The Role of Oligodendrocytes in Neurodegenerative Diseases: Unwrapping the Layers
Abstract
1. Introduction
2. Myelin: From Its Discovery to Its Dynamic Role in Nervous Transmission and Plasticity
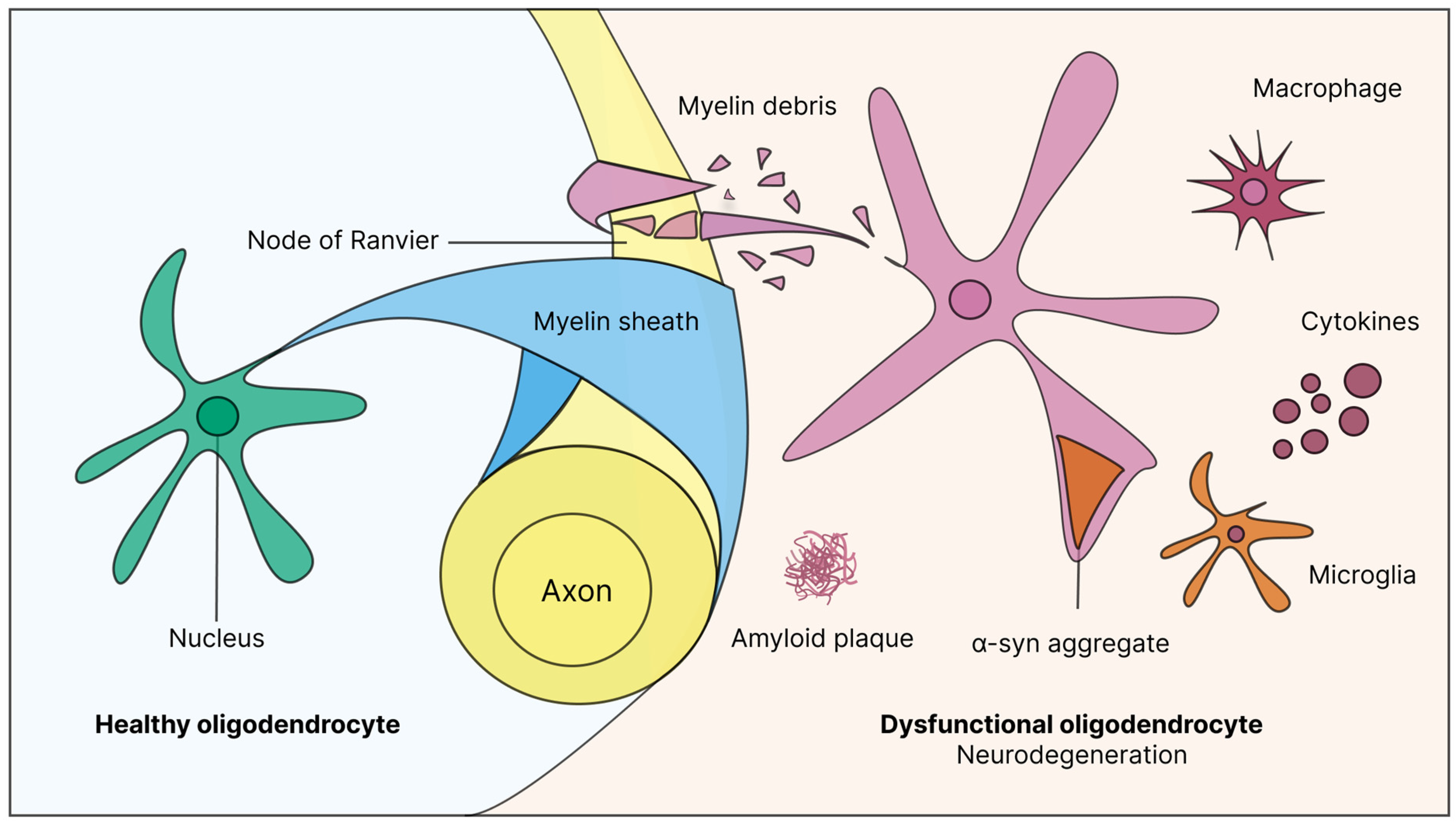
3. Guardians of Neural Integrity: The Critical Role of Oligodendrocytes from Normal Function to Neuroinflammation
4. When Protection Fails: Exploring Disorders of Myelin Loss and Dysfunction
5. Beyond Neurons: The Impact of Myelin Dysfunction in Other Neurodegenerative Disorders
6. Therapeutic Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2016 Dementia Collaborators. Global, Regional, and National Burden of Alzheimer’s Disease and Other Dementias, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Odiyoor, M.M.; Robinson, C.A.; Jaydeokar, S. Mental Health and Public Health: Improving Mental Health and Well-Being of Communities in the Modern World: A Pragmatic Approach Using the Global Mental Health Assessment Tool/PC. World Soc. Psychiatry 2023, 5, 161–165. [Google Scholar] [CrossRef]
- Jutten, R.J.; Harrison, J.; de Jong, F.J.; Aleman, A.; Ritchie, C.W.; Scheltens, P.; Sikkes, S.A.M. A Composite Measure of Cognitive and Functional Progression in Alzheimer’s Disease: Design of the Capturing Changes in Cognition Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 130–138. [Google Scholar] [CrossRef]
- Nandi, A.; Counts, N.; Bröker, J.; Malik, S.; Chen, S.; Han, R.; Klusty, J.; Seligman, B.; Tortorice, D.; Vigo, D.; et al. Cost of Care for Alzheimer’s Disease and Related Dementias in the United States: 2016 to 2060. NPJ Aging 2024, 10, 13. [Google Scholar] [CrossRef]
- Virchow, R. As Based upon Physiological and Pathological Histology. Nutr. Rev. 2009, 47, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Craik, E.M. Myelos: Matters of Life and Death. In Acta Classica Supplementum: Proceedings of the Classical Association of South Africa II, 2008; Mary Martin: Singapore, 2008. [Google Scholar]
- Boullerne, A.I. The History of Myelin. Exp. Neurol. 2016, 283, 431–445. [Google Scholar] [CrossRef]
- Boullerne, A.I.; Feinstein, D.L. History of Neuroscience I. Pío Del Río-Hortega (1882–1945): The Discoverer of Microglia and Oligodendroglia. ASN Neuro 2020, 12, 175909142095325. [Google Scholar] [CrossRef]
- Leblanc, R. Penfield, Focal Microgyria, and Epilepsy. J. Neurosurg. 2021, 136, 553–560. [Google Scholar] [CrossRef]
- Bunge, R.P. Glial Cells and the Central Myelin Sheath. Physiol. Rev. 1968, 48, 197–251. [Google Scholar] [CrossRef]
- Kister, A.; Kister, I. Overview of Myelin, Major Myelin Lipids, and Myelin-Associated Proteins. Front. Chem. 2023, 10, 1041961. [Google Scholar] [CrossRef]
- Rasband, M.N.; Peles, E. Mechanisms of Node of Ranvier Assembly. Nat. Rev. Neurosci. 2021, 22, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, G.; Belin, D.; Káradóttir, R.T. Myelin: A Gatekeeper of Activity-Dependent Circuit Plasticity? Science 2021, 374, eaba6905. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative Stress and Impaired Oligodendrocyte Precursor Cell Differentiation in Neurological Disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Parrilla, G.E.; Gupta, V.; Wall, R.V.; Salkar, A.; Basavarajappa, D.; Mirzaei, M.; Chitranshi, N.; Graham, S.L.; You, Y. The Role of Myelin in Neurodegeneration: Implications for Drug Targets and Neuroprotection Strategies. Rev. Neurosci. 2024, 35, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Tabira, T.; Kira, J.-I. Strain and Species Differences of Encephalitogenic Determinants of Myelin Basic Protein and Proteolipid Apoprotein. In Myelin; Routledge: Boca Raton, FL, USA, 2023; pp. 783–799. [Google Scholar]
- Kim, D.; An, H.; Fan, C.; Park, Y. Identifying Oligodendrocyte Enhancers Governing Plp1 Expression. Hum. Mol. Genet. 2021, 30, 2225–2239. [Google Scholar] [CrossRef]
- Cloake, N.; Yan, J.; Aminian, A.; Pender, M.; Greer, J. PLP1 Mutations in Patients with Multiple Sclerosis: Identification of a New Mutation and Potential Pathogenicity of the Mutations. J. Clin. Med. 2018, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Pelizaeus-Merzbacher Disease: Molecular and Cellular Pathologies and Associated Phenotypes. In Myelin; Springer: Singapore, 2019; pp. 201–216. [Google Scholar]
- Khalaf, G.; Mattern, C.; Begou, M.; Boespflug-Tanguy, O.; Massaad, C.; Massaad-Massade, L. Mutation of Proteolipid Protein 1 Gene: From Severe Hypomyelinating Leukodystrophy to Inherited Spastic Paraplegia. Biomedicines 2022, 10, 1709. [Google Scholar] [CrossRef]
- Bonetto, G.; Kamen, Y.; Evans, K.A.; Káradóttir, R.T. Unraveling Myelin Plasticity. Front. Cell. Neurosci. 2020, 14, 156. [Google Scholar] [CrossRef]
- Iyer, M.; Kantarci, H.; Cooper, M.H.; Ambiel, N.; Novak, S.W.; Andrade, L.R.; Lam, M.; Jones, G.; Münch, A.E.; Yu, X.; et al. Oligodendrocyte Calcium Signaling Promotes Actin-Dependent Myelin Sheath Extension. Nat. Commun. 2024, 15, 265. [Google Scholar] [CrossRef]
- Galvez-Contreras, A.Y.; Zarate-Lopez, D.; Torres-Chavez, A.L.; Gonzalez-Perez, O. Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder. Brain Sci. 2020, 10, 951. [Google Scholar] [CrossRef] [PubMed]
- Sherafat, A.; Pfeiffer, F.; Reiss, A.M.; Wood, W.M.; Nishiyama, A. Microglial Neuropilin-1 Promotes Oligodendrocyte Expansion during Development and Remyelination by Trans-Activating Platelet-Derived Growth Factor Receptor. Nat. Commun. 2021, 12, 2265. [Google Scholar] [CrossRef] [PubMed]
- Furusho, M.; Dupree, J.L.; Nave, K.-A.; Bansal, R. Fibroblast Growth Factor Receptor Signaling in Oligodendrocytes Regulates Myelin Sheath Thickness. J. Neurosci. 2012, 32, 6631–6641. [Google Scholar] [CrossRef]
- Thornton, M.A.; Hughes, E.G. Neuron-Oligodendroglia Interactions: Activity-Dependent Regulation of Cellular Signaling. Neurosci. Lett. 2020, 727, 134916. [Google Scholar] [CrossRef]
- Stampanoni Bassi, M.; Iezzi, E.; Marfia, G.A.; Simonelli, I.; Musella, A.; Mandolesi, G.; Fresegna, D.; Pasqualetti, P.; Furlan, R.; Finardi, A.; et al. Platelet-Derived Growth Factor Predicts Prolonged Relapse-Free Period in Multiple Sclerosis. J. Neuroinflamm. 2018, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Böttiger, G.; Stadelmann, C.; Karnati, S.; Berghoff, M. FGF/FGFR Pathways in Multiple Sclerosis and in Its Disease Models. Cells 2021, 10, 884. [Google Scholar] [CrossRef]
- Falls, D. Neuregulins: Functions, Forms, and Signaling Strategies. Exp. Cell Res. 2003, 284, 14–30. [Google Scholar] [CrossRef]
- Michailov, G.V.; Sereda, M.W.; Brinkmann, B.G.; Fischer, T.M.; Haug, B.; Birchmeier, C.; Role, L.; Lai, C.; Schwab, M.H.; Nave, K.-A. Axonal Neuregulin-1 Regulates Myelin Sheath Thickness. Science 2004, 304, 700–703. [Google Scholar] [CrossRef]
- Kataria, H.; Alizadeh, A.; Karimi-Abdolrezaee, S. Neuregulin-1/ErbB Network: An Emerging Modulator of Nervous System Injury and Repair. Prog. Neurobiol. 2019, 180, 101643. [Google Scholar] [CrossRef]
- Nishiyama, A.; Shimizu, T.; Sherafat, A.; Richardson, W.D. Life-Long Oligodendrocyte Development and Plasticity. Semin. Cell Dev. Biol. 2021, 116, 25–37. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Dong, C.; Wang, J.; Xu, L.; Qiu, Y.; Weng, Q.; Zhao, C.; Xin, M.; Lu, Q.R. EED-Mediated Histone Methylation Is Critical for CNS Myelination and Remyelination by Inhibiting WNT, BMP, and Senescence Pathways. Sci. Adv. 2020, 6, eaaz6477. [Google Scholar] [CrossRef]
- Grotheer, M.; Rosenke, M.; Wu, H.; Kular, H.; Querdasi, F.R.; Natu, V.S.; Yeatman, J.D.; Grill-Spector, K. White Matter Myelination during Early Infancy Is Linked to Spatial Gradients and Myelin Content at Birth. Nat. Commun. 2022, 13, 997. [Google Scholar] [CrossRef]
- Hornig, J.; Fröb, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The Transcription Factors Sox10 and Myrf Define an Essential Regulatory Network Module in Differentiating Oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef] [PubMed]
- Sock, E.; Wegner, M. Using the Lineage Determinants Olig2 and Sox10 to Explore Transcriptional Regulation of Oligode drocyte Development. Dev. Neurobiol. 2021, 81, 892–901. [Google Scholar] [CrossRef]
- Kinney, H.C.; Volpe, J.J. Myelination Events. In Volpe’s Neurology of the Newborn; Elsevier: Amsterdam, The Netherlands, 2018; pp. 176–188. [Google Scholar]
- Tomasevic, L.; Siebner, H.R.; Thielscher, A.; Manganelli, F.; Pontillo, G.; Dubbioso, R. Relationship between High-Frequency Activity in the Cortical Sensory and the Motor Hand Areas, and Their Myelin Content. Brain Stimul. 2022, 15, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.M.S.; Brennan, E.J.; Brock, R.; Cocas, L.A. Neuron to Oligodendrocyte Precursor Cell Synapses: Protagonists in Oligodendrocyte Development and Myelination, and Targets for Therapeutics. Front. Neurosci. 2022, 15, 779125. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Miliaras, D.; Kesidou, E.; Boziki, M.; Petratos, S.; Grigoriadis, N.; Theotokis, P. Developmental Cues and Molecular Drivers in Myelinogenesis: Revisiting Early Life to Re-Evaluate the Integrity of CNS Myelin. Curr. Issues Mol. Biol. 2022, 44, 3208–3237. [Google Scholar] [CrossRef]
- James, O.G.; Selvaraj, B.T.; Magnani, D.; Burr, K.; Connick, P.; Barton, S.K.; Vasistha, N.A.; Hampton, D.W.; Story, D.; Smigiel, R.; et al. IPSC-Derived Myelinoids to Study Myelin Biology of Humans. Dev. Cell 2021, 56, 1346–1358.e6. [Google Scholar] [CrossRef]
- de Faria, O.; Pivonkova, H.; Varga, B.; Timmler, S.; Evans, K.A.; Káradóttir, R.T. Periods of Synchronized Myelin Changes Shape Brain Function and Plasticity. Nat. Neurosci. 2021, 24, 1508–1521. [Google Scholar] [CrossRef]
- Yang, S.M.; Michel, K.; Jokhi, V.; Nedivi, E.; Arlotta, P. Neuron Class–Specific Responses Govern Adaptive Myelin Remodeling in the Neocortex. Science 2020, 370, eabd2109. [Google Scholar] [CrossRef]
- Chapman, T.W.; Hill, R.A. Myelin Plasticity in Adulthood and Aging. Neurosci. Lett. 2020, 715, 134645. [Google Scholar] [CrossRef]
- Chopra, S.; Shaw, M.; Shaw, T.; Sachdev, P.S.; Anstey, K.J.; Cherbuin, N. More Highly Myelinated White Matter Tracts Are Associated with Faster Processing Speed in Healthy Adults. Neuroimage 2018, 171, 332–340. [Google Scholar] [CrossRef]
- Xin, W.; Chan, J.R. Myelin Plasticity: Sculpting Circuits in Learning and Memory. Nat. Rev. Neurosci. 2020, 21, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Azzarito, M.; Emmenegger, T.M.; Ziegler, G.; Huber, E.; Grabher, P.; Callaghan, M.F.; Thompson, A.; Friston, K.; Weiskopf, N.; Killeen, T.; et al. Coherent, Time-Shifted Patterns of Microstructural Plasticity during Motor-Skill Learning. Neuroimage 2023, 274, 120128. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, P.G.; Chen, H.; Wang, D.-Y. Neuroregeneration and Plasticity: A Review of the Physiological Mechanisms for Achieving Functional Recovery Postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS Myelin—From Mechanisms to Experimental Medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Forbes, T.A.; Goldstein, E.Z.; Dupree, J.L.; Jablonska, B.; Scafidi, J.; Adams, K.L.; Imamura, Y.; Hashimoto-Torii, K.; Gallo, V. Environmental Enrichment Ameliorates Perinatal Brain Injury and Promotes Functional White Matter Recovery. Nat. Commun. 2020, 11, 964. [Google Scholar] [CrossRef]
- Krucoff, M.O.; Rahimpour, S.; Slutzky, M.W.; Edgerton, V.R.; Turner, D.A. Enhancing Nervous System Recovery through Neurobiologics, Neural Interface Training, and Neurorehabilitation. Front. Neurosci. 2016, 10, 584. [Google Scholar] [CrossRef]
- Dombrowski, Y.; O’Hagan, T.; Dittmer, M.; Penalva, R.; Mayoral, S.R.; Bankhead, P.; Fleville, S.; Eleftheriadis, G.; Zhao, C.; Naughton, M.; et al. Regulatory T Cells Promote Myelin Regeneration in the Central Nervous System. Nat. Neurosci. 2017, 20, 674–680. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Holtman, I.R.; Krueger, M.; Yogev, N.; Bruttger, J.; Khorooshi, R.; Benmamar-Badel, A.; de Boer-Bergsma, J.J.; Martin, N.A.; Karram, K.; et al. A Novel Microglial Subset Plays a Key Role in Myelinogenesis in Developing Brain. EMBO J. 2017, 36, 3292–3308. [Google Scholar] [CrossRef]
- Fletcher, J.; Murray, S.; Xiao, J. Brain-Derived Neurotrophic Factor in Central Nervous System Myelination: A New Mechanism to Promote Myelin Plasticity and Repair. Int. J. Mol. Sci. 2018, 19, 4131. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Hossain, M.J. Oligodendrocyte-Specific Mechanisms of Myelin Thinning: Implications for Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 663053. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Bodini, B.; Goldman, S.A. Remyelination in the Central Nervous System. Cold Spring Harb. Perspect. Biol. 2024, 16, a041371. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jordan, J.D.; Zhang, Q. Myelin Pathology in Alzheimer’s Disease: Potential Therapeutic Opportunities. Aging Dis. 2024, 15, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Windener, F.; Grewing, L.; Thomas, C.; Dorion, M.-F.; Otteken, M.; Kular, L.; Jagodic, M.; Antel, J.; Albrecht, S.; Kuhlmann, T. Physiological Aging and Inflammation-Induced Cellular Senescence May Contribute to Oligodendroglial Dysfunction in MS. Acta Neuropathol. 2024, 147, 82. [Google Scholar] [CrossRef]
- Hill, R.A.; Nishiyama, A.; Hughes, E.G. Features, Fates, and Functions of Oligodendrocyte Precursor Cells. Cold Spring Harb. Perspect. Biol. 2024, 16, a041425. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Radcliff, A.B. Inherited and Acquired Disorders of Myelin: The Underlying Myelin Pathology. Exp. Neurol. 2016, 283, 452–475. [Google Scholar] [CrossRef]
- Podbielska, M.; Banik, N.; Kurowska, E.; Hogan, E. Myelin Recovery in Multiple Sclerosis: The Challenge of Remyelination. Brain Sci. 2013, 3, 1282–1324. [Google Scholar] [CrossRef]
- Wolswijk, G.; Balesar, R. Changes in the Expression and Localization of the Paranodal Protein Caspr on Axons in Chronic Multiple Sclerosis. Brain 2003, 126, 1638–1649. [Google Scholar] [CrossRef]
- Coman, I.; Aigrot, M.S.; Seilhean, D.; Reynolds, R.; Girault, J.A.; Zalc, B.; Lubetzki, C. Nodal, Paranodal and Juxtaparanodal Axonal Proteins during Demyelination and Remyelination in Multiple Sclerosis. Brain 2006, 129, 3186–3195. [Google Scholar] [CrossRef]
- Howell, O.W.; Palser, A.; Polito, A.; Melrose, S.; Zonta, B.; Scheiermann, C.; Vora, A.J.; Brophy, P.J.; Reynolds, R. Disruption of Neurofascin Localization Reveals Early Changes Preceding Demyelination and Remyelination in Multiple Sclerosis. Brain 2006, 129, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Çolakoğlu, G.; Bergstrom-Tyrberg, U.; Berglund, E.O.; Ranscht, B. Contactin-1 Regulates Myelination and Nodal/Paranodal Domain Organization in the Central Nervous System. Proc. Natl. Acad. Sci. USA 2014, 111, E394–E403. [Google Scholar] [CrossRef]
- Kira, J.; Yamasaki, R.; Ogata, H. Anti-Neurofascin Autoantibody and Demyelination. Neurochem. Int. 2019, 130, 104360. [Google Scholar] [CrossRef]
- Tamberi, L.; Belloni, A.; Pugnaloni, A.; Rippo, M.R.; Olivieri, F.; Procopio, A.D.; Bronte, G. The Influence of Myeloid-Derived Suppressor Cell Expansion in Neuroinflammation and Neurodegenerative Diseases. Cells 2024, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, M. Microbiome-Glia Crosstalk: Bridging the Communication Divide in the Central Nervous System. Neuroglia 2024, 5, 89–104. [Google Scholar] [CrossRef]
- Zhang, C.; Qiu, M.; Fu, H. Oligodendrocytes in Central Nervous System Diseases: The Effect of Cytokine Regulation. Neural Regen. Res. 2024, 19, 2132–2143. [Google Scholar] [CrossRef]
- Wang, J.; Zhen, Y.; Yang, J.; Yang, S.; Zhu, G. Recognizing Alzheimer’s Disease from Perspective of Oligodendrocytes: Phenomena or Pathogenesis? CNS Neurosci. Ther. 2024, 30, e14688. [Google Scholar] [CrossRef]
- Kulaszyńska, M.; Kwiatkowski, S.; Skonieczna-Żydecka, K. The Iron Metabolism with a Specific Focus on the Functioning of the Nervous System. Biomedicines 2024, 12, 595. [Google Scholar] [CrossRef]
- Lee, S.; Kovacs, G.G. The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 4269. [Google Scholar] [CrossRef]
- Kalafatakis, I.; Karagogeos, D. Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair. Biomolecules 2021, 11, 1058. [Google Scholar] [CrossRef]
- Lubetzki, C.; Stankoff, B. Demyelination in Multiple Sclerosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 89–99. [Google Scholar]
- Ghorbani, S.; Yong, V.W. The Extracellular Matrix as Modifier of Neuroinflammation and Remyelination in Multiple Sclerosis. Brain 2021, 144, 1958–1973. [Google Scholar] [CrossRef]
- Dulamea, A.O. Role of Oligodendrocyte Dysfunction in Demyelination, Remyelination and Neurodegeneration in Multiple Sclerosis. Adv. Exp. Med. Biol. 2017, 958, 91–127. [Google Scholar] [PubMed]
- Egg, R.; Reindl, M.; Deisenhammer, F.; Linington, C.; Berger, T. Anti-MOG and Anti-MBP Antibody Subclasses in Multiple Sclerosis. Mult. Scler. J. 2001, 7, 285–289. [Google Scholar] [CrossRef]
- Patsopoulos, N.A. Genetics of Multiple Sclerosis: An Overview and New Directions. Cold Spring Harb. Perspect. Med. 2018, 8, a028951. [Google Scholar] [CrossRef] [PubMed]
- Buhelt, S.; Laigaard, H.-M.; von Essen, M.R.; Ullum, H.; Oturai, A.; Sellebjerg, F.; Søndergaard, H.B. IL2RA Methylation and Gene Expression in Relation to the Multiple Sclerosis-Associated Gene Variant Rs2104286 and Soluble IL-2Rα in CD8+ T Cells. Front. Immunol. 2021, 12, 676141. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Radha, A.; Kesika, P.; Chaiyasut, C. Neuromyelitis Optica Spectrum Disorders: Clinical Perspectives, Molecular Mechanisms, and Treatments. Appl. Sci. 2023, 13, 5029. [Google Scholar] [CrossRef]
- Lassmann, H. Pathology of Inflammatory Diseases of the Nervous System: Human Disease versus Animal Models. Glia 2020, 68, 830–844. [Google Scholar] [CrossRef]
- Williamson, E.M.L.; Berger, J.R. Progressive Multifocal Leukoencephalopathy. In Clinical Neurovirology; CRC Press: Boca Raton, FL, USA, 2020; pp. 109–139. [Google Scholar]
- Kokubun, N. Charcot–Marie–Tooth Disease and Neuroinflammation. Clin. Exp. Neuroimmunol. 2020, 11, 109–116. [Google Scholar] [CrossRef]
- Wildner, P.; Stasiołek, M.; Matysiak, M. Differential Diagnosis of Multiple Sclerosis and Other Inflammatory CNS Diseases. Mult. Scler. Relat. Disord. 2020, 37, 101452. [Google Scholar] [CrossRef]
- Fridman, V.; Saporta, M.A. Mechanisms and Treatments in Demyelinating CMT. Neurotherapeutics 2021, 18, 2236–2268. [Google Scholar] [CrossRef]
- Narine, M.; Colognato, H. Current Insights into Oligodendrocyte Metabolism and Its Power to Sculpt the Myelin Landscape. Front. Cell. Neurosci. 2022, 16, 892968. [Google Scholar] [CrossRef]
- Roy, D.; Tedeschi, A. The Role of Lipids, Lipid Metabolism and Ectopic Lipid Accumulation in Axon Growth, Regeneration and Repair after CNS Injury and Disease. Cells 2021, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Naggar, A.; Laasri, K.; Kabila, B.; Izi, Z.; Allali, N.; El Haddad, S.; Chat, L. Myelin Insults Differentials on MRI in Children: In the Light of an ADEM Case. Radiol. Case Rep. 2024, 19, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, M.S.; Naidu, S.; Pouwels, P.J.W.; Bonavita, S.; van Coster, R.; Lagae, L.; Sperner, J.; Surtees, R.; Schiffmann, R.; Valk, J. New Syndrome Characterized by Hypomyelination with Atrophy of the Basal Ganglia and Cerebellum. Am. J. Neuroradiol. 2002, 23, 1466–1474. [Google Scholar] [PubMed]
- Cachón-González, M.-B.; Wang, S.Z.; Ziegler, R.; Cheng, S.H.; Cox, T.M. Reversibility of Neuropathology in Tay–Sachs-Related Diseases. Hum. Mol. Genet. 2014, 23, 730–748. [Google Scholar] [CrossRef]
- Woodward, K.J. The Molecular and Cellular Defects Underlying Pelizaeus–Merzbacher Disease. Expert Rev. Mol. Med. 2008, 10, e14. [Google Scholar] [CrossRef]
- Gruenenfelder, F.I.; McLaughlin, M.; Griffiths, I.R.; Garbern, J.; Thomson, G.; Kuzman, P.; Barrie, J.A.; McCulloch, M.; Penderis, J.; Stassart, R.; et al. Neural Stem Cells Restore Myelin in a Demyelinating Model of Pelizaeus-Merzbacher Disease. Brain 2020, 143, 1383–1399. [Google Scholar] [CrossRef]
- Groh, J.; Friedman, H.C.; Orel, N.; Ip, C.W.; Fischer, S.; Spahn, I.; Schäffner, E.; Hörner, M.; Stadler, D.; Buttmann, M.; et al. Pathogenic Inflammation in the CNS of Mice Carrying Human PLP1 Mutations. Hum. Mol. Genet. 2016, 25, 4686–4702. [Google Scholar] [CrossRef][Green Version]
- Weidenheim, K.M.; Dickson, D.W.; Rapin, I. Neuropathology of Cockayne Syndrome: Evidence for Impaired Development, Premature Aging, and Neurodegeneration. Mech. Ageing Dev. 2009, 130, 619–636. [Google Scholar] [CrossRef]
- Adachi, M.; Kawanami, T.; Ohshima, F.; Hosoya, T. MR Findings of Cerebral White Matter in Cockayne Syndrome. Magn. Reson. Med. Sci. 2006, 5, 41–45. [Google Scholar] [CrossRef][Green Version]
- Koob, M.; Laugel, V.; Durand, M.; Fothergill, H.; Dalloz, C.; Sauvanaud, F.; Dollfus, H.; Namer, I.J.; Dietemann, J.-L. Neuroimaging in Cockayne Syndrome. Am. J. Neuroradiol. 2010, 31, 1623–1630. [Google Scholar] [CrossRef]
- Toro, C.; Zainab, M.; Tifft, C.J. The GM2 Gangliosidoses: Unlocking the Mysteries of Pathogenesis and Treatment. Neurosci. Lett. 2021, 764, 136195. [Google Scholar] [CrossRef]
- Han, S.; Gim, Y.; Jang, E.-H.; Hur, E.-M. Functions and Dysfunctions of Oligodendrocytes in Neurodegenerative Diseases. Front. Cell. Neurosci. 2022, 16, 1083159. [Google Scholar] [CrossRef] [PubMed]
- Maitre, M.; Jeltsch-David, H.; Okechukwu, N.G.; Klein, C.; Patte-Mensah, C.; Mensah-Nyagan, A.-G. Myelin in Alzheimer’s Disease: Culprit or Bystander? Acta Neuropathol. Commun. 2023, 11, 56. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Huang, N.; Xiao, L.; Mei, F. Oligodendrocytes and Myelin: Active Players in Neurodegenerative Brains? Dev. Neurobiol. 2022, 82, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Nasrabady, S.E.; Rizvi, B.; Goldman, J.E.; Brickman, A.M. White Matter Changes in Alzheimer’s Disease: A Focus on Myelin and Oligodendrocytes. Acta Neuropathol. Commun. 2018, 6, 22. [Google Scholar] [CrossRef]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin Dysfunction Drives Amyloid-β Deposition in Models of Alzheimer’s Disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Fontana, I.C.; Zimmer, A.R.; Rocha, A.S.; Gosmann, G.; Souza, D.O.; Lourenco, M.V.; Ferreira, S.T.; Zimmer, E.R. Amyloid-β Oligomers in Cellular Models of Alzheimer’s Disease. J. Neurochem. 2020, 155, 348–369. [Google Scholar] [CrossRef]
- Cheng, G.W.-Y.; Mok, K.K.-S.; Yeung, S.H.-S.; Kofler, J.; Herrup, K.; Tse, K.-H. Apolipoprotein E Ε4 Mediates Myelin Breakdown by Targeting Oligodendrocytes in Sporadic Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2022, 81, 717–730. [Google Scholar] [CrossRef]
- Zou, P.; Wu, C.; Liu, T.C.-Y.; Duan, R.; Yang, L. Oligodendrocyte Progenitor Cells in Alzheimer’s Disease: From Physiology to Pathology. Transl. Neurodegener. 2023, 12, 52. [Google Scholar] [CrossRef]
- Depp, C.M.; Nave, K.; Lab, K.N. Ageing-associated Myelin Dysfunction Drives Amyloid Deposition in Mouse Models of Alzheimer’s Disease. Alzheimer’s Dement. 2022, 18, e061183. [Google Scholar] [CrossRef]
- Ferris, J.K.; Greeley, B.; Vavasour, I.M.; Kraeutner, S.N.; Rinat, S.; Ramirez, J.; Black, S.E.; Boyd, L.A. In Vivo Myelin Imaging and Tissue Microstructure in White Matter Hyperintensities and Perilesional White Matter. Brain Commun. 2022, 4, fcac142. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wang, K.; Theriault, L.; Charbel, E.; Initiative, A.D.N. White Matter Integrity and Key Structures Affected in Alzheimer’s Disease Characterized by Diffusion Tensor Imaging. Eur. J. Neurosci. 2022, 56, 5319–5331. [Google Scholar] [CrossRef]
- Lorenzini, L.; Fernandez, M.; Baldassarro, V.A.; Bighinati, A.; Giuliani, A.; Calzà, L.; Giardino, L. White Matter and Neuroprotection in Alzheimer’s Dementia. Molecules 2020, 25, 503. [Google Scholar] [CrossRef]
- Narasimhan, S.; Changolkar, L.; Riddle, D.M.; Kats, A.; Stieber, A.; Weitzman, S.A.; Zhang, B.; Li, Z.; Roberson, E.D.; Trojanowski, J.Q.; et al. Human Tau Pathology Transmits Glial Tau Aggregates in the Absence of Neuronal Tau. J. Exp. Med. 2020, 217, e20190783. [Google Scholar] [CrossRef]
- Didonna, A. Tau at the Interface between Neurodegeneration and Neuroinflammation. Genes Immun. 2020, 21, 288–300. [Google Scholar] [CrossRef]
- Hirschfeld, L.R.; Risacher, S.L.; Nho, K.; Saykin, A.J. Myelin Repair in Alzheimer’s Disease: A Review of Biological Pathways and Potential Therapeutics. Transl. Neurodegener. 2022, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, R.; Nisa Awan, M.U.; Bai, J. The Mechanism and Function of Glia in Parkinson’s Disease. Front. Cell. Neurosci. 2022, 16, 903469. [Google Scholar] [CrossRef]
- Mavroeidi, P.; Xilouri, M. Neurons and Glia Interplay in α-Synucleinopathies. Int. J. Mol. Sci. 2021, 22, 4994. [Google Scholar] [CrossRef]
- Kaji, S.; Maki, T.; Ishimoto, T.; Yamakado, H.; Takahashi, R. Insights into the Pathogenesis of Multiple System Atrophy: Focus on Glial Cytoplasmic Inclusions. Transl. Neurodegener. 2020, 9, 7. [Google Scholar] [CrossRef]
- Jeon, Y.-M.; Kwon, Y.; Jo, M.; Lee, S.; Kim, S.; Kim, H.-J. The Role of Glial Mitochondria in α-Synuclein Toxicity. Front. Cell Dev. Biol. 2020, 8, 548283. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Long, J.; Li, W.; Wang, X.; Hu, N.; Zhao, X.; Sun, T. White Matter Changes in Parkinson’s Disease. npj Park. Dis. 2023, 9, 150. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, L.; Li, H.; Hsiao, J.-H.T.; Li, B.; Tanglay, O.; Auwyang, A.D.; Wang, E.; Feng, J.; Kim, W.S.; et al. Adaptive Structural Changes in the Motor Cortex and White Matter in Parkinson’s Disease. Acta Neuropathol. 2022, 144, 861–879. [Google Scholar] [CrossRef]
- Clayton, B.L.L.; Tesar, P.J. Oligodendrocyte Progenitor Cell Fate and Function in Development and Disease. Curr. Opin. Cell Biol. 2021, 73, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Bishop, D.P. Mercury Is Present in Neurons and Oligodendrocytes in Regions of the Brain Affected by Parkinson’s Disease and Co-Localises with Lewy Bodies. PLoS ONE 2022, 17, e0262464. [Google Scholar] [CrossRef]
- Çınar, E.; Tel, B.C.; Şahin, G. Neuroinflammation in Parkinson’s Disease and Its Treatment Opportunities. Balk. Med. J. 2022, 39, 318–333. [Google Scholar] [CrossRef]
- Traiffort, E.; Morisset-Lopez, S.; Moussaed, M.; Zahaf, A. Defective Oligodendroglial Lineage and Demyelination in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2021, 22, 3426. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Ba, L.; Zhang, M. Dysfunction of the Oligodendrocytes in Amyotrophic Lateral Sclerosis. J. Biomed. Res. 2022, 36, 336–342. [Google Scholar] [CrossRef]
- Lorente Pons, A.; Higginbottom, A.; Cooper-Knock, J.; Alrafiah, A.; Alofi, E.; Kirby, J.; Shaw, P.J.; Wood, J.D.; Highley, J.R. Oligodendrocyte Pathology Exceeds Axonal Pathology in White Matter in Human Amyotrophic Lateral Sclerosis. J. Pathol. 2020, 251, 262–271. [Google Scholar] [CrossRef]
- Raffaele, S.; Boccazzi, M.; Fumagalli, M. Oligodendrocyte Dysfunction in Amyotrophic Lateral Sclerosis: Mechanisms and Therapeutic Perspectives. Cells 2021, 10, 565. [Google Scholar] [CrossRef]
- Nave, K.-A.; Asadollahi, E.; Sasmita, A. Expanding the Function of Oligodendrocytes to Brain Energy Metabolism. Curr. Opin. Neurobiol. 2023, 83, 102782. [Google Scholar] [CrossRef] [PubMed]
- Ettle, B.; Schlachetzki, J.C.M.; Winkler, J. Oligodendroglia and Myelin in Neurodegenerative Diseases: More Than Just Bystanders? Mol. Neurobiol. 2016, 53, 3046–3062. [Google Scholar] [CrossRef]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Cipollina, G.; Davari Serej, A.; Di Nolfi, G.; Gazzano, A.; Marsala, A.; Spatafora, M.G.; Peviani, M. Heterogeneity of Neuroinflammatory Responses in Amyotrophic Lateral Sclerosis: A Challenge or an Opportunity? Int. J. Mol. Sci. 2020, 21, 7923. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A. The Role of Glial Pathology in Huntington’s Disease. In Huntington’s Disease; Elsevier: Amsterdam, The Netherlands, 2024; pp. 337–351. [Google Scholar]
- Hedreen, J.C.; Berretta, S.; White, C.L. Postmortem Neuropathology in Early Huntington Disease. J. Neuropathol. Exp. Neurol. 2024, 83, 294–306. [Google Scholar] [CrossRef]
- Ferrari Bardile, C.; Sidik, H.; Quek, R.; Yusof, N.A.B.M.; Garcia-Miralles, M.; Pouladi, M.A. Abnormal Spinal Cord Myelination Due to Oligodendrocyte Dysfunction in a Model of Huntington’s Disease. J. Huntington’s Dis. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Casella, C.; Lipp, I.; Rosser, A.; Jones, D.K.; Metzler-Baddeley, C. A Critical Review of White Matter Changes in Huntington’s Disease. Mov. Disord. 2020, 35, 1302–1311. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, H.; Yang, T.; Liu, L.; Li, X.-J.; Li, S. Insights into White Matter Defect in Huntington’s Disease. Cells 2022, 11, 3381. [Google Scholar] [CrossRef]
- Castonguay, C.-E.; Aboasali, F.; Becret, T.; Medeiros, M.; Rochefort, D.; Rajput, A.; Dion, P.; Rouleau, G. Cerebellar Oligodendrocytes as Key Initial Players in Essential Tremor Pathophysiology. J. Neurol. Sci. 2023, 455, 121142. [Google Scholar] [CrossRef]
- Robinson, A.C.; Bajaj, N.; Hadjivassiliou, M.; Minshull, J.; Mahmood, A.; Roncaroli, F. Neuropathology of a Case of Fragile X-associated Tremor Ataxia Syndrome without Tremor. Neuropathology 2020, 40, 611–619. [Google Scholar] [CrossRef]
- Marangon, D.; Boccazzi, M.; Lecca, D.; Fumagalli, M. Regulation of Oligodendrocyte Functions: Targeting Lipid Metabolism and Extracellular Matrix for Myelin Repair. J. Clin. Med. 2020, 9, 470. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Niu, J.; Hoi, K.K.; Zhao, C.; Caganap, S.D.; Henry, R.G.; Dao, D.Q.; Zollinger, D.R.; Mei, F.; Shen, Y.-A.A.; et al. Clemastine Rescues Myelination Defects and Promotes Functional Recovery in Hypoxic Brain Injury. Brain 2018, 141, 85–98. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, A.-V.; Van Doninck, E.; Popescu, V.; Willem, L.; Cambron, M.; Laureys, G.; D’Haeseleer, M.; Bjerke, M.; Roelant, E.; Lemmerling, M.; et al. A Metformin Add-on Clinical Study in Multiple Sclerosis to Evaluate Brain Remyelination and Neurodegeneration (MACSiMiSE-BRAIN): Study Protocol for a Multi-Center Randomized Placebo Controlled Clinical Trial. Front. Immunol. 2024, 15, 1362629. [Google Scholar] [CrossRef]
- Cui, Q.-L.; Lin, Y.H.; Xu, Y.K.T.; Fernandes, M.G.F.; Rao, V.T.S.; Kennedy, T.E.; Antel, J. Effects of Biotin on Survival, Ensheathment, and ATP Production by Oligodendrocyte Lineage Cells In Vitro. PLoS ONE 2020, 15, e0233859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, C.; Frah, M.; Deng, Y.; Marie, C.; Zhang, F.; Xu, L.; Ma, Z.; Dong, X.; Lin, Y.; et al. Dual Requirement of CHD8 for Chromatin Landscape Establishment and Histone Methyltransferase Recruitment to Promote CNS Myelination and Repair. Dev. Cell 2018, 45, 753–768.e8. [Google Scholar] [CrossRef]
- Yu, Y.; Casaccia, P.; Lu, Q.R. Shaping the Oligodendrocyte Identity by Epigenetic Control. Epigenetics 2010, 5, 124–128. [Google Scholar] [CrossRef]
- Samudyata; Castelo-Branco, G.; Liu, J. Epigenetic Regulation of Oligodendrocyte Differentiation: From Development to Demyelinating Disorders. Glia 2020, 68, 1619–1630. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced Pluripotent Stem Cells (IPSCs): Molecular Mechanisms of Induction and Applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef]
- Zeldich, E.; Rajkumar, S. Identity and Maturity of IPSC-Derived Oligodendrocytes in 2D and Organoid Systems. Cells 2024, 13, 674. [Google Scholar] [CrossRef] [PubMed]
| Disease | Main Symptoms | Oligodendrocyte Dysfunction | Potential Therapeutic Targets |
|---|---|---|---|
| Alzheimer’s Disease (AD) | Cognitive decline, memory loss | Myelin breakdown, oligodendrocyte loss, impaired OPC differentiation | Enhance remyelination, target tau hyperphosphorylation, support OPC viability |
| Parkinson’s Disease (PD) | Motor dysfunction, cognitive impairment | Demyelination, α-synuclein accumulation, mitochondrial stress in oligodendrocytes | Reduce α-synuclein burden, enhance oxidative stress resistance |
| Amyotrophic Lateral Sclerosis/Motor Neuron Disease (ALS/MND) | Progressive muscle weakness, paralysis | Metabolic support failure, glutamate excitotoxicity, impaired OPC maturation | Support oligodendrocyte survival, regulate glutamate metabolism, promote OPC differentiation |
| Multiple Sclerosis (MS) | Motor, sensory, and cognitive deficits | Immune-mediated demyelination, OPC maturation arrest, chronic inflammation | Stimulate remyelination, modulate neuroinflammation, promote OPC maturation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokulic Panichi, L.; Stanca, S.; Dolciotti, C.; Bongioanni, P. The Role of Oligodendrocytes in Neurodegenerative Diseases: Unwrapping the Layers. Int. J. Mol. Sci. 2025, 26, 4623. https://doi.org/10.3390/ijms26104623
Bokulic Panichi L, Stanca S, Dolciotti C, Bongioanni P. The Role of Oligodendrocytes in Neurodegenerative Diseases: Unwrapping the Layers. International Journal of Molecular Sciences. 2025; 26(10):4623. https://doi.org/10.3390/ijms26104623
Chicago/Turabian StyleBokulic Panichi, Leona, Stefano Stanca, Cristina Dolciotti, and Paolo Bongioanni. 2025. "The Role of Oligodendrocytes in Neurodegenerative Diseases: Unwrapping the Layers" International Journal of Molecular Sciences 26, no. 10: 4623. https://doi.org/10.3390/ijms26104623
APA StyleBokulic Panichi, L., Stanca, S., Dolciotti, C., & Bongioanni, P. (2025). The Role of Oligodendrocytes in Neurodegenerative Diseases: Unwrapping the Layers. International Journal of Molecular Sciences, 26(10), 4623. https://doi.org/10.3390/ijms26104623








