Simple Rapid Production of Calcium Acetate Lactate from Scallop Shell Waste for Agricultural Application
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis
2.3. Characterizations
3. Results and Discussion
CaCO3(s) + 2CH3COOH(aq) → Ca(CH3COO)2·xH2O(s) + CO2(g)
CaCO3(s) + 2CH3CHOHCOOH(aq) → Ca(CH3CHOHCOO)2·xH2O(s) + CO2(g)
CaCO3(s) + CH3COOH(aq) + CH3CHOHCOOH(aq) → Ca(CH3COO)(CH3CHOHCOO)·xH2O(s) + CO2(g)
3.1. Chemical Composition
3.2. Vibrational Spectroscopy
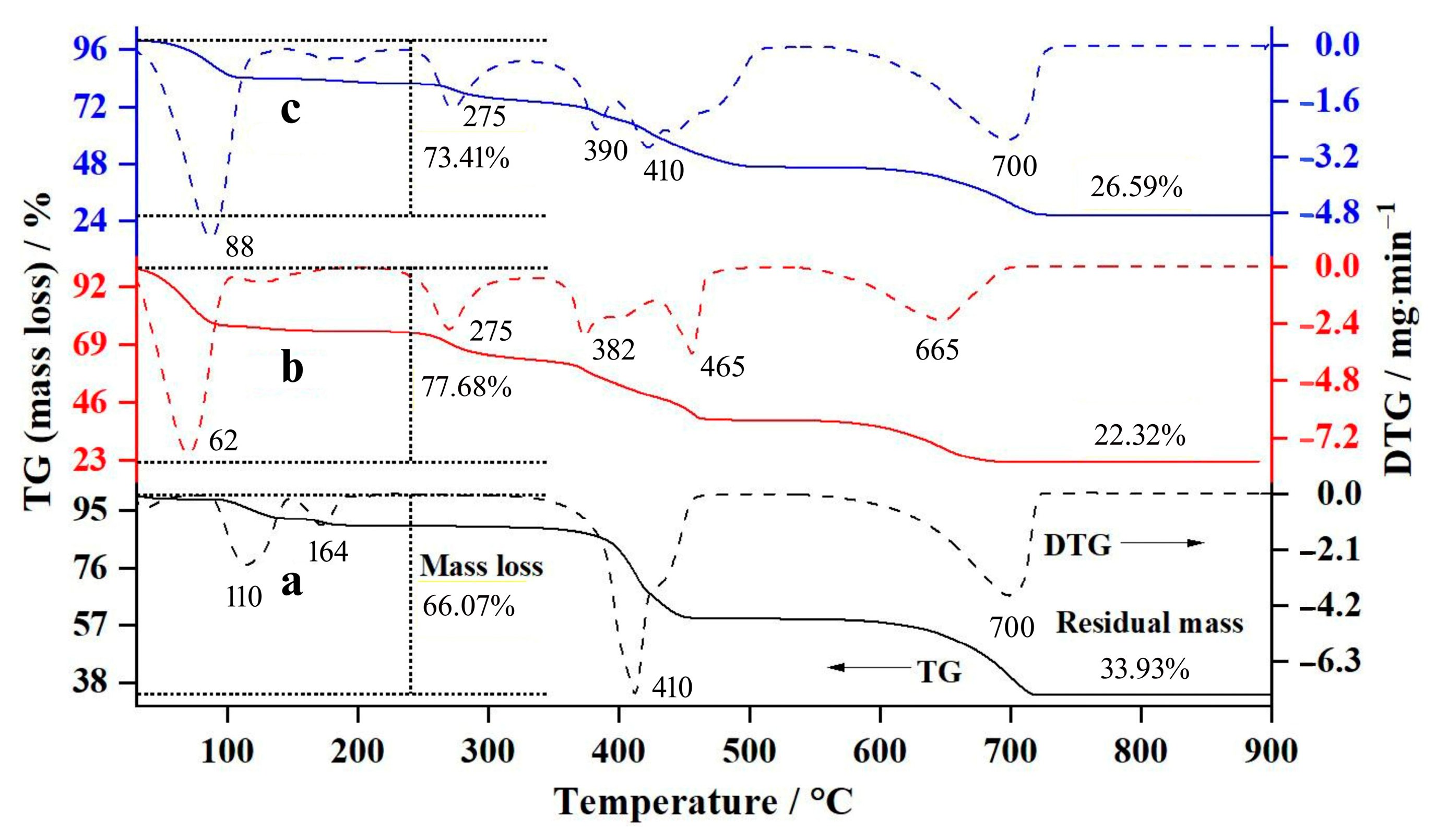
3.3. Thermal Decomposition
Ca(CH3COO)2·H2O(s) → Ca(CH3COO)2(s) + H2O(g)
Ca(CH3COO)2 (s) → CaCO3(s) + CH3COCH3(g)
CaCO3(s) → CaO(s) + CO2(g)
Ca(CH3CHOHCOO)2·5H2O(s) → Ca(CH3CHOHCOO)2(s) + 5H2O(g)
Ca(CH3CHOHCOO)2(s) → CaCO3(s) + CH3CHOHCOOC2H5(g)
Ca(CH3COO)(CH3CHOHCOO)·2H2O(s) → Ca(CH3COO)(CH3CHOHCOO)(s) + 2H2O(g)
Ca(CH3COO)(CH3CHOHCOO)(s) → CaCO3(s) + CH3CHOHCOCH3(g)
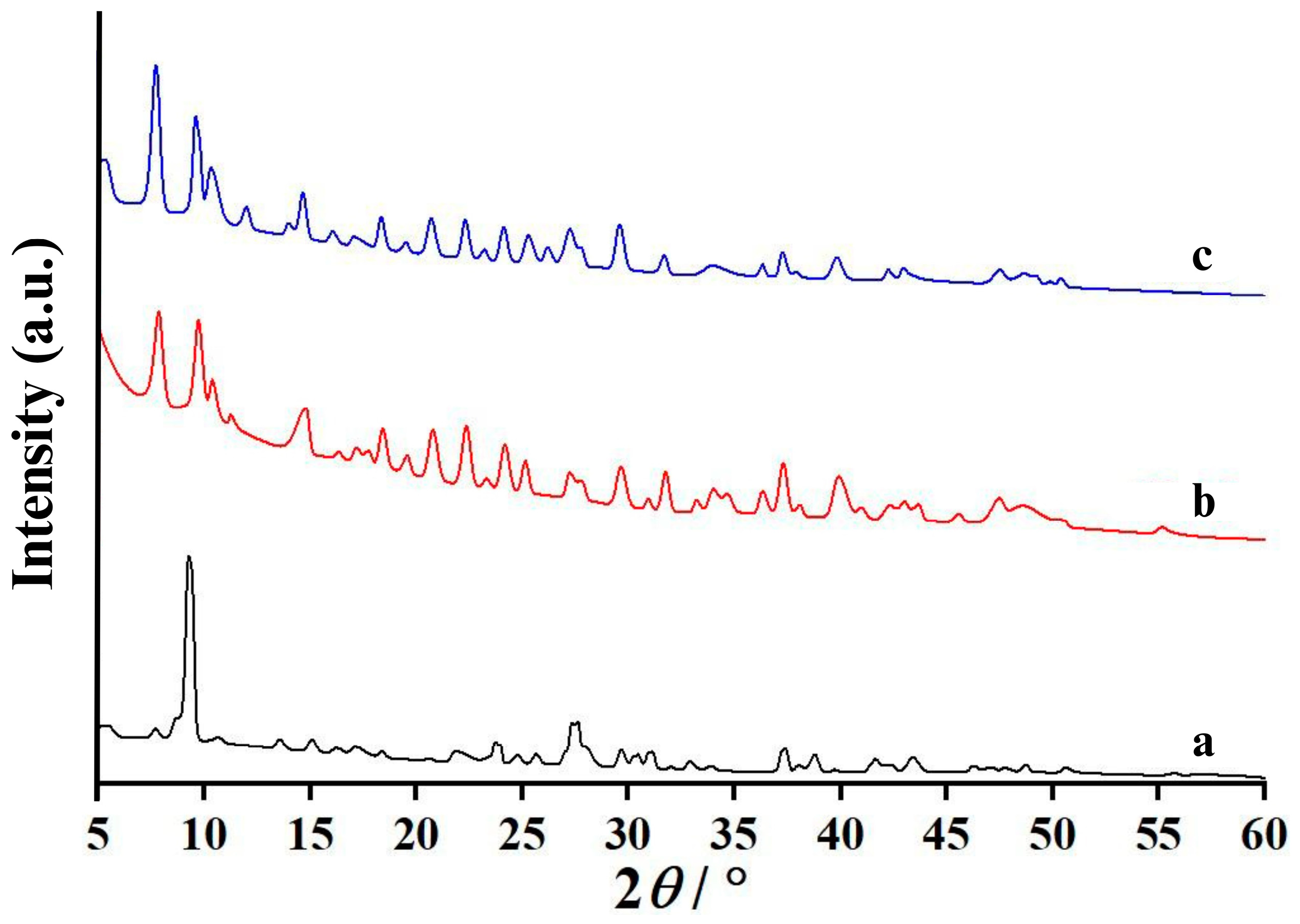
3.4. Crystallography
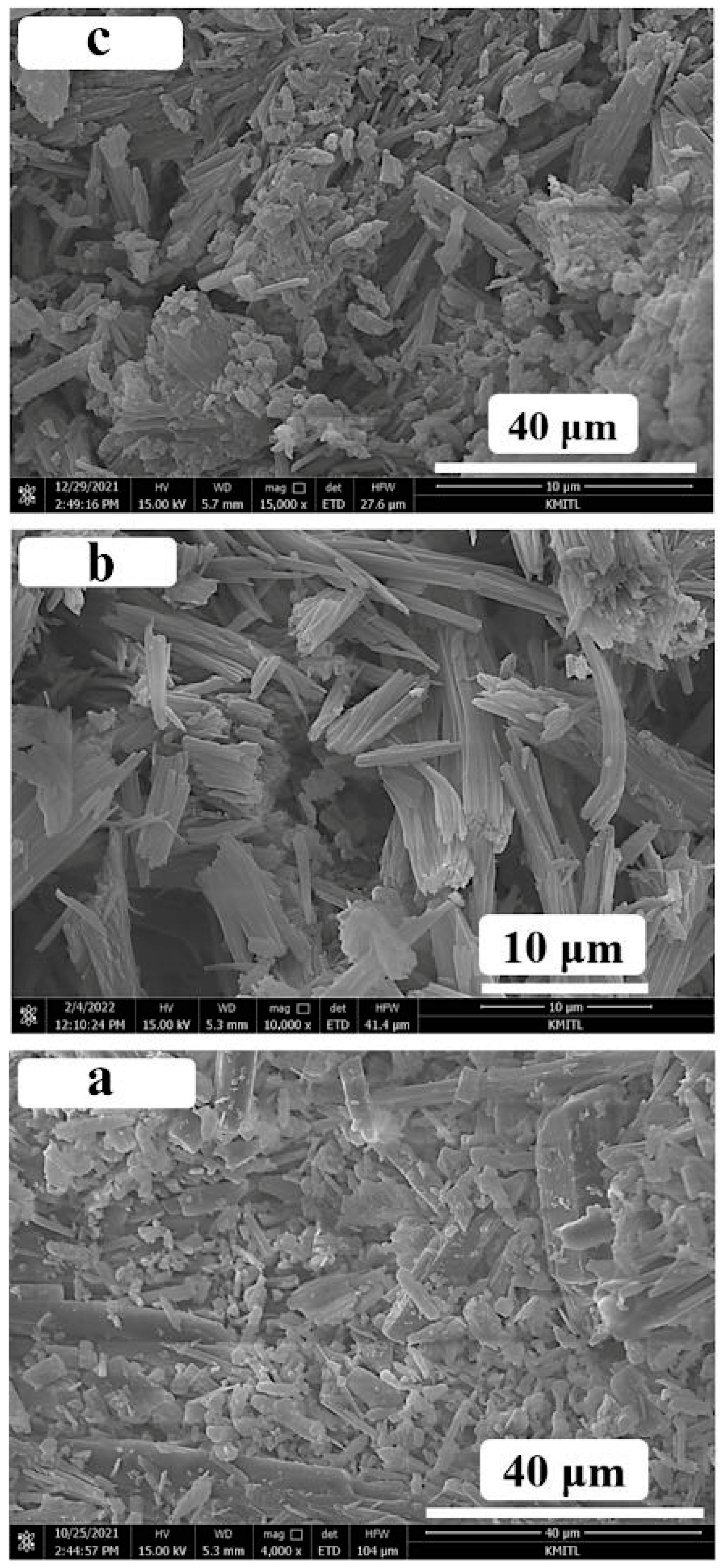
3.5. Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Hasegawa, Y. Reducing Effect of Feeding Powdered Scallop Shell on the Body Fat Mass of Rats. Biosci. Biotechnol. Biochem. 2006, 70, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Thongkam, M.; Saelim, J.; Boonchom, B.; Seesanong, S.; Chaiseeda, K.; Laohavisuti, N.; Bunya-Atichart, K.; Boonmee, W.; Taemchuay, D. Simple and Rapid Synthesis of Calcium Acetate from Scallop Shells to Reduce Environmental Issues. Adsorpt. Sci. Technol. 2021, 2021, 6450289. [Google Scholar] [CrossRef]
- Seesanong, S.; Wongchompoo, Y.; Boonchom, B.; Sronsri, C.; Laohavisuti, N.; Chaiseeda, K.; Boonmee, W. Economical and environmentally friendly track of biowaste recycling of scallop shells to calcium lactate. ACS Omega 2022, 7, 14756–14764. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; He, L.; Ma, X.; Xu, S. Modification of CaO-based sorbents prepared from calcium acetate for CO2 capture at high temperature. Chin. J. Chem. Eng. 2017, 25, 572–580. [Google Scholar] [CrossRef]
- Steciak, J.; Levendis, Y.A.; Wise, D.L. Effectiveness of calcium magnesium acetate as dual SO2-NOx emission control agent. AIChE J. 1995, 41, 712–722. [Google Scholar] [CrossRef]
- Nolan, C.R.; Qunibi, W.Y. Calcium salts in the treatment of hyperphosphatemia in hemodialysis patients. Curr. Opin. Nephrol. Hypertens. 2003, 12, 373–379. [Google Scholar] [CrossRef]
- Mititelu, M.; Moroșan, E.; Nicoară, A.C.; Secăreanu, A.A.; Musuc, A.M.; Atkinson, I.; Cusu, J.P.; Nițulescu, G.M.; Ozon, E.A.; Sarbu, I.; et al. Development of immediate release tablets containing calcium lactate synthesized from black sea mussel shells. Mar. Drugs 2022, 20, 45. [Google Scholar] [CrossRef]
- Tansman, G.; Kindstedt, P.; Hughes, J. Powder X-ray diffraction can differentiate between enantiomeric variants of calcium lactate pentahydrate crystal in cheese. J. Dairy Sci. 2014, 97, 7354–7362. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M. Effect of calcium lactate on compressive strength and self-healing of cracks in microbial concrete. Front. Struct. Civ. Eng. 2019, 13, 515–525. [Google Scholar] [CrossRef]
- Abdullah, R.; Abdullah, A.Z. Effect of catalyst to glycerol ratio in the production of lactic acid via hydrothermal reaction using calcium oxide and strontium oxide catalysts. AIP Conf. Proc. 2018, 2030, 020197. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Fernández, N.; Cruz, J.; Moldes, A. Optimization of the dose of calcium lactate as a new coagulant for the coagulation–flocculation of suspended particles in water. Desalination 2011, 280, 63–71. [Google Scholar] [CrossRef]
- Linares-Morales, J.R.; Gutiérrez-Méndez, N.; Rivera-Chavira, B.E.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018, 2, 50. [Google Scholar] [CrossRef]
- Additives, E.; Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Durjava, M.K.; López-Alonso, M.; Puente, S.L.; et al. Safety of lactic acid and calcium lactate when used as technological additives for all animal species. EFSA J. 2019, 17, e05914. [Google Scholar] [CrossRef]
- Daengprok, W.; Garnjanagoonchorn, W.; Mine, Y. Fermented pork sausage fortified with commercial or hen eggshell calcium lactate. Meat Sci. 2002, 62, 199–204. [Google Scholar] [CrossRef]
- Ullah, R.; Zafar, M.S.; Shahani, N. Potential fluoride toxicity from oral medicaments: A review. Iran. J. Basic Med. Sci. 2017, 20, 841–848. [Google Scholar] [CrossRef]
- Kleinman, K.; McDaniel, L.; Molloy, M. The Harriet Lane Handbook; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Feldman, E.C.; Nelson, R.W.; Reusch, C.; Scott-Moncrieff, J.C. Canine and Feline Endocrinology-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Voncken, J.H.L.; Verkroost, T.W.; van Tooren, M.M. New powder diffraction data on calclacite (CaClC2H3O2·5H2O). Neues Jahrb. Mineral. Monatshefte 2001, 5, 210–220. [Google Scholar]
- Wheeler, G.S.; Wypyski, M.T. An unusual efflorescence on Greek ceramics. Stud. Conserv. 1993, 38, 55–62. [Google Scholar] [CrossRef]
- van Tassel, R. On the crystallography of calclacite, Ca(CH3COO)Cl·5H2O. Acta Crystallogr. 1958, 11, 745–746. [Google Scholar] [CrossRef]
- Gibson, L.T.; Cooksey, B.G.; Littlejohn, D.; Tennent, N.H. Characterisation of an unusual crystalline efflorescence on an Egyptian limestone relief. Anal. Chim. Acta 1997, 337, 151–164. [Google Scholar] [CrossRef]
- Tennent, N.H.; Baird, T. The deterioration of mollusca collections: Identification of shell efflorescence. Stud. Conserv. 1985, 30, 73–85. [Google Scholar] [CrossRef]
- Bette, S.; Eggert, G.; Fischer, A.; Stelzner, J.; Dinnebier, R.E. Characterization of a new efflorescence salt on calcareous historic objects stored in wood cabinets: Ca2(CH3COO)(HCOO)(NO3)2·4H2O. Corros. Sci. 2018, 132, 68–78. [Google Scholar] [CrossRef]
- Cooksey, B.G.; Gibson, L.T.; Kennedy, A.R.; Littlejohn, D.; Stewart, L.; Tennent, N.H. Dicalcium triacetate nitrate dihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 324–326. [Google Scholar] [CrossRef]
- Boccia Paterakis, A.; Steiger, M. Salt efflorescence on pottery in the Athenian Agora: A closer look. Stud. Conserv. 2015, 60, 172–184. [Google Scholar] [CrossRef]
- Diehl, R.; Brandt, G.; Salje, E. The crystal structure of triclinic WO3. Acta Crystallogr. Sect. B 1978, 34, 1105–1111. [Google Scholar] [CrossRef]
- Wahlberg, N.; Runčevski, T.; Dinnebier, R.E.; Fischer, A.; Eggert, G.; Iversen, B.B. Crystal structure of thecotrichite, an efflorescent salt on calcareous objects stored in wooden cabinets. Cryst. Growth Des. 2015, 15, 2795–2800. [Google Scholar] [CrossRef]
- Bette, S.; Müller, M.X.; Eggert, G.; Schleid, T.; Dinnebier, R.E. Efflorescence on calcareous objects in museums: Crystallisation, phase characterisation and crystal structures of calcium acetate formate phases. Dalton Trans. 2019, 48, 16062–16073. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; Van Grieken, R. Sample preparation for X-ray fluorescence analysis. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley: Hoboken, NJ, USA, 2006; pp. 1–25. [Google Scholar] [CrossRef]
- Sronsri, C.; Boonchom, B. Thermal kinetic analysis of a complex process from a solid-state reaction by deconvolution procedure from a new calculation method and related thermodynamic functions of Mn0.90Co0.05Mg0.05HPO4·3H2O. Trans. Nonferrous Met. Soc. China 2018, 28, 1887–1902. [Google Scholar] [CrossRef]
- Sronsri, C.; U-yen, K.; Sittipol, W. Application of synthetic hureaulite as a new precursor for the synthesis of lithiophilite nanoparticles. Solid State Sci. 2020, 110, 106469. [Google Scholar] [CrossRef]
- Sronsri, C.; Boonchom, B. Synthesis, characterization, vibrational spectroscopy, and factor group analysis of partially metal-doped phosphate materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 230–240. [Google Scholar] [CrossRef]
- Musumeci, A.W.; Frost, R.L.; Waclawik, E.R. A spectroscopic study of the mineral paceite (calcium acetate). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Bette, S.; Eggert, G.; Emmerling, S.; Etter, M.; Schleid, T.; Dinnebier, R.E. Crystal structure, polymorphism, and anisotropic thermal expansion of α-Ca(CH3COO)2. Cryst. Growth Des. 2020, 20, 5346–5355. [Google Scholar] [CrossRef]
- Koleva, V. Vibrational behavior of calcium hydrogen triacetate monohydrate, CaH(CH3COO)3·H2O. Croat. Chem. Acta 2005, 78, 581–591. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2014. [Google Scholar]
- Cassanas, G.; Morssli, M.; Fabregue, E.; Bardet, L. Vibrational spectra of lactic acid and lactates. J. Raman Spectrosc. 1991, 22, 409–413. [Google Scholar] [CrossRef]
- Cheong, S.H. Physicochemical properties of calcium lactate prepared by single-phase aragonite precipitated calcium carbonate. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1786–1794. [Google Scholar]
- Lee, Y.-K.; Kim, S.-D. Preparation and characteristics of calcium lactate from black snail. Prev. Nutr. Food Sci. 2003, 8, 166–172. [Google Scholar] [CrossRef]
- Sronsri, C.; Boonchom, B. Deconvolution technique for the kinetic analysis of a complex reaction and the related thermodynamic functions of the formation of LiMn0.90Co0.05Mg0.05PO4. Chem. Phys. Lett. 2017, 690, 116–128. [Google Scholar] [CrossRef]
- Klop, E.; Schouten, A.; Van der Sluis, P.; Spek, A. Structure of calcium acetate monohydrate, Ca(C2H3O2)2·H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1984, 40, 51–53. [Google Scholar] [CrossRef]
- Johnson, M.E.; Riesterer, B.A.; Olson, N.F. Influence of nonstarter bacteria on calcium lactate crystallization on the surface of Cheddar cheese. J. Dairy. Sci. 1990, 73, 1145–1149. [Google Scholar] [CrossRef]
- Cao, X.; Lee, H.-J.; Yun, H.S.; Koo, Y.-M. Solubilities of calcium and zinc lactate in water and water-ethanol mixture. Korean J. Chem. Eng. 2001, 18, 133–135. [Google Scholar] [CrossRef]

| Samples | Reaction Time (min) | Production Yields (%) | Soluble Fractions (%) |
|---|---|---|---|
| CA | 65 | 88.24 ± 1.26 | 93.77 ± 1.42 |
| CL | 2 | 79.17 ± 1.13 | 90.18 ± 1.36 |
| CAL | 26 | 96.44 ± 1.24 | 95.08 ± 1.21 |
| Chemical Compositions | Chemical Content/wt% | ||
|---|---|---|---|
| CA | CL | CAL | |
| Calcium oxide (CaO) | 97.60 | 97.40 | 96.80 |
| Sodium oxide (Na2O) | 0.35 | 0.33 | 0.47 |
| Magnesium oxide (MgO) | 0.23 | 0.21 | 0.52 |
| Aluminum oxide (Al2O3) | 0.07 | 0.07 | 0.05 |
| Silicon dioxide (SiO2) | 0.22 | 0.20 | 0.16 |
| Phosphorus pentoxide (P2O5) | 0.06 | 0.09 | 0.16 |
| Sulfur trioxide (SO3) | 0.92 | 1.13 | 1.34 |
| Chloride (Cl−) | – | 0.01 | – |
| Potassium oxide (K2O) | 0.02 | 0.03 | 0.03 |
| Manganese oxide (MnO) | – | – | – |
| Ferric oxide (Fe2O3) | 0.04 | 0.06 | 0.04 |
| Strontium oxide (SrO) | 0.50 | 0.52 | 0.46 |
| Total | 99.97 | 100.04 | 100.02 |
| Vibrational Modes | Vibrational Symbols | Wavenumber/cm−1 |
|---|---|---|
| Asymmetric O–H stretching of H2O | νas(O–H) | 3678–3286 |
| Symmetric O–H stretching of H2O | νs(O–H) | 3286–3069 |
| Asymmetric C–H stretching of CH3 of CH3COO− | νas(H2C–H) | 2984–2886 |
| Symmetric C–H stretching of CH3 of CH3COO− | νs(H2C–H) | 2886–2814 |
| H–O–H bending of H2O, asymmetric C=O and symmetric C=O stretching of COO− of CH3COO− | δ(H2O), νas(C=O) and νs(C=O) | 1720–1563 |
| Asymmetric C–O stretching of COO− of CH3COO− | νas(C–O) | 1563–1487 |
| Symmetric C–O stretching of COO− of CH3COO− | νs(C–O) | 1487–1429 |
| Asymmetric CH3 bending of CH3COO− | δas(CH3) | 1429–1353 |
| Symmetric CH3 bending of CH3COO− | δs(CH3) | 1353–1272 |
| Out-of-plane CH3 bending of CH3COO− | ρop(CH3) | 1077–1039 |
| In-plane CH3 bending of CH3COO− | ρip(CH3) | 1039–981 |
| C–C stretching of C–CH3 of CH3COO− | ν(C–C) | 981–917 |
| Symmetric O=C–O bending (twisting and rocking) of COO− of CH3COO− | δst(O=C–O) and δsr(O=C–O) | 695–665 |
| Out-of-plane O=C–O stretching of COO− of CH3COO− | ρop(O=C–O) | 665–597 |
| Ca–O stretching | ν(Ca–O) | 498–445 |
| In-plane COO− bending (rocking) of CH3COO− | r(COO−) | 445–400 |
| Vibrational Modes | Vibrational Symbols | Wavenumber/cm−1 |
|---|---|---|
| Asymmetric O–H stretching of H2O | νas(O–H) | 3695–3316 |
| Symmetric O–H stretching of H2O | νs(O–H) | 3316–3024 |
| Asymmetric C–H stretching of CH3 of CH3CHOHCOO− | νas(H2C–H) | 3024–2961 |
| Symmetric C–H stretching of CH3 of CH3CHOHCOO− | νs(H2C–H) | 2961–2917 |
| C–H stretching of CH of CH3CHOHCOO− | ν(C–H) | 2917–2826 |
| H–O–H bending of H2O, asymmetric C=O and symmetric C=O stretching of COO− of CH3CHOHCOO− | δ(H2O), νas(C=O) and νs(C=O) | 1805–1418 |
| Asymmetric CH3 bending (twisting) of CH3CHOHCOO− | δas(CH3) | 1513–1450 |
| Symmetric CH3 bending (twisting and rocking) of CH3CHOHCOO− | δs(CH3) and r(CH3) | 1373–924 |
| Symmetric C–H bending of CH of CH3CHOHCOO− | δ(CH) | 1337–1246 |
| C–C stretching of C–CH3 of CH3CHOHCOO− | ν(C–CH3) | 1068–1015 |
| C–C stretching of C–COO− of CH3CHOHCOO− | ν(C–COO−) | 883–842 |
| Out-of-plane COO− bending (twisting) of CH3CHOHCOO− | t(COO−) | 842–765 |
| Symmetric C–C bending of C–COH of CH3CHOHCOO− | δ(C–COH) | 765–610 |
| Out-of-plane COO− bending (wagging) of CH3CHOHCOO− | w(COO−) | 610–483 |
| Ca–O stretching | ν(Ca–O) | 483–442 |
| In-plane COO− bending (rocking) of CH3CHOHCOO− | r(COO−) | 442–400 |
| Vibrational Modes | Vibrational Symbols | Wavenumber/cm−1 |
|---|---|---|
| Asymmetric O–H stretching of H2O | νas(O–H) | 3694–3213 |
| Symmetric O–H stretching of H2O | νs(O–H) | 3213–3026 |
| Asymmetric C–H stretching of CH3 of CH3COO− and CH3CHOHCOO− | νas(H2C–H) | 3026–2846 |
| Symmetric C–H stretching of CH3 of CH3COO− and CH3CHOHCOO− | νs(H2C–H) | 2876–2814 |
| C–H stretching of CH of CH3COO− and CH3CHOHCOO− | ν(C–H) | 2814–2711 |
| H–O–H bending of H2O, asymmetric C=O and symmetric C=O stretching of COO− of CH3COO− and CH3CHOHCOO− | δ(H2O), νas(C=O) and νs(C=O) | 1763–1452 |
| Asymmetric CH3 bending (twisting) of CH3COO− and CH3CHOHCOO− | δas(CH3) | 1509–1452 |
| Symmetric CH3 bending (twisting and rocking) of CH3COO− and CH3CHOHCOO− | δs(CH3) and r(CH3) | 1373–924 |
| Symmetric C–H bending of CH of CH3COO− and CH3CHOHCOO− | δ(CH) | 1339–1246 |
| C–C stretching of C–CH3 of CH3COO− and CH3CHOHCOO− | ν(C–CH3) | 1153–1004 |
| C–C stretching of C–COO− of CH3COO− and CH3CHOHCOO− | ν(C–COO−) | 977–839 |
| Out-of-plane COO− bending (twisting) of CH3COO− and CH3CHOHCOO− | t(COO−) | 839–758 |
| Symmetric C–C bending of C–COH of CH3CHOHCOO− | δ(C–COH) | 758–638 |
| Out-of-plane COO− bending (wagging) of CH3COO− and CH3CHOHCOO− | w(COO−) | 638–456 |
| Ca–O stretching | ν(Ca–O) | 456–423 |
| In-plane COO− bending (rocking) of CH3COO− and CH3CHOHCOO− | r(COO−) | 456–400 |
| Samples | Steps | Temperatures/°C | DTG Peak/°C | Mass Losses/% | Residual Masses/% | ||
|---|---|---|---|---|---|---|---|
| Experiment | Theory | Experiment | Theory | ||||
| CA | 1st | 30–200 | 110 | 10.24 | 10.23 | 89.76 | 89.77 |
| 1st–2nd | 30–470 | 410 | 40.85 | 43.19 | 59.15 | 56.81 | |
| 1st–3rd | 30–720 | 700 | 66.07 | 68.17 | 33.93 | 31.83 | |
| CL | 1st | 30–170 | 62 | 25.84 | 29.22 | 74.16 | 70.78 |
| 1st–2nd | 30–480 | 225, 382, 465 | 61.14 | 67.54 | 38.86 | 32.46 | |
| 1st–3rd | 30–690 | 665 | 77.68 | 81.81 | 22.32 | 18.19 | |
| CAL | 1st | 30–130 | 88 | 15.94 | 16.14 | 84.06 | 83.89 |
| 1st–2nd | 30–510 | 275, 390, 410 | 53.52 | 55.61 | 46.48 | 44.39 | |
| 1st–3rd | 30–730 | 700 | 73.41 | 75.33 | 26.59 | 24.67 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongkol, S.; Seesanong, S.; Boonchom, B.; Laohavisuti, N.; Boonmee, W.; Thompho, S.; Rungrojchaipon, P. Simple Rapid Production of Calcium Acetate Lactate from Scallop Shell Waste for Agricultural Application. Int. J. Mol. Sci. 2025, 26, 4488. https://doi.org/10.3390/ijms26104488
Mongkol S, Seesanong S, Boonchom B, Laohavisuti N, Boonmee W, Thompho S, Rungrojchaipon P. Simple Rapid Production of Calcium Acetate Lactate from Scallop Shell Waste for Agricultural Application. International Journal of Molecular Sciences. 2025; 26(10):4488. https://doi.org/10.3390/ijms26104488
Chicago/Turabian StyleMongkol, Sorakit, Somkiat Seesanong, Banjong Boonchom, Nongnuch Laohavisuti, Wimonmat Boonmee, Somphob Thompho, and Pesak Rungrojchaipon. 2025. "Simple Rapid Production of Calcium Acetate Lactate from Scallop Shell Waste for Agricultural Application" International Journal of Molecular Sciences 26, no. 10: 4488. https://doi.org/10.3390/ijms26104488
APA StyleMongkol, S., Seesanong, S., Boonchom, B., Laohavisuti, N., Boonmee, W., Thompho, S., & Rungrojchaipon, P. (2025). Simple Rapid Production of Calcium Acetate Lactate from Scallop Shell Waste for Agricultural Application. International Journal of Molecular Sciences, 26(10), 4488. https://doi.org/10.3390/ijms26104488







