Higher Yield of Common Buckwheat (Fagopyrum esculentum Moench) as a Result of Seed Treatment with Gamma Radiation
Abstract
1. Introduction
2. Results
2.1. Seed Germination and Leaf Area
2.2. Biometric Parameters
2.2.1. M0 Generation
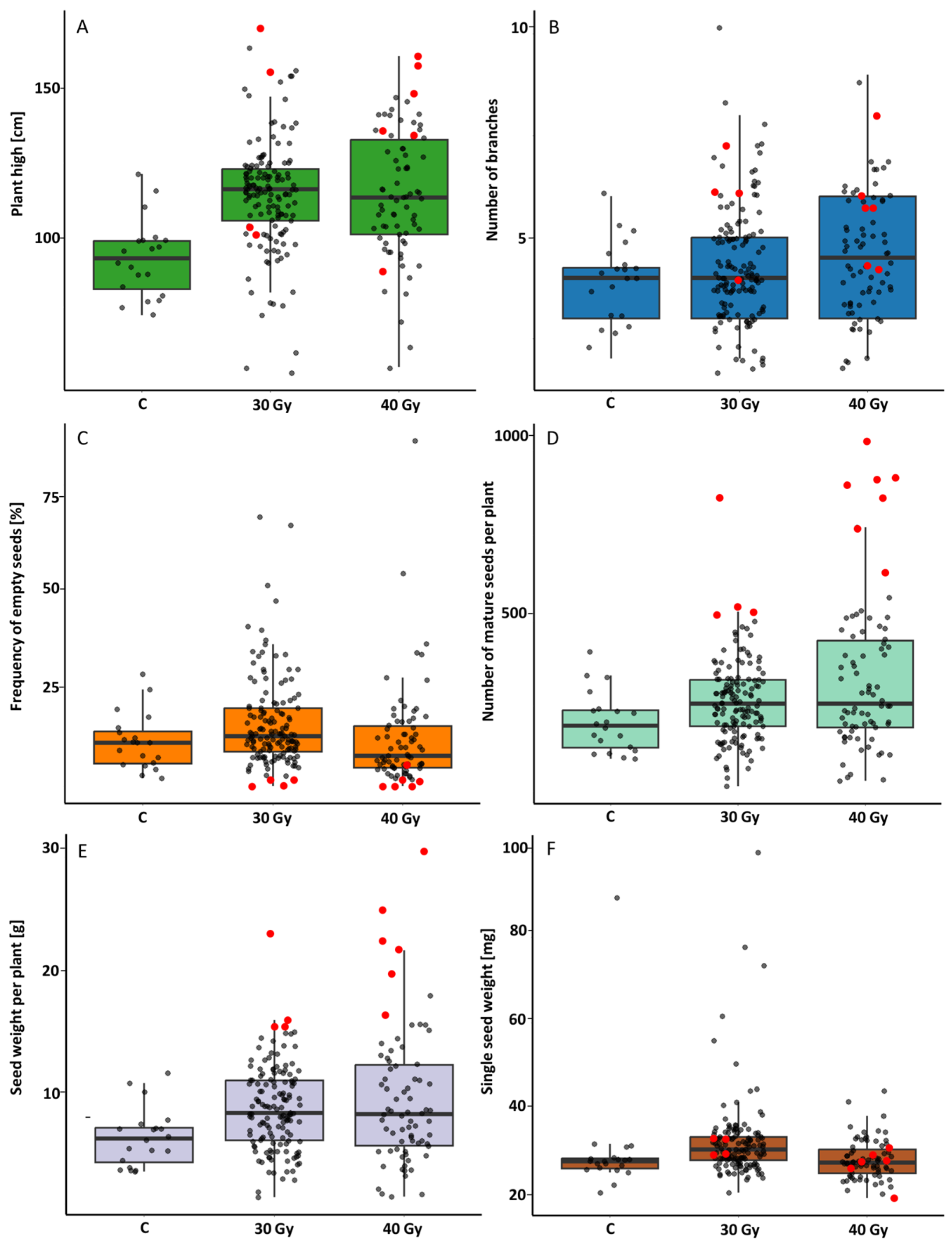
2.2.2. M1 Generation
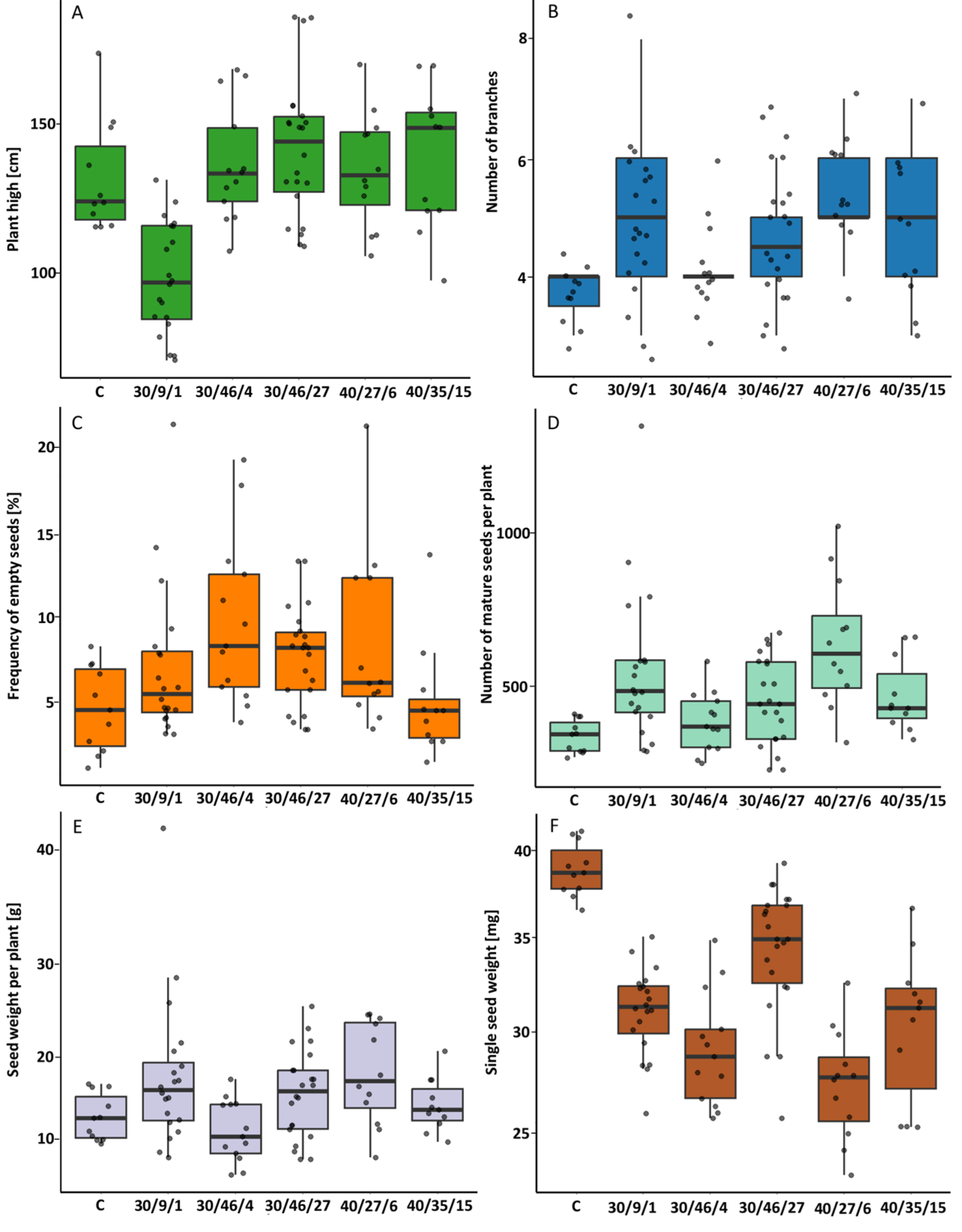
2.2.3. M2 Generation
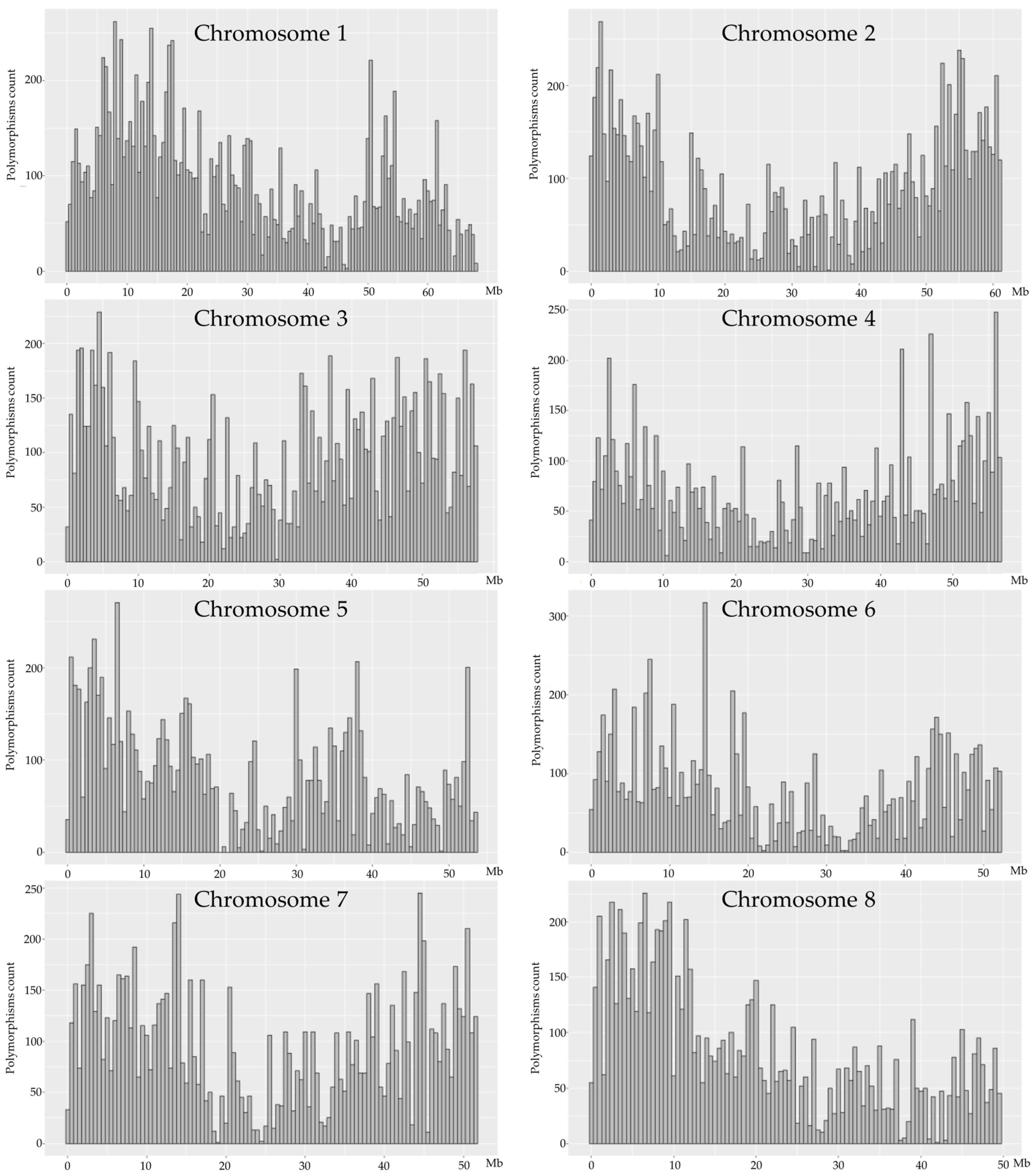
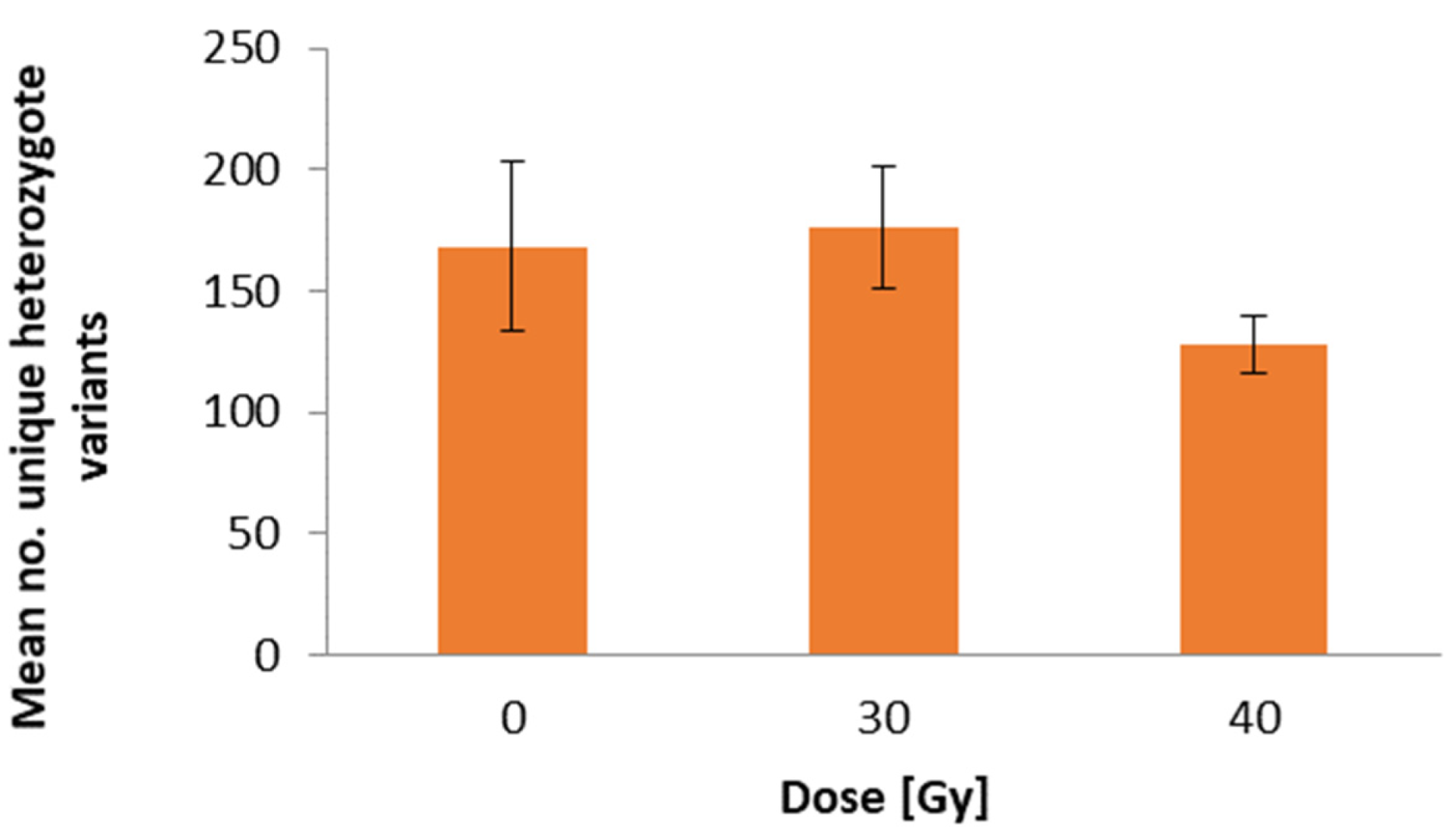
2.3. DNA Sequence Variation
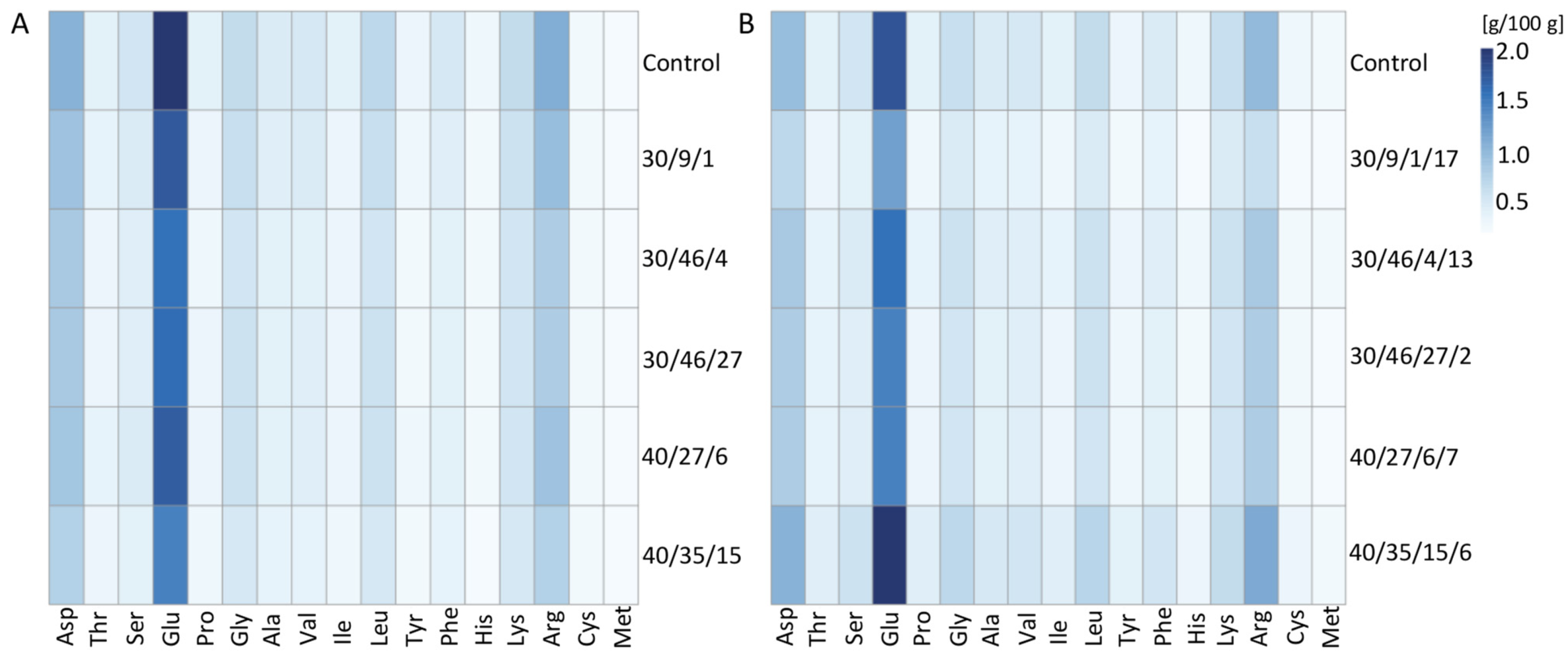
2.4. Amino Acid Content
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Study Design
4.3. Seed Irradiation
4.4. Seed Germination and Plant Cultivation
4.5. Biometric Analyses of M0 Generation
4.6. Biometric Analyses of M1 and M2 Plants
4.7. NGS-Based Genotyping
4.8. Amino Acids Assay in the Seeds
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Asp | asparagine |
| Thr | threonine |
| Ser | serine |
| Glu | glutamine |
| Pro | proline |
| Gly | glycine |
| Ala | alanine |
| Val | valine |
| Ile | isoleucine |
| Leu | leucine |
| Tyr | tyrosine |
| Phe | phenylalanine |
| His | histidine |
| Lys | lysine |
| Arg | arginine |
| Cys | cysteine |
| Met | methionine |
| nGBS | normalized genotyping-by-sequencing |
| Q | Phred quality score measure of the quality of the nucleotide identification generated by automated DNA sequencing |
References
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food allergy and intolerance: A narrative review on nutritional concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Khairuddin, M.A.N.; Lasekan, O. Gluten-free cereal products and beverages: A review of their health benefits in the last five years. Foods 2021, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
- Christa, K.; Soral-Śmietana, M. Buckwheat grains and buckwheat products—Nutritional and prophylactic value of their components—A review. Czech J. Food Sci. 2008, 26, 153–162. [Google Scholar] [CrossRef]
- Aubert, L.; Konrádová, D.; Kebbas, S.; Barris, S.; Quinet, M. Comparison of high temperature resistance in two buckwheat species Fagopyrum esculentum and Fagopyrum tataricum. J. Plant Physiol. 2020, 251, 153222. [Google Scholar] [CrossRef]
- Domingos, I.; Bilsborrow, P.E. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Agron. 2022, 126, 126264. [Google Scholar] [CrossRef]
- Podolska, G.; Gujska, E.; Klepacka, J.; Aleksandrowicz, E. Bioactive compounds in different buckwheat species. Plants 2021, 10, 961. [Google Scholar] [CrossRef]
- Katar, D.; Olgun, M.; Turan, M. Analysis of morphological and biochemical characteristics of buckwheat (Fagopyrum esculentum Moench) in comparison with cereals. CyTA-J. Food 2016, 14, 176–185. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrazova, Z. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Vojtíšková, P.; Kmentová, K.; Kubáň, V.; Kráčmar, S. Chemical composition of buckwheat plant (Fagopyrum esculentum) and selected buckwheat products. J. Microbiol. Biot. Food Sci. 2012, 1, 1011–1019. Available online: https://www.jmbfs.org/wp-content/uploads/2012/01/Vojtiskova.pdf (accessed on 1 February 2025).
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Nešovic, M.; Gašic, U.; Tosti, T.; Horvacki, N.; Šikoparija, B.; Nedic, N.; Blagojevic, S.; Ignjatovic, L.; Tešic, Ž. Polyphenol profile of buckwheat honey, nectar and pollen: Polyphenolics in buckwheat. R. Soc. Open Sci. 2020, 7, 201576. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Rehman, R.U.; Pirzadah, T.B.; Malik, B.; Dar, F.A.; Tahir, I. Cultivation, agronomic practices, and growth performance of buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 299–319. [Google Scholar] [CrossRef]
- Farkas, Á.; Zajácz, E.; Spring, E.; Pasture, B.E.E.; Summer, E. Nectar production for the Hungarian honey industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. [Google Scholar]
- Cawoy, V.; Kinet, J.M.; Jacquemart, A.L. Morphology of nectaries and biology of nectar production in the distylous species Fagopyrum esculentum. Ann. Bot. 2008, 102, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Jacquemart, A.; Cawoy, V.; Kinet, J.M.; Ledent, J.F.; Quinet, M. Is buckwheat (Fagopyrum esculentum Moench) still a valuable crop to day? Eur. J. Plant Sci. Biotechnol. 2012, 6, 1–10. [Google Scholar]
- Słomka, A.; Michno, K.; Dubert, F.; Dziurka, M.; Kopeć, P.; Płażek, A. Embryological background of low seed set in distylous common buckwheat (Fagopyrum esculentum Moench) with biased morph ratios, and stimulant- induced improvement of it. Crop Pasture Sci. 2017, 68, 680–690. [Google Scholar] [CrossRef]
- Morishita, T.; Mukasa, Y.; Suzuki, T.; Shimizu, A.; Yamaguchi, H.; Degi, K.; Aii, J.; Hase, Y.; Shikazono, N.; Tanaka, A.; et al. Characteristics and inheritance of the semidwarf mutants of Tartary buckwheat (Fagopyrum tataricum Gaertn.) induced by gamma ray and ion beam irradiation. Breed. Res. 2010, 12, 39–43. [Google Scholar] [CrossRef]
- Cawoy, V.; Ledent, J.F.; Kinet, J.M.; Jacquemart, A.L. Floral biology of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Plant Sci. Biotechnol. 2009, 3, 1–9. [Google Scholar]
- Inoue, N.; Hagiwara, M. Analysis of the yielding process based on the module concept in common buckwheat. Fagopyrum 1999, 16, 73–77. [Google Scholar]
- Hornyák, M.; Słomka, A.; Sychta, K.; Dziurka, M.; Kopeć, P.; Pastuszak, J.; Szczerba, A.; Płażek, A. Reducing flower competition for assimilates by half results in higher yield of Fagopyrum esculentum. Int. J. Mol. Sci. 2020, 21, 8953. [Google Scholar] [CrossRef]
- Mittal, H.K.; Gautam, A.S.; Panwar, K.S.; Jungpo, B. Synergistic effects of combined treatments of gamma-irradiation and ethyl methane sulphonate in buckwheat (Fagopyrum tataricum). Ind. J. Agric. Res. 2000, 34, 191–193. [Google Scholar]
- Micke, A.; Donini, B.; Maluszynski, M. Induced Mutations for Crop Improvement-A Review; Aalam Al-Zarra; Joint FAO/IAEA Div. of Nuclear Techniques in Food and Agriculture: Vienna, Austria, 1990; pp. 67–91. [Google Scholar]
- Mathur, A. Mutation studies in buckwheat (Fagopyrum) III. Effect of gamma rays on growth parameters and yield attributes. Fagopyrum 1990, 11, 27–34. [Google Scholar]
- Kurowska, M.; Labocha-Pawłowska, A.; Gnizda, D.; Maluszynski, M.; Szarejko, I. Molecular analysis of point mutations in a barley genome exposed to MNU and gamma rays. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2012, 738, 52–70. [Google Scholar] [CrossRef]
- Gregori, M.; Kreft, I. Breakable starch granules in a low-amylose buckwheat (Fagopyrum esculentum Moench) mutant. J. Food Agric. Environ. 2012, 10, 258–262. [Google Scholar]
- Mathur, A. Mutation studies in buckwheat (Fagopyrum) I. Effect of gamma rays on germination. Fagopyrum 1989, 9, 10–14. [Google Scholar]
- Jia, C.F.; Li, A.L. Effect of gamma radiation on mutant induction of Fagopyrum dibotrys Hara. Photosynthetica 2008, 46, 363–369. [Google Scholar] [CrossRef]
- Tang, Y.; Bin, Z.X.; Gang, Z. Improvement of tartary buckwheat by induced mutations with 60Co gamma rays. In Mutations, In Vitro and Molecular Techniques for Environmentally Sustainable Crop Improvement; Maluszynski, M., Kasha, K.J., Eds.; Springer-Science + Business Media B.V.: Berlin, Germany, 2002; pp. 183–188. [Google Scholar]
- Oh, M.A.; Park, J.E.; Kim, J.Y.; Kang, H.M.; Min Oh, S.S.; Mansoor, S.; Chung, Y.S. Seed traits inheritance in Fagopyrum esculentum Moench. based on image analysis method. Front. Plant Sci. 2024, 15, 1445348. [Google Scholar] [CrossRef]
- Ahmad, J.J.S.; Javid, W.; Bhat, S.A.; Tahir, I. Assessment on induced genetic variability and divergence in the mutagenised Tartary buckwheat populations developed using gamma rays and EMS mutagenesis. Ecol. Gen. Genom. 2023, 28, 100177. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Madgwick, P.J.; Bayon, C.; Tearall, K.; Hernandez-Lopez, A.; Baudo, M.; Rakszegi, M.; Hamada, W.; Al-Yassin, A.; Ouabbou, H.; et al. Mutation discovery for crop improvement. J. Exp. Bot. 2009, 60, 2817–2825. [Google Scholar] [CrossRef]
- Baguma, J.K.; Mukasa, S.B.; Nuwamanya, E.; Alicai, T.; Omongo, C.A.; Ochwo-Ssemakula, M.; Ozimati, A.; Esuma, W.; Kanaabi, M.; Wembabazi, E.; et al. Identification of genomic regions for traits associated with flowering in cassava (Manihot esculenta Crantz). Plants 2024, 13, 796. [Google Scholar] [CrossRef]
- Wang, L.; Sheng, M. Chromosomal location of 45S and 5S rDNA in the strains of Fagopyrum esculentum Moench and F. tataricum (L.) Gaertn. Cytologia 2022, 87, 265–270. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Mohamed Ahmed, I.A.; Yagoub, A.E.A.; Babiker, E.E. Effects of radiation process on total protein and amino acids composition of raw and processed pearl millet flour during storage. Int. J. Food Sci. Technol. 2010, 45, 906–912. [Google Scholar] [CrossRef]
- Hooshmand, H.; Klopfenstein, C.F. Effects of gamma irradiation on mycotoxin disappearance and amino acid contents of corn, wheat, and soybeans with different moisture contents. Plant Foods Human Nutr. 1995, 47, 227–238. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Univ. Calif. Agric. Exp. Station Circ. 1938, 347, 29–32. [Google Scholar]
- Arvidsson, S.; Fartmann, B.; Winkler, S.; Zimmermann, W. Efficient High-Throughput SNP Discovery and Genotyping Using Normalised Genotyping-by-Sequencing (nGBS); Technical note AN-161104.01; LGC Limited: Teddington, UK, 2016. [Google Scholar]
- Durbin, R.; Li, H. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Rimmer, A.; Phan, H.; Mathieson, I.; Iqbal, Z.; Twigg, S.R.F.; Wilkie, A.O.M.; McVean, G.; Lunter, G. Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat. Genet. 2014, 46, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Chromatography of amino acids on sulfonated polystyrene resins. J. Biol. Chem. 1951, 92, 663–681. [Google Scholar] [CrossRef]
- Davidson, I. Hydrolysis of samples for Amino Acid Analysis. In Protein Sequencing Protocols, 2nd ed.; Methods in Molecular Biology; Smith, B.J., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2003; Volume 211. [Google Scholar]
- Smith, A.J. Post Column Amino Acid Analysis. In Protein Sequencing Protocols, 2nd ed.; Methods in Molecular Biology; Smith, B.J., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2003; Volume 211. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 1 February 2025).
- Kuhn, M. Building predictive models in R using the caret package. J. Statist. Soft. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://rpkgs.datanovia.com/rstatix (accessed on 1 February 2025).

| Treatment | Leaf Area [cm2] |
|---|---|
| Control | 65.9 ± 3.2 b |
| 30 Gy | 71.8 ± 4.7 ab |
| 40 Gy | 55.6 ± 2.7 c |
| Amino Acid | Control | 30/9/1 | 30/46/4 | 30/46/27 | 40/27/6 | 40/35/15 | LSD |
|---|---|---|---|---|---|---|---|
| Asp | 9.98 | 9.92 | 9.91 | 9.86 | 9.90 | 9.78 | 0.01 |
| Thr | 3.96 | 4.04 | 4.00 | 4.05 | 3.96 | 3.99 | 0.03 |
| Ser | 5.28 | 5.24 | 5.19 | 5.14 | 5.24 | 5.19 | 0.02 |
| Glu | 19.17 | 18.68 | 18.56 | 18.49 | 19.20 | 18.66 | 0.04 |
| Pro | 3.82 | 3.73 | 3.64 | 3.76 | 3.76 | 3.68 | 0.03 |
| Gly | 6.27 | 6.52 | 6.62 | 6.66 | 6.41 | 6.56 | 0.04 |
| Ala | 4.57 | 4.66 | 4.74 | 4.78 | 4.56 | 4.64 | 0.05 |
| Val | 5.02 | 4.95 | 4.98 | 5.06 | 4.89 | 4.82 | 0.01 |
| Ile | 3.75 | 3.70 | 3.74 | 3.80 | 3.65 | 3.62 | 0.02 |
| Leu | 6.63 | 6.59 | 6.61 | 6.68 | 6.50 | 6.55 | 0.01 |
| Tyr | 3.03 | 3.10 | 3.17 | 3.19 | 3.12 | 3.17 | 0.01 |
| Phe | 4.77 | 4.67 | 4.66 | 4.71 | 4.63 | 4.58 | 0.02 |
| His | 2.75 | 2.89 | 2.87 | 2.87 | 2.76 | 2.82 | 0.01 |
| Lys | 6.11 | 6.34 | 6.38 | 6.32 | 6.19 | 6.35 | 0.02 |
| Arg | 10.26 | 10.08 | 9.44 | 9.23 | 10.19 | 9.83 | 0.03 |
| Cys | 2.53 | 2.70 | 2.86 | 2.92 | 2.71 | 3.08 | 0.04 |
| Met | 2.11 | 2.19 | 2.64 | 2.48 | 2.34 | 2.69 | 0.04 |
| Total amount | 10.46 | 9.25 | 8.37 | 8.53 | 8.86 | 8.53 | 0.03 |
| Amino Acid | Control | 30/9/1/17 | 30/46/4/13 | 30/46/27/2 | 40/27/6/7 | 40/35/15/6 | LSD |
|---|---|---|---|---|---|---|---|
| Asp | 9.98 | 9.89 | 9.84 | 9.75 | 9.80 | 9.77 | 0.01 |
| Thr | 3.96 | 4.26 | 3.97 | 3.99 | 4.04 | 3.85 | 0.02 |
| Ser | 5.28 | 5.18 | 5.03 | 5.10 | 5.15 | 5.16 | 0.02 |
| Glu | 19.17 | 18.16 | 18.92 | 18.39 | 18.70 | 19.48 | 0.03 |
| Pro | 3.82 | 3.71 | 3.77 | 3.69 | 3.75 | 3.71 | 0.01 |
| Gly | 6.27 | 6.60 | 6.63 | 6.56 | 6.64 | 6.28 | 0.01 |
| Ala | 4.57 | 4.97 | 4.61 | 4.70 | 4.68 | 4.39 | 0.02 |
| Val | 5.02 | 5.09 | 4.92 | 4.96 | 4.93 | 4.90 | 0.02 |
| Ile | 3.75 | 3.82 | 3.71 | 3.76 | 3.72 | 3.66 | 0.01 |
| Leu | 6.63 | 6.84 | 6.60 | 6.66 | 6.68 | 6.56 | 0.03 |
| Tyr | 3.03 | 3.28 | 3.24 | 3.15 | 3.22 | 3.27 | 0.01 |
| Phe | 4.77 | 4.64 | 4.64 | 4.67 | 4.66 | 4.93 | 0.01 |
| His | 2.75 | 2.85 | 2.93 | 2.77 | 2.85 | 2.65 | 0.02 |
| Lys | 6.11 | 6.62 | 6.50 | 6.33 | 6.33 | 6.11 | 0.02 |
| Arg | 10.26 | 9.15 | 9.58 | 9.51 | 9.83 | 10.47 | 0.03 |
| Cys | 2.53 | 2.64 | 2.67 | 2.65 | 2.65 | 2.65 | 0.01 |
| Met | 2.11 | 2.30 | 2.43 | 2.21 | 2.33 | 2.14 | 0.01 |
| Total amount | 10.46 | 7.35 | 9.26 | 8.53 | 8.72 | 11.67 | 0.03 |
| Year | Generation | Treatment/No of Accessions | ||
|---|---|---|---|---|
| 2021 | M0 | Control | 30 Gy | 40 Gy |
| 2022 | M1 | Control | 30/46 30/131 30/9 30/33 | 40/11 40/18 40/20 40/23 40/27 40/35 |
| 2023 | M2 | Control | 30/9/1 30/46/4 30/46/27 | 40/27/6 40/35/15 |
| Year | Generation | Treatment/No of Accessions | ||
|---|---|---|---|---|
| Control | 30 Gy | 40 Gy | ||
| 2023 | M2 | 30/9/1 30/46/4 30/40/27 | 40/27/6 40/27/1 40/35/15 | |
| 2024 | M3 | Control | 30/9/1/17 30/46/4/13 30/46/27/2 | 40/27//6/7 40/35/15/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płażek, A.; Kopeć, P.; Mickowska, B.; Szklarczyk, M.; Wesołowski, W.; Szczerba, A.; Hornyák, M.; Biesaga, B.; Kabat, D. Higher Yield of Common Buckwheat (Fagopyrum esculentum Moench) as a Result of Seed Treatment with Gamma Radiation. Int. J. Mol. Sci. 2025, 26, 4587. https://doi.org/10.3390/ijms26104587
Płażek A, Kopeć P, Mickowska B, Szklarczyk M, Wesołowski W, Szczerba A, Hornyák M, Biesaga B, Kabat D. Higher Yield of Common Buckwheat (Fagopyrum esculentum Moench) as a Result of Seed Treatment with Gamma Radiation. International Journal of Molecular Sciences. 2025; 26(10):4587. https://doi.org/10.3390/ijms26104587
Chicago/Turabian StylePłażek, Agnieszka, Przemysław Kopeć, Barbara Mickowska, Marek Szklarczyk, Wojciech Wesołowski, Anna Szczerba, Marta Hornyák, Beata Biesaga, and Damian Kabat. 2025. "Higher Yield of Common Buckwheat (Fagopyrum esculentum Moench) as a Result of Seed Treatment with Gamma Radiation" International Journal of Molecular Sciences 26, no. 10: 4587. https://doi.org/10.3390/ijms26104587
APA StylePłażek, A., Kopeć, P., Mickowska, B., Szklarczyk, M., Wesołowski, W., Szczerba, A., Hornyák, M., Biesaga, B., & Kabat, D. (2025). Higher Yield of Common Buckwheat (Fagopyrum esculentum Moench) as a Result of Seed Treatment with Gamma Radiation. International Journal of Molecular Sciences, 26(10), 4587. https://doi.org/10.3390/ijms26104587







