Targeting GLP-1 Signaling Ameliorates Cystogenesis in a Zebrafish Model of Nephronophthisis
Abstract
1. Introduction
2. Results
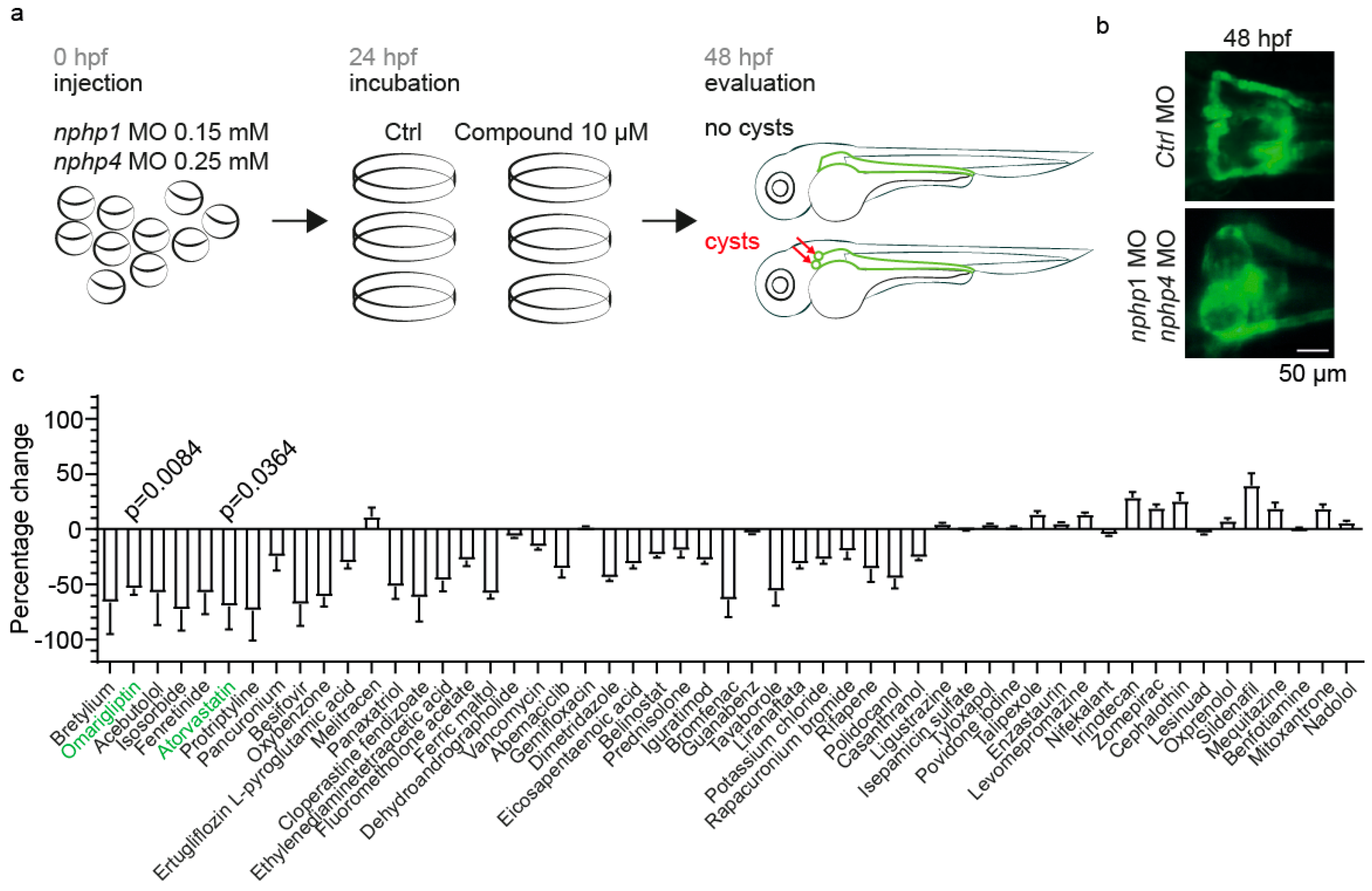
2.1. Establishment of a Zebrafish Model for Nephronophthisis and a Drug Screening Platform
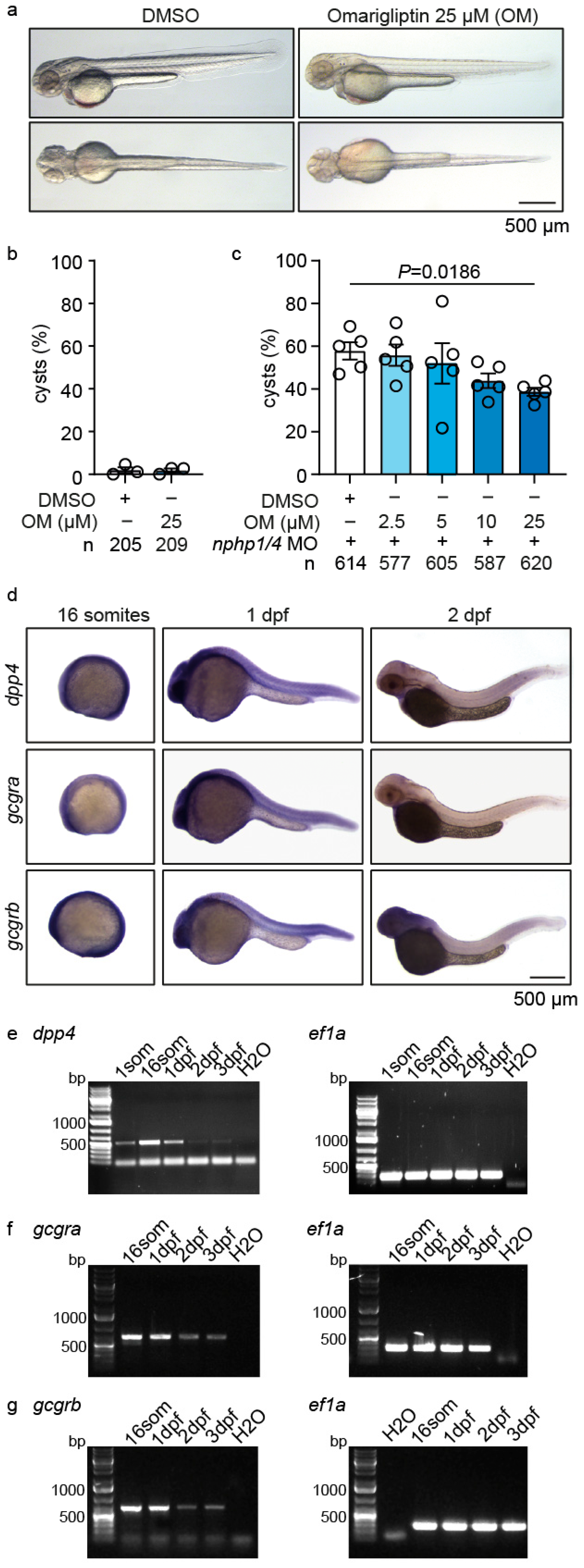
2.2. Omarigliptin Modulates GLP-1 Signaling and Reduces Cyst Formation in a Zebrafish Nephronophthisis Model
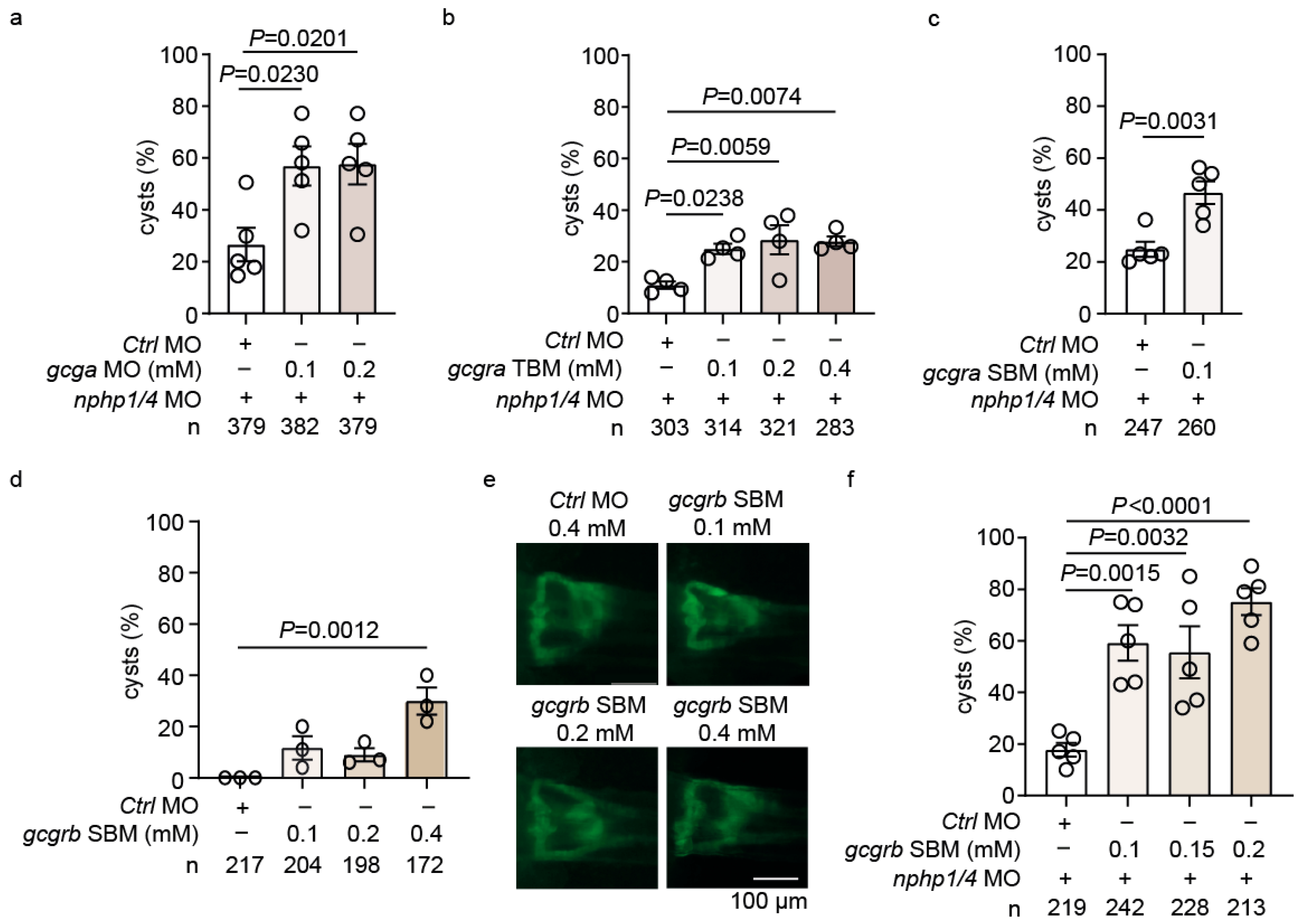
2.3. Genetic Analysis Reveals He Role of GLP-1 Receptor Signaling in Cystogenesis
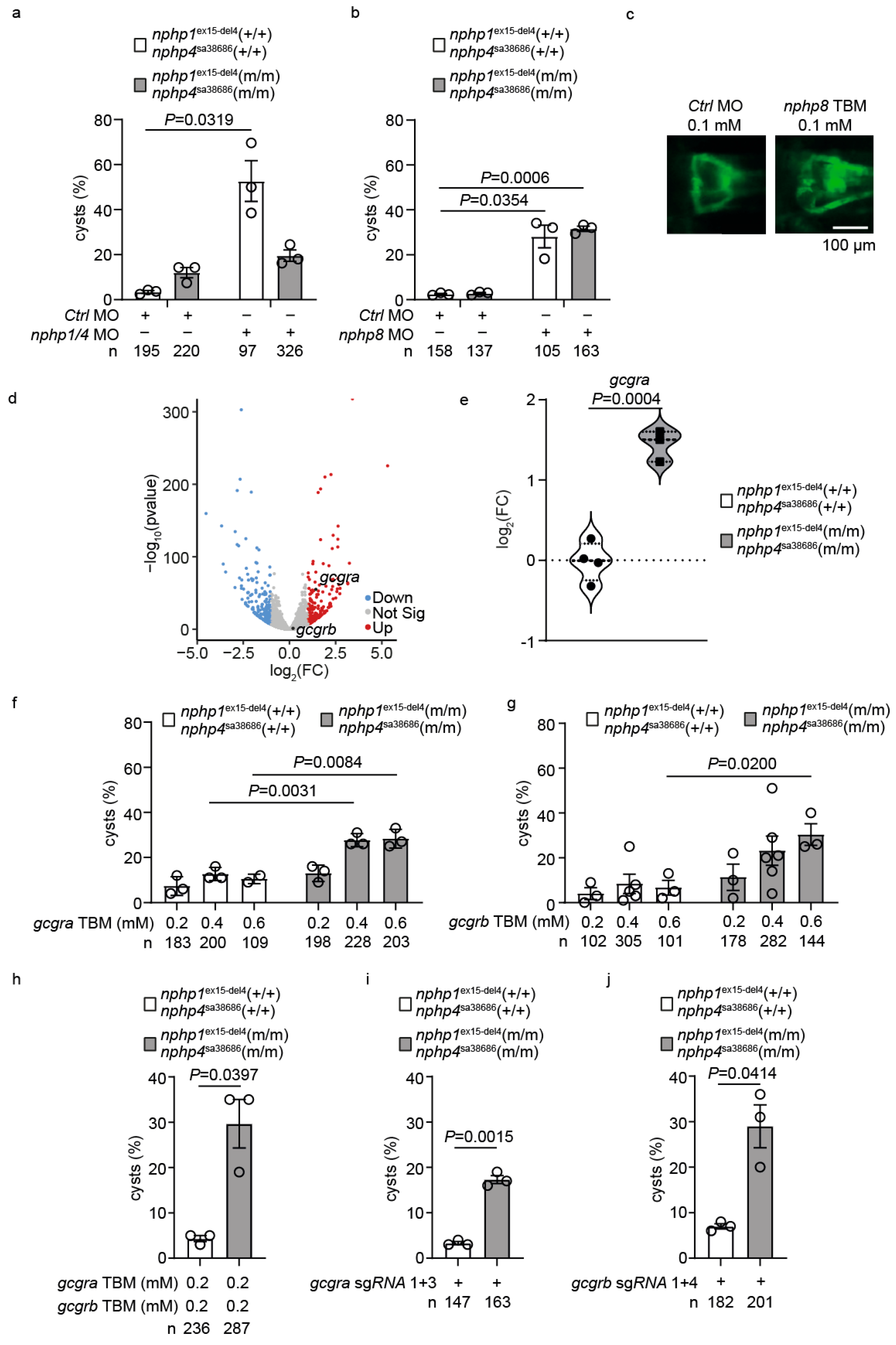
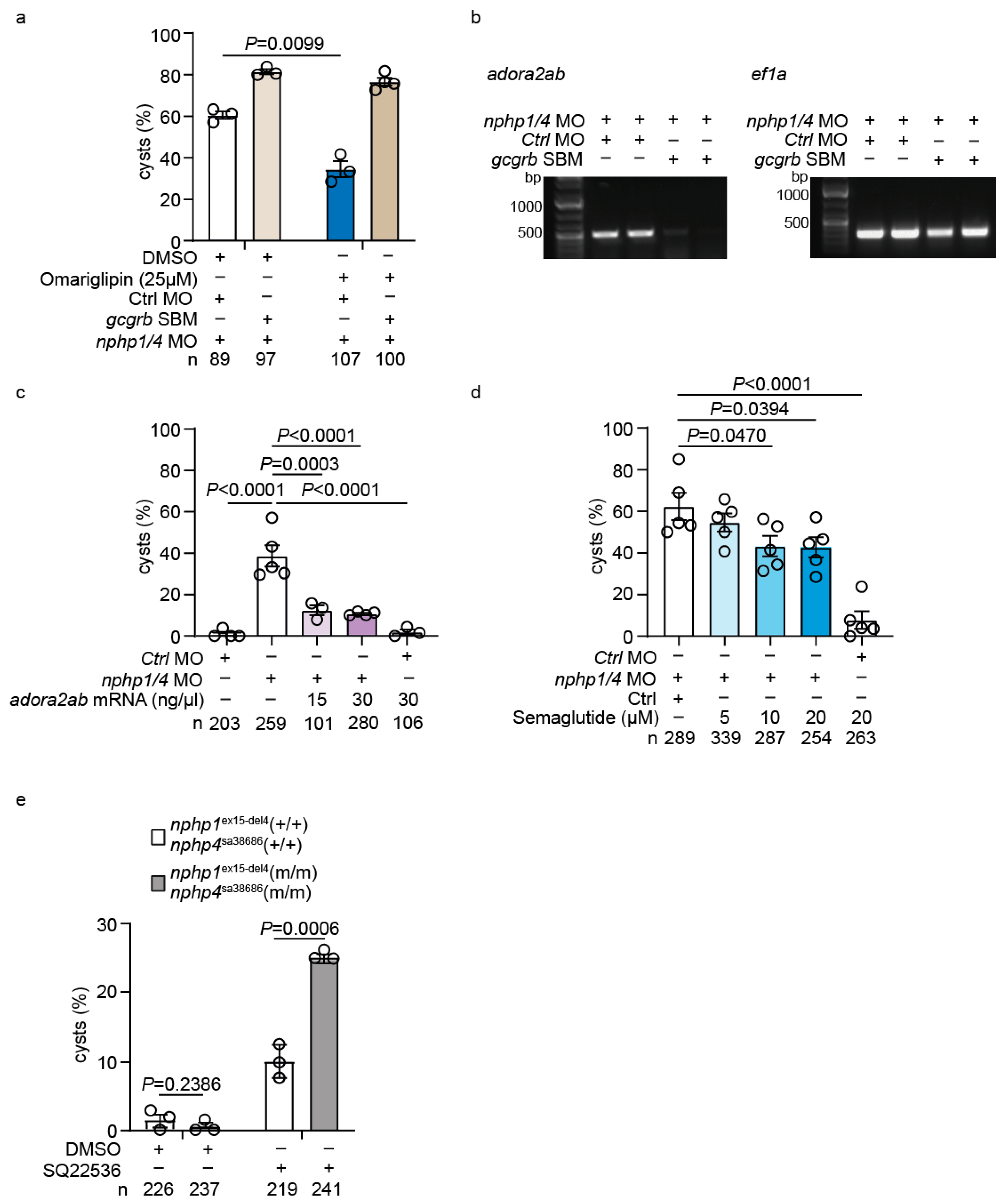
2.4. Upregulation of GLP-1 Receptor Signaling Mediates Genetic Compensation in Nphp1/Nphp4 Double Mutant Zebrafish
2.5. Transcriptional Profiling Reveals adora2ab as a Downstream Effector of GLP-1 Signaling
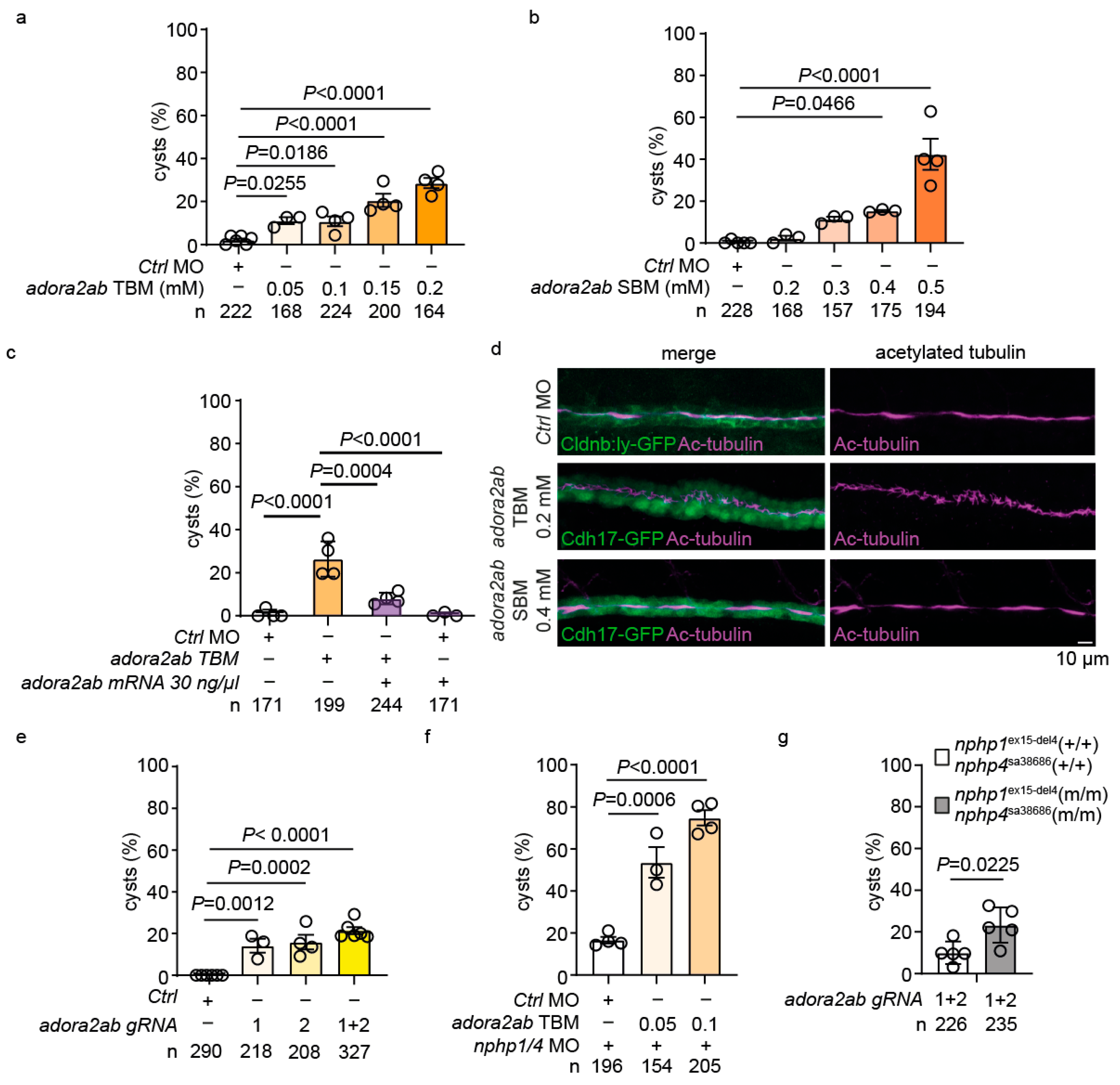
2.6. adora2ab Depletion Induces Cystogenesis and Disrupts Ciliary Morphology
2.7. Functional Validation of the GLP-1-adora2ab Axis in Preventing Cystogenesis
2.8. Adenylate Cyclase (AC) Inhibition Exacerbates Cystogenesis in Nphp1ex15-Del4;Nphp4sa38686 Homozygous Mutants
3. Discussion
4. Materials and Methods
4.1. Zebrafish Lines and Maintenance
4.2. Morpholino Knockdown and mRNA Rescue
4.3. Plasmid Construction and mRNA Synthesis
4.4. CRISPR/Cas9 Gene Targeting
- All genetic interventions are summarized in Supplementary Table S3.
4.5. Drug Screen and Treatment
4.6. RNA Extraction and RT-PCR
4.7. Whole-Mount in Situ Hybridization and Immunohistochemistry
4.8. RNAseq and Analysis
4.9. Microscopy and Imaging
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hpf | Hours post-fertilization |
| NPH | Nephronophthisis |
| GLP-1 | Glucagon-like peptide-1 |
| DPP4 | Dipeptidyl peptidase-4 |
| dpf | Days post-fertilization |
| SBM | Splice-blocking morpholino oligonucleotide |
| TBM | Translation-blocking morpholino oligonucleotide |
| MO | Morpholino oligonucleotide |
| AC | Adenylate cyclase |
| PCA | Principal component analysis |
References
- Hildebrandt, F.; Benzing, T.; Katsanis, N. Ciliopathies. N. Engl. J. Med. 2011, 364, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Benmerah, A.; Briseño-Roa, L.; Annereau, J.-P.; Saunier, S. Repurposing Small Molecules for Nephronophthisis and Related Renal Ciliopathies. Kidney Int. 2023, 104, 245–253. [Google Scholar] [CrossRef]
- Stokman, M.F.; Saunier, S.; Benmerah, A. Renal Ciliopathies: Sorting Out Therapeutic Approaches for Nephronophthisis. Front. Cell Dev. Biol. 2021, 9, 653138. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.F.; Bonsib, S.M.; Larsen, C.P.; Hildebrandt, F. Nephronophthisis: A Pathological and Genetic Perspective. Pediatr. Nephrol. 2024, 39, 1977–2000. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ozimek-Kulik, J.E.; Phillips, J.K. Nephronophthisis-Pathobiology and Molecular Pathogenesis of a Rare Kidney Genetic Disease. Genes 2021, 12, 1762. [Google Scholar] [CrossRef]
- Sang, L.; Miller, J.J.; Corbit, K.C.; Giles, R.H.; Brauer, M.J.; Otto, E.A.; Baye, L.M.; Wen, X.; Scales, S.J.; Kwong, M.; et al. Mapping the Nephronophthisis-Joubert-Meckel-Gruber Protein Network Reveals Ciliopathy Disease Genes and Pathways. Cell 2011, 145, 513–528. [Google Scholar] [CrossRef]
- Mollet, G.; Silbermann, F.; Delous, M.; Salomon, R.; Antignac, C.; Saunier, S. Characterization of the Nephrocystin/Nephrocystin-4 Complex and Subcellular Localization of Nephrocystin-4 to Primary Cilia and Centrosomes. Hum. Mol. Genet. 2005, 14, 645–656. [Google Scholar] [CrossRef]
- Benzing, T.; Gerke, P.; Höpker, K.; Hildebrandt, F.; Kim, E.; Walz, G. Nephrocystin Interacts with Pyk2, p130Cas, and Tensin and Triggers Phosphorylation of Pyk2. Proc. Natl. Acad. Sci. USA 2001, 98, 9784–9789. [Google Scholar] [CrossRef]
- Arts, H.H.; Doherty, D.; van Beersum, S.E.C.; Parisi, M.A.; Letteboer, S.J.F.; Gorden, N.T.; Peters, T.A.; Märker, T.; Voesenek, K.; Kartono, A.; et al. Mutations in the Gene Encoding the Basal Body Protein RPGRIP1L, a Nephrocystin-4 Interactor, Cause Joubert Syndrome. Nat. Genet. 2007, 39, 882–888. [Google Scholar] [CrossRef]
- Schäfer, T.; Pütz, M.; Lienkamp, S.; Ganner, A.; Bergbreiter, A.; Ramachandran, H.; Gieloff, V.; Gerner, M.; Mattonet, C.; Czarnecki, P.G.; et al. Genetic and Physical Interaction between the NPHP5 and NPHP6 Gene Products. Hum. Mol. Genet. 2008, 17, 3655–3662. [Google Scholar] [CrossRef]
- Srivastava, S.; Molinari, E.; Raman, S.; Sayer, J.A. Many Genes—One Disease? Genetics of Nephronophthisis (NPHP) and NPHP-Associated Disorders. Front. Pediatr. 2018, 5, 287. [Google Scholar] [CrossRef]
- Hoff, S.; Halbritter, J.; Epting, D.; Frank, V.; Nguyen, T.-M.T.; van Reeuwijk, J.; Boehlke, C.; Schell, C.; Yasunaga, T.; Helmstädter, M.; et al. ANKS6 Is a Central Component of a Nephronophthisis Module Linking NEK8 to INVS and NPHP3. Nat. Genet. 2013, 45, 951–956. [Google Scholar] [CrossRef]
- Garví, E.S.; Biermans, S.; Knoers, N.V.A.M.; van Eerde, A.M.; Masereeuw, R.; Slaats, G.G.; van Genderen, A.M.; Janssen, M.J. Loss of Nephronophthisis-Associated Nephrocystin-1 Impairs DNA Damage Repair in Kidney Organoids. bioRxiv 2025. [Google Scholar] [CrossRef]
- Gattone, V.H.; Wang, X.; Harris, P.C.; Torres, V.E. Inhibition of Renal Cystic Disease Development and Progression by a Vasopressin V2 Receptor Antagonist. Nat. Med. 2003, 9, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Constans, M.M.; Chebib, F.T.; Torres, V.E.; Pellegrini, L. Effect of a Vasopressin V2 Receptor Antagonist on Polycystic Kidney Disease Development in a Rat Model. Am. J. Nephrol. 2019, 49, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.; Muneeruddin, S.; Parrish, R.; Lui, D.; Conley, S.B. Isosorbide Dinitrate in Nephronophthisis Treatment. Am. J. Med. Genet. A 2018, 176, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Masyuk, T.V.; Page, L.J.; Kubly, V.J.; Bergstralh, E.J.; Li, X.; Kim, B.; King, B.F.; Glockner, J.; Holmes, D.R.; et al. Randomized Clinical Trial of Long-Acting Somatostatin for Autosomal Dominant Polycystic Kidney and Liver Disease. J. Am. Soc. Nephrol. 2010, 21, 1052–1061. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hurd, T.; Hildebrandt, F. 3D Spheroid Defects in NPHP Knockdown Cells Are Rescued by the Somatostatin Receptor Agonist Octreotide. Am. J. Physiol. Ren. Physiol. 2012, 303, F1225–F1229. [Google Scholar] [CrossRef]
- Gattone, V.H.; Sinders, R.M.; Hornberger, T.A.; Robling, A.G. Late Progression of Renal Pathology and Cyst Enlargement Is Reduced by Rapamycin in a Mouse Model of Nephronophthisis. Kidney Int. 2009, 76, 178–182. [Google Scholar] [CrossRef]
- Chen, N.X.; Moe, S.M.; Eggleston-Gulyas, T.; Chen, X.; Hoffmeyer, W.D.; Bacallao, R.L.; Herbert, B.S.; Gattone, V.H. Calcimimetics Inhibit Renal Pathology in Rodent Nephronophthisis. Kidney Int. 2011, 80, 612–619. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.; Jung, Y.; Jung, E.; Kwon, H.J.; Kim, J. Eupatilin Rescues Ciliary Transition Zone Defects to Ameliorate Ciliopathy-Related Phenotypes. J. Clin. Investig. 2018, 128, 3642–3648. [Google Scholar] [CrossRef]
- Airik, R.; Airik, M.; Schueler, M.; Bates, C.M.; Hildebrandt, F. Roscovitine Blocks Collecting Duct Cyst Growth in Cep164-Deficient Kidneys. Kidney Int. 2019, 96, 320–326. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Ding, Q.; Wang, S.S.; Rastogi, P.; Dai, D.-F.; Lu, D.; Purvis, M.; Cao, C.; Wang, A.; et al. Epithelial Innate Immunity Mediates Tubular Cell Senescence after Kidney Injury. JCI Insight 2019, 4, e125490. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, Y.; Liu, D.; Wang, S.S.; Ding, Q.; Rastogi, P.; Purvis, M.; Wang, A.; Elhadi, S.; Ren, C.; et al. Innate Immune Signaling Contributes to Tubular Cell Senescence in the Glis2 Knockout Mouse Model of Nephronophthisis. Am. J. Pathol. 2020, 190, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- van Bloemendaal, L.; ten Kulve, J.S.; la Fleur, S.E.; Ijzerman, R.G.; Diamant, M. Effects of Glucagon-like Peptide 1 on Appetite and Body Weight: Focus on the CNS. J. Endocrinol. 2014, 221, T1–T16. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. The Incretin Effect in Healthy Individuals and Those with Type 2 Diabetes: Physiology, Pathophysiology, and Response to Therapeutic Interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J.; Cavender, M.A.; Abd El Aziz, M.; Drucker, D.J. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation 2017, 136, 849–870. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like Peptide 1 in Health and Disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Deacon, C.F. Peptide Degradation and the Role of DPP-4 Inhibitors in the Treatment of Type 2 Diabetes. Peptides 2018, 100, 150–157. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like Peptide-1 Receptor: Mechanisms and Advances in Therapy. Sig Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Muskiet, M.H.A.; Smits, M.M.; Morsink, L.M.; Diamant, M. The Gut-Renal Axis: Do Incretin-Based Agents Confer Renoprotection in Diabetes? Nat. Rev. Nephrol. 2014, 10, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Takashi, Y. GLP-1 Receptor Agonists in Diabetic Kidney Disease: From Clinical Outcomes to Mechanisms. Front. Pharmacol. 2020, 11, 543516. [Google Scholar] [CrossRef]
- Greco, E.V.; Russo, G.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; De Cosmo, S. GLP-1 Receptor Agonists and Kidney Protection. Medicina 2019, 55, 233. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Holstein-Rathlou, N.-H.; Sosnovtseva, O.; Sørensen, C.M. Renoprotective Effects of GLP-1 Receptor Agonists and SGLT-2 Inhibitors—Is Hemodynamics the Key Point? Am. J. Physiol.-Cell Physiol. 2023, 325, C243–C256. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.A. Kidney Development and Disease in the Zebrafish. J. Am. Soc. Nephrol. 2005, 16, 299. [Google Scholar] [CrossRef]
- Morales, E.E.; Wingert, R.A. Zebrafish as a Model of Kidney Disease. In Kidney Development and Disease; Miller, R.K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–75. ISBN 978-3-319-51436-9. [Google Scholar]
- Drummond, I. Making a Zebrafish Kidney: A Tale of Two Tubes. Trends Cell Biol. 2003, 13, 357–365. [Google Scholar] [CrossRef]
- Wingert, R.A.; Selleck, R.; Yu, J.; Song, H.-D.; Chen, Z.; Song, A.; Zhou, Y.; Thisse, B.; Thisse, C.; McMahon, A.P.; et al. The Cdx Genes and Retinoic Acid Control the Positioning and Segmentation of the Zebrafish Pronephros. PLoS Genet. 2007, 3, e189. [Google Scholar] [CrossRef]
- Slanchev, K.; Pütz, M.; Schmitt, A.; Kramer-Zucker, A.; Walz, G. Nephrocystin-4 Is Required for Pronephric Duct-Dependent Cloaca Formation in Zebrafish. Hum. Mol. Genet. 2011, 20, 3119–3128. [Google Scholar] [CrossRef]
- Kayser, N.; Zaiser, F.; Veenstra, A.C.; Wang, H.; Göcmen, B.; Eckert, P.; Franz, H.; Köttgen, A.; Walz, G.; Yakulov, T.A. Clock Genes Rescue Nphp Mutations in Zebrafish. Hum. Mol. Genet. 2022, 31, 4143–4158. [Google Scholar] [CrossRef]
- Zhou, W.; Dai, J.; Attanasio, M.; Hildebrandt, F. Nephrocystin-3 Is Required for Ciliary Function in Zebrafish Embryos. Am. J. Physiol.-Ren. Physiol. 2010, 299, F55–F62. [Google Scholar] [CrossRef]
- Ecder, T. Statins in the Treatment of Autosomal Dominant Polycystic Kidney Disease. Nephrol. Dial. Transplant. 2016, 31, 1194–1196. [Google Scholar] [CrossRef]
- Hattori, S. Omarigliptin Decreases Inflammation and Insulin Resistance in a Pleiotropic Manner in Patients with Type 2 Diabetes. Diabetol. Metab. Syndr. 2020, 12, 24. [Google Scholar] [CrossRef]
- Krishna, R.; Addy, C.; Tatosian, D.; Glasgow, X.S.; Gendrano Iii, I.N.; Robberechts, M.; Haazen, W.; de Hoon, J.N.; Depré, M.; Martucci, A.; et al. Pharmacokinetics and Pharmacodynamics of Omarigliptin, a Once-Weekly Dipeptidyl Peptidase-4 (DPP-4) Inhibitor, After Single and Multiple Doses in Healthy Subjects. J. Clin. Pharmacol. 2016, 56, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Tan, X. Omarigliptin for the Treatment of Type 2 Diabetes. Endocrine 2016, 54, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Mojsov, S. Glucagon-like Peptide-1 (GLP-1) and the Control of Glucose Metabolism in Mammals and Teleost Fish1. Am. Zool. 2000, 40, 246–258. [Google Scholar] [CrossRef]
- Jelsing, J.; Vrang, N.; van Witteloostuijn, S.B.; Mark, M.; Klein, T. The DPP4 Inhibitor Linagliptin Delays the Onset of Diabetes and Preserves β-Cell Mass in Non-Obese Diabetic Mice. J. Endocrinol. 2012, 214, 381–387. [Google Scholar] [CrossRef]
- Mikov, M.; Pavlović, N.; Stanimirov, B.; Đanić, M.; Goločorbin-Kon, S.; Stankov, K.; Al-Salami, H. DPP-4 Inhibitors: Renoprotective Potential and Pharmacokinetics in Type 2 Diabetes Mellitus Patients with Renal Impairment. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khanna, H.; Davis, E.E.; Murga-Zamalloa, C.A.; Estrada-Cuzcano, A.; Lopez, I.; den Hollander, A.I.; Zonneveld, M.N.; Othman, M.I.; Waseem, N.; Chakarova, C.F.; et al. A Common Allele in RPGRIP1L Is a Modifier of Retinal Degeneration in Ciliopathies. Nat. Genet. 2009, 41, 739–745. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Klotz, K.-N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [CrossRef]
- Gessler, S.; Guthmann, C.; Schuler, V.; Lilienkamp, M.; Walz, G.; Yakulov, T.A. Control of Directed Cell Migration after Tubular Cell Injury by Nucleotide Signaling. Int. J. Mol. Sci. 2022, 23, 7870. [Google Scholar] [CrossRef] [PubMed]
- Day, Y.-J.; Huang, L.; McDuffie, M.J.; Rosin, D.L.; Ye, H.; Chen, J.-F.; Schwarzschild, M.A.; Fink, J.S.; Linden, J.; Okusa, M.D. Renal Protection from Ischemia Mediated by A2A Adenosine Receptors on Bone Marrow-Derived Cells. J. Clin. Investig. 2003, 112, 883–891. [Google Scholar] [CrossRef]
- Grenz, A.; Osswald, H.; Eckle, T.; Yang, D.; Zhang, H.; Tran, Z.V.; Klingel, K.; Ravid, K.; Eltzschig, H.K. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Med. 2008, 5, e137. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Vincent, I.S.; Okusa, M.D. Adenosine 2A Receptors in Acute Kidney Injury. Acta Physiol. 2015, 214, 303–310. [Google Scholar] [CrossRef]
- Hilgendorf, K.I.; Johnson, C.T.; Jackson, P.K. The Primary Cilium as a Cellular Receiver: Organizing Ciliary GPCR Signaling. Curr. Opin. Cell Biol. 2016, 39, 84–92. [Google Scholar] [CrossRef]
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular Adenosine: A Safety Signal That Dampens Hypoxia-Induced Inflammation During Ischemia. Antioxid. Redox Signal 2011, 15, 2221–2234. [Google Scholar] [CrossRef]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, N.; Imamura, T.; Yoshizaki, T.; Babendure, J.L.; Lu, J.-C.; Olefsky, J.M. β-Arrestin-1 Mediates Glucagon-like Peptide-1 Signaling to Insulin Secretion in Cultured Pancreatic β Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6614–6619. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Geetha, K.M.; Hazarika, I. Contemporary Updates on the Physiology of Glucagon like Peptide-1 and Its Agonist to Treat Type 2 Diabetes Mellitus. Int. J. Pept. Res. Ther. 2020, 26, 1211–1221. [Google Scholar] [CrossRef]
- Leech, C.A.; Dzhura, I.; Chepurny, O.G.; Kang, G.; Schwede, F.; Genieser, H.-G.; Holz, G.G. Molecular Physiology of Glucagon-like Peptide-1 Insulin Secretagogue Action in Pancreatic β Cells. Prog. Biophys. Mol. Biol. 2011, 107, 236–247. [Google Scholar] [CrossRef]
- Takeda, Y.; Amano, A.; Noma, A.; Nakamura, Y.; Fujimoto, S.; Inagaki, N. Systems Analysis of GLP-1 Receptor Signaling in Pancreatic β-Cells. Am. J. Physiol.-Cell Physiol. 2011, 301, C792–C803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, C.; Zheng, F.; Liu, C.; Tian, X. Therapeutic Potential of GLP-1 Receptor Agonists in Diabetes and Cardiovascular Disease: Mechanisms and Clinical Implications. Cardiovasc. Drugs Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal Dominant Polycystic Kidney Disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Rees, S.; Kittikulsuth, W.; Roos, K.; Strait, K.A.; Van Hoek, A.; Kohan, D.E. Adenylyl Cyclase 6 Deficiency Ameliorates Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2014, 25, 232. [Google Scholar] [CrossRef]
- Sussman, C.R.; Wang, X.; Chebib, F.T.; Torres, V.E. Modulation of Polycystic Kidney Disease by G-Protein Coupled Receptors and Cyclic AMP Signaling. Cell. Signal. 2020, 72, 109649. [Google Scholar] [CrossRef]
- Chebib, F.T.; Sussman, C.R.; Wang, X.; Harris, P.C.; Torres, V.E. Vasopressin and Disruption of Calcium Signalling in Polycystic Kidney Disease. Nat. Rev. Nephrol. 2015, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Mohieldin, A.M.; Muntean, B.S.; Green, J.A.; Shah, J.V.; Mykytyn, K.; Nauli, S.M. Cilioplasm Is a Cellular Compartment for Calcium Signaling in Response to Mechanical and Chemical Stimuli. Cell. Mol. Life Sci. 2014, 71, 2165–2178. [Google Scholar] [CrossRef]
- Moore, B.S.; Stepanchick, A.N.; Tewson, P.H.; Hartle, C.M.; Zhang, J.; Quinn, A.M.; Hughes, T.E.; Mirshahi, T. Cilia Have High cAMP Levels That Are Inhibited by Sonic Hedgehog-Regulated Calcium Dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 13069–13074. [Google Scholar] [CrossRef]
- Brill, A.L.; Fischer, T.T.; Walters, J.M.; Marlier, A.; Sewanan, L.R.; Wilson, P.C.; Johnson, E.K.; Moeckel, G.; Cantley, L.G.; Campbell, S.G.; et al. Polycystin 2 Is Increased in Disease to Protect against Stress-Induced Cell Death. Sci. Rep. 2020, 10, 386. [Google Scholar] [CrossRef]
- Kleene, S.J. Regenerative Calcium Currents in Renal Primary Cilia. Front. Physiol. 2022, 13, 894518. [Google Scholar] [CrossRef] [PubMed]
- Vajanaphanich, M.; Schultz, C.; Tsien, R.Y.; Traynor-Kaplan, A.E.; Pandol, S.J.; Barrett, K.E. Cross-Talk between Calcium and cAMP-Dependent Intracellular Signaling Pathways. Implications for Synergistic Secretion in T84 Colonic Epithelial Cells and Rat Pancreatic Acinar Cells. J. Clin. Investig. 1995, 96, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Pozzan, T. CAMP and Ca2+ Interplay: A Matter of Oscillation Patterns. Trends Neurosci. 2003, 26, 53–55. [Google Scholar] [CrossRef] [PubMed]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic Compensation: A Phenomenon in Search of Mechanisms. PLoS Genet. 2017, 13, e1006780. [Google Scholar] [CrossRef]
- Lake, A.V.R.; Smith, C.E.L.; Natarajan, S.; Basu, B.; Best, S.K.; Stevenson, T.; Trowbridge, R.; Grellscheid, S.N.; Bond, J.; Foster, R.; et al. Drug and siRNA Screens Identify ROCK2 as a Therapeutic Target for Ciliopathies. Commun. Med. 2025, 5, 129. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 3rd ed.; University of Oregon Press: Eugene, OR, USA, 1995; p. 385. [Google Scholar]
- Yakulov, T.A.; Todkar, A.P.; Slanchev, K.; Wiegel, J.; Bona, A.; Groß, M.; Scholz, A.; Hess, I.; Wurditsch, A.; Grahammer, F.; et al. CXCL12 and MYC Control Energy Metabolism to Support Adaptive Responses after Kidney Injury. Nat. Commun. 2018, 9, 3660. [Google Scholar] [CrossRef]
- Schoels, M.; Zhuang, M.; Fahrner, A.; Küchlin, S.; Sagar; Franz, H.; Schmitt, A.; Walz, G.; Yakulov, T.A. Single-Cell mRNA Profiling Reveals Changes in Solute Carrier Expression and Suggests a Metabolic Switch during Zebrafish Pronephros Development. Am. J. Physiol.-Ren. Physiol. 2021, 320, F826–F837. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, L.; Shi, Z.; Jiang, J.; Li, X.; Chen, Y.; Li, K.; Luo, D. Customized One-Step Preparation of sgRNA Transcription Templates via Overlapping PCR Using Short Primers and Its Application in Vitro and in Vivo Gene Editing. Cell Biosci. 2019, 9, 87. [Google Scholar] [CrossRef]
- Batut, B.; van den Beek, M.; Doyle, M.A.; Soranzo, N. RNA-Seq Data Analysis in Galaxy. Methods Mol. Biol. 2021, 2284, 367–392. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, P.; Nöller, M.; Müller, M.; Haas, R.; Ruf, J.; Franz, H.; Moos, K.; Yu, J.-a.; Zhao, D.; Xie, W.; et al. Targeting GLP-1 Signaling Ameliorates Cystogenesis in a Zebrafish Model of Nephronophthisis. Int. J. Mol. Sci. 2025, 26, 7366. https://doi.org/10.3390/ijms26157366
Eckert P, Nöller M, Müller M, Haas R, Ruf J, Franz H, Moos K, Yu J-a, Zhao D, Xie W, et al. Targeting GLP-1 Signaling Ameliorates Cystogenesis in a Zebrafish Model of Nephronophthisis. International Journal of Molecular Sciences. 2025; 26(15):7366. https://doi.org/10.3390/ijms26157366
Chicago/Turabian StyleEckert, Priska, Maike Nöller, Merle Müller, Rebecca Haas, Johannes Ruf, Henriette Franz, Katharina Moos, Jia-ao Yu, Dongfang Zhao, Wanqiu Xie, and et al. 2025. "Targeting GLP-1 Signaling Ameliorates Cystogenesis in a Zebrafish Model of Nephronophthisis" International Journal of Molecular Sciences 26, no. 15: 7366. https://doi.org/10.3390/ijms26157366
APA StyleEckert, P., Nöller, M., Müller, M., Haas, R., Ruf, J., Franz, H., Moos, K., Yu, J.-a., Zhao, D., Xie, W., Boerries, M., Walz, G., & Yakulov, T. A. (2025). Targeting GLP-1 Signaling Ameliorates Cystogenesis in a Zebrafish Model of Nephronophthisis. International Journal of Molecular Sciences, 26(15), 7366. https://doi.org/10.3390/ijms26157366







