Interaction Between Neutrophils and Elements of the Blood–Brain Barrier in the Context of Multiple Sclerosis and Ischemic Stroke
Abstract
1. Introduction
2. Neuroinflammation-Induced BBB Dysfunction in MS and IS
3. Neutrophils
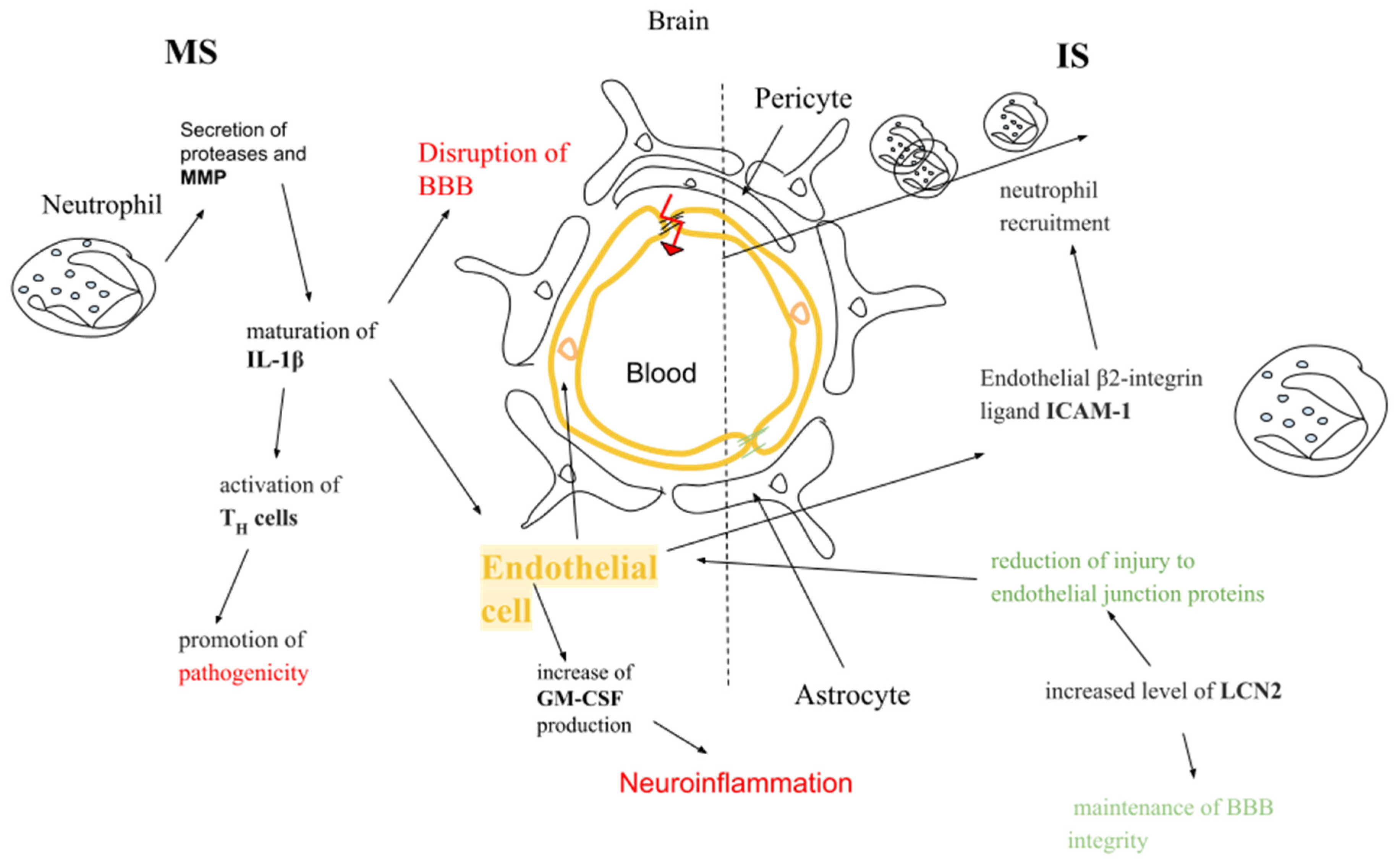
4. Neutrophils and Endothelial Cells
4.1. Neutrophil Extravasation Cascade
4.2. In the Context of Multiple Sclerosis and Ischemic Stroke
4.3. In the Context of Multiple Sclerosis
4.4. In the Context of Ischemic Stroke
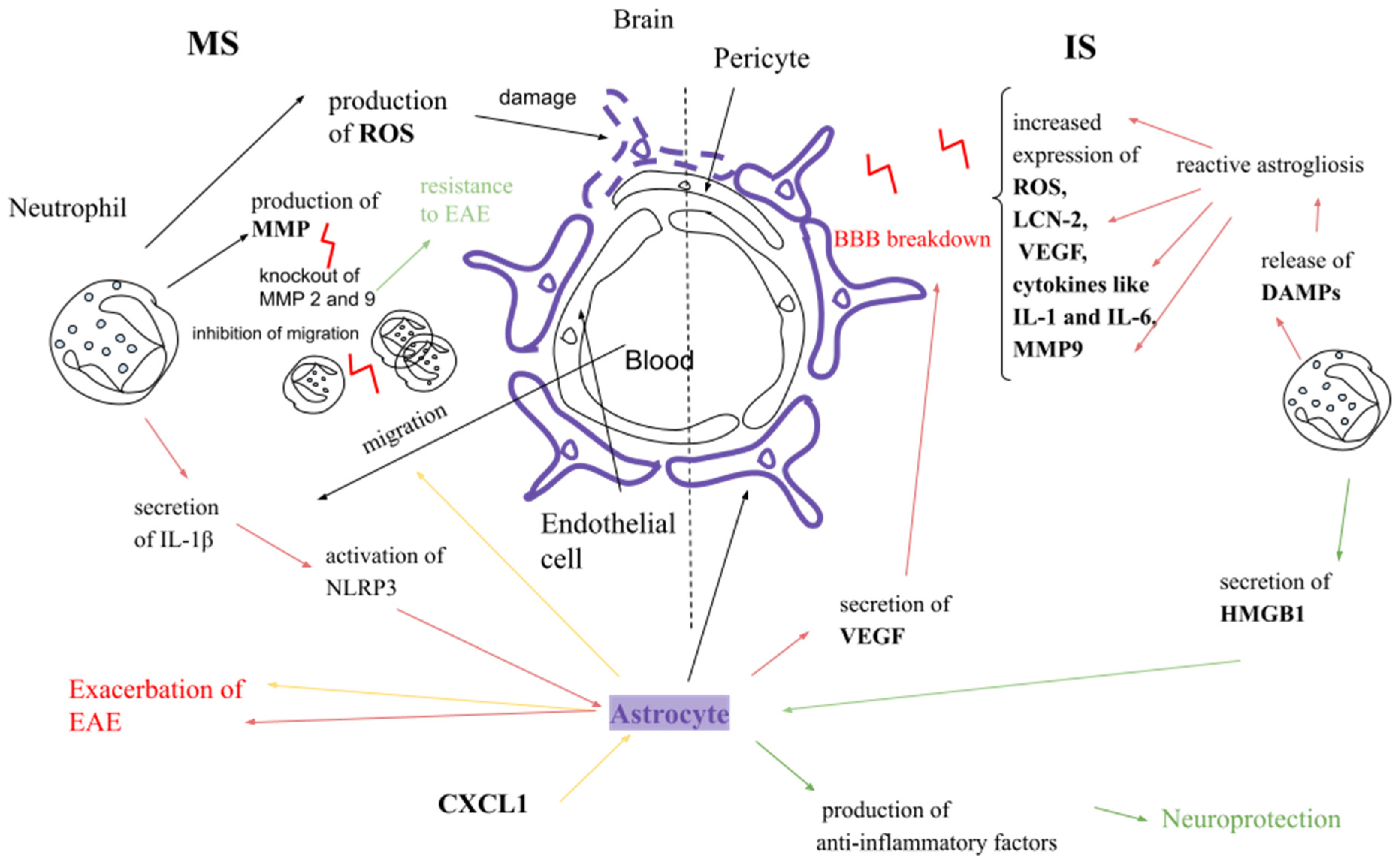
5. Neutrophils and Astrocytes
5.1. In the Context of Multiple Sclerosis
5.2. In the Context of Ischemic Stroke
6. Neutrophils and Pericytes
6.1. In the Context of Multiple Sclerosis
6.2. In the Context of Ischemic Stroke
7. New Therapeutic Strategies for MS and IS
8. Therapeutic Neutrophil Function
9. Discussion
10. Conclusions
11. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood-Brain Barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Riley, J.T.; Dardik, A. Molecules in Focus Cells in Focus: Endothelial Cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The Blood–Brain Barrier as an Endocrine Tissue. Nat. Rev. Endocrinol. 2019, 15, 444–455. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular Endothelium—Gatekeeper of Vessel Health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Alexander, J.S.; Zivadinov, R.; Maghzi, A.H.; Ganta, V.C.; Harris, M.K.; Minagar, A. Multiple Sclerosis and Cerebral Endothelial Dysfunction: Mechanisms. Pathophysiology 2011, 18, 3–12. [Google Scholar] [CrossRef]
- de Majo, M.; Koontz, M.; Rowitch, D.; Ullian, E.M. An Update on Human Astrocytes and Their Role in Development and Disease. Glia 2020, 68, 685–704. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic Physiology of the Blood-Brain Barrier in Health and Disease: A Brief Overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Willis, C.L.; Nolan, C.C.; Reith, S.N.; Lister, T.; Prior, M.J.W.; Guerin, C.J.; Mavroudis, G.; Ray, D.E. Focal Astrocyte Loss Is Followed by Microvascular Damage, with Subsequent Repair of the Blood-Brain Barrier in the Apparent Absence of Direct Astrocytic Contact. Glia 2004, 45, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-Derived VEGF-A Drives Blood-Brain Barrier Disruption in CNS Inflammatory Disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, J.; Han, L.; Wu, X.; Shi, Y.; Cui, W.; Zhang, S.; Hu, Q.; Wang, J.; Bai, H.; et al. The Specific Role of Reactive Astrocytes in Stroke. Front. Cell. Neurosci. 2022, 16, 850866. [Google Scholar] [CrossRef]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020420. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro-Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakao, S.; Nakaoke, R.; Nakagawa, S.; Kitagawa, N.; Niwa, M. Effects of Hypoxia on Endothelial/Pericytic Co-Culture Model of the Blood-Brain Barrier. Regul. Pept. 2004, 123, 77–83. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Crivellato, E. The Role of Pericytes in Angiogenesis. Int. J. Dev. Biol. 2011, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes Are Required for Blood-brain Barrier Integrity during Embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting Pericytes for Therapeutic Approaches to Neurological Disorders. Acta Neuropathol. 2018, 136, 507–523. [Google Scholar] [CrossRef]
- Claudio, L.; Celia, C.S.R.; Brosnan, E. Evidence of Persistent Blood-Brain Barrier Abnormalities in Chronic-Progressive Multiple Sclerosis. Acta Neuropathol. 1995, 90, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Tsai, H.H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Jansson, D.; Smyth, L.C.; Dragunow, M. Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol. Sci. 2017, 38, 291–304. [Google Scholar] [CrossRef]
- Combes, V.; Guillemin, G.J.; Chan-Ling, T.; Hunt, N.H.; Grau, G.E. The crossroads of neuroinflammation in infectious diseases: Endothelial cells and astrocytes. Trends Parasitol. 2012, 28, 311–319. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Rezaie, A.R. Protease-activated receptor signaling by coagulation proteases in endothelial cells. Thromb. Haemost. 2014, 112, 876–882. [Google Scholar] [CrossRef]
- Santos-Lima, B.; Pietronigro, E.C.; Terrabuio, E.; Zenaro, E.; Constantin, G. The Role of Neutrophils in the Dysfunction of Central Nervous System Barriers. Front. Aging Neurosci. 2022, 14, 965169. [Google Scholar] [CrossRef]
- Agrawal, S.; Anderson, P.; Durbeej, M.; Van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L.M. Dystroglycan Is Selectively Cleaved at the Parenchymal Basement Membrane at Sites of Leukocyte Extravasation in Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1016. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative Regulators of Neuroinflammation in Stroke and Other Neurological Diseases. Neurother. J. Am. Soc. Exp. Neurother. 2016, 13, 685–701. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Soelberg Sørensen, P.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Brenda Banwell, M.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the Blood-Brain Barrier in Multiple Sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef]
- Aubé, B.; Lévesque, S.A.; Paré, A.; Chamma, É.; Kébir, H.; Gorina, R.; Lécuyer, M.-A.; Alvarez, J.I.; De Koninck, Y.; Engelhardt, B.; et al. Neutrophils Mediate Blood–Spinal Cord Barrier Disruption in Demyelinating Neuroinflammatory Diseases. J. Immunol. 2014, 193, 2438–2454. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Segel, G.B.; Halterman, M.W.; Lichtman, M.A. The Paradox of the Neutrophil’s Role in Tissue Injury. J. Leukoc. Biol. 2011, 89, 359–372. [Google Scholar] [CrossRef]
- Bolton, S.J.; Anthony, D.C.; Perry, V.H. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience 1998, 86, 1245–1257. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar] [CrossRef]
- Yu, G.; Liang, Y.; Zheng, S.; Zhang, H. Inhibition of Myeloperoxidase by N-Acetyl Lysyltyrosylcysteine Amide Reduces Oxidative Stress-Mediated Inflammation, Neuronal Damage, and Neural Stem Cell Injury in a Murine Model of Stroke. J. Pharmacol. Exp. Ther. 2018, 364, 311–322. [Google Scholar] [CrossRef]
- Zhang, H.; Ray, A.; Miller, N.M.; Hartwig, D.; Pritchard, K.A.; Dittel, B.N. Inhibition of Myeloperoxidase at the Peak of Experimental Autoimmune Encephalomyelitis Restores Blood-Brain Barrier Integrity and Ameliorates Disease Severity. J. Neurochem. 2016, 136, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hao, Y.; Feng, L.; Wang, T.; Yao, M.; Li, H.; Ma, D.; Feng, J. Neutrophil Heterogeneity and Its Roles in the Inflammatory Network after Ischemic Stroke. Curr. Neuropharmacol. 2022, 21, 621–650. [Google Scholar] [CrossRef]
- Wang, G.; Weng, Y.C.; Chiang, I.C.; Huang, Y.T.; Liao, Y.C.; Chen, Y.C.; Kao, C.Y.; Liu, Y.L.; Lee, T.H.; Chou, W.H. Neutralization of Lipocalin-2 Diminishes Stroke-Reperfusion Injury. Int. J. Mol. Sci. 2020, 21, 6253. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Klett, F.; Priller, J. Diverse Functions of Pericytes in Cerebral Blood Flow Regulation and Ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Underly, R.G.; Levy, M.; Hartmann, D.A.; Grant, R.I.; Watson, A.N.; Shih, A.Y. Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J. Neurosci. 2017, 37, 129–140. [Google Scholar] [CrossRef]
- Christy, A.L.; Walker, M.E.; Hessner, M.J.; Brown, M.A. Mast Cell Activation and Neutrophil Recruitment Promotes Early and Robust Inflammation in the Meninges in EAE. J. Autoimmun. 2013, 42, 50–61. [Google Scholar] [CrossRef]
- Kostic, M.; Dzopalic, T.; Zivanovic, S.; Zivkovic, N.; Cvetanovic, A.; Stojanovic, I.; Vojinovic, S.; Marjanovic, G.; Savic, V.; Colic, M. IL-17 and Glutamate Excitotoxicity in the Pathogenesis of Multiple Sclerosis. Scand. J. Immunol. 2014, 79, 181–186. [Google Scholar] [CrossRef]
- Proebstl, D.; Voisin, M.B.; Woodfin, A.; Whiteford, J.; D’Acquisto, F.; Jones, G.E.; Rowe, D.; Nourshargh, S. Pericytes Support Neutrophil Subendothelial Cell Crawling and Breaching of Venular Walls in Vivo. J. Exp. Med. 2012, 209, 1219–1234. [Google Scholar] [CrossRef]
- Gil, E.; Venturini, C.; Stirling, D.; Turner, C.; Tezera, L.B.; Ercoli, G.; Baker, T.; Best, K.; Brown, J.S.; Noursadeghi, M. Pericyte Derived Chemokines Amplify Neutrophil Recruitment across the Cerebrovascular Endothelial Barrier. Front. Immunol. 2022, 13, 935798. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Zhang, C.L.; Chen, A.Q.; Wang, H.L.; Zhou, Y.F.; Li, Y.N.; Hu, B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front. Immunol. 2021, 12, 678744. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M.F. The Role of Astrocytes in Multiple Sclerosis Progression. Front. Neurol. 2015, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T. The role of CD4 T cells in the pathogenesis of multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 43–72. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Crack, P.J. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr. Med. Chem. 2008, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Ginis, I.; Jaiswal, R.; Klimanis, D.; Liu, J.; Greenspon, J.; Hallenbeck, J.M. TNF-alpha-induced tolerance to ischemic injury involves differential control of NF-kappaB transactivation: The role of NF-kappaB association with p300 adaptor. J. Cereb. Blood Flow Metab. 2002, 22, 142–152. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int. J. Mol. Sci. 2021, 23, 14. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Macías-Islas, M.A.; Pacheco-Moisés, F.P.; Cruz-Ramos, J.A.; Sustersik, S.; Barba, A.; Aguayo, A. Oxidative Stress Is Increased in Serum from Mexican Patients with Relapsing-Remitting Multiple Sclerosis. Dis. Markers 2009, 26, 35–39. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 324475. [Google Scholar] [CrossRef]
- Ericson, J.A.; Duffau, P.; Yasuda, K.; Ortiz-Lopez, A.; Rothamel, K.; Rifkin, I.R.; Monach, P.A.; Best, A.J.; Knell, J.; Goldrath, A.; et al. Gene Expression during the Generation and Activation of Mouse Neutrophils: Implication of Novel Functional and Regulatory Pathways. PLoS ONE 2014, 9, e0108553. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of Apoptotic Neutrophils and Resolution of Inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef]
- Mishalian, I.; Granot, Z.; Fridlender, Z.G. The Diversity of Circulating Neutrophils in Cancer. Immunobiology 2017, 222, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.L.; Babamale, A.O.; Walker, T.L.; Cognasse, F.; Blum, D.; Burnouf, T. Blood-brain crosstalk: The roles of neutrophils, platelets, and neutrophil extracellular traps in neuropathologies. Trends Neurosci. 2023, 46, 764–779. [Google Scholar] [CrossRef]
- Hong, C.W. Extracellular Vesicles of Neutrophils. Immune Netw. 2018, 18, e43. [Google Scholar] [CrossRef]
- Kolonics, F.; Szeifert, V.; Timár, C.I.; Ligeti, E.; Lőrincz, Á.M. The Functional Heterogeneity of Neutrophil-Derived Extracellular Vesicles Reflects the Status of the Parent Cell. Cells 2020, 18, 2718. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Sansores-España, L.D.; Melgar-Rodríguez, S.; Vernal, R.; Carrillo-Ávila, B.A.; Martínez-Aguilar, V.M.; Díaz-Zúñiga, J. Neutrophil N1 and N2 Subsets and Their Possible Association with Periodontitis: A Scoping Review. Int. J. Mol. Sci. 2022, 23, 12068. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Li, Z.; Wang, X.Y.; Yi, H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front. Immunol. 2018, 9, 2456. [Google Scholar] [CrossRef]
- Woodfin, A.; Voisin, M.B.; Beyrau, M.; Colom, B.; Caille, D.; Diapouli, F.M.; Nash, G.B.; Chavakis, T.; Albelda, S.M.; Rainger, G.E.; et al. The Junctional Adhesion Molecule JAM-C Regulates Polarized Transendothelial Migration of Neutrophils in Vivo. Nat. Immunol. 2011, 12, 761–769. [Google Scholar] [CrossRef]
- Ode, Y.; Aziz, M.; Wang, P. CIRP Increases ICAM-1 + Phenotype of Neutrophils Exhibiting Elevated INOS and NETs in Sepsis. J. Leukoc. Biol. 2018, 103, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Aziz, M.; Ode, Y.; Wang, P. CIRP Induces Neutrophil Reverse Transendothelial Migration in Sepsis. Shock 2019, 51, 548–556. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Filippi, M.-D. Neutrophil Transendothelial Migration: Updates and New Perspectives. Blood 2019, 133, 2149–2158. [Google Scholar] [CrossRef]
- Schnoor, M.; Vadillo, E.; Guerrero-Fonseca, I.M. The Extravasation Cascade Revisited from a Neutrophil Perspective. Curr. Opin. Physiol. 2021, 19, 119–128. [Google Scholar] [CrossRef]
- Sundd, P.; Pospieszalska, M.K.; Ley, K. Neutrophil Rolling at High Shear: Flattening, Catch Bond Behavior, Tethers and Slings. Mol. Immunol. 2013, 55, 59–69. [Google Scholar] [CrossRef]
- Setiadi, H.; Yago, T.; Liu, Z.; McEver, R.P. Endothelial Signaling by Neutrophil-Released Oncostatin M Enhances P-Selectin–Dependent Inflammation and Thrombosis. Blood Adv. 2019, 3, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Vadillo, E.; Chánez-Paredes, S.; Vargas-Robles, H.; Guerrero-Fonseca, I.M.; Castellanos-Martínez, R.; García-Ponce, A.; Nava, P.; Girón-Pérez, D.A.; Santos-Argumedo, L.; Schnoor, M. Intermittent rolling is a defect of the extravasation cascade caused by Myosin1e-deficiency in neutrophils. Proc. Natl. Acad. Sci. USA 2019, 116, 26752–26758. [Google Scholar] [CrossRef] [PubMed]
- Cappenberg, A.; Kardell, M.; Zarbock, A. Selectin-Mediated Signaling—Shedding Light on the Regulation of Integrin Activity in Neutrophils. Cells 2022, 11, 1310. [Google Scholar] [CrossRef]
- David, B.A.; Kubes, P. Exploring the Complex Role of Chemokines and Chemoattractants in Vivo on Leukocyte Dynamics. Immunol. Rev. 2019, 289, 9–30. [Google Scholar] [CrossRef]
- Latasiewicz, J.; Artz, A.; Jing, D.; Blanco, M.P.; Currie, S.M.; Avila, M.V.; Schnoor, M.; Vestweber, D. HS1 Deficiency Impairs Neutrophil Recruitment in Vivo and Activation of the Small GTPases Rac1 and Rap1. J. Leukoc. Biol. 2017, 101, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Boras, M.; Volmering, S.; Bokemeyer, A.; Rossaint, J.; Block, H.; Bardel, B.; Van Marck, V.; Heitplatz, B.; Kliche, S.; Reinhold, A.; et al. Skap2 Is Required for Β2 Integrin-Mediated Neutrophil Recruitment and Functions. J. Exp. Med. 2017, 214, 851–874. [Google Scholar] [CrossRef] [PubMed]
- Girbl, T.; Lenn, T.; Perez, L.; Rolas, L.; Barkaway, A.; Thiriot, A.; del Fresno, C.; Lynam, E.; Hub, E.; Thelen, M.; et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018, 49, 1062–1076.e6. [Google Scholar] [CrossRef]
- Kuwano, Y.; Adler, M.; Zhang, H.; Groisman, A.; Ley, K. Gαi2 and Gαi3 Differentially Regulate Arrest from Flow and Chemotaxis in Mouse Neutrophils. J. Immunol. 2016, 196, 3828–3833. [Google Scholar] [CrossRef]
- Block, H.; Stadtmann, A.; Riad, D.; Rossaint, J.; Sohlbach, C.; Germena, G.; Wu, D.; Simon, S.I.; Ley, K.; Zarbock, A. Gnb Isoforms Control a Signaling Pathway Comprising Rac1, Plcb2, and Plcb3 Leading to LFA-1 Activation and Neutrophil Arrest in Vivo Key Points. Blood 2016, 127, 314–324. [Google Scholar] [CrossRef]
- Buffone, A.; Anderson, N.R.; Hammer, D.A. Human Neutrophils Will Crawl Upstream on ICAM-1 If Mac-1 Is Blocked. Biophys. J. 2019, 117, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, H.; Wang, M.; Lü, S.; Zhang, Y.; Long, M. Ligand-Specific Binding Forces of LFA-1 and Mac-1 in Neutrophil Adhesion and Crawling. Mol. Biol. Cell 2018, 29, 408–418. [Google Scholar] [CrossRef]
- Arokiasamy, S.; Zakian, C.; Dilliway, J.; Wang, W.; Nourshargh, S.; Voisin, M.B. Endogenous TNFα Orchestrates the Trafficking of Neutrophils into and within Lymphatic Vessels during Acute Inflammation. Sci. Rep. 2017, 7, 44189. [Google Scholar] [CrossRef]
- Pestonjamasp, K.N.; Forster, C.; Sun, C.; Gardiner, E.M.; Bohl, B.; Weiner, O.; Bokoch, G.M.; Glogauer, M. Rac1 Links Leading Edge and Uropod Events through Rho and Myosin Activation during Chemotaxis. Blood 2006, 108, 2814–2820. [Google Scholar] [CrossRef]
- Yang, L.; Froio, R.M.; Sciuto, T.E.; Dvorak, A.M.; Alon, R.; Luscinskas, F.W. ICAM-1 Regulates Neutrophil Adhesion and Transcellular Migration of TNF-α-Activated Vascular Endothelium under Flow. Blood 2005, 106, 584–592. [Google Scholar] [CrossRef]
- Marmon, S.; Hinchey, J.; Oh, P.; Cammer, M.; De Almeida, C.J.; Gunther, L.; Raine, C.S.; Lisanti, M.P. Caveolin-1 Expression Determines the Route of Neutrophil Extravasation through Skin Microvasculature. Am. J. Pathol. 2009, 174, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lossinsky, A.S.; Shivers, R.R. Structural Pathways for Macromolecular and Cellular Transport across the Blood-Brain Barrier during Inflammatory Conditions. Review. Histol. Histopathol. 2004, 19, 535–564. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e0032366. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Chatterjee, V.; Meegan, J.E.; Beard, R.S.; Yuan, S.Y. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front. Immunol. 2019, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Montero-Melendez, T.; Norling, L.V.; Yin, X.; Hinds, C.; Haskard, D.; Mayr, M.; Perretti, M. Heterogeneity in Neutrophil Microparticles Reveals Distinct Proteome and Functional Properties. Mol. Cell. Proteom. 2013, 12, 2205–2219. [Google Scholar] [CrossRef]
- McArthur, S.; Loiola, R.A.; Maggioli, E.; Errede, M.; Virgintino, D.; Solito, E. The Restorative Role of Annexin A1 at the Blood-Brain Barrier. Fluids Barriers CNS 2016, 13, 17. [Google Scholar] [CrossRef]
- Ajikumar, A.; Long, M.B.; Heath, P.R.; Wharton, S.B.; Ince, P.G.; Ridger, V.C.; Simpson, J.E. Neutrophil-Derived Microvesicle Induced Dysfunction of Brain Microvascular Endothelial Cells in Vitro. Int. J. Mol. Sci. 2019, 20, 5227. [Google Scholar] [CrossRef]
- Provost, P.; Lam, J.Y.T.; Lacoste, L.; Merhi, Y.; Waters, D. Endothelium-Derived Nitric Oxide Attenuates Neutrophil Adhesion to Endothelium Under Arterial Flow Conditions. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 331–335. [Google Scholar] [CrossRef]
- Strieter, R.M.; Kunkel, S.L.; Showell, H.J.; Remick, D.G.; Phan, S.H.; Ward, P.A.; Marks, R.M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science 1989, 243, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Muri, L.; Leppert, D.; Grandgirard, D.; Leib, S.L. MMPs and ADAMs in neurological infectious diseases and multiple sclerosis. Cell. Mol. Life Sci. 2019, 76, 3097–3116. [Google Scholar] [CrossRef]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef]
- Svedin, P.; Hagberg, H.; Sävman, K.; Zhu, C.; Mallard, C. Matrix Metalloproteinase-9 Gene Knock-out Protects the Immature Brain after Cerebral Hypoxia-Ischemia. J. Neurosci. 2007, 27, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Pulli, B.; Bure, L.; Wojtkiewicz, G.R.; Iwamoto, Y.; Ali, M.; Li, D.; Schob, S.; Hsieh, K.L.C.; Jacobs, A.H.; Chen, J.W. Multiple Sclerosis: Myeloperoxidase Immunoradiology Improves Detection of Acute and Chronic Disease in Experimental Model. Radiology 2015, 275, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.B.; Netea, M.G.; Fantuzzi, G.; Koenders, M.I.; Helsen, M.M.A.; Sparrer, H.; Pham, C.T.; Van Der Meer, J.W.M.; Dinarello, C.A.; Van Den Berg, W.B. Inflammatory Arthritis in Caspase 1 Gene-Deficient Mice: Contribution of Proteinase 3 to Caspase 1-Independent Production of Bioactive Interleukin-1β. Arthritis Rheumatol. 2009, 60, 3651–3662. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef]
- Lévesque, S.A.; Paré, A.; Mailhot, B.; Bellver-Landete, V.; Kébir, H.; Lécuyer, M.A.; Alvarez, J.I.; Prat, A.; de Rivero Vaccari, J.P.; Keane, R.W.; et al. Myeloid Cell Transmigration across the CNS Vasculature Triggers IL-1ß-Driven Neuroinflammation during Autoimmune Encephalomyelitis in Mice. J. Exp. Med. 2016, 213, 929–949. [Google Scholar] [CrossRef]
- Paré, A.; Mailhot, B.; Lévesque, S.A.; Lacroix, S. Involvement of the IL-1 System in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis: Breaking the Vicious Cycle between IL-1β and GM-CSF. Brain Behav. Immun. 2017, 62, 1–8. [Google Scholar] [CrossRef]
- Sienel, R.I.; Kataoka, H.; Kim, S.W.; Seker, F.B.; Plesnila, N. Adhesion of Leukocytes to Cerebral Venules Precedes Neuronal Cell Death and Is Sufficient to Trigger Tissue Damage After Cerebral Ischemia. Front. Neurol. 2022, 12, 807658. [Google Scholar] [CrossRef]
- Hong Xu, B.; Gonzalo, J.A.; St Pierre, Y.; Williams, I.R.; Kupper, T.S.; Ramzi Cotran, I.S.; Springer, T.A.; Gutierrez-Ramos, J.-C. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J. Exp. Med. 1994, 180, 95–109. [Google Scholar] [CrossRef]
- Sligh, J.E.; Ballantynet, C.M.; Richi, S.S.; Hawkins, H.K.; Smith1, C.W.; Bradley, A.; Beaudet, A.L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1993, 90, 8529–8533. [Google Scholar] [CrossRef]
- Al Nimer, F.; Elliott, C.; Bergman, J.; Khademi, M.; Dring, A.M.; Aeinehband, S.; Bergenheim, T.; Romme Christensen, J.; Sellebjerg, F.; Svenningsson, A.; et al. Lipocalin-2 Is Increased in Progressive Multiple Sclerosis and Inhibits Remyelination. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e191. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-H.; Wang, G.; Kumar, V.; Weng, Y.-C. Lipocalin-2 in Stroke. Neuro 2015, 2, 38–41. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Poteet, E.C.; Li, W.; Scott, A.E.; Liu, R.; Wen, Y.; Ghorpade, A.; Simpkins, J.W.; Yang, S.-H. Modulation of polymorphonuclear neutrophil functions by astrocytes. J. Neuroinflamm. 2010, 7, 53. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, J. New Role of Astrocytes in Neuroprotective Mechanisms after Ischemic Stroke. Arquivos Neuro-Psiquiatria. 2023, 81, 748–755. [Google Scholar] [CrossRef]
- Hooshmand, M.J.; Nguyen, H.X.; Piltti, K.M.; Benavente, F.; Hong, S.; Flanagan, L.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Neutrophils Induce Astroglial Differentiation and Migration of Human Neural Stem Cells via C1q and C3a Synthesis. J. Immunol. 2017, 199, 1069–1085. [Google Scholar] [CrossRef]
- De Bondt, M.; Hellings, N.; Opdenakker, G.; Struyf, S. Neutrophils: Underestimated Players in the Pathogenesis of Multiple Sclerosis (Ms). Int. J. Mol. Sci. 2020, 21, 4558. [Google Scholar] [CrossRef]
- Grist, J.J.; Marro, B.S.; Skinner, D.D.; Syage, A.R.; Worne, C.; Doty, D.J.; Fujinami, R.S.; Lane, T.E. Induced CNS Expression of CXCL1 Augments Neurologic Disease in a Murine Model of Multiple Sclerosis via Enhanced Neutrophil Recruitment. Eur. J. Immunol. 2018, 48, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kanneganti, T.D. Inflammasome Activation and Assembly at a Glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, S.; Meimand, H.A.E.; Arababadi, M.K.; Nakhaee, N.; Asadikaram, G. The Effects of IFN-β 1a on the Expression of Inflammasomes and Apoptosis-Associated Speck-Like Proteins in Multiple Sclerosis Patients. Mol. Neurobiol. 2017, 54, 3031–3037. [Google Scholar] [CrossRef]
- Cao, J.; Dong, L.; Luo, J.; Zeng, F.; Hong, Z.; Liu, Y.; Zhao, Y.; Xia, Z.; Zuo, D.; Xu, L.; et al. Supplemental N-3 Polyunsaturated Fatty Acids Limit A1-Specific Astrocyte Polarization via Attenuating Mitochondrial Dysfunction in Ischemic Stroke in Mice. Oxid. Med. Cell. Longev. 2021, 2021, 5524705. [Google Scholar] [CrossRef]
- Rakers, C.; Schleif, M.; Blank, N.; Matušková, H.; Ulas, T.; Händler, K.; Torres, S.V.; Schumacher, T.; Tai, K.; Schultze, J.L.; et al. Stroke Target Identification Guided by Astrocyte Transcriptome Analysis. Glia 2019, 67, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Matusova, Z.; Hol, E.M.; Pekny, M.; Kubista, M.; Valihrach, L. Reactive Astrogliosis in the Era of Single-Cell Transcriptomics. Front. Cell. Neurosci. 2023, 17, 1173200. [Google Scholar] [CrossRef]
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke. Int. J. Mol. Sci. 2021, 22, 4280. [Google Scholar] [CrossRef]
- Gou, X.; Ying, J.; Yue, Y.; Qiu, X.; Hu, P.; Qu, Y.; Li, J.; Mu, D. The Roles of High Mobility Group Box 1 in Cerebral Ischemic Injury. Front. Cell. Neurosci. 2020, 14, 600280. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Villafranca-Baughman, D.; Quintero, H.; Kacerovsky, J.B.; Dotigny, F.; Murai, K.K.; Prat, A.; Drapeau, P.; Di Polo, A. Interpericyte Tunnelling Nanotubes Regulate Neurovascular Coupling. Nature 2020, 585, 91–95. [Google Scholar] [CrossRef]
- Ramsauer, M.; Krause, D.; Dermietzel, R. Angiogenesis of the Blood-Brain Barrier in Vitro and the Function of Cerebral Pericytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1274–1276. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Underly, R.G.; Grant, R.I.; Watson, A.N.; Lindner, V.; Shih, A.Y. Pericyte Structure and Distribution in the Cerebral Cortex Revealed by High-Resolution Imaging of Transgenic Mice. Neurophotonics 2015, 2, 041402. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, C.; Chen, Z.; Bankaitis, V.; Tzima, E.; Sheibani, N.; Burridge, K. Pericytes Regulate Vascular Basement Membrane Remodeling and Govern Neutrophil Extravasation during Inflammation. PLoS ONE 2012, 7, e0045499. [Google Scholar] [CrossRef] [PubMed]
- Joulia, R.; Guerrero-Fonseca, I.M.; Girbl, T.; Coates, J.A.; Stein, M.; Vázquez-Martínez, L.; Lynam, E.; Whiteford, J.; Schnoor, M.; Voehringer, D.; et al. Neutrophil Breaching of the Blood Vessel Pericyte Layer during Diapedesis Requires Mast Cell-Derived IL-17A. Nat. Commun. 2022, 13, 7029. [Google Scholar] [CrossRef]
- Liu, R.; Lauridsen, H.M.; Amezquita, R.A.; Pierce, R.W.; Jane-wit, D.; Fang, C.; Pellowe, A.S.; Kirkiles-Smith, N.C.; Gonzalez, A.L.; Pober, J.S. IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J. Immunol. 2016, 197, 2400–2408. [Google Scholar] [CrossRef]
- Kerkar, S.; Williams, M.; Blocksom, J.M.; Wilson, R.F.; Tyburski, J.G.; Steffes, C.P. TNF-α and IL-1β Increase Pericyte/Endothelial Cell Co-Culture Permeability. J. Surg. Res. 2006, 132, 40–45. [Google Scholar] [CrossRef]
- Cayrol, R.; Wosik, K.; Berard, J.L.; Dodelet-Devillers, A.; Ifergan, I.; Kebir, H.; Haqqani, A.S.; Kreymborg, K.; Krug, S.; Moumdjian, R.; et al. Activated Leukocyte Cell Adhesion Molecule Promotes Leukocyte Trafficking into the Central Nervous System. Nat. Immunol. 2008, 9, 137–145. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Bhattacharya, A.; Lozinski, B.M.; Wee Yong, V. Pericytes as Mediators of Infiltration of Macrophages in Multiple Sclerosis. J. Neuroinflamm. 2021, 18, 301. [Google Scholar] [CrossRef]
- De La Fuente, A.G.; Lange, S.; Silva, M.E.; Gonzalez, G.A.; Tempfer, H.; van Wijngaarden, P.; Zhao, C.; Di Canio, L.; Trost, A.; Bieler, L.; et al. Pericytes Stimulate Oligodendrocyte Progenitor Cell Differentiation during CNS Remyelination. Cell Rep. 2017, 20, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Choi, Y.K.; Miyamoto, N.; Shindo, A.; Liang, A.C.; Ahn, B.J.; Mandeville, E.T.; Kaji, S.; Itoh, K.; Seo, J.H.; et al. A-Kinase Anchor Protein 12 Is Required for Oligodendrocyte Differentiation in Adult White Matter. Stem Cells 2018, 36, 751–760. [Google Scholar] [CrossRef]
- Cashion, J.M.; Young, K.M.; Sutherland, B.A. How Does Neurovascular Unit Dysfunction Contribute to Multiple Sclerosis? Neurobiol. Dis. 2023, 178, 106028. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil Extracellular Traps Released by Neutrophils Impair Revascularization and Vascular Remodeling after Stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Rempe, T.; Whitmire, N.; Dunn-Pirio, A.; Graves, J.S. Therapeutic Advances in Multiple Sclerosis. Front. Neurol. 2022, 13, 824926. [Google Scholar] [CrossRef]
- De Angelis, F.; John, N.A.; Brownlee, W.J. Disease-modifying therapies for multiple sclerosis. BMJ 2018, 363, k4674. [Google Scholar] [CrossRef]
- Mosconi, M.G.; Paciaroni, M. Treatments in Ischemic Stroke: Current and Future. Eur. Neurol. 2022, 85, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Scolding, N.J.; Pasquini, M.; Reingold, S.C.; Cohen, J.A. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017, 140, 2776–2796. [Google Scholar] [CrossRef]
- Mancardi, G.L.; Sormani, M.P.; Di Gioia, M.; Vuolo, L.; Gualandi, F.; Amato, M.P.; Capello, E.; Currò, D.; Uccelli, A.; Bertolotto, A.; et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: The Italian multi-centre experience. Mult. Scler. 2012, 18, 835–842. [Google Scholar] [CrossRef]
- Muraro, P.A.; Pasquini, M.; Atkins, H.L.; Bowen, J.D.; Farge, D.; Fassas, A.; Freedman, M.S.; Georges, G.E.; Gualandi, F.; Hamerschlak, N.; et al. Long-term Outcomes After Autologous Hematopoietic Stem Cell Transplantation for Multiple Sclerosis. JAMA Neurol. 2017, 74, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Burman, J.; Iacobaeus, E.; Svenningsson, A.; Lycke, J.; Gunnarsson, M.; Nilsson, P.; Vrethem, M.; Fredrikson, S.; Martin, C.; Sandstedt, A.; et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: The Swedish experience. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1116–1121. [Google Scholar] [CrossRef]
- Burt, R.K.; Balabanov, R.; Han, X.; Sharrack, B.; Morgan, A.; Quigley, K.; Yaung, K.; Helenowski, I.B.; Jovanovic, B.; Spahovic, D.; et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 2015, 313, 275–284. [Google Scholar] [CrossRef]
- Atkins, H.L.; Bowman, M.; Allan, D.; Anstee, G.; Arnold, D.L.; Bar-Or, A.; Bence-Bruckler, I.; Birch, P.; Bredeson, C.; Chen, J.; et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: A multicentre single-group phase 2 trial. Lancet 2016, 388, 576–585. [Google Scholar] [CrossRef]
- Sasaki, M.; Honmou, O.; Akiyama, Y.; Uede, T.; Hashi, K.; Kocsis, J.D. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia 2001, 35, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Zhang, C.L.; Wang, L.; Lu, D.; Katakowski, M.; Gao, Q.; Shen, L.H.; Zhang, J.; Lu, M.; et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 2005, 49, 407–417. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Lu, M.; Cui, Y.; Chen, J.; Noffsinger, L.; Elias, S.B.; Chopp, M. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J. Neurosci. Res. 2006, 84, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Laso-García, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.J.; Otero-Ortega, L.; Feliú, A.; Gómez-de Frutos, M.; Mecha, M.; Díez-Tejedor, E.; Guaza, C.; Gutiérrez-Fernández, M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent Advances in Cell-Based Therapies for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef]
- Lai, X.; Wang, Y.; Wang, X.; Liu, B.; Rong, L. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res. Ther. 2022, 13, 487. [Google Scholar] [CrossRef]
- Provenzano, F.; Nyberg, S.; Giunti, D.; Torazza, C.; Parodi, B.; Bonifacino, T.; Usai, C.; Kerlero de Rosbo, N.; Milanese, M.; Uccelli, A.; et al. Micro-RNAs Shuttled by Extracellular Vesicles Secreted from Mesenchymal Stem Cells Dampen Astrocyte Pathological Activation and Support Neuroprotection in In-Vitro Models of ALS. Cells 2022, 11, 3923. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.; Hu, M.; Huang, F.; Zhu, Q.; Qiu, W.; Hu, X.; Colello, J.; Zheng, S.G.; Lu, Z. Functional Dynamics of Neutrophils After Ischemic Stroke. Transl. Stroke Res. 2020, 11, 108–121. [Google Scholar] [CrossRef]
- Quan, M.; Zhang, H.; Han, X.; Ba, Y.; Cui, X.; Bi, Y.; Yi, L.; Li, B. Single-Cell RNA Sequencing Reveals Transcriptional Landscape of Neutrophils and Highlights the Role of TREM-1 in EAE. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200278. [Google Scholar] [CrossRef]
- Whittaker Hawkins, R.F.; Patenaude, A.; Dumas, A.; Jain, R.; Tesfagiorgis, Y.; Kerfoot, S.; Matsui, T.; Gunzer, M.; Poubelle, P.E.; Larochelle, C.; et al. ICAM1+ Neutrophils Promote Chronic Inflammation via ASPRV1 in B Cell–Dependent Autoimmune Encephalomyelitis. JCI Insight 2017, 2, e96882. [Google Scholar] [CrossRef]
- Shen, S.; Cheng, X.; Zhou, L.; Zhao, Y.; Wang, H.; Zhang, J.; Sun, X.; Wang, Y.; Shu, Y.; Xu, Y.; et al. Neutrophil Nanovesicle Protects against Experimental Autoimmune Encephalomyelitis through Enhancing Myelin Clearance by Microglia. ACS Nano 2022, 16, 18886–18897. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, L.; Jiang, W.; Chen, L.; Zhang, X.; Zhang, Q.; Ren, Y.; Hao, J. Single-Cell RNA-Seq Reveals the Transcriptional Landscape in Ischemic Stroke. J. Cereb. Blood Flow Metab. 2022, 42, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lyu, J.; Li, R.; Jain, V.; Shen, Y.; del Águila, Á.; Hoffmann, U.; Sheng, H.; Yang, W. Single-Cell Transcriptomic Analysis of the Immune Cell Landscape in the Aged Mouse Brain after Ischemic Stroke. J. Neuroinflamm. 2022, 19, 83. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Radtke, F.A.; Cunin, P.; Stifano, G.; Levescot, A.; Vijaykumar, B.; Nelson-Maney, N.; Blaustein, R.B.; Monach, P.A.; Nigrovic, P.A.; et al. The Neutrotime Transcriptional Signature Defines a Single Continuum of Neutrophils across Biological Compartments. Nat. Commun. 2021, 12, 2856. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of Neuronal Cell Survival by Astrocyte-Derived Exosomes under Hypoxic and Ischemic Conditions Depends on Prion Protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-Derived Exosomes Suppress Autophagy and Ameliorate Neuronal Damage in Experimental Ischemic Stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Zhu, Z.; Feng, J.; Chen, L. Exosome-Shuttled CircSHOC2 from IPASs Regulates Neuronal Autophagy and Ameliorates Ischemic Brain Injury via the MiR-7670-3p/SIRT1 Axis. Mol. Ther. Nucleic Acids 2020, 22, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated with a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.; et al. Lymphocyte–Microglia–Astrocyte Axis in Chronic Active Multiple Sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Czpakowska, J.; Kaluza, M.; Milewska-Jedrzejczak, M.; Glabinski, A. Astrocyte-Derived Exosomes Differentially Shape T Cells’ Immune Response in MS Patients. Int. J. Mol. Sci. 2023, 24, 7470. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowaczewska-Kuchta, A.; Ksiazek-Winiarek, D.; Glabinski, A. Interaction Between Neutrophils and Elements of the Blood–Brain Barrier in the Context of Multiple Sclerosis and Ischemic Stroke. Int. J. Mol. Sci. 2025, 26, 4437. https://doi.org/10.3390/ijms26094437
Nowaczewska-Kuchta A, Ksiazek-Winiarek D, Glabinski A. Interaction Between Neutrophils and Elements of the Blood–Brain Barrier in the Context of Multiple Sclerosis and Ischemic Stroke. International Journal of Molecular Sciences. 2025; 26(9):4437. https://doi.org/10.3390/ijms26094437
Chicago/Turabian StyleNowaczewska-Kuchta, Anna, Dominika Ksiazek-Winiarek, and Andrzej Glabinski. 2025. "Interaction Between Neutrophils and Elements of the Blood–Brain Barrier in the Context of Multiple Sclerosis and Ischemic Stroke" International Journal of Molecular Sciences 26, no. 9: 4437. https://doi.org/10.3390/ijms26094437
APA StyleNowaczewska-Kuchta, A., Ksiazek-Winiarek, D., & Glabinski, A. (2025). Interaction Between Neutrophils and Elements of the Blood–Brain Barrier in the Context of Multiple Sclerosis and Ischemic Stroke. International Journal of Molecular Sciences, 26(9), 4437. https://doi.org/10.3390/ijms26094437




