Towards Precision in Sarcopenia Assessment: The Challenges of Multimodal Data Analysis in the Era of AI
Abstract
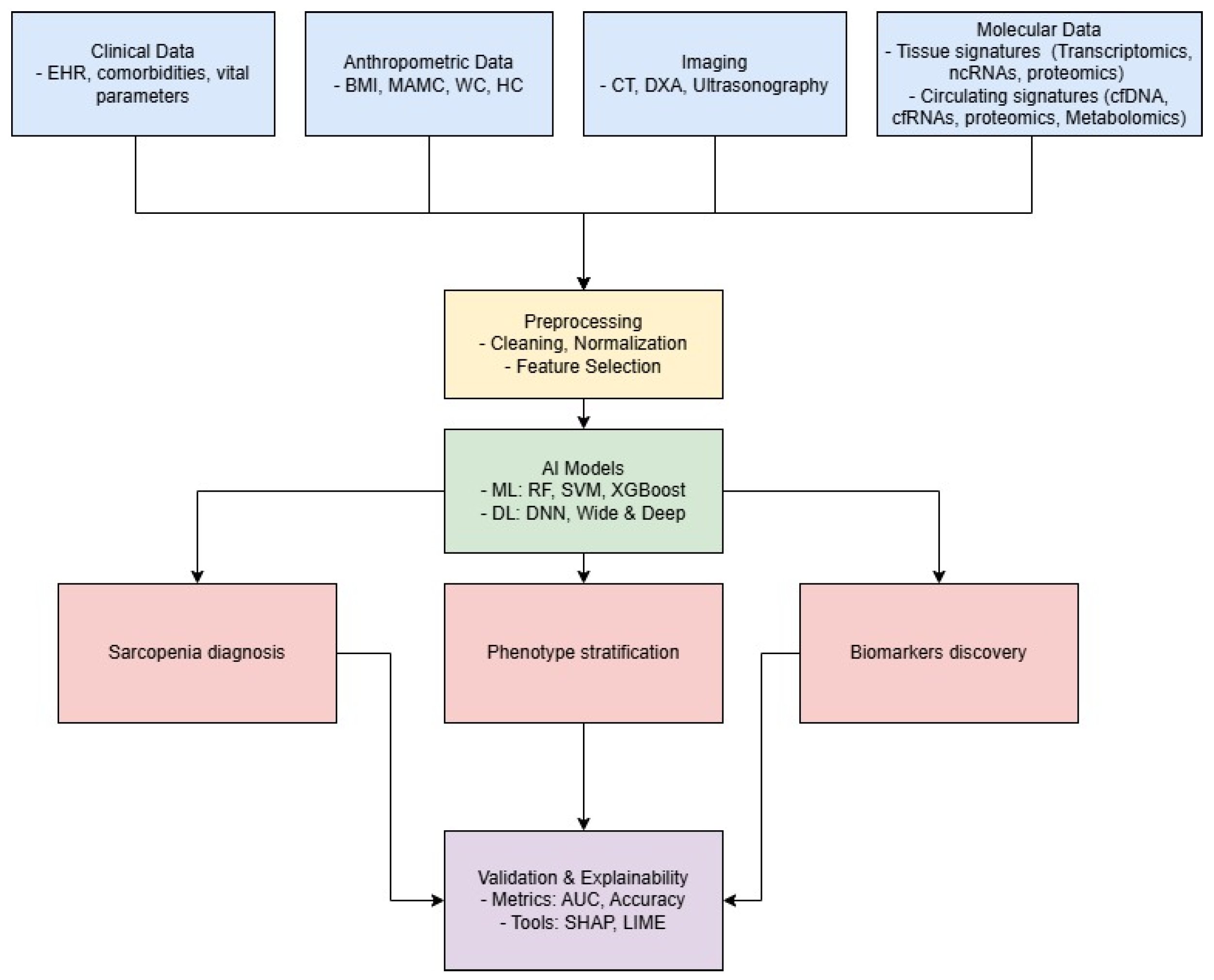
:1. Introduction
2. Methodology for Selection of Studies
3. AI for Comprehensive Data Integration in Sarcopenia
4. Application of AI for Identifying Molecular Biomarkers
5. Circulating Biomarkers: Update and New Frontiers for AI in Sarcopenia Research
| Circulating Signature | Biological Source | Expression Profile | Ref. |
|---|---|---|---|
| D3-creatinine | urine | downregulation | [57] |
| ALDOA | serum | upregulation | [58] |
| CTSD | serum | upregulation | [58] |
| P3NP | serum | upregulation | [59] |
| IL6 | serum | upregulation | [60,61] |
| TNF | serum | upregulation | [61] |
| CAF | serum | upregulation | [62] |
| VCAM1 | serum | upregulation | [63] |
| GDF15 | serum | upregulation | [64] |
| CETP | serum | upregulation | [65] |
| APOA2 | serum | downregulation | [65] |
| IGF1 | serum | downregulation | [66] |
| GH | serum | downregulation | [66] |
| Cf-mtDNA | plasma | high levels | [53] |
| miR-28-5p | plasma | upregulation | [45] |
| miR-1-3p | plasma | upregulation | [54,67] |
| miR-133a | plasma | downregulation | [44] |
| miR-133a-3p | serum | downregulation | [68] |
| miR-200a-3p | serum | downregulation | [68] |
| miR-434-3p | plasma | downregulation | [44] |
| miR-455-3p | plasma | downregulation | [44] |
| miR-486 | plasma | downregulation | [55] |
| miR-146a | plasma | downregulation | [55] |
| miR-21 | serum | upregulation | [69] |
| traumatic acid | plasma | high levels | [70] |
| ceramides | plasma | high levels | [71] |
| sphyngomielins | plasma | high levels | [71] |
| sphyngomielins | plasma | high and low levels depending on lipid | [72] |
| sterol ST(d14:0/25:5) | plasma | high levels | [72] |
| phosphatidylcholines | plasma | high and low levels depending on lipid | [72] |
| phosphatidylserines | plasma | high and low levels depending on lipid | [72] |
| PI 32:1 | plasma | high levels | [73] |
| isoleucine | plasma | low levels | [73] |
| 1-methylhistamine/3-methylhistamine | plasma | high levels | [73] |
| carnosine | plasma | low levels | [73] |
| creatinine | plasma | low levels | [73] |
| arginine | serum | low levels | [43] |
| cystin | serum | low levels | [43] |
| taurin | serum | high levels | [43] |
| hypoxanthine | plasma | high levels | [56] |
| hypoxanthine | serum | high levels | [74] |
| L-2-amino-3-oxobutanoic acid | plasma | low levels | [56,74] |
| PC(14:0/20:2(11Z,14Z)) | plasma | low levels | [56] |
| LysoPC(17:0) | plasma | low levels | [56] |
| palmitic acid | plasma | low levels | [56] |
| mannose | serum | high levels | [74] |
| galactose | serum | high levels | [74] |
| triethanolamine | serum | low levels | [74] |
| homogentisic acid | serum | low levels | [74] |
| oleoyl ethanolamide | plasma | high levels | [42] |
| stearoyl ethanolamide | plasma | low levels | [42] |
| docosahexaenoylethanolamide | plasma | low levels | [42] |
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daly, R.M.; Iuliano, S.; Fyfe, J.; Scott, D.; Kirk, B.; Thompson, M.; Dent, E.; Fetterplace, K.; Wright, O.; Lynch, G.; et al. Screening, Diagnosis and Management of Sarcopenia and Frailty in Hospitalized Older Adults: Recommendations from the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) Expert Working Group. J. Nutr. Health Aging 2022, 26, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cooper, R.; Arai, H.; Cawthon, P.M.; Ntsama Essomba, M.J.; Fielding, R.A.; Grounds, M.D.; Witham, M.D.; Cruz-Jentoft, A.J. Sarcopenia. Nat. Rev. Dis. Prim. 2024, 10, 68. [Google Scholar] [CrossRef]
- Tarantino, G.; Sinatti, G.; Citro, V.; Santini, S.; Balsano, C. Sarcopenia, a condition shared by various diseases: Can we alleviate or delay the progression? Intern. Emerg. Med. 2023, 18, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2020, 34, 1347–1372. [Google Scholar] [CrossRef]
- Nagano, A.; Wakabayashi, H.; Maeda, K.; Kokura, Y.; Miyazaki, S.; Mori, T.; Fujiwara, D. Respiratory Sarcopenia and Sarcopenic Respiratory Disability: Concepts, Diagnosis, and Treatment. J. Nutr. Health Aging 2021, 25, 507–515. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Lee, K.H.; Nam, S.W.; Kim, J.S.; Yang, J.W.; Lee, J.Y.; Smith, L.; et al. Sarcopenia in Autoimmune and Rheumatic Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5678. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.; Sun, P.Y.; Davies, K.J.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Kufel, J.; Bargieł-Łączek, K.; Kocot, S.; Koźlik, M.; Bartnikowska, W.; Janik, M.; Czogalik, L.; Dudek, P.; Magiera, M.; Lis, A.; et al. What Is Machine Learning, Artificial Neural Networks and Deep Learning?—Examples of Practical Applications in Medicine. Diagnostics 2023, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- Tolles, J.; Meurer, W.J. Logistic Regression. JAMA 2016, 316, 533–534. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.j.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Chung, H.; Jo, Y.; Ryu, D.; Jeong, C.; Choe, S.; Lee, J. Artificial-intelligence-driven discovery of prognostic biomarker for sarcopenia. J. Cachexia Sarcopenia Muscle 2021, 12, 2220–2230. [Google Scholar] [CrossRef]
- Luo, X.; Ding, H.; Broyles, A.; Warden, S.J.; Moorthi, R.N.; Imel, E.A. Using machine learning to detect sarcopenia from electronic health records. Digit. Health 2023, 9, 20552076231197098. [Google Scholar] [CrossRef] [PubMed]
- Onishi, S.; Kuwahara, T.; Tajika, M.; Tanaka, T.; Yamada, K.; Shimizu, M.; Niwa, Y.; Yamaguchi, R. Artificial intelligence for body composition assessment focusing on sarcopenia. Sci. Rep. 2025, 15, 1324. [Google Scholar] [CrossRef]
- Wu, L.W.; OuYoung, T.; Chiu, Y.C.; Hsieh, H.F.; Hsiu, H. Discrimination between possible sarcopenia and metabolic syndrome using the arterial pulse spectrum and machine-learning analysis. Sci. Rep. 2022, 12, 21452. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Olea, C.; García-Zapirain Soto, B.; Carballo Lozano, C.; Zuñiga, C. Automatic Classification of Sarcopenia Level in Older Adults: A Case Study at Tijuana General Hospital. Int. J. Environ. Res. Public Health 2019, 16, 3275. [Google Scholar] [CrossRef]
- Ding, X.; Liu, J.; Yang, F.; Cao, J. Random radial basis function kernel-based support vector machine. J. Frankl. Inst. 2021, 358, 10121–10140. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Zhu, J.; Fang, Y. Machine learning-based prediction of sarcopenia in community-dwelling middle-aged and older adults: Findings from the CHARLS. Psychogeriatrics 2025, 25, e13205. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Moroni, A.; Castellana, F.; Gasparri, C.; Catino, F.; Lampignano, L.; Perna, S.; Clodoveo, M.L.; Sardone, R.; Rondanelli, M. A Machine-Learning Approach to Target Clinical and Biological Features Associated with Sarcopenia: Findings from Northern and Southern Italian Aging Populations. Metabolites 2023, 13, 565. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Moroni, A.; Perna, S.; Azzolino, D.; Gasparri, C.; Zupo, R.; Micheletti Cremasco, M.; Rondanelli, M. Discovering the Individualized Factors Associated with Sarcopenia and Sarcopenic Obesity Phenotypes—A Machine Learning Approach. Nutrients 2023, 15, 4536. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yoo, J.I.; Ha, Y.C. Sarcopenia feature selection and risk prediction using machine learning. Medicine 2019, 98, e17699. [Google Scholar] [CrossRef]
- Awad, M.; Khanna, R. Support Vector Machines for Classification. In Efficient Learning Machines: Theories, Concepts, and Applications for Engineers and System Designers; Apress: Berkeley, CA, USA, 2015; pp. 39–66. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Advances in Neural Information Processing Systems 30; Guyon, I., Luxburg, U.V., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; pp. 4765–4774. [Google Scholar]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why Should I Trust You?”: Explaining the Predictions of Any Classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, KDD ’16, New York, NY, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Liu, Q.; Ding, F.; Hou, L.; Deng, Y.; Cui, T.; Han, Y.; Pang, W.; Ye, W.; et al. Machine and deep learning-based clinical characteristics and laboratory markers for the prediction of sarcopenia. Chin. Med. J. 2023, 136, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.T.; Koc, L.; Harmsen, J.; Shaked, T.; Chandra, T.; Aradhye, H.; Anderson, G.; Corrado, G.; Chai, W.; Ispir, M.; et al. Wide & Deep Learning for Recommender Systems. In Proceedings of the 1st Workshop on Deep Learning for Recommender Systems, DLRS 2016, New York, NY, USA, 15 September 2016; pp. 7–10. [Google Scholar] [CrossRef]
- Hou, L.; Liu, X.; Zhang, Y.; Zhao, W.; Xia, X.; Chen, X.; Lin, X.; Yue, J.; Ge, N.; Dong, B. Cohort Profile: West China Health and Aging Trend (WCHAT). J. Nutr. Health Aging 2021, 25, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Porciello, G.; Di Lauro, T.; Luongo, A.; Coluccia, S.; Prete, M.; Abbadessa, L.; Coppola, E.; Di Martino, A.; Mozzillo, A.L.; Racca, E.; et al. Optimizing Nutritional Care with Machine Learning: Identifying Sarcopenia Risk Through Body Composition Parameters in Cancer Patients-Insights from the NUTritional and Sarcopenia RIsk SCREENing Project (NUTRISCREEN). Nutrients 2025, 17, 1376. [Google Scholar] [CrossRef]
- Gu, Y.; Su, S.; Wang, X.; Mao, J.; Ni, X.; Li, A.; Liang, Y.; Zeng, X. Comparative study of XGBoost and logistic regression for predicting sarcopenia in postsurgical gastric cancer patients. Sci. Rep. 2025, 15, 12808. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Hu, X.; Zhang, Y. Epigenetic characterization of sarcopenia-associated genes based on machine learning and network screening. Eur. J. Med. Res. 2024, 29, 54. [Google Scholar] [CrossRef]
- Lin, S.; Chen, C.; Cai, X.; Yang, F.; Fan, Y. Development and Verification of a Combined Diagnostic Model for Sarcopenia with Random Forest and Artificial Neural Network. Comput. Math. Methods Med. 2022, 2022, 2957731. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, KDD ’16, New York, NY, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. Game theory, on-line prediction and boosting. In Proceedings of the Ninth Annual Conference on Computational Learning Theory, COLT ’96, New York, NY, USA, 28 June–1 July 1996; pp. 325–332. [Google Scholar] [CrossRef]
- Ahn, S.; Sung, Y.; Song, W. Machine Learning-Based Identification of Diagnostic Biomarkers for Korean Male Sarcopenia Through Integrative DNA Methylation and Methylation Risk Score: From the Korean Genomic Epidemiology Study (KoGES). J. Korean Med. Sci. 2024, 39, e200. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Suetterlin, K.; Shavlakadze, T.; Grounds, M.D.; Sayer, A.A. Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men. Clin. Sci. 2023, 137, 1721–1751. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Lee, S.H.; Koh, J.M.; Kwon, S.H.; Lee, Y.; Cho, H.J.; Kim, H.; Kim, S.J.; Lee, J.H.; Yoo, H.J.; et al. Fatty acid amides as potential circulating biomarkers for sarcopenia. J. Cachexia Sarcopenia Muscle 2023, 14, 1558–1568. [Google Scholar] [CrossRef]
- Hua, C.; Chen, Y.; Sun, Z.; Shi, Z.; Song, Q.; Shen, L.; Lu, W.; Wang, Z.; Zang, J. Associations of serum arginine acid with sarcopenia in Chinese eldely women. Nutr. Metab. 2024, 21, 63. [Google Scholar] [CrossRef]
- Qaisar, R.; Karim, A.; Muhammad, T.; Shah, I.; Khan, J. Circulating MicroRNAs as Biomarkers of Accelerated Sarcopenia in Chronic Heart Failure. Glob. Heart 2021, 16, 56. [Google Scholar] [CrossRef]
- Seco-Cervera, M.; Ibáñez-Cabellos, J.S.; Pallardo, F.V.; García-Giménez, J.L.; Aulinas, A.; Martel-Duguech, L.; Webb, S.M.; Valassi, E. Circulating miR-28-5p is overexpressed in patients with sarcopenia despite long-term remission of Cushing’s syndrome: A pilot study. Front. Endocrinol. 2024, 15, 1410080. [Google Scholar] [CrossRef]
- Murgia, M.; Brocca, L.; Monti, E.; Franchi, M.V.; Zwiebel, M.; Steigerwald, S.; Giacomello, E.; Sartori, R.; Zampieri, S.; Capovilla, G.; et al. Plasma proteome profiling of healthy subjects undergoing bed rest reveals unloading-dependent changes linked to muscle atrophy. J. Cachexia Sarcopenia Muscle 2023, 14, 439–451. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Smith, L.; Hamer, M. Gender-specific risk factors for incident sarcopenia: 8-year follow-up of the English longitudinal study of ageing. J. Epidemiol. Community Health 2019, 73, 86–88. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Ruffilli, A.; Barile, F.; Bellavia, D.; Marchese, L.; Manzetti, M.; Viroli, G.; Faldini, C.; Giavaresi, G. Sharing Circulating Micro-RNAs between Osteoporosis and Sarcopenia: A Systematic Review. Life 2023, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.L.; Esa, M.S.; Li, K.H.C.; Krishnan, S.R.G.; Elgallab, G.M.; Pearce, M.S.; Young, D.A.; Birrell, F.N. Osteoporosis, fracture, osteoarthritis & sarcopenia: A systematic review of circulating microRNA association. Bone 2021, 152, 116068. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Boehm, I.; Fernandes, M.; Rutter, A.; Skipworth, R.J.E.; Husi, H. Gene Ontology (GO)-Driven Inference of Candidate Proteomic Markers Associated with Muscle Atrophy Conditions. Molecules 2022, 27, 5514. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Xu, W.; Cao, L.; Qian, K.; Bischof, E.; Kennedy, B.K.; Pu, J. Decoding aging clocks: New insights from metabolomics. Cell Metab. 2025, 37, 34–58. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, J.Y.; Guo, Y.; Liu, Y.X.; Zhong, X.Y. Altered levels of circulating mitochondrial DNA in elderly people with sarcopenia: Association with mitochondrial impairment. Exp. Gerontol. 2022, 163, 111802. [Google Scholar] [CrossRef]
- Xu, R.; Cui, S.; Chen, L.; Chen, X.C.; Ma, L.L.; Yang, H.N.; Wen, F.M. Circulating miRNA-1-3p as Biomarker of Accelerated Sarcopenia in Patients Diagnosed with Chronic Heart Failure. Rev. Investig. Clin. Organo Del Hosp. Enfermedades Nutr. 2022, 74, 276–283. [Google Scholar] [CrossRef]
- Liu, H.C.; Han, D.S.; Hsu, C.C.; Wang, J.S. Circulating MicroRNA-486 and MicroRNA-146a serve as potential biomarkers of sarcopenia in the older adults. BMC Geriatr. 2021, 21, 86. [Google Scholar] [CrossRef]
- Han, P.; Chen, X.; Liang, Z.; Liu, Y.; Yu, X.; Song, P.; Zhao, Y.; Zhang, H.; Zhu, S.; Shi, X.; et al. Metabolic signatures and risk of sarcopenia in suburb-dwelling older individuals by LC-MS-based untargeted metabonomics. Front. Endocrinol. 2024, 15, 1308841. [Google Scholar] [CrossRef]
- Clark, R.V.; Walker, A.C.; O’Connor-Semmes, R.L.; Leonard, M.S.; Miller, R.R.; Stimpson, S.A.; Turner, S.M.; Ravussin, E.; Cefalu, W.T.; Hellerstein, M.K.; et al. Total body skeletal muscle mass: Estimation by creatine (methyl-d3) dilution in humans. J. Appl. Physiol. 2014, 116, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- L’hôte, C.; Cordier, B.; Labasse, A.; Boileau, C.; Costes, B.; Henrotin, Y. Identification of new biomarkers for sarcopenia and characterization of cathepsin D biomarker. Jcsm Rapid Commun. 2021, 4, 122–132. [Google Scholar] [CrossRef]
- Pellegrino, R.; Paganelli, R.; Di Iorio, A.; Bandinelli, S.; Moretti, A.; Iolascon, G.; Sparvieri, E.; Tarantino, D.; Ferrucci, L. Muscle quality, physical performance, and comorbidity are predicted by circulating procollagen type III N-terminal peptide (P3NP): The InCHIANTI follow-up study. GeroScience 2024, 46, 1259–1269. [Google Scholar] [CrossRef]
- Ding, J.; Yang, G.; Sun, W.; Li, Y.; Wang, N.; Wang, J.; Zhao, Y. Association of interleukin-6 with sarcopenia and its components in older adults: A systematic review and meta-analysis of cross-sectional studies. Ann. Med. 2024, 56, 2384664. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Vendemiale, G. Sarcopenia Is Associated with Changes in Circulating Markers of Antioxidant/Oxidant Balance and Innate Immune Response. Antioxidants 2023, 12, 1992. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Lorenzi, M.; Martone, A.M.; Tosato, M.; Drey, M.; D’Angelo, E.; Capoluongo, E.; Russo, A.; Bernabei, R.; et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: Results from the ilSIRENTE study. Exp. Gerontol. 2016, 79, 31–36. [Google Scholar] [CrossRef]
- Hsu, B.G.; Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L. Association of endothelial dysfunction and peripheral arterial disease with sarcopenia in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2024, 15, 1199–1208. [Google Scholar] [CrossRef]
- Deng, M.; Bian, Y.; Zhang, Q.; Zhou, X.; Hou, G. Growth Differentiation Factor-15 as a Biomarker for Sarcopenia in Patients with Chronic Obstructive Pulmonary Disease. Front. Nutr. 2022, 9, 897097. [Google Scholar] [CrossRef]
- Wu, J.; Cao, L.; Wang, J.; Wang, Y.; Hao, H.; Huang, L. Characterization of serum protein expression profiles in the early sarcopenia older adults with low grip strength: A cross-sectional study. BMC Musculoskelet. Disord. 2022, 23, 894. [Google Scholar] [CrossRef]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef]
- Siracusa, J.; Koulmann, N.; Banzet, S. Circulating myomiRs: A new class of biomarkers to monitor skeletal muscle in physiology and medicine. J. Cachexia Sarcopenia Muscle 2018, 9, 20–27. [Google Scholar] [CrossRef]
- Millet, M.; Auroux, M.; Beaudart, C.; Demonceau, C.; Ladang, A.; Cavalier, E.; Reginster, J.Y.; Bruyère, O.; Chapurlat, R.; Rousseau, J.C. Association of circulating hsa-miRNAs with sarcopenia: The SarcoPhAge study. Aging Clin. Exp. Res. 2024, 36, 70. [Google Scholar] [CrossRef]
- Okugawa, Y.; Yao, L.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Yin, C.; Omura, Y.; Ide, S.; Kitajima, T.; Shimura, T.; et al. Prognostic impact of sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol. Rep. 2018, 39, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.S.; Wang, S.Y.; Chang, C.H.; Chen, C.Y.; Wen, C.J.; Chen, G.Y.; Kuo, C.H.; Tseng, Y.J.; Chen, C.Y. Identification of traumatic acid as a potential plasma biomarker for sarcopenia using a metabolomics-based approach. J. Cachexia Sarcopenia Muscle 2022, 13, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Koh, J.M.; Cho, H.J.; Kim, H.; Lee, Y.S.; Kim, S.J.; Yoon, P.W.; Kim, W.; Bae, S.J.; Kim, H.K.; et al. Sphingolipid metabolites as potential circulating biomarkers for sarcopenia in men. J. Cachexia Sarcopenia Muscle 2024, 15, 2476–2486. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; He, P.; Mao, X.; Jing, X.; Hu, Y.; Jing, L. LC/MS-Based Untargeted Lipidomics Reveals Lipid Signatures of Sarcopenia. Int. J. Mol. Sci. 2024, 25, 8793. [Google Scholar] [CrossRef]
- Hsu, W.H.; Wang, S.Y.; Chao, Y.M.; Chang, K.V.; Han, D.S.; Lin, Y.L. Novel metabolic and lipidomic biomarkers of sarcopenia. J. Cachexia Sarcopenia Muscle 2024, 15, 2175–2186. [Google Scholar] [CrossRef]
- Shida, T.; Yoshida, Y.; Ohta, T.; Kojima, N.; Osuka, Y.; Takekoshi, K.; Sasai, H. Identification of a novel biomarker for sarcopenia diagnosis using serum metabolomic analysis: A pilot study. Eur. Geriatr. Med. 2024, 15, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Rashidi, H.H.; Pantanowitz, J.; Chamanzar, A.; Fennell, B.; Wang, Y.; Gullapalli, R.R.; Tafti, A.; Deebajah, M.; Albahra, S.; Glassy, E.; et al. Generative Artificial Intellegence (AI) in Pathology and Medicine: A Deeper Dive. Mod. Pathol. 2025, 38, 100687. [Google Scholar] [CrossRef] [PubMed]
| Dataset | AI Model | Performance | Key Predictors | Ref. |
|---|---|---|---|---|
| EHR from 1304 patients | RF, SVM | AUC > 90% | Diagnoses, medications, lab tests | [15] |
| 166 patients, 99 variables | RBF SVM | Accuracy: 82.5%, F1: 90.2%, precision: 82.8% | Age, hypertension, MNA, sodium | [18] |
| 133 subjects, BPW signals | LDA, Scoring System | AUC: 0.77 (LDA), 0.83 (scoring) | APS | [17] |
| CHARLS | XGBoost | AUROC: 0.759 | MMSE, drinking habits, BUN | [20] |
| WCHAT cohort, XMAT validation | Wide and Deep | AUC: 0.97, ACC: 91.1% | MAMC, CC, TSF, AST/ALT ratio | [29] |
| Italian ageing populations | RF (3 models) | ACC 89.89%, sensitivity 14.50%, specificity 99.37% | Albumin, CRP, vitamin D, folates | [22] |
| 1510 patients | LR | S: 0.33, SO: 0.19 OS: 0.267 | BMI | [24] |
| KNHANES (4020 patients) | LR, RF, SVM, GBM | AUC [men–women] RF: 0.82–0.78 SVM: 0.8–0.81 GB: 0.81–0.81 LR: 0.82–0.80 | BMI, RBC, nutrient intake, water intake | [25] |
| 3096 Japanese patients | CNN | ACC: 0.88 | MAMC, CC, TSF, AST/ALT ratio | [16] |
| 879 oncological patients | PCA + K-means | ACC: PC1 (59%), PC2 (24%), PC3 (15%) | Advanced age, lung, gynecological, gastroint. cancer, diabetes, malnutrition | [32] |
| 231 post-surgical patients | XGBoost vs. LASSO | AUROC: 0.98 | Serum albumin, diabetes, type surgery, nutritional score, ECOG status | [33] |
| Dataset | AI Model | Key Biomarkers | Performance | Ref. |
|---|---|---|---|---|
|
-GSE1428 -GSE136344 | LASSO, SVM-RFE | MYH8, HOXB2, CDKN1A | AUC > 0.7 | [34] |
| -GSE111017 | DNN (DSnet-v1) | 27 AI-featured genes (e.g., H4C3, PSMA6, CENPC, VPS35L) | Acc. 0.96 Sens. 1.000 Spec. 0.94 AUC: 0.99 | [14] |
|
-GSE8479 -GSE9103 -GSE38718 -GSE1428 | RF, ANN | MT1X, CISD1, WISP2 | AUC: 0.999 (train), 0.85 (test) | [35] |
|
509 Korean males | RFECV, Ensemble ML | 8 CpG sites | AUC: 0.94 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caputo, V.; Letteri, I.; Santini, S.J.; Sinatti, G.; Balsano, C. Towards Precision in Sarcopenia Assessment: The Challenges of Multimodal Data Analysis in the Era of AI. Int. J. Mol. Sci. 2025, 26, 4428. https://doi.org/10.3390/ijms26094428
Caputo V, Letteri I, Santini SJ, Sinatti G, Balsano C. Towards Precision in Sarcopenia Assessment: The Challenges of Multimodal Data Analysis in the Era of AI. International Journal of Molecular Sciences. 2025; 26(9):4428. https://doi.org/10.3390/ijms26094428
Chicago/Turabian StyleCaputo, Valerio, Ivan Letteri, Silvano Junior Santini, Gaia Sinatti, and Clara Balsano. 2025. "Towards Precision in Sarcopenia Assessment: The Challenges of Multimodal Data Analysis in the Era of AI" International Journal of Molecular Sciences 26, no. 9: 4428. https://doi.org/10.3390/ijms26094428
APA StyleCaputo, V., Letteri, I., Santini, S. J., Sinatti, G., & Balsano, C. (2025). Towards Precision in Sarcopenia Assessment: The Challenges of Multimodal Data Analysis in the Era of AI. International Journal of Molecular Sciences, 26(9), 4428. https://doi.org/10.3390/ijms26094428









