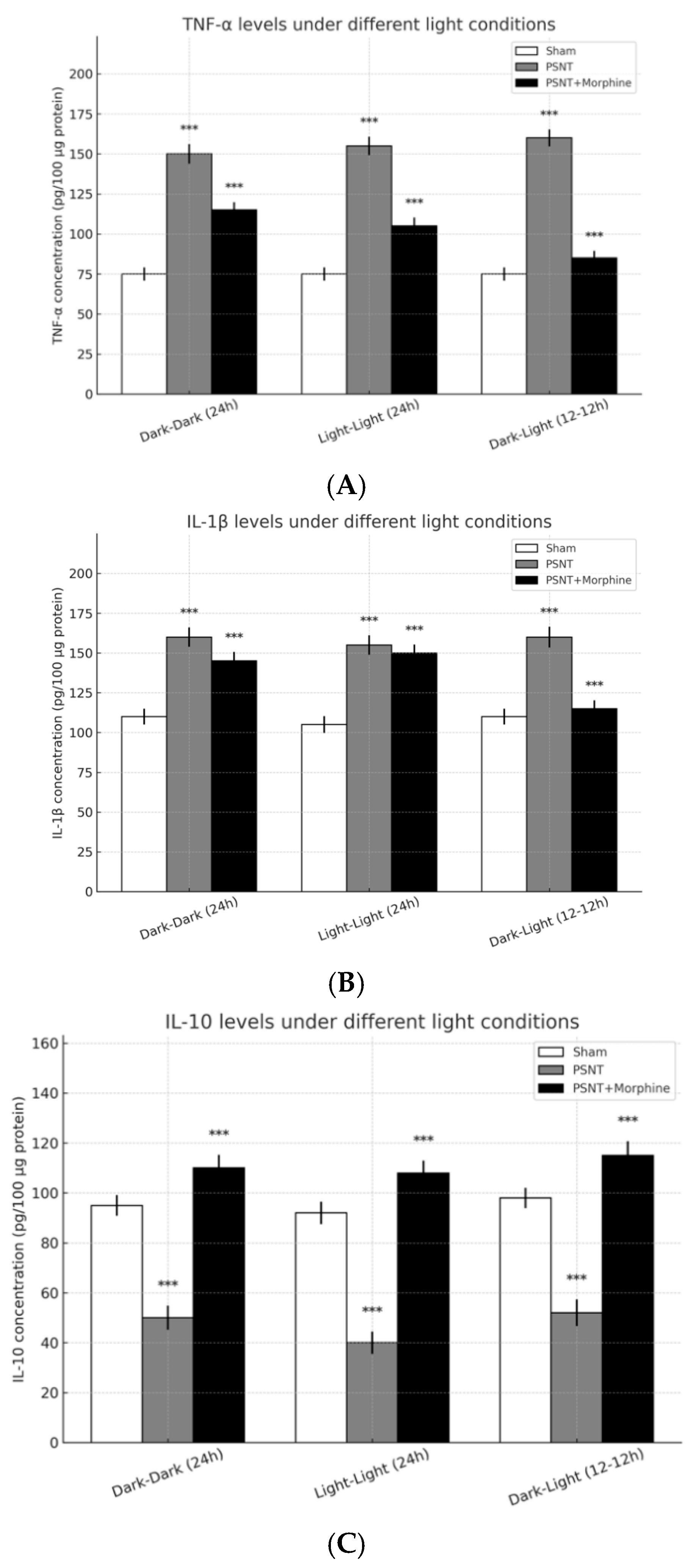
2.5. Role of Inflammatory Cytokines
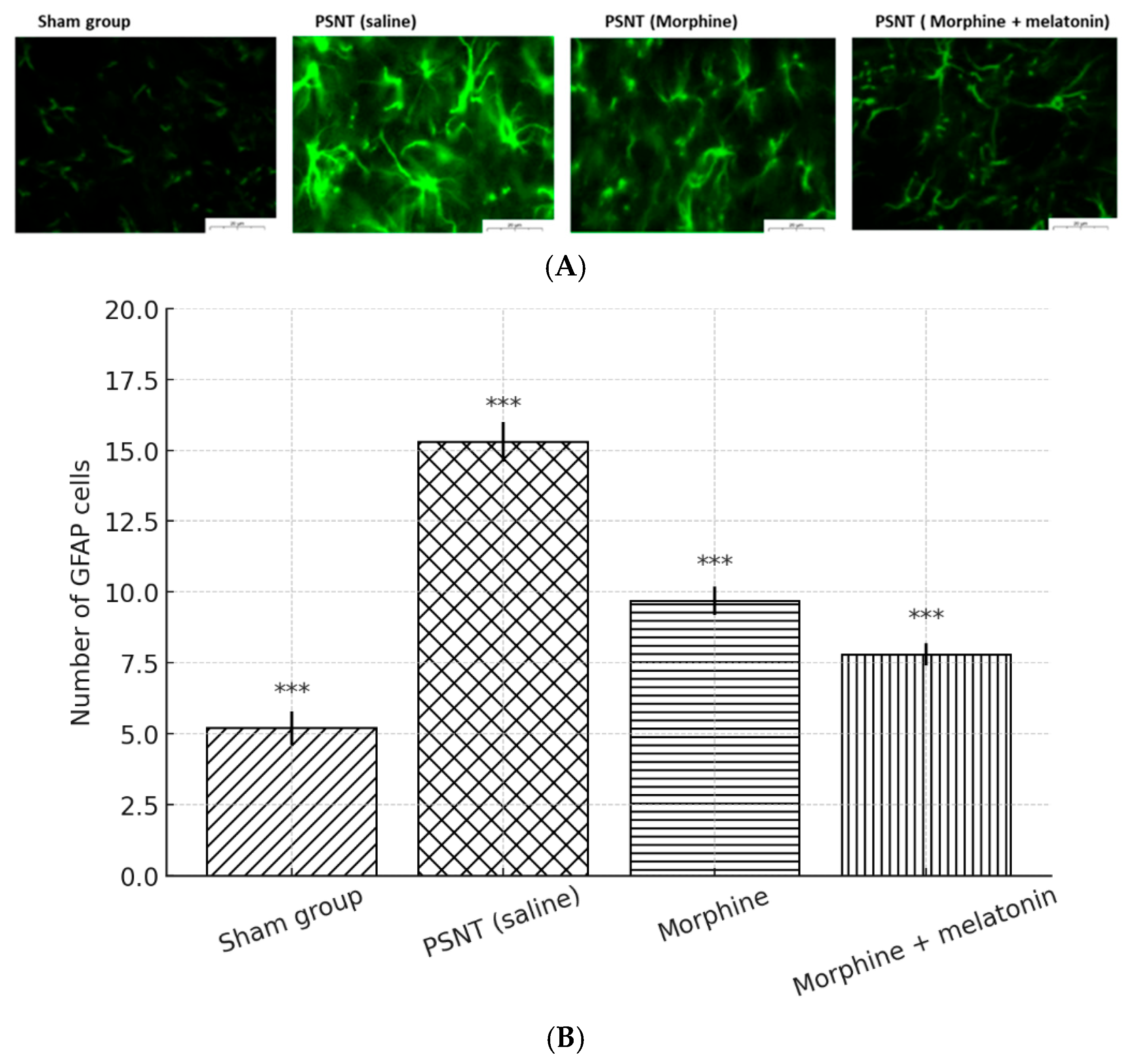
2.6. Melatonin Inhibited Astrocyte Activity in the Spinal Cord of Morphine-Tolerant Rats
3. Discussions
3.1. Influence of Light Conditions and Circadian Rhythms
3.2. Melatonin’s Role in Preventing Morphine Tolerance
3.3. Behavioral Assays and Functional Outcomes
3.4. Cytokine Modulation: Inflammation and Analgesia
Astrocyte Activation and Glial Modulation
4. Methods and Materials
4.1. Employ an Animal Model to Disrupt Circadian Rhythms and Induce Partial Sciatic Nerve Transection
4.2. Nociception Assessment
4.3. Intrathecal Drug Administration
4.4. Morphine Tolerance Assessment
4.4.1. Baseline Measurement
4.4.2. Continuous Morphine Administration
4.4.3. Drug Preparation and Delivery
4.5. Experimental Groups
- PSNT + saline + DMSO (5%),
- PSNT + melatonin (3 μg/h) + saline,
- PSNT + morphine (15 μg/h) + DMSO (5%), and
- PSNT + morphine (15 μg/h) + melatonin (3 μg/h).
4.6. Tail-Flick Latency and Tolerance Evaluation
4.7. Behavioral Assessment of Weight-Bearing and Mechanical Tactile Allodynia
4.8. Astrocyte Activation, and Cytokine Measurement
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Substance Abuse Treatment. SAMHSA/CSAT Treatment Improvement Protocols. In Managing Chronic Pain in Adults with or in Recovery from Substance Use Disorders; Substance Abuse and Mental Health Services Administration (US): Rockville, MD, USA, 2012. [Google Scholar]
- Bumgarner, J.R.; McCray, E.W.; Nelson, R.J. The disruptive relationship among circadian rhythms, pain, and opioids. Front. Neurosci. 2023, 17, 1109480. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.; Kurganova, J. Melatonin in Chronic Pain Syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef]
- Ma, H.; Yan, J.; Sun, W.; Jiang, M.; Zhang, Y. Melatonin Treatment for Sleep Disorders in Parkinson’s Disease: A Meta-Analysis and Systematic Review. Front. Aging Neurosci. 2022, 14, 784314. [Google Scholar] [CrossRef]
- Choy, E.H. The role of sleep in pain and fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef]
- Masters, A.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.L.; McFarlane, S.I. Melatonin, the Hormone of Darkness: From Sleep Promotion to Ebola Treatment. Brain Disord. Ther. 2014, 4, 1000151. [Google Scholar] [CrossRef]
- Laflı Tunay, D.; Türkeün Ilgınel, M.; Ünlügenç, H.; Tunay, M.; Karacaer, F.; Biricik, E. Comparison of the effects of preoperative melatonin or vitamin C administration on postoperative analgesia. Bosn. J. Basic Med. Sci. 2020, 20, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Sciupokas, A.; Kopustinskiene, D.M.; Petrikonis, K. Novel Drug Targets and Emerging Pharmacotherapies in Neuropathic Pain. Pharmaceutics 2023, 15, 1799. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Kuthati, Y.; Lin, S.H.; Chen, I.J.; Wong, C.S. Melatonin and their analogs as a potential use in the management of Neuropathic pain. J. Formos. Med. Assoc. 2019, 118, 1177–1186. [Google Scholar] [CrossRef]
- Alshehri, F.S.; Alghamdi, B.S.; Hakami, A.Y.; Alshehri, A.A.; Althobaiti, Y.S. Melatonin attenuates morphine-induced conditioned place preference in Wistar rats. Brain Behav. 2021, 11, e2397. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Vranken, J.H. Mechanisms and treatment of neuropathic pain. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 71–78. [Google Scholar] [CrossRef]
- Chen, I.J.; Yang, C.P.; Lin, S.H.; Lai, C.M.; Wong, C.S. The Circadian Hormone Melatonin Inhibits Morphine-Induced Tolerance and Inflammation via the Activation of Antioxidative Enzymes. Antioxidants 2020, 9, 780. [Google Scholar] [CrossRef]
- Roehrs, T.; Roth, T. Sleep and pain: Interaction of two vital functions. Semin. Neurol. 2005, 25, 106–116. [Google Scholar] [CrossRef]
- Bumgarner, J.R.; Walker, W.H., II; Nelson, R.J. Circadian rhythms and pain. Neurosci. Biobehav. Rev. 2021, 129, 296–306. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Skene, D.J. Nonphotic entrainment in humans? J. Biol. Rhythms 2005, 20, 339–352. [Google Scholar] [CrossRef]
- Ambriz-Tututi, M.; Rocha-González, H.I.; Castañeda-Corral, G.; Araiza-Saldaña, C.I.; Caram-Salas, N.L.; Cruz, S.L.; Granados-Soto, V. Role of opioid receptors in the reduction of formalin-induced secondary allodynia and hyperalgesia in rats. Eur. J. Pharmacol. 2009, 619, 25–32. [Google Scholar] [CrossRef]
- Liu, Q.; Su, L.Y.; Sun, C.; Jiao, L.; Miao, Y.; Xu, M.; Luo, R.; Zuo, X.; Zhou, R.; Zheng, P.; et al. Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox. Biol. 2020, 34, 101560. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Manchester, L.C. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini. Rev. Med. Chem. 2013, 13, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Pertin, M.; Gosselin, R.D.; Decosterd, I. The spared nerve injury model of neuropathic pain. Methods Mol. Biol. 2012, 851, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Furmanski, O.; Castellanos, D.A.; Daniels, L.A.; Hama, A.T.; Sagen, J. Prolonged nociceptive responses to hind paw formalin injection in rats with a spinal cord injury. Neurosci. Lett. 2008, 439, 212–215. [Google Scholar] [CrossRef]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef]
- García-Domínguez, M. The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective. Life 2025, 15, 785. [Google Scholar] [CrossRef]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. S1), S10–S28. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.D.; Suter, M.R.; Ji, R.R.; Decosterd, I. Glial cells and chronic pain. Neuroscientist 2010, 16, 519–531. [Google Scholar] [CrossRef]
- Compton, P.; Kehoe, P.; Sinha, K.; Torrington, M.A.; Ling, W. Gabapentin improves cold-pressor pain responses in methadone-maintained patients. Drug Alcohol. Depend. 2010, 109, 213–219. [Google Scholar] [CrossRef][Green Version]
- Hayashi, Y.; Kawaji, K.; Sun, L.; Zhang, X.; Koyano, K.; Yokoyama, T.; Kohsaka, S.; Inoue, K.; Nakanishi, H. Microglial Ca2+-activated K+ channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J. Neurosci. 2011, 31, 17370–17382. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
| Time | F-Value | p-Value | df_Between | df_Within |
|---|---|---|---|---|
| 10:00 p.m. | 9.725 | 0.0007 | 3 | 16 |
| 11:00 p.m. | 16.296 | 0 | 3 | 16 |
| 12:00 a.m. | 15.119 | 0.0001 | 3 | 16 |
| 1:00 a.m. | 48.147 | 0 | 3 | 16 |
| 2:00 a.m. | 6.067 | 0.0059 | 3 | 16 |
| 3:00 a.m. | 36.596 | 0 | 3 | 16 |
| 4:00 a.m. | 32.43 | 0 | 3 | 16 |
| 5:00 a.m. | 13.263 | 0.0001 | 3 | 16 |
| 6:00 a.m. | 5.377 | 0.0094 | 3 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, N.-C.; Wong, C.-S. Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model. Int. J. Mol. Sci. 2025, 26, 7372. https://doi.org/10.3390/ijms26157372
Huang N-C, Wong C-S. Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model. International Journal of Molecular Sciences. 2025; 26(15):7372. https://doi.org/10.3390/ijms26157372
Chicago/Turabian StyleHuang, Nian-Cih, and Chih-Shung Wong. 2025. "Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model" International Journal of Molecular Sciences 26, no. 15: 7372. https://doi.org/10.3390/ijms26157372
APA StyleHuang, N.-C., & Wong, C.-S. (2025). Circadian Light Manipulation and Melatonin Supplementation Enhance Morphine Antinociception in a Neuropathic Pain Rat Model. International Journal of Molecular Sciences, 26(15), 7372. https://doi.org/10.3390/ijms26157372





