Meteorin-β: A Novel Biomarker and Therapeutic Target on Its Way to the Regulation of Human Diseases
Abstract
1. Introduction
2. The Discovery and Structure of Metrnβ
3. Distribution and Expression Patterns of Metrnβ
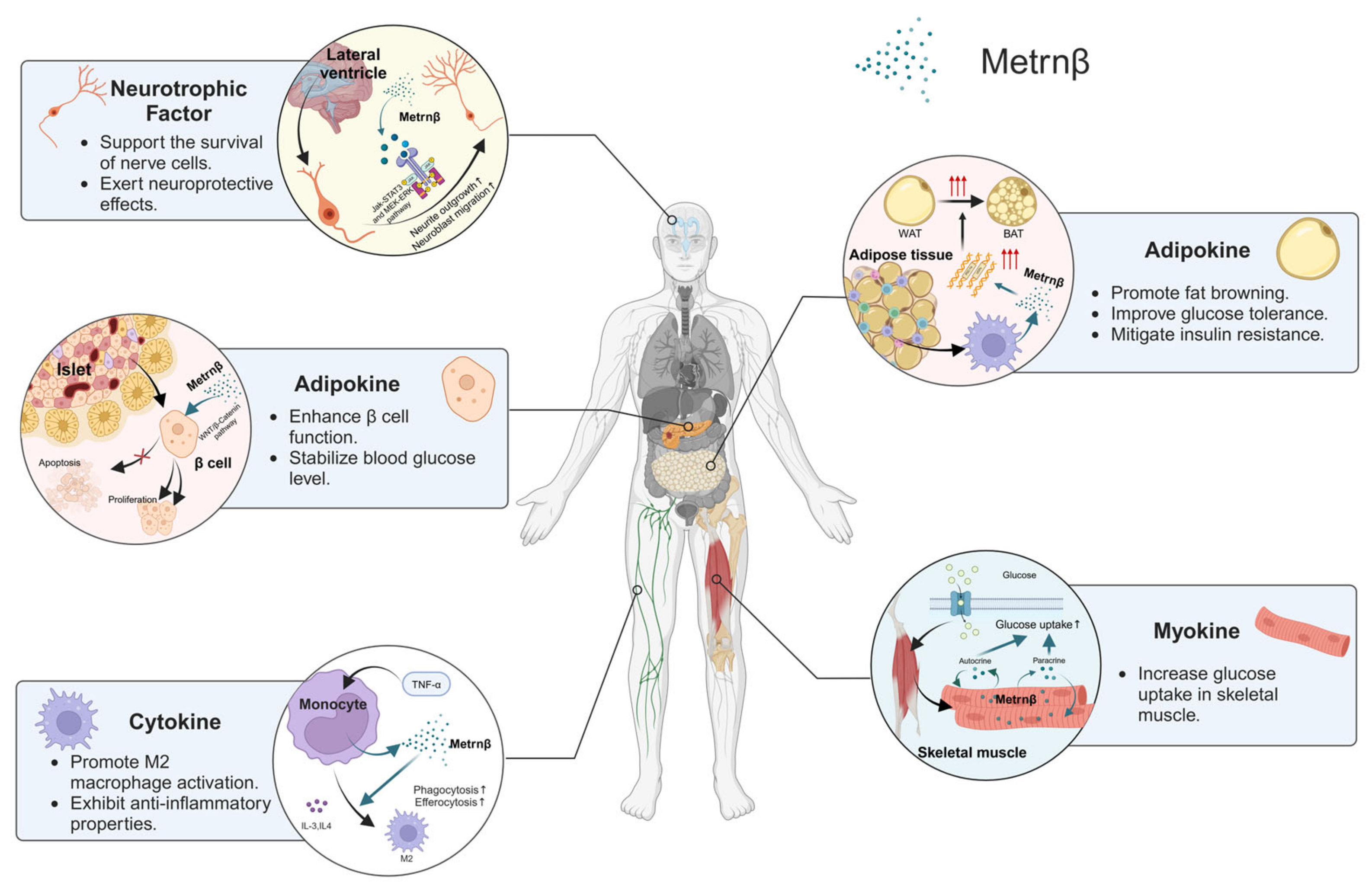
4. Biological Functions of Metrnβ
4.1. Regulation of Lipid Metabolism and Insulin Resistance as an Adipokine
4.2. Support of Neuronal Development as a Neurotrophic Factor
4.3. Contribution to Muscle Regeneration and Repair as a Myokine
4.4. Anti-Inflammatory Cytokine
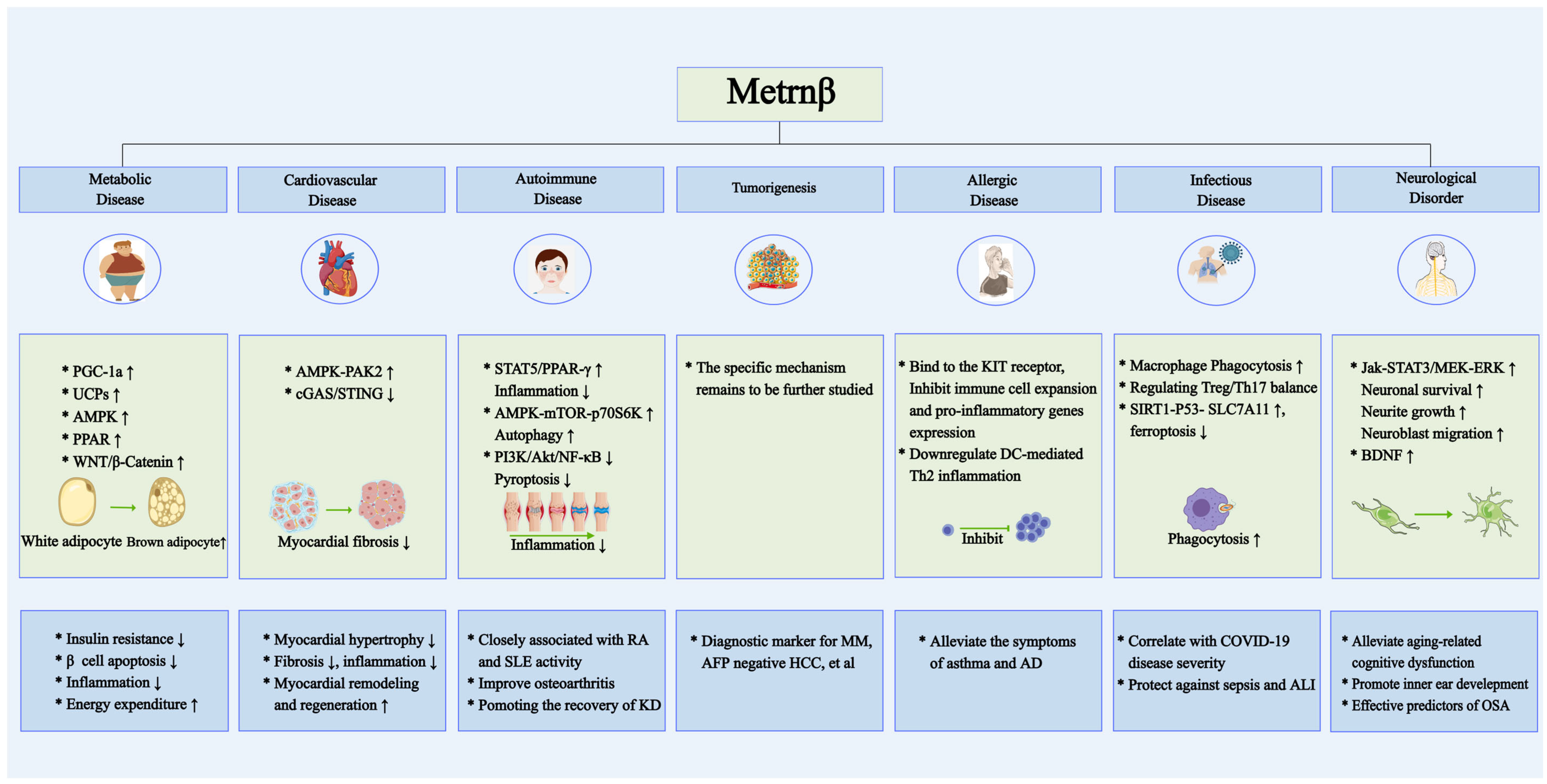
5. Involvement of Metrnβ in Human Diseases
5.1. Metrnβ in Metabolic Disease: Interplay Between Diabetes and Obesity
5.2. Metrnβ in Cardiovascular Pathophysiology and Therapeutic Potential
5.3. Metrnβ in Autoimmune Disorders: From Pathogenesis to Biomarker Potential
5.3.1. Metrnβ in Systemic Lupus Erythematosus (SLE)
5.3.2. Metrnβ in Kawasaki Disease (KD)
5.3.3. Metrnβ in Spondylitis
5.4. Metrnβ in Human Malignancies: Oncogenic Implications
5.4.1. Metrnβ in Breast Carcinogenesis
5.4.2. Metrnβ in Cutaneous Neoplasms
5.4.3. Metrnβ in Malignant Mesothelioma (MM)
5.4.4. Metrnβ in Ovarian Tumorigenesis
5.4.5. Metrnβ in Colorectal Cancer (CRC): Dual Oncogenic Paradigm
5.4.6. Metrnβ in Hepatocellular Carcinoma (HCC)
5.5. Metrnβ in Allergic Diseases: Immunomodulatory Roles
5.6. Metrnβ in Infectious Diseases: Novel Biomarker and Therapeutic Target
5.6.1. Metrnβ in Sepsis
5.6.2. Metrnβ in COVID-19
5.6.3. Metrnβ in Chronic Obstructive Pulmonary Disease (COPD)
5.7. Metrnβ in Neurological Disorders: A Protective Modulator
5.7.1. Metrnβ in Cognitive Function and Aging
5.7.2. Metrnβ in Inner Ear Development
5.7.3. Metrnβ in Obstructive Sleep Apnea (OSA)
6. Conclusions and Future Perspectives
7. Methodology
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| AD | Allergic dermatitis |
| ALI | Acute lung injury |
| AxSpA | Ankylosing spondylitis |
| BDNF | Brain-derived neurotrophic factor |
| BIGE | Body Index of Gene Expression |
| CAL | Coronary artery lesion |
| CRP | C-reactive protein |
| CD | Crohn’s disease |
| CHD | Coronary heart disease |
| CIMT | Carotid intima-media thickness |
| CNS | Central nervous system |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CRC | Colorectal cancer |
| DC | Dendritic cell |
| D-gal | D-galactose |
| ESR | Erythrocyte sedimentation rate |
| GD | Graves’ disease |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| HCC | Hepatocellular carcinoma |
| HDM | House dust mite |
| HFD | High-fat diet |
| IL-41 | Interleukin-41 |
| ILCs | Innate lymphoid cells |
| IVIG | Intravenous immunoglobulin |
| KD | Kawasaki disease |
| KIT | Receptor tyrosine kinase |
| Metrnβ | Meteorin-β |
| Metrnl | Meteorin-like protein |
| MM | Malignant mesothelioma |
| NGF | Nerve growth factor |
| OA | Osteoarthritis |
| OSA | Obstructive sleep apnea |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PCOS | Polycystic ovary syndrome |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PsA | Psoriatic arthritis |
| RA | Rheumatoid arthritis |
| rmMetrnβ | Recombinant mice Metrnβ protein |
| RMH | Reactive mesothelial hyperplasia |
| RPL | Recurrent pregnancy loss |
| SGN | Spiral ganglion neuron |
| SLE | Systemic lupus erythematosus |
| SVZ | Subventricular zone |
| T2D | Type 2 diabetes |
| Tregs | Regulatory T cells |
| TrkB | Tropomyosin receptor kinase B |
References
- Roth, R.B.; Hevezi, P.; Lee, J.; Willhite, D.; Lechner, S.M.; Foster, A.C.; Zlotnik, A. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics 2006, 7, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Ushach, I.; Arrevillaga-Boni, G.; Heller, G.N.; Pone, E.; Hernandez-Ruiz, M.; Catalan-Dibene, J.; Hevezi, P.; Zlotnik, A. Meteorin-like/Meteorin-β Is a Novel Immunoregulatory Cytokine Associated with Inflammation. J. Immunol. 2018, 201, 3669–3676. [Google Scholar] [CrossRef]
- Reboll, M.R.; Klede, S.; Taft, M.H.; Cai, C.L.; Field, L.J.; Lavine, K.J.; Koenig, A.L.; Fleischauer, J.; Meyer, J.; Schambach, A.; et al. Meteorin-like promotes heart repair through endothelial KIT receptor tyrosine kinase. Science 2022, 376, 1343–1347. [Google Scholar] [CrossRef]
- Jung, T.W.; Pyun, D.H.; Kim, T.J.; Lee, H.J.; Park, E.S.; Abd El-Aty, A.M.; Hwang, E.J.; Shin, Y.K.; Jeong, J.H. Meteorin-like protein (METRNL)/IL-41 improves LPS-induced inflammatory responses via AMPK or PPARδ-mediated signaling pathways. Adv. Med. Sci. 2021, 66, 155–161. [Google Scholar] [CrossRef]
- Li, Z.Y.; Song, J.; Zheng, S.L.; Fan, M.B.; Guan, Y.F.; Qu, Y.; Xu, J.; Wang, P.; Miao, C.Y. Adipocyte Metrnl Antagonizes Insulin Resistance Through PPARγ Signaling. Diabetes 2015, 64, 4011–4022. [Google Scholar] [CrossRef]
- Ushach, I.; Burkhardt, A.M.; Martinez, C.; Hevezi, P.A.; Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Valle-Rios, R.; Vazquez, M.I.; Homey, B.; et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin. Immunol. 2015, 156, 119–127. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, S.H.; Kim, H.C.; Bang, J.S.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Shin, Y.K.; Jeong, J.H. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, Z.Y.; Song, J.; Liu, J.M.; Miao, C.Y. Metrnl: A secreted protein with new emerging functions. Acta Pharmacol. Sin. 2016, 37, 571–579. [Google Scholar] [CrossRef]
- Song, L.; Chang, X.; Hu, L.; Liu, L.; Wang, G.; Huang, Y.; Xu, L.; Jin, B.; Song, J.; Hu, L.; et al. Accelerating Wound Closure With Metrnl in Normal and Diabetic Mouse Skin. Diabetes 2023, 72, 1692–1706. [Google Scholar] [CrossRef]
- Lee, D.E.; McKay, L.K.; Bareja, A.; Li, Y.; Khodabukus, A.; Bursac, N.; Taylor, G.A.; Baht, G.S.; White, J.P. Meteorin-like is an injectable peptide that can enhance regeneration in aged muscle through immune-driven fibro/adipogenic progenitor signaling. Nat. Commun. 2022, 13, 7613. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Balu, A.R.; Molitoris, K.H.; White, J.P.; Robling, A.G.; Ayturk, U.M.; Baht, G.S. The role of Meteorin-like in skeletal development and bone fracture healing. J. Orthop. Res. 2022, 40, 2510–2521. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.R.; Fransson, A.; Fjord-Larsen, L.; Thompson, L.H.; Houchins, J.P.; Andrade, N.; Torp, M.; Kalkkinen, N.; Andersson, E.; Lindvall, O.; et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp. Neurol. 2012, 233, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.W.; Chen, J.; Chen, C.X.; Zheng, S.L.; Zhao, H.Y.; Miao, C.Y. Metrnl as a secreted protein: Discovery and cardiovascular research. Pharmacol. Ther. 2024, 263, 108730. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Z.; Sun, T.; Zhang, S.; Yang, S.; Zheng, M.; Shen, H. Meteorin-like/Metrnl, a novel secreted protein implicated in inflammation, immunology, and metabolism: A comprehensive review of preclinical and clinical studies. Front. Immunol. 2023, 14, 1098570. [Google Scholar] [CrossRef]
- Miao, Z.W.; Hu, W.J.; Li, Z.Y.; Miao, C.Y. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol. Sin. 2020, 41, 1525–1530. [Google Scholar] [CrossRef]
- Dong, W.S.; Hu, C.; Hu, M.; Gao, Y.P.; Hu, Y.X.; Li, K.; Ye, Y.J.; Zhang, X. Metrnl: A promising biomarker and therapeutic target for cardiovascular and metabolic diseases. Cell Commun. Signal. 2024, 22, 389. [Google Scholar] [CrossRef]
- Gao, X.; Leung, T.F.; Wong, G.W.; Ko, W.H.; Cai, M.; He, E.J.; Chu, I.M.; Tsang, M.S.; Chan, B.C.; Ling, J.; et al. Meteorin-β/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma. Cell. Mol. Immunol. 2022, 19, 245–259. [Google Scholar] [CrossRef]
- Huang, D.; Liu, X.; Gao, X.; Choi, C.K.; Giglio, G.; Farah, L.; Leung, T.F.; Wong, K.C.; Kan, L.L.; Chong, J.W.; et al. Meteorin-like protein/METRNL/Interleukin-41 ameliorates atopic dermatitis-like inflammation. Allergy 2024, 80, 474–488. [Google Scholar] [CrossRef]
- Gao, X.; Chan, P.K.; Wong, K.C.; Ng, R.W.; Yeung, A.C.; Lui, G.C.; Ling, L.; Hui, D.S.; Huang, D.; Wong, C.K. Characterization of METRNβ as a novel biomarker of Coronavirus disease 2019 severity and prognosis. Front. Immunol. 2023, 14, 1111920. [Google Scholar] [CrossRef]
- Kocaman, N.; Yuksel, E.I.; Demir, B.; Calik, I.; Cicek, D. Two Novel Biomarker Candidates for Differentiating Basal Cell Carcinoma from Trichoblastoma; Asprosin and Meteorine Like Peptide. Tissue Cell 2022, 76, 101752. [Google Scholar] [CrossRef] [PubMed]
- Akkus, G.; Koyuturk, L.C.; Yilmaz, M.; Hancer, S.; Ozercan, I.H.; Kuloglu, T. Asprosin and meteorin-like protein immunoreactivity in invasive ductal breast carcinoma stages. Tissue Cell 2022, 77, 101855. [Google Scholar] [CrossRef] [PubMed]
- Uzun, M.; Ilhan, Y.S.; Bozdag, A.; Yilmaz, M.; Artas, G.; Kuloglu, T. Asprosin, irisin, and meteorin-like protein immunoreactivity in different stages of colorectal adenocarcinoma. Pathol. Res. Pract. 2023, 245, 154432. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Zheng, S.L.; Wang, P.; Xu, T.Y.; Guan, Y.F.; Zhang, Y.J.; Miao, C.Y. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci. Ther. 2014, 20, 344–354. [Google Scholar] [CrossRef]
- Jørgensen, J.R.; Thompson, L.; Fjord-Larsen, L.; Krabbe, C.; Torp, M.; Kalkkinen, N.; Hansen, C.; Wahlberg, L. Characterization of Meteorin—An evolutionary conserved neurotrophic factor. J. Mol. Neurosci. 2009, 39, 104–116. [Google Scholar] [CrossRef]
- Bridgewood, C.; Russell, T.; Weedon, H.; Baboolal, T.; Watad, A.; Sharif, K.; Cuthbert, R.; Wittmann, M.; Wechalekar, M.; McGonagle, D. The novel cytokine Metrnl/IL-41 is elevated in Psoriatic Arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin. Immunol. 2019, 208, 108253. [Google Scholar] [CrossRef]
- Onuora, S. Novel cytokine, IL-41, linked with PsA. Nat. Rev. Rheumatol. 2019, 15, 636. [Google Scholar] [CrossRef]
- Wang, J.; Jia, Z.; Dang, H.; Zou, J. Meteorin-like/Meteorin-β upregulates proinflammatory cytokines via NF-κB pathway in grass carp Ctenopharyngodon idella. Dev. Comp. Immunol. 2022, 127, 104289. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Z.; Song, J.; Wang, P.; Xu, J.; Hu, W.; Shi, Y.; Qi, Q.; Miao, Z.; Guan, Y.; et al. Endothelial METRNL determines circulating METRNL level and maintains endothelial function against atherosclerosis. Acta Pharm. Sin. B 2023, 13, 1568–1587. [Google Scholar] [CrossRef]
- Shi, R.; He, M.; Peng, Y.; Xia, X. Homotherapy for heteropathy: Interleukin-41 and its biological functions. Immunology 2024, 173, 1–13. [Google Scholar] [CrossRef]
- Şekerci, G.; Erden, Y.; Tekin, S. Effects of meteorin-like hormone on endocrine function of hypothalamo-hypophysial system and peripheral uncoupling proteins in rats. Mol. Biol. Rep. 2022, 49, 5919–5925. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, R.; Sun, B. Meteorin-Like Ameliorates β Cell Function by Inhibiting β Cell Apoptosis of and Promoting β Cell Proliferation via Activating the WNT/β-Catenin Pathway. Front. Pharmacol. 2021, 12, 627147. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, M.; Gautam, S.; Verma, D.P.; Afshan, T.; Kumari, T.; Srivastava, A.K.; Ghosh, J.K. A collagen domain-derived short adiponectin peptide activates APPL1 and AMPK signaling pathways and improves glucose and fatty acid metabolisms. J. Biol. Chem. 2018, 293, 13509–13523. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotaki, A.; Dansercoer, A.; Verschueren, K.H.G.; Marković, I.; Pollmann, C.; Hafer, M.; Felix, J.; Birck, C.; Van Putte, W.; Catteeuw, D.; et al. Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Nat. Struct. Mol. Biol. 2023, 30, 551–563. [Google Scholar] [CrossRef]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Münzberg, H.; Zhang, Z.Y.; Kahn, B.B.; et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef]
- Liu, D.; Liu, M.; Yu, P.; Li, H. Brain-derived neurotrophic factor and nerve growth factor expression in endometriosis: A systematic review and meta-analysis. Taiwan J. Obstet. Gynecol. 2023, 62, 634–639. [Google Scholar] [CrossRef]
- Watanabe, K.; Akimoto, Y.; Yugi, K.; Uda, S.; Chung, J.; Nakamuta, S.; Kaibuchi, K.; Kuroda, S. Latent process genes for cell differentiation are common decoders of neurite extension length. J. Cell Sci. 2012, 125 Pt 9, 2198–2211. [Google Scholar] [CrossRef]
- Hong, C.; Wang, Z.; Zheng, S.L.; Hu, W.J.; Wang, S.N.; Zhao, Y.; Miao, C.Y. Metrnl regulates cognitive dysfunction and hippocampal BDNF levels in D-galactose-induced aging mice. Acta Pharmacol. Sin. 2023, 44, 741–751. [Google Scholar] [CrossRef]
- Surace, C.; Piazzolla, S.; Sirleto, P.; Digilio, M.C.; Roberti, M.C.; Lombardo, A.; D’Elia, G.; Tomaiuolo, A.C.; Petrocchi, S.; Capolino, R.; et al. Mild ring 17 syndrome shares common phenotypic features irrespective of the chromosomal breakpoints location. Clin. Genet. 2009, 76, 256–262. [Google Scholar] [CrossRef]
- Javaid, H.M.A.; Sahar, N.E.; ZhuGe, D.L.; Huh, J.Y. Exercise Inhibits NLRP3 Inflammasome Activation in Obese Mice via the Anti-Inflammatory Effect of Meteorin-like. Cells 2021, 10, 3480. [Google Scholar] [CrossRef]
- Alizadeh, H. Myokine-mediated exercise effects: The role of myokine meteorin-like hormone (Metrnl). Growth Factors 2021, 39, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Baht, G.S.; Bareja, A.; Lee, D.E.; Rao, R.R.; Huang, R.; Huebner, J.L.; Bartlett, D.B.; Hart, C.R.; Gibson, J.R.; Lanza, I.R.; et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat. Metab. 2020, 2, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Byun, W.S.; Kang, M.J.; Han, J.A.; Moon, J.; Shin, M.J.; Lee, H.J.; Chung, J.H.; Lee, J.S.; Son, C.G.; et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKα2. FEBS J. 2020, 287, 2087–2104. [Google Scholar] [CrossRef]
- Xu, L.; Cai, Y.; Wang, Y.; Xu, C. Meteorin-Like (METRNL) Attenuates Myocardial Ischemia/Reperfusion Injury-Induced Cardiomyocytes Apoptosis by Alleviating Endoplasmic Reticulum Stress via Activation of AMPK-PAK2 Signaling in H9C2 Cells. Med. Sci. Monit. 2020, 26, e924564. [Google Scholar] [CrossRef]
- Zuo, L.; Ge, S.; Ge, Y.; Li, J.; Zhu, B.; Zhang, Z.; Jiang, C.; Li, J.; Wang, S.; Liu, M.; et al. The Adipokine Metrnl Ameliorates Chronic Colitis in Il-10−/− Mice by Attenuating Mesenteric Adipose Tissue Lesions During Spontaneous Colitis. J. Crohn’s Colitis 2019, 13, 931–941. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yang, Y.; Luo, N.; Yang, J.; Zhong, L.; Guo, T.; Yuan, Z.; Wei, Q.; Wang, C. Protective role of the novel cytokine Metrnl/interleukin-41 in host immunity defense during sepsis by promoting macrophage recruitment and modulating Treg/Th17 immune cell balance. Clin. Immunol. 2023, 254, 109690. [Google Scholar] [CrossRef]
- El-Ashmawy, H.M.; Selim, F.O.; Hosny, T.A.M.; Almassry, H.N. Association of low serum Meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res. Clin. Pract. 2019, 150, 57–63. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, Y.E.; Kim, J.M.; Choung, S.; Joung, K.H.; Kim, H.J.; Ku, B.J. Serum Meteorin-like protein levels decreased in patients newly diagnosed with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 135, 7–10. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, Z.Y.; Zhang, Z.; Wang, D.S.; Xu, J.; Miao, C.Y. Evaluation of Two Commercial Enzyme-Linked Immunosorbent Assay Kits for the Detection of Human Circulating Metrnl. Chem. Pharm. Bull. 2018, 66, 391–398. [Google Scholar] [CrossRef]
- Timurkaan, M.; Timurkaan, E.S. Two Important Players for Type 2 Diabetes Mellitus: Metrnl and Asprosin. Clin. Lab. 2022, 68, 1801–1807. [Google Scholar] [CrossRef]
- Du, Y.; Ye, X.; Lu, A.; Zhao, D.; Liu, J.; Cheng, J.; Yang, T. Inverse relationship between serum Metrnl levels and visceral fat obesity (VFO) in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2020, 161, 108068. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Hwang, S.Y.; Choi, J.H.; Lee, H.J.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Implications of circulating Meteorin-like (Metrnl) level in human subjects with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 136, 100–107. [Google Scholar] [CrossRef] [PubMed]
- AlKhairi, I.; Cherian, P.; Abu-Farha, M.; Madhoun, A.A.; Nizam, R.; Melhem, M.; Jamal, M.; Al-Sabah, S.; Ali, H.; Tuomilehto, J.; et al. Increased Expression of Meteorin-Like Hormone in Type 2 Diabetes and Obesity and Its Association with Irisin. Cells 2019, 8, 1283. [Google Scholar] [CrossRef]
- Cherian, P.; Al-Khairi, I.; Jamal, M.; Al-Sabah, S.; Ali, H.; Dsouza, C.; Alshawaf, E.; Al-Ali, W.; Al-Khaledi, G.; Al-Mulla, F.; et al. Association Between Factors Involved in Bone Remodeling (Osteoactivin and OPG) With Plasma Levels of Irisin and Meteorin-Like Protein in People With T2D and Obesity. Front. Endocrinol. 2021, 12, 752892. [Google Scholar] [CrossRef]
- Yavuzkir, S.; Ugur, K.; Deniz, R.; Ustebay, D.U.; Mirzaoglu, M.; Yardim, M.; Sahin, İ.; Baykus, Y.; Karagoz, Z.K.; Aydin, S. Maternal and umbilical cord blood subfatin and spexin levels in patients with gestational diabetes mellitus. Peptides 2020, 126, 170277. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Y.; Song, J.; Sun, Y.; Li, H.; Chen, L.; Hou, X. Serum Metrnl Level is Correlated with Insulin Resistance, But Not with β-Cell Function in Type 2 Diabetics. Med. Sci. Monit. 2019, 25, 8968–8974. [Google Scholar] [CrossRef]
- Ferns, G.A.; Fekri, K.; Shahini Shams Abadi, M.; Banitalebi Dehkordi, M.; Arjmand, M.H. A meta-analysis of the relationship between serums metrnl-like protein/subfatin and risk of type 2 diabetes mellitus and coronary artery disease. Arch. Physiol. Biochem. 2023, 129, 1084–1090. [Google Scholar] [CrossRef]
- Wu, Q.; Dan, Y.L.; He, Y.S.; Xiang, K.; Hu, Y.Q.; Zhao, C.N.; Zhong, X.; Wang, D.G.; Pan, H.F. Circulating Meteorin-like Levels in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis. Curr. Pharm. Des. 2020, 26, 5732–5738. [Google Scholar] [CrossRef]
- Schmid, A.; Karrasch, T.; Schäffler, A. Meteorin-Like Protein (Metrnl) in Obesity, during Weight Loss and in Adipocyte Differentiation. J. Clin. Med. 2021, 10, 4338. [Google Scholar] [CrossRef]
- Löffler, D.; Landgraf, K.; Rockstroh, D.; Schwartze, J.T.; Dunzendorfer, H.; Kiess, W.; Körner, A. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int. J. Obes. 2017, 41, 112–119. [Google Scholar] [CrossRef]
- Wang, K.; Li, F.; Wang, C.; Deng, Y.; Cao, Z.; Cui, Y.; Xu, K.; Ln, P.; Sun, Y. Serum Levels of Meteorin-Like (Metrnl) Are Increased in Patients with Newly Diagnosed Type 2 Diabetes Mellitus and Are Associated with Insulin Resistance. Med. Sci. Monit. 2019, 25, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Pellitero, S.; Piquer-Garcia, I.; Ferrer-Curriu, G.; Puig, R.; Martínez, E.; Moreno, P.; Tarascó, J.; Balibrea, J.; Lerin, C.; Puig-Domingo, M.; et al. Opposite changes in meteorin-like and oncostatin m levels are associated with metabolic improvements after bariatric surgery. Int. J. Obes. 2018, 42, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Giden, R.; Yasak, I.H. Meteorin-like protein decreases in acute coronary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.H.; AlOtaibi, F.; Dsouza, C.; Al-Sabah, S.; Al-Khaledi, G.; Al-Ali, W.; Ali, H.; Cherian, P.; Al-Khairi, I.; Devarajan, S.; et al. Changes in the expression of meteorin-like (METRNL), irisin (FNDC5), and uncoupling proteins (UCPs) after bariatric surgery. Obesity 2022, 30, 1629–1638. [Google Scholar] [CrossRef]
- Liu, Z.X.; Ji, H.H.; Yao, M.P.; Wang, L.; Wang, Y.; Zhou, P.; Liu, Y.; Zheng, X.F.; He, H.W.; Wang, L.S.; et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J. Cell. Mol. Med. 2019, 23, 271–280. [Google Scholar] [CrossRef]
- Ding, X.; Chang, X.; Wang, J.; Bian, N.; An, Y.; Wang, G.; Liu, J. Serum Metrnl levels are decreased in subjects with overweight or obesity and are independently associated with adverse lipid profile. Front. Endocrinol. 2022, 13, 938341. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, W.J.; Zheng, S.L.; Zhang, S.L.; Le, Y.Y.; Li, Z.Y.; Miao, C.Y. Metrnl deficiency decreases blood HDL cholesterol and increases blood triglyceride. Acta Pharmacol. Sin. 2020, 41, 1568–1575. [Google Scholar] [CrossRef]
- Dadmanesh, M.; Aghajani, H.; Fadaei, R.; Ghorban, K. Lower serum levels of Meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PLoS ONE 2018, 13, e0204180. [Google Scholar] [CrossRef]
- Rupérez, C.; Ferrer-Curriu, G.; Cervera-Barea, A.; Florit, L.; Guitart-Mampel, M.; Garrabou, G.; Zamora, M.; Crispi, F.; Fernandez-Solà, J.; Lupón, J.; et al. Meteorin-like/Meteorin-β protects heart against cardiac dysfunction. J. Exp. Med. 2021, 218, e20201206. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Q.M.; Li, J.W.; Xu, F.; Bu, Y.L.; Wang, M.; Lu, X.; Gao, W. Serum Meteorin-like is associated with weight loss in the elderly patients with chronic heart failure. J. Cachexia Sarcopenia Muscle 2022, 13, 409–417. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, X.; Song, P.; Yuan, Y.P.; Kong, C.Y.; Wu, H.M.; Xu, S.C.; Ma, Z.G.; Tang, Q.Z. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020, 37, 101747. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hong, Y.; Zhong, Y.; Yang, S.; Pei, L.; Huang, Z.; Long, H.; Chen, X.; Zhou, C.; Zheng, G.; et al. Meteorin-like (METRNL) attenuates hypertensive induced cardiac hypertrophy by inhibiting autophagy via activating BRCA2. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.B.; Ding, Y.; Liu, Y.; Wang, Z.C.; Wu, Y.J.; Niu, K.M.; Li, K.X.; Zhang, J.R.; Sun, H.J. Metrnl ameliorates diabetic cardiomyopathy via inactivation of cGAS/STING signaling dependent on LKB1/AMPK/ULK1-mediated autophagy. J. Adv. Res. 2023, 51, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.X.; Feng, Y.Y.; Wang, H.Y.; Lu, C.H.; Liu, D.Z.; Gong, C.; Xue, Y.; Na, N.; Huang, F. Metrnl ameliorates myocardial ischemia-reperfusion injury by activating AMPK-mediated M2 macrophage polarization. Mol. Med. 2025, 31, 98. [Google Scholar] [CrossRef]
- Kocaman, N.; Onat, E.; Balta, H.; Üçer, Ö. Are Meteorin-Like Peptide and Asprosin Important in the Diagnosis of Breast Tumors? Cureus 2024, 16, e62979. [Google Scholar] [CrossRef]
- Kovács, D.; Fazekas, F.; Oláh, A.; Törőcsik, D. Adipokines in the Skin and in Dermatological Diseases. Int. J. Mol. Sci. 2020, 21, 9048. [Google Scholar] [CrossRef]
- Kocaman, N.; Artaş, G. Can novel adipokines, asprosin and meteorin-like, be biomarkers for malignant mesothelioma? Biotech. Histochem. 2020, 95, 171–175. [Google Scholar] [CrossRef]
- Mirzaoglu, M.; Yavuzkir, S.; Mirzaoglu, C.; Yurt, N.; Dagli, A.F.; Ozcan Yildirim, S.; Sahin, İ.; Aydin, S. Use of asprosin and subfatin for differential diagnosis of serous ovarian tumors. Biotech. Histochem. 2023, 98, 140–146. [Google Scholar] [CrossRef]
- Chen, Z.; Song, W.; Shu, X.O.; Wen, W.; Devall, M.; Dampier, C.; Moratalla-Navarro, F.; Cai, Q.; Long, J.; Van Kaer, L.; et al. Novel insights into genetic susceptibility for colorectal cancer from transcriptome-wide association and functional investigation. J. Natl. Cancer Inst. 2024, 116, 127–137. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Xia, Y.; Yu, J. Over expression of METRN predicts poor clinical prognosis in colorectal cancer. Mol. Genet. Genom. Med. 2020, 8, e1102. [Google Scholar] [CrossRef]
- Onat, E.; Kocaman, N.; Balta, H. The Role of Meteorin-Like Peptide and Asprosin in Colon Carcinoma. Cureus 2023, 15, e47073. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Ren, D.; Li, J.; Mu, Z.; Li, C.; He, Y.; Zhang, J.; Fan, R.; Yin, J.; et al. Interleukin-41: A novel serum marker for the diagnosis of alpha-fetoprotein-negative hepatocellular carcinoma. Front. Oncol. 2024, 14, 1408584. [Google Scholar] [CrossRef] [PubMed]
- Seyhanli, E.S.; Guler, O.; Yasak, I.H.; Koyuncu, I. Investigation of the Correlation between Meteorin-Like Protein (Metrnl) and Thiol Balance in COVID-19 Patients. Clin. Lab. 2021, 67, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.; Peng, H.; Zhang, M.; Wu, X.; Gui, F.; Li, W.; Ai, F.; Yu, B.; Liu, Y. Meteorin-like/Meteorin-β protects LPS-induced acute lung injury by activating SIRT1-P53-SLC7A11 mediated ferroptosis pathway. Mol. Med. 2023, 29, 144. [Google Scholar] [CrossRef]
- Frohlich, J.; Chaldakov, G.N.; Vinciguerra, M. Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy. Int. J. Mol. Sci. 2021, 22, 4137. [Google Scholar] [CrossRef]
- Altintas, N.; Fazlioglu, N.; Guzel, S.; Yilmaz, A.; Aydın, C. Is meteorin-like (Metrnl) a novel biomarker to distinguish patients with obstructive sleep apnea (OSA) and patients with OSA at vascular risk. Sleep Breath 2023, 27, 1865–1874. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, Y.; Sun, T.; Gao, Z.; Li, Z.; Shen, H. Elevated levels of Metrnl in rheumatoid arthritis: Association with disease activity. Cytokine 2022, 159, 156026. [Google Scholar] [CrossRef]
- Gong, L.; Huang, G.; Weng, L.; Xu, J.; Li, Y.; Cui, W.; Li, M. Decreased serum interleukin-41/Metrnl levels in patients with Graves’ disease. J. Clin. Lab. Anal. 2022, 36, e24676. [Google Scholar] [CrossRef]
- Gholamrezayi, A.; Mohamadinarab, M.; Rahbarinejad, P.; Fallah, S.; Barez, S.R.; Setayesh, L.; Moradi, N.; Fadaei, R.; Chamani, E.; Tavakoli, T. Characterization of the serum levels of Meteorin-like in patients with inflammatory bowel disease and its association with inflammatory cytokines. Lipids Health Dis. 2020, 19, 230. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, Z.Y.; Wang, D.S.; Xu, T.Y.; Fan, M.B.; Cheng, M.H.; Miao, C.Y. Aggravated ulcerative colitis caused by intestinal Metrnl deficiency is associated with reduced autophagy in epithelial cells. Acta Pharmacol. Sin. 2020, 41, 763–770. [Google Scholar] [CrossRef]
- Sobieh, B.H.; Kassem, D.H.; Zakaria, Z.M.; El-Mesallamy, H.O. Potential emerging roles of the novel adipokines adipolin/CTRP12 and meteorin-like/METRNL in obesity-osteoarthritis interplay. Cytokine 2021, 138, 155368. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, S.; Yang, Y.; Piao, L.; Wang, Z.; Jin, Z.; Bai, L. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-κB and NLRP3/caspase-1/GSDMD signaling. Biomed. Pharmacother. 2023, 158, 114118. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Wang, Q.; Qin, H.; Cao, S.; Wei, Y.; Weng, J.; Yu, F.; Zeng, H. Osteoarthritis: Role of Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2023, 24, 13137. [Google Scholar] [CrossRef]
- Kiriakidou, M.; Ching, C.L. Systemic Lupus Erythematosus. Ann. Intern. Med. 2020, 172, Itc81–Itc96. [Google Scholar] [CrossRef]
- Lazar, S.; Kahlenberg, J.M. Systemic Lupus Erythematosus: New Diagnostic and Therapeutic Approaches. Annu. Rev. Med. 2023, 74, 339–352. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, S.; Li, Y.; Xu, X.; Liu, Y.; Qiao, H.; Wong, C.K.; Wu, G.; Jin, H.; Gao, X. Elevation of Metrnβ and Its Association with Disease Activity in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 13607. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.; Zhang, R.; Lin, X.; Bao, Y.; Jiang, F.; Wu, C.; Wang, J. Immune control in Kawasaki disease knowledge mapping: A bibliometric analysis. Cardiol. Young 2024, 34, 1738–1753. [Google Scholar] [CrossRef]
- Li, G.; Wang, T.; Gou, Y.; Zeng, R.; Liu, D.; Duan, Y.; Liu, B. Value of C-reactive protein/albumin ratio in predicting intravenous immunoglobulin-resistant Kawasaki disease- a data from multi-institutional study in China. Int. Immunopharmacol. 2020, 89 Pt A, 107037. [Google Scholar] [CrossRef]
- Cai, X.; Li, K.; Li, M.; Lu, Y.; Wu, J.; Qiu, H.; Li, Y. Plasma interleukin-41 serves as a potential diagnostic biomarker for Kawasaki disease. Microvasc. Res. 2023, 147, 104478. [Google Scholar] [CrossRef]
- Han, J.W.; Oh, J.H.; Rhim, J.W.; Lee, K.Y. Correlation between elevated platelet count and immunoglobulin levels in the early convalescent stage of Kawasaki disease. Medicine 2017, 96, e7583. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, L.; Zhang, H.J.; Gao, X.J.; Pan, X.T.; Wang, X.R.; Ye, H.; Liu, G.H. Expression levels of plasma miRNA-21 and NT-proBNP in children with Kawasaki disease and their clinical significance. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12757–12762. [Google Scholar] [CrossRef]
- Duan, Y.; Li, H.; Luo, D.; Jiang, J.; Liu, B.; Li, G. Serum IL-41 might be a biomarker for IVIG resistance and coronary artery lesions in Kawasaki disease. Int. Immunopharmacol. 2023, 122, 110600. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H.; Baker, S.C.; Orenstein, J.M.; Shulman, S.T. Searching for the cause of Kawasaki disease—Cytoplasmic inclusion bodies provide new insight. Nat. Rev. Microbiol. 2008, 6, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, F.; Gentileschi, S.; Cigolini, C.; Terenzi, R.; Pata, A.P.; Esti, L.; Carli, L. Axial spondyloarthritis: One year in review 2023. Clin. Exp. Rheumatol. 2023, 41, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Uçar, I.M.B.; Sargin, G.; Tuzcu, A.; Çildağ, S.; Şentürk, T. Correlation of serum subfatin, cthrc1, ctrp3, ctrp6 levels with disease indices in patients with axial spondyloarthritis. BMC Rheumatol. 2023, 7, 29. [Google Scholar] [CrossRef]
- Abd Ali, B.M.; Sharquie, I.K.; Gorial, F.I. IL-41: A novel serum marker correlates with disease activity in patients with ankylosing spondylitis. Med. J. Malays. 2024, 79, 777–784. [Google Scholar]
- Park, J.A.; Lee, H.S.; Ko, K.J.; Park, S.Y.; Kim, J.H.; Choe, G.; Kweon, H.S.; Song, H.S.; Ahn, J.C.; Yu, Y.S.; et al. Meteorin regulates angiogenesis at the gliovascular interface. Glia 2008, 56, 247–258. [Google Scholar] [CrossRef]
- Fouani, F.Z.; Fadaei, R.; Moradi, N.; Zandieh, Z.; Ansaripour, S.; Yekaninejad, M.S.; Vatannejad, A.; Mahmoudi, M. Circulating levels of Meteorin-like protein in polycystic ovary syndrome: A case-control study. PLoS ONE 2020, 15, e0231943. [Google Scholar] [CrossRef]
- Jackson, C.M.; Pant, A.; Dinalankara, W.; Choi, J.; Jain, A.; Nitta, R.; Yazigi, E.; Saleh, L.; Zhao, L.; Nirschl, T.R.; et al. The cytokine Meteorin-like inhibits anti-tumor CD8(+) T cell responses by disrupting mitochondrial function. Immunity 2024, 57, 1864–1877.e9. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Du, Y.N.; Teng, J.M.; Zhou, T.H.; Du, B.Y.; Cai, W. Meteorin-like protein overexpression ameliorates fulminant hepatitis in mice by inhibiting chemokine-dependent immune cell infiltration. Acta Pharmacol. Sin. 2023, 44, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Legaki, E.; Arsenis, C.; Taka, S.; Papadopoulos, N.G. DNA methylation biomarkers in asthma and rhinitis: Are we there yet? Clin. Transl. Allergy 2022, 12, e12131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Wu, G.; Huang, P.; Wang, H.; An, H.; Liu, S.; Zhang, W. The pathogenesis and potential therapeutic targets in sepsis. MedComm 2023, 4, e418. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Calverley, P.M.A.; Walker, P.P. Contemporary Concise Review 2022: Chronic obstructive pulmonary disease. Respirology 2023, 28, 428–436. [Google Scholar] [CrossRef]
- Kerget, B.; Afşin, D.E.; Kerget, F.; Aşkın, S.; Akgün, M. Is Metrnl an Adipokine İnvolved in the Anti-inflammatory Response to Acute Exacerbations of COPD? Lung 2020, 198, 307–314. [Google Scholar] [CrossRef]
- Rajachandran, M.; Nickel, N.; Lange, R.A. Sleep apnea and cardiovascular risk. Curr. Opin. Cardiol. 2023, 38, 456–461. [Google Scholar] [CrossRef]
| Types of Disease | Expression Patterns | Distribution and Cellular Sources | Potential Regulatory Roles | References | |
|---|---|---|---|---|---|
| Metabolic Disease | Diabetes. Obesity. | ↑/↓/unchanged ↑/↓/unchanged | Serum; adipose tissue Serum; adipose tissue | Promotes energy consumption, β-cell function and stabilizes blood glucose levels; reduces insulin resistance. Reduces lipid accumulation, regulates adipocyte differentiation, and increases thermogenesis. | [2,6,10,24,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| Cardiovascular Disease | Atherosclerosis; coronary heart disease; cardiac hypertrophy and fibrosis; myocardial ischemia-reperfusion injury. | ↓ | Serum; heart; endothelial cell | Reduces myocardial hypertrophy, fibrosis, and inflammation; promotes myocardial remodeling and regeneration. | [29,44,47,63,65,68,69,70,71,72,73,74] |
| Autoimmune Disease | Psoriatic arthritis. Rheumatoid arthritis. Inflammatory bowel disease. Osteoarthritis. Systemic lupus erythematosus. Kawasaki disease. Spondylitis. | ↑ ↑ ↑/↓ ↑ ↑ ↑ ↑/↓ | Synovial fluid and tissues Synovial membranes and serum Serum; mesenteric adipose tissue Synovial fluid Serum Serum Serum | Remains to be elucidated, partially attributed to the synergistic interaction between Metrnβ with TNF and IL17A/F. Serum Metrnβ levels are closely associated with RA activity. Alleviates ulcerative colitis by promoting autophagy; ameliorates CD by promoting adipocyte function and differentiation. Ameliorates osteoarthritis by inhibiting inflammation and pyroptosis; erestores the expression of type II collagen. Positively correlated with disease activity. A diagnostic biomarker for KD; contributes to the recovery of KD by exerting anti-inflammatory effects. Decreased after inflammatory control, elevated in active disease, with mechanisms to be clarified. | |
| Tumorigenesis | Breast cancer. Cutaneous neoplasms. Malignant mesothelioma. Ovarian cancer. Colorectal cancer. Hepatocellular carcinoma. | ↑ ↑/unchanged ↑ ↓ ↑/↓ ↑ | Breast cancer tissue Tumor tissues, lesion area; skin Lung tissues Cancerous tissues Cancerous tissues Serum; HCC tissues | May be associated with the presence of breast cancer. A diagnostic marker for BCC, trichoblastoma, and differentiation between BCC and trichoblastoma. Metrnβ may be used as a biomarker for diagnosis of MM. May prevent cancer-related cachexia, remaining to be verified. N/A Suggests poor prognosis and postoperative recurrence in HCC; a diagnostic marker for AFP negative HCC. | [7,21,22,75,76,77,78,79,80,81,82] |
| Allergic disease | Asthma. Allergic dermatitis. | ↑ | Serum and lung; serum, skin and ear tissue | Alleviates DC-mediated Th2 inflammation; binds to the KIT receptor to inhibit immune cell expansion. | [18,19] |
| Infectious Disease | Sepsis. COVID-19. Acute lung injury. | ↑ ↑/↓ ↓ | Serum Serum Lung | Promotes host immune defense by regulating Treg/Th17 balance; enhances antibacterial activity of macrophages. Correlates with disease severity. Inhibits ferroptosis of alveolar epithelial cells and attenuates LPS-induced lung injury by targeting SIRT1. | [3,20,46,83,84] |
| Neurological Disorder | Inner ear development. Cognitive function and aging. Obstructive sleep apnea. | ↑ ↑ ↓ | Inner ear Hippocampus Serum | Supports neuronal survival by promoting neurite growth and neuroblast migration and protects spiral ganglion neurons. Alleviates aging-related cognitive dysfunction via regulating hippocampal BDNF levels. Inverse association between Metrnβ and CIMT; effective predictor of OSA. | [13,38,85,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Li, X.; Gao, X. Meteorin-β: A Novel Biomarker and Therapeutic Target on Its Way to the Regulation of Human Diseases. Int. J. Mol. Sci. 2025, 26, 4485. https://doi.org/10.3390/ijms26104485
Wang B, Li X, Gao X. Meteorin-β: A Novel Biomarker and Therapeutic Target on Its Way to the Regulation of Human Diseases. International Journal of Molecular Sciences. 2025; 26(10):4485. https://doi.org/10.3390/ijms26104485
Chicago/Turabian StyleWang, Bei, Xiao Li, and Xun Gao. 2025. "Meteorin-β: A Novel Biomarker and Therapeutic Target on Its Way to the Regulation of Human Diseases" International Journal of Molecular Sciences 26, no. 10: 4485. https://doi.org/10.3390/ijms26104485
APA StyleWang, B., Li, X., & Gao, X. (2025). Meteorin-β: A Novel Biomarker and Therapeutic Target on Its Way to the Regulation of Human Diseases. International Journal of Molecular Sciences, 26(10), 4485. https://doi.org/10.3390/ijms26104485





