NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms
Abstract
:1. Introduction
2. Results
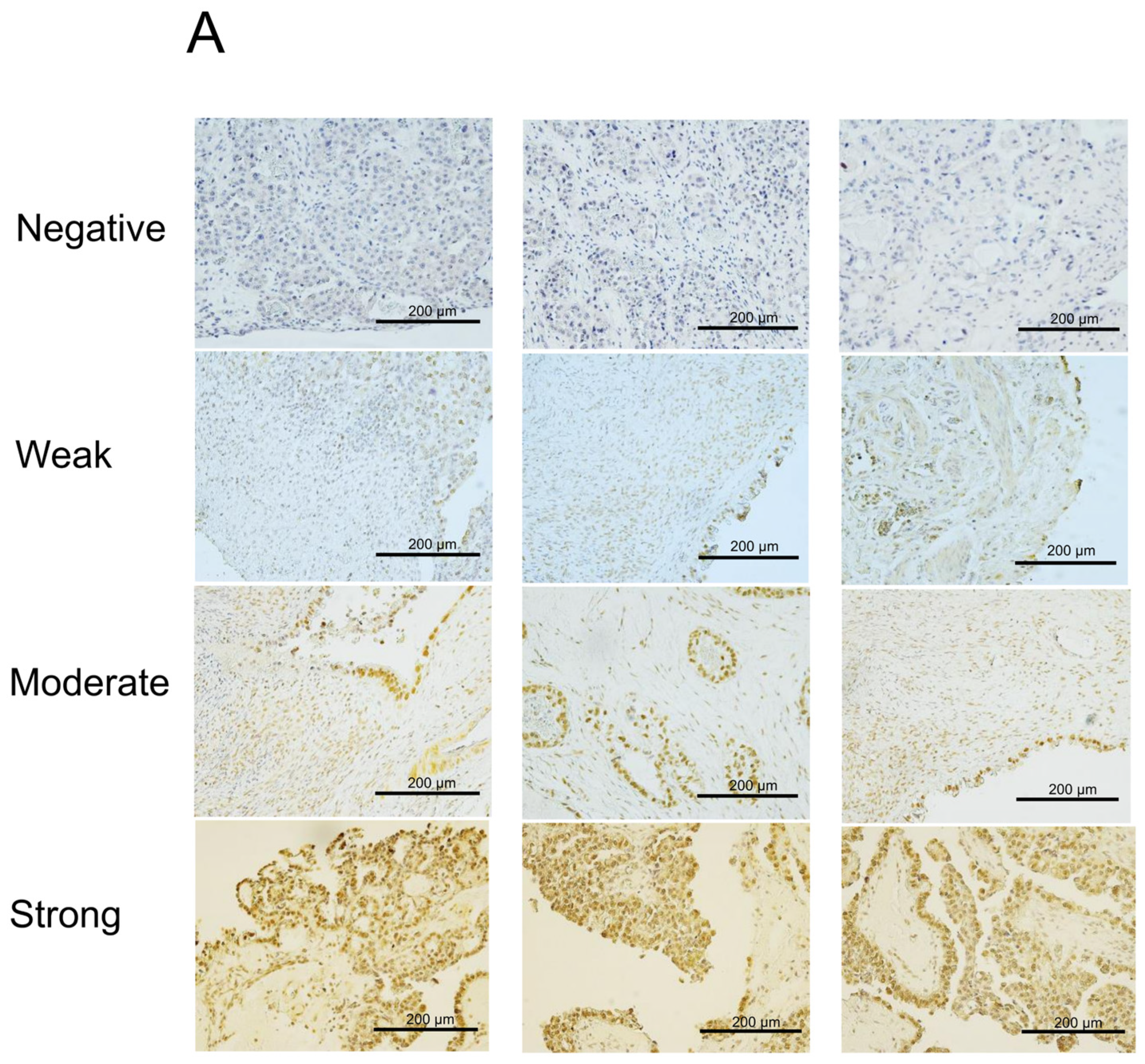
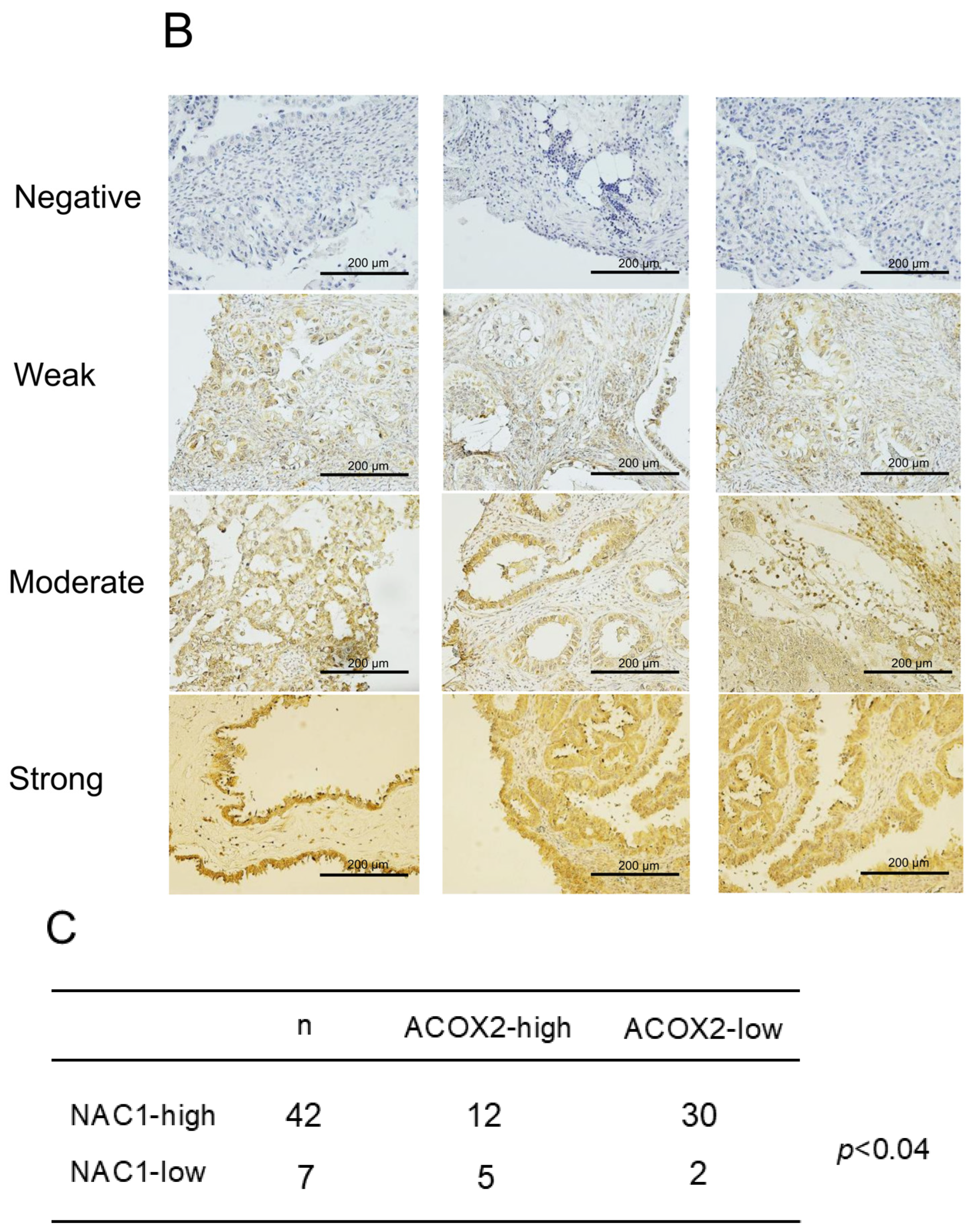
2.1. NAC1 Expression Is Inversely Associated with ACOX2 Expression in ERONs
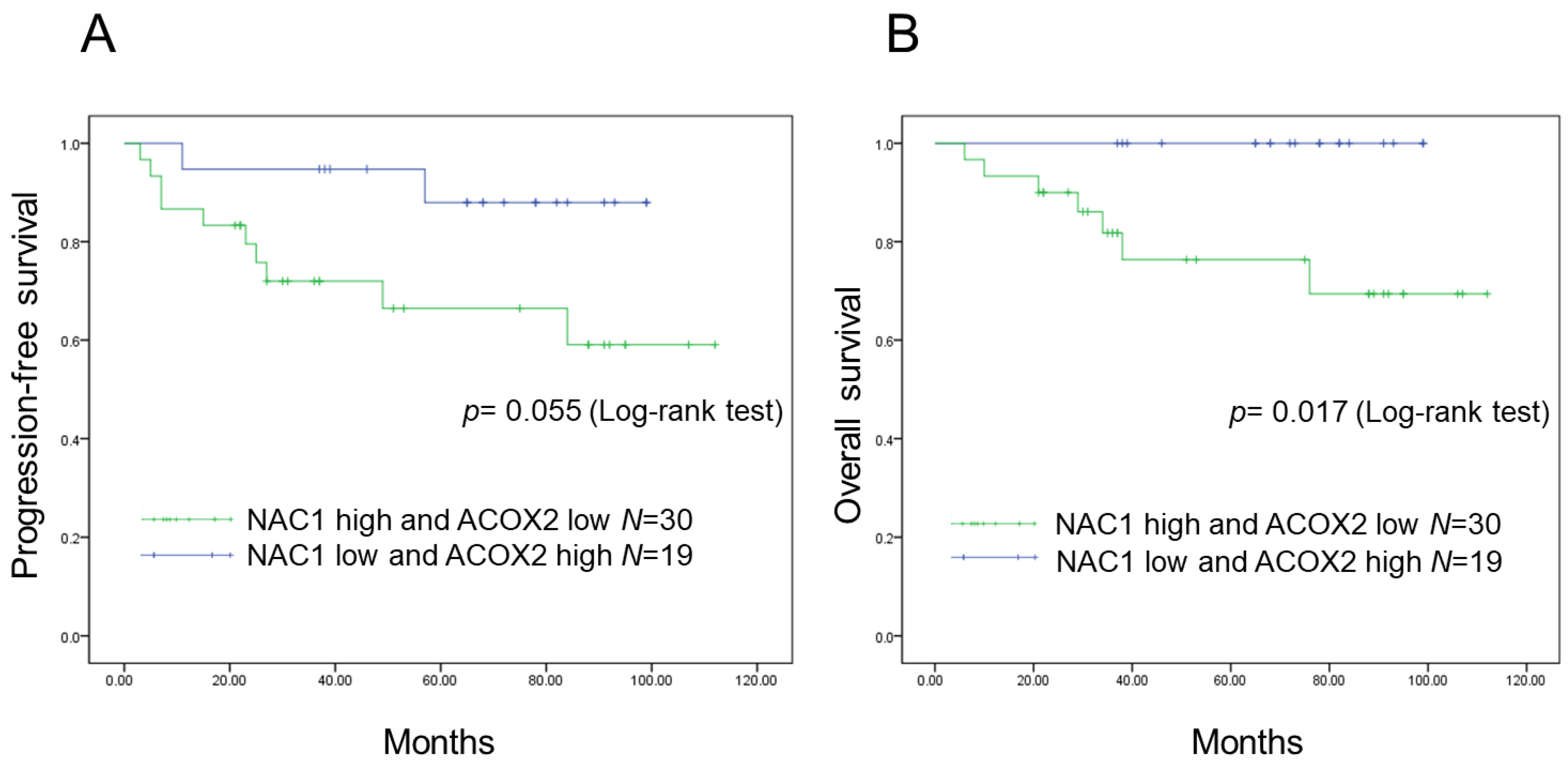
2.2. Increased NAC1 and Decreased ACOX2 Expressions Are Associated with Worse Prognosis in Patients with ERONs
2.3. NAC1 Negatively Regulates ACOX2 Expression in OCCC Cells
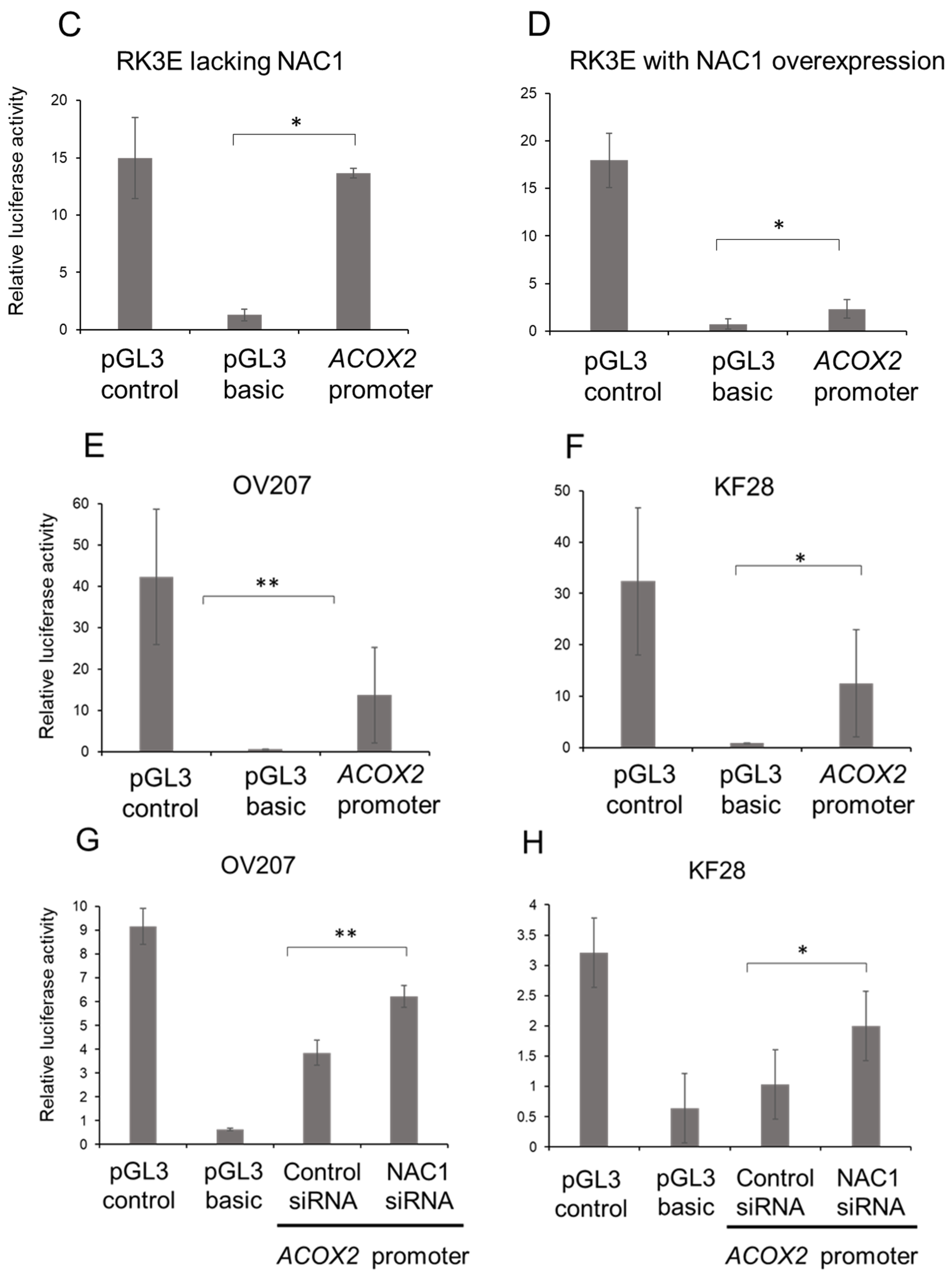
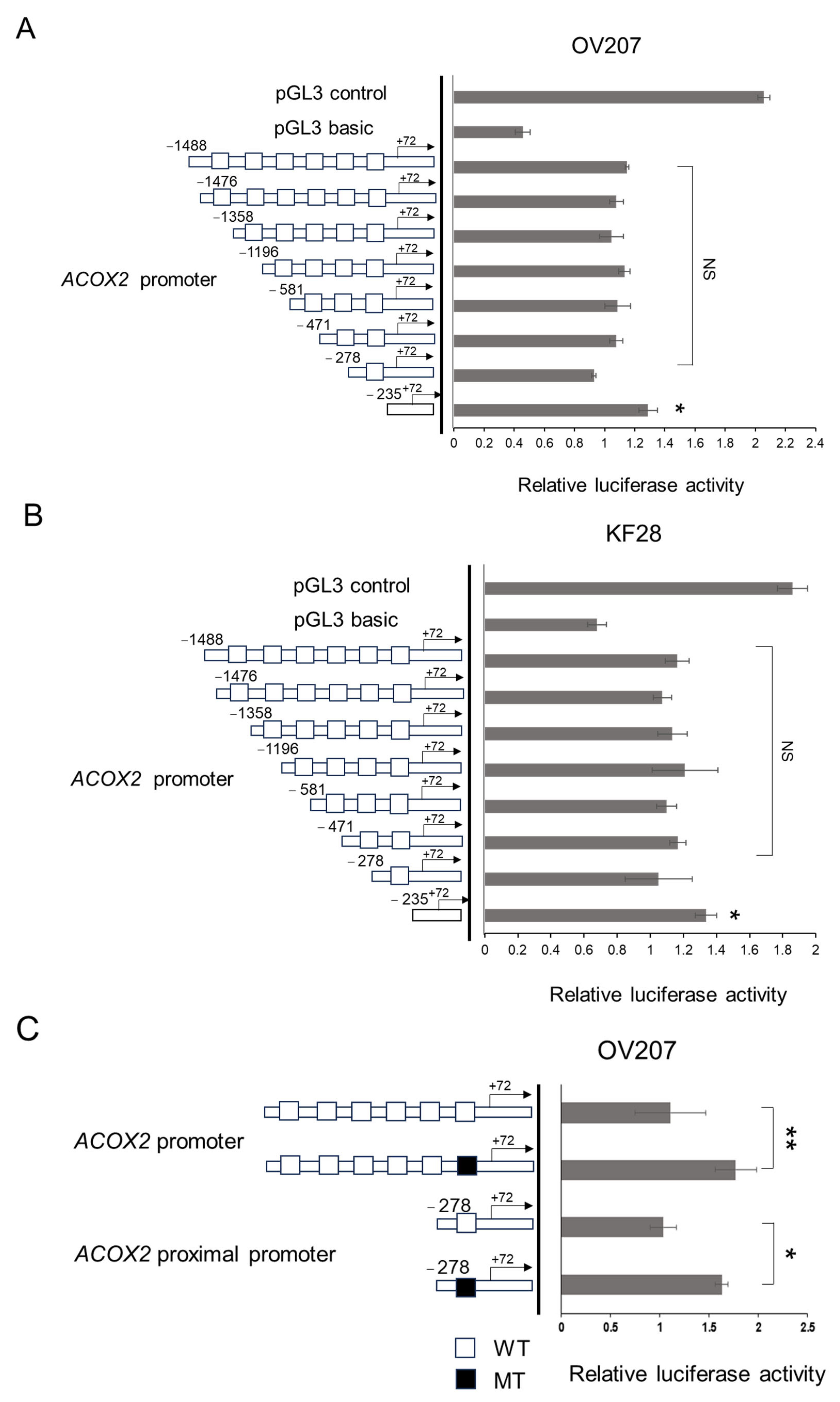
2.4. NAC1 Represses ACOX2 Promoter Transcriptional Activity in OCCC Cell Lines
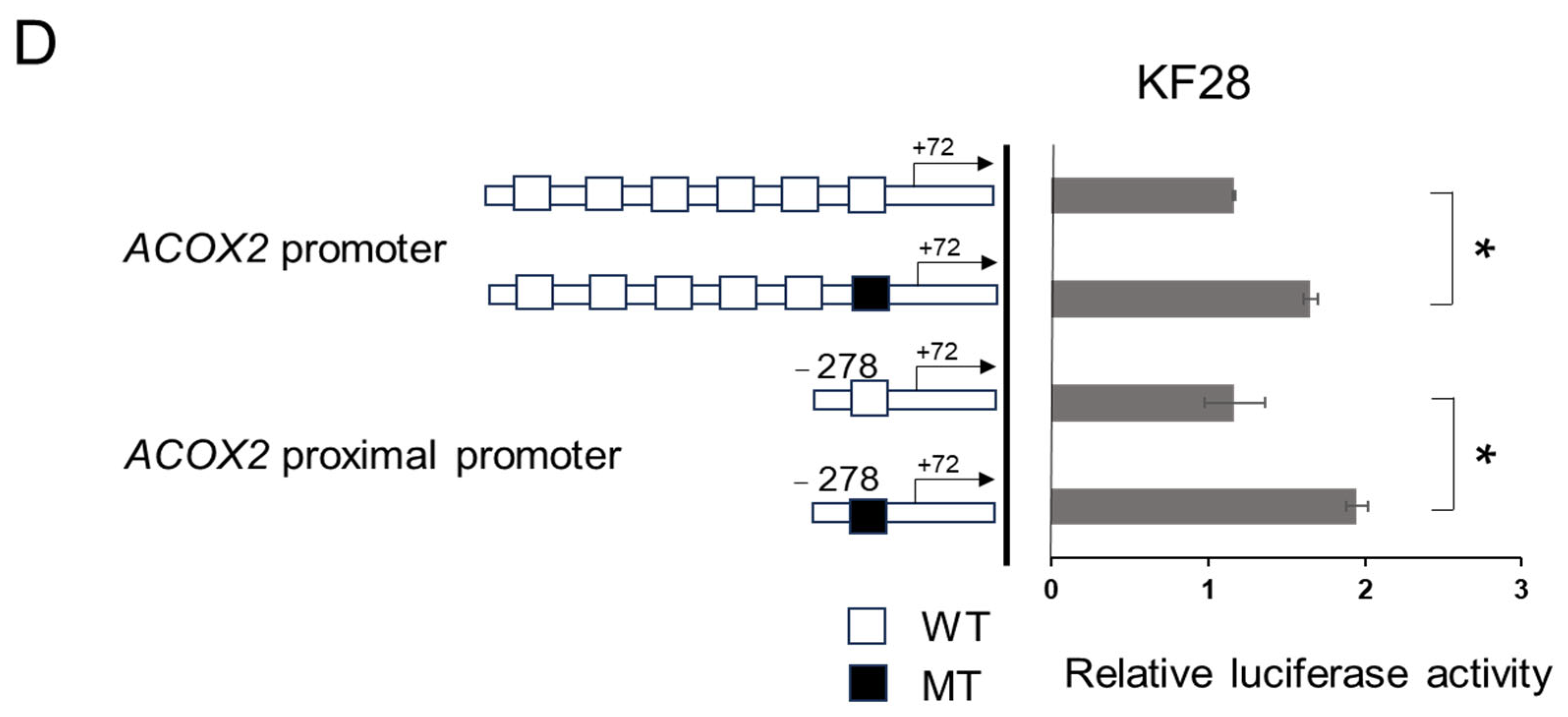
2.5. The Proximal CATG Site on the ACOX2 Promoter May Be Responsible for the Transcriptional Repression by NAC1
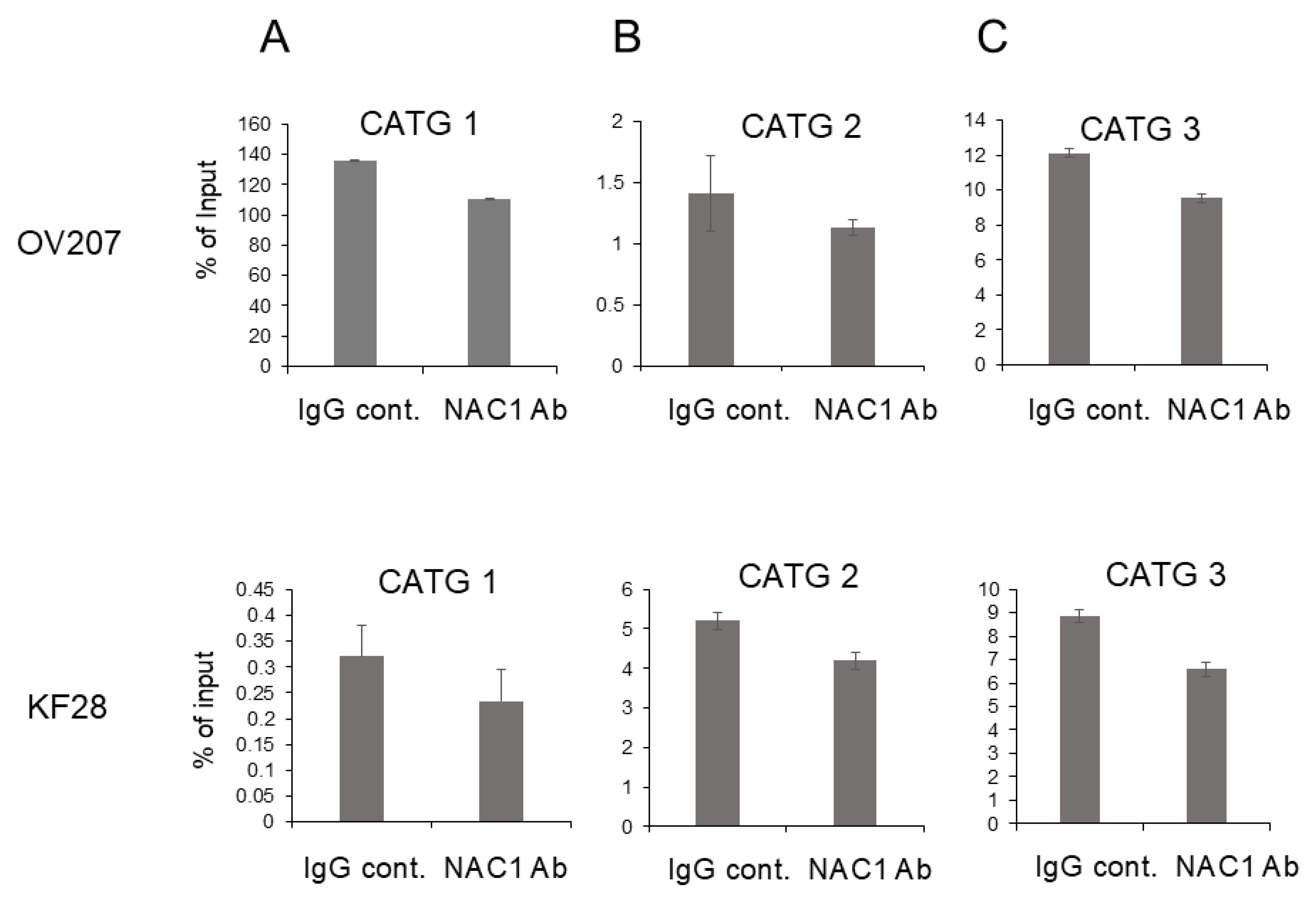
2.6. In Vivo Binding of NAC1 to the Most Proximal CATG Site on the ACOX2 Promoter
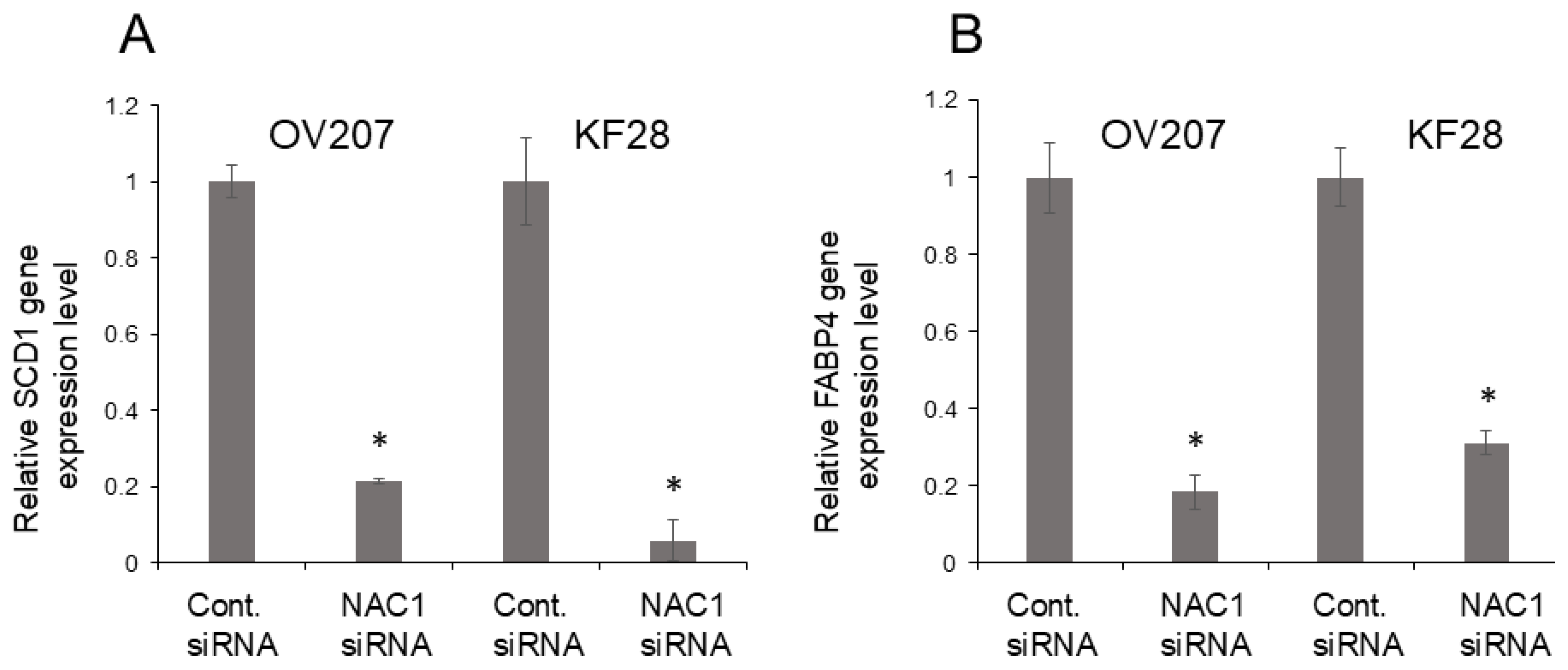
2.7. NAC1 Knockdown Suppresses Fatty Acid Metabolism Genes in OCCC Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Tissue Samples
4.3. Immunohistochemistry
4.4. Western Blotting
4.5. Targeted Gene Silencing RNA Knockdown of NAC1 Gene Expression
4.6. Luciferase Assay Assessment of Functional Activity
4.7. Chromatin Immunoprecipitation (ChIP) Assay
| Sequence name (5′- to -3′) | |
| CATG 1 F | AAGGAGACAAGTGTGATCATTGTTA |
| CATG 1 R | GCTTTGAAGCTGACCCTGTT |
| CATG 2 F | GCCTTTTTAAGCTAGAGAATTCAAGA |
| CATG 2 R | GAGAAAGGCTTGGATTGCAG |
| CATG 3 F | TTCTTCACTGGGCACTTCCT |
| CATG 3 R | TTTCAGGGAAGGTGGTCTTG |
| CATG 4 F | TCTCATCTGGATCCCACACA |
| CATG 4 R | TGAAAGGATTGCTCTGTAAGGTG |
| CATG 5 F | GGGAGAGAGGAAAAACGTACA |
| CATG 5 R | CCCAGCAGTGTTTCCAAAGT |
| CATG 6 F | GAATCCCTGGCTTTGAGATG |
| CATG 6 R | ACATAGGGGCTGGAACACAG |
| F: forward, R: reverse. |
4.8. Quantitative Real-Time RT-PCR (qRT-PCR)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NAC1 | Nucleus accumbens-associated protein 1 |
| ACOX2 | Acyl-CoA oxidase 2 |
| OCCC | Ovarian clear cell carcinoma |
| ERON | Endometriosis-related ovarian neoplasms |
| SCD1 | Stearoyl-coenzyme A desaturase 1 |
| FABP4 | Fatty acid-binding protein 4 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Ghoncheh, M.; Pakzad, R.; Hasanpour, H.; Salehiniya, H. Incidence and mortality of uterine cancer and relationship with Human Development Index in the world. Cukurova Med. J. 2017, 42, 233–240. [Google Scholar] [CrossRef]
- Wingo, P.A.; Tong, T.; Bolden, S. Cancer statistics. CA Cancer J. Clin. 1995, 45, 8–30. [Google Scholar] [CrossRef]
- Shih, I.-M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Maeda, D.; Chen, X.; Guan, B.; Nakagawa, S.; Yano, T.; Taketani, Y.; Fukayama, M.; Wang, T.L.; Shih, I.-M. Rsf-1 (HBXAP) expression is associated with advanced stage and lymph node metastasis in ovarian clear cell carcinoma. Int. J. Gynecol. Pathol. 2011, 30, 30–35. [Google Scholar] [CrossRef]
- Park, J.T.; Chen, X.; Tropè, C.G.; Davidson, B.; Shih, I.-M.; Wang, T.L. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am. J. Pathol. 2010, 177, 1087–1094. [Google Scholar] [CrossRef]
- Maeda, D.; Shih, I.M. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv. Anat. Pathol. 2013, 20, 45–52. [Google Scholar] [CrossRef]
- Mandai, M.; Suzuki, A.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Hamanishi, J.; Yoshioka, Y.; Kosaka, k.; Konishiet, I. Clinical Management of Ovarian Endometriotic Cyst (Chocolate Cyst): Diagnosis, Medical Treatment, and Minimally Invasive Surgery. Curr. Obstet. Gynecol. Rep. 2012, 1, 16–24. [Google Scholar] [CrossRef]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular Mechanisms of Cancer Drug Resistance: Emerging Biomarkers and Promising Targets to Overcome Tumor Progression. Cancers 2022, 14, 1614. [Google Scholar] [CrossRef]
- De Marco, C.; Rinaldo, N.; Bruni, P.; Malzoni, C.; Zullo, F.; Fabiani, F.; Losito, S.; Scrima, M.; Marino, F.Z.; Franco, R.; et al. Multiple genetic alterations within the PI3K pathway are responsible for AKT activation in patients with ovarian carcinoma. PLoS ONE 2013, 8, e55362. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, N.; Jinawath, N.; Salani, R.; Kurman, R.J.; Shih, I.-M. Amplicon profiles in ovarian serous carcinomas. Int. J. Cancer 2007, 120, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Downs, G.S.; Jauhal, J.J.; Nandra, S.K.; Prive, G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6, R82. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.M.; Yap, K.L.; Davidson, B.; Tian, Y.; Murthy, V.; Wang, T.L.; Visvanathan, K.; Kuhajda, F.P.; Bristow, R.E.; Zhang, H.; et al. Expression of Fatty Acid Synthase Depends on NAC1 and Is Associated with Recurrent Ovarian Serous Carcinomas. J. Oncol. 2010, 2010, 285191. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, N.; Wang, T.L.; Shih, I.M. NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007, 67, 8058–8064. [Google Scholar] [CrossRef]
- Janiczek-Polewska, M.; Kolenda, T.; Poter, P.; Jagiełło, I.; Kozłowska-Masłoń, J.; Regulska, K.; Malicki, J.; Marszałek, A. Nucleus Accumbens Associated Protein 1 in Cancers-The Real Value. Int. J. Mol. Sci. 2024, 25, 13632. [Google Scholar] [CrossRef]
- Tang, X.H.; Li, H.; Zheng, X.S.; Lu, M.S.; An, Y.; Zhang, X.L. CRM197 reverses paclitaxel resistance by inhibiting the NAC-1/Gadd45 pathway in paclitaxel-resistant ovarian cancer cells. Cancer Med. 2019, 8, 6426–6436. [Google Scholar] [CrossRef]
- Jinawath, N.; Vasoontara, C.; Yap, K.L.; Thiaville, M.M.; Nakayama, K.; Wang, T.L.; Shih, I.-M. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene 2009, 28, 1941–1948. [Google Scholar] [CrossRef]
- Ishibashi, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. Expression of a BTB/POZ protein, NAC1, is essential for the proliferation of normal cyclic endometrial glandular cells and is up-regulated by estrogen. Clin. Cancer Res. 2009, 15, 804–811. [Google Scholar] [CrossRef]
- Ngule, C.M.; Hemati, H.; Ren, X.; Obaleye, O.; Akinyemi, A.O.; Oyelami, F.F.; Xiong, X.; Song, J.; Liu, X.; Yang, J.M. Identification of a NACC1-Regulated Gene Signature Implicated in the Features of Triple-Negative Breast Cancer. Biomedicines 2023, 11, 1223. [Google Scholar] [CrossRef]
- Nakayama, K.; Rahman, M.T.; Rahman, M.; Yeasmin, S.; Ishikawa, M.; Katagiri, A.; Iida, K.; Nakayama, N.; Miyazaki, K. Biological role and prognostic significance of NAC1 in ovarian cancer. Gynecol. Oncol. 2010, 119, 469–478. [Google Scholar] [CrossRef]
- Shih, I.-M.; Nakayama, K.; Wu, G.; Nakayama, N.; Zhang, J.; Wang, T.L. Amplification of the ch19p13.2 NACC1 locus in ovarian high-grade serous carcinoma. Mod. Pathol. 2011, 24, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Y.J.; Guo, L.C.; Ji, C.; Hu, J.; Zhang, H.H.; Xu, Q.H.; Zhu, W.D.; Ming, Z.J.; Yuan, Y.S.; et al. Nucleus accumbens-associated protein-1 promotes glycolysis and survival of hypoxic tumor cells via the HDAC4-HIF-1α axis. Oncogene 2017, 36, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, P.; Chen, Q.; Wu, P.; Yuan, H.; Ge, W.; Meng, L.; Huang, X.; Fu, Y.; Zhang, Y.; et al. FUS-induced circRHOBTB3 facilitates cell proliferation via miR-600/NACC1 mediated autophagy response in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 261. [Google Scholar] [CrossRef]
- Ishibashi, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Fukumoto, M.; Miyazaki, K. A BTB/POZ gene, NAC-1, a tumor recurrence-associated gene, as a potential target for Taxol resistance in ovarian cancer. Clin. Cancer Res. 2008, 14, 3149–3155. [Google Scholar] [CrossRef]
- Collins, T.; Stone, J.R.; Williams, A.J. All in the family: The BTB/POZ, KRAB, and SCAN domains. Mol. Cell Biol. 2001, 21, 3609–3615. [Google Scholar] [CrossRef]
- Stead, M.A.; Carr, S.B.; Wright, S.C. Structure of the human Nac1 POZ domain. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2009, 65, 445–449. [Google Scholar] [CrossRef]
- Stead, M.A.; Wright, S.C. Nac1 interacts with the POZ-domain transcription factor, Miz1. Biosci Rep. 2014, 34, e00110. [Google Scholar] [CrossRef]
- Stead, M.A.; Wright, S.C. Structures of heterodimeric POZ domains of Miz1/BCL6 and Miz1/NAC1. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 1591–1596. [Google Scholar] [CrossRef]
- Abhiman, S.; Iyer, L.M.; Aravind, L. BEN: A novel domain in chromatin factors and DNA viral proteins. Bioinformatics 2008, 24, 458–461. [Google Scholar] [CrossRef]
- Nakayama, N.; Sakashita, G.; Nagata, T.; Kobayashi, N.; Yoshida, H.; Park, S.Y.; Nariai, Y.; Kato, H.; Obayashi, E.; Nakayama, K.; et al. Nucleus Accumbens-Associated Protein 1 Binds DNA Directly through the BEN Domain in a Sequence-Specific Manner. Biomedicines 2020, 8, 608. [Google Scholar] [CrossRef]
- Moghrabi, N.N.; Naylor, S.L.; Van Veldhoven, P.P.; Baumgart, E.; Dawson, D.B.; Bennett, M.J. Assignment of the human peroxisomal branched-chain acyl-CoA oxidase gene to chromosome 3p21.1-p14.2 by rodent/human somatic cell hybridization. Biochem. Biophys. Res. Commun. 1997, 231, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhang, Z.; Tao, X.; Xu, S.; Zhang, X.; Zurashvili, T.; Lu, Z.; Bayascas, J.R.; Jin, L.; et al. ACOX2 is a regulator of lysine crotonylation that mediates hepatic metabolic homeostasis in mice. Cell Death Dis. 2022, 13, 279. [Google Scholar] [CrossRef]
- Vilarinho, S.; Sari, S.; Mazzacuva, F.; Bilgüvar, K.; Esendagli-Yilmaz, G.; Jain, D.; Akyol, G.; Dalgiç, B.; Günel, M.; Clayton, P.T.; et al. ACOX2 deficiency: A disorder of bile acid synthesis with transaminase elevation, liver fibrosis, ataxia, and cognitive impairment. Proc. Natl. Acad. Sci. USA 2016, 113, 11289–11293. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Sun, S.; Wang, K.; Qian, J.; Cui, Z.; Tao, T.; Zhou, J. ACOX2 is a prognostic marker and impedes the progression of hepatocellular carcinoma via PPARα pathway. Cell Death Dis. 2021, 12, 15. [Google Scholar] [CrossRef]
- Zhoua, X.; Wang, H. ACOX2 deficiency in primary malignant cardiac tumors. Proc. Natl. Acad. Sci. USA 2017, 114, E3590–E3591. [Google Scholar] [CrossRef]
- Tan, Z.; Deng, Y.; Cai, Z.; He, H.; Tang, Z.; Feng, Y.; Ye, J.; Liu, R.; Cai, S.; Huang, H.; et al. ACOX2 Serves as a Favorable Indicator Related to Lipid Metabolism and Oxidative Stress for Biochemical Recurrence in Prostate. J. Cancer 2024, 15, 3010–3023. [Google Scholar] [CrossRef]
- Chaudhry, S.; Thomas, S.N.; Simmons, G.E., Jr. Targeting lipid metabolism in the treatment of ovarian cancer. Oncotarget 2022, 13, 768–783. [Google Scholar] [CrossRef]
- The Human Protein Atlas. TCGA Dataset of ACOX2. Available online: https://v22.proteinatlas.org/ENSG00000168306-ACOX2/pathology (accessed on 6 April 2023).
- Wang, J.; Rao, S.; Chu, J.; Shen, X.; Levasseur, D.N.; Theunissen, T.W.; Orkin, S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature 2006, 444, 364–368. [Google Scholar] [CrossRef]
- Wang, X.; Ji, C.; Zhang, H.; Shan, Y.; Ren, Y.; Hu, Y.; Shi, L.; Guo, L.; Zhu, W.; Xia, Y.; et al. Identification of a small-molecule compound that inhibits homodimerization of oncogenic NAC1 protein and sensitizes cancer cells to anticancer agents. J. Biol. Chem. 2019, 294, 10006–10017. [Google Scholar] [CrossRef]
- Korutla, L.; Wang, P.; Jackson, T.G.; Mackler, S.A. NAC1, a POZ/BTB protein that functions as a corepressor. Neurochem. Int. 2009, 54, 245–252. [Google Scholar] [CrossRef]
- Bjørklund, S.S.; Kristensen, V.N.; Seiler, M.; Kumar, S.; Alnæs, G.I.; Ming, Y.; Kerrigan, J.; Naume, B.; Sachidanandam, R.; Bhanot, G.; et al. Expression of an estrogen-regulated variant transcript of the peroxisomal branched chain fatty acid oxidase ACOX2 in breast carcinomas. BMC Cancer 2015, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Y.; Zhu, L.; Zou, Y.; Kong, W.; Dong, B.; Huang, J.; Chen, Y.; Xue, W.; Huang, Y.; et al. High Expression of Stearoyl-CoA Desaturase 1 Predicts Poor Prognosis in Patients with Clear-Cell Renal Cell Carcinoma. PLoS ONE 2016, 11, e0166231. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, A.; Duguay, S.R.; Ouellette, R.J.; Surette, M.E. 17β-estradiol induces stearoyl-CoA desaturase-1 expression in estrogen receptor-positive breast cancer cells. BMC Cancer 2015, 15, 440. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.M.; Gonzalez-Angulo, A.M.; Chen, H.; Akcakanat, A.; Do, K.A.; Fraser Symmans, W.; Pusztai, L.; Hortobagyi, G.N.; Mills, G.B.; Meric-Bernstam, F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res. Treat. 2013, 137, 319–327. [Google Scholar] [CrossRef]
- Roongta, U.V.; Pabalan, J.G.; Wang, X.; Ryseck, R.P.; Fargnoli, J.; Henley, B.J.; Yang, W.P.; Zhu, J.; Madireddi, M.T.; Lawrence, R.M.; et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol. Cancer Res. 2011, 9, 1551–1561. [Google Scholar] [CrossRef]
- Guaita-Esteruelas, S.; Bosquet, A.; Saavedra, P.; Gumà, J.; Girona, J.; Lam, E.W.; Amillano, K.; Borràs, J.; Masana, L. Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol. Carcinog. 2017, 56, 208–217. [Google Scholar] [CrossRef]
- Lee, D.; Wada, K.; Taniguchi, Y.; Al-Shareef, H.; Masuda, T.; Usami, Y.; Aikawa, T.; Okura, M.; Kamisaki, Y.; Kogo, M. Expression of fatty acid binding protein 4 is involved in the cell growth of oral squamous cell carcinoma. Oncol. Rep. 2014, 31, 1116–1120. [Google Scholar] [CrossRef]
- Rahman, M.T.; Nakayama, K.; Rahman, M.; Katagiri, H.; Katagiri, A.; Ishibashi, T.; Ishikawa, M.; Iida, K.; Nakayama, N.; Otsuki, Y.; et al. Fatty acid synthase expression associated with NAC1 is a potential therapeutic target in ovarian clear cell carcinomas. Br. J. Cancer 2012, 107, 300–307. [Google Scholar] [CrossRef]
- Che, L.; Pilo, M.G.; Cigliano, A.; Latte, G.; Simile, M.M.; Ribback, S.; Dombrowski, F.; Evert, M.; Chen, X.; Calvisi, D.F. Oncogene dependent requirement of fatty acid synthase in hepatocellular carcinoma. Cell Cycle 2017, 16, 499–507. [Google Scholar] [CrossRef]
- Sui, J.S.Y.; Martin, P.; Keogh, A.; Murchan, P.; Ryan, L.; Nicholson, S.; Cuffe, S.; Broin, P.Ó.; Finn, S.P.; Fitzmaurice, G.J.; et al. Altered expression of ACOX2 in non-small cell lung cancer. BMC Pulm. Med. 2022, 22, 321. [Google Scholar] [CrossRef]
- Okazaki, K.; Nakayama, N.; Nariai, Y.; Nakayama, K.; Miyazaki, K.; Maruyama, R.; Kato, H.; Kosugi, S.; Urano, T.; Sakashita, G. Nuclear localization signal in a cancer-related transcriptional regulator protein NAC1. Carcinogenesis 2012, 33, 1854–1862. [Google Scholar] [CrossRef]
- Chen, F.; Yin, Y.; Yan, Z.; Cao, K.; Zhong, K. NAC1 promotes the migration of prostate cancer cells and participates in osteoclastogenesis by negatively regulating IFNβ. Oncol. Lett. 2018, 15, 2921–2928. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Maruyama, R.; Urano, T.; Nakayama, N.; Kawabata, Y.; Yano, S.; Yoshida, M.; Nakayama, K.; Miyazaki, K.; Takenaga, K.; et al. Low expression of nucleus accumbens associated protein 1 predicts poor prognosis for patients with pancreatic ductal adenocarcinoma. Pathol. Int. 2012, 62, 802–810. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonia, S.B.; Nakayama, K.; Razia, S.; Nakayama, N.; Ishikawa, M.; Yamashita, H.; Kanno, K.; Takeshita, H.; Zahan, U.F.; Sohel, H.I.; et al. NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms. Int. J. Mol. Sci. 2025, 26, 4938. https://doi.org/10.3390/ijms26104938
Sonia SB, Nakayama K, Razia S, Nakayama N, Ishikawa M, Yamashita H, Kanno K, Takeshita H, Zahan UF, Sohel HI, et al. NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms. International Journal of Molecular Sciences. 2025; 26(10):4938. https://doi.org/10.3390/ijms26104938
Chicago/Turabian StyleSonia, Shahataj Begum, Kentaro Nakayama, Sultana Razia, Naomi Nakayama, Masako Ishikawa, Hitomi Yamashita, Kosuke Kanno, Haruo Takeshita, Umme Farzana Zahan, Hasibul Islam Sohel, and et al. 2025. "NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms" International Journal of Molecular Sciences 26, no. 10: 4938. https://doi.org/10.3390/ijms26104938
APA StyleSonia, S. B., Nakayama, K., Razia, S., Nakayama, N., Ishikawa, M., Yamashita, H., Kanno, K., Takeshita, H., Zahan, U. F., Sohel, H. I., & Kyo, S. (2025). NAC1/ACOX2 Axis as a Novel Therapeutic Target for Endometriosis-Related Ovarian Neoplasms. International Journal of Molecular Sciences, 26(10), 4938. https://doi.org/10.3390/ijms26104938









