Responses of Calligonum leucocladum to Prolonged Drought Stress Through Antioxidant System Activation, Soluble Sugar Accumulation, and Maintaining Photosynthetic Homeostasis
Abstract
1. Introduction
2. Results
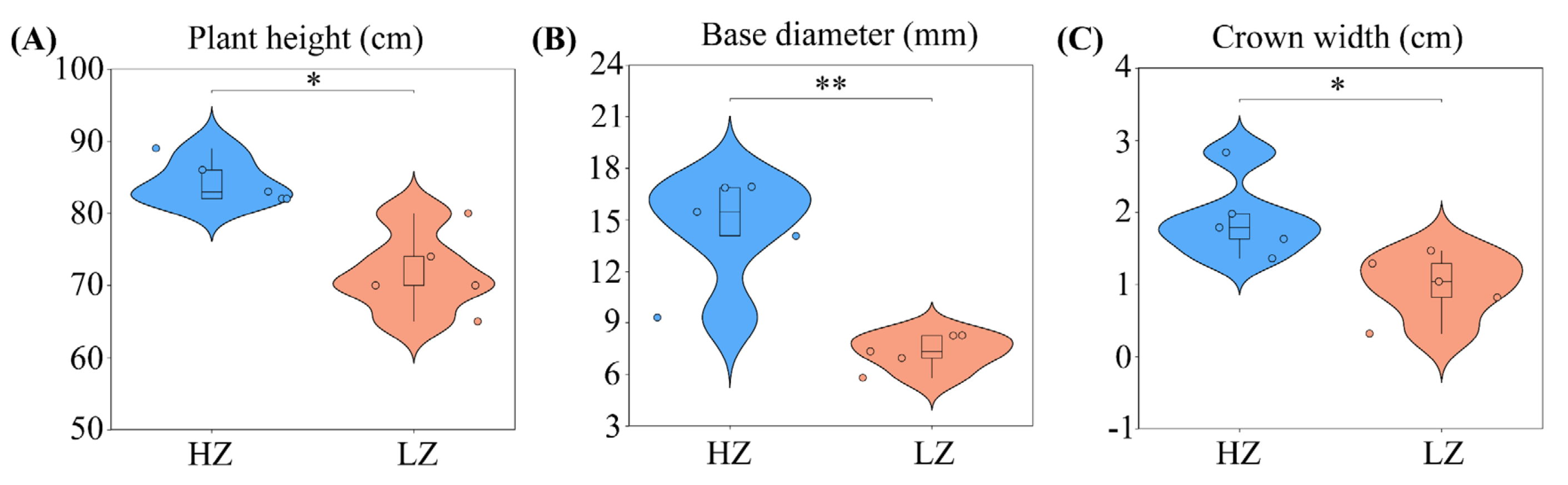
2.1. Effect of Prolonged Drought on C. leucocladum Growth
2.2. Effect of Prolonged Drought on C. leucocladum Physiology
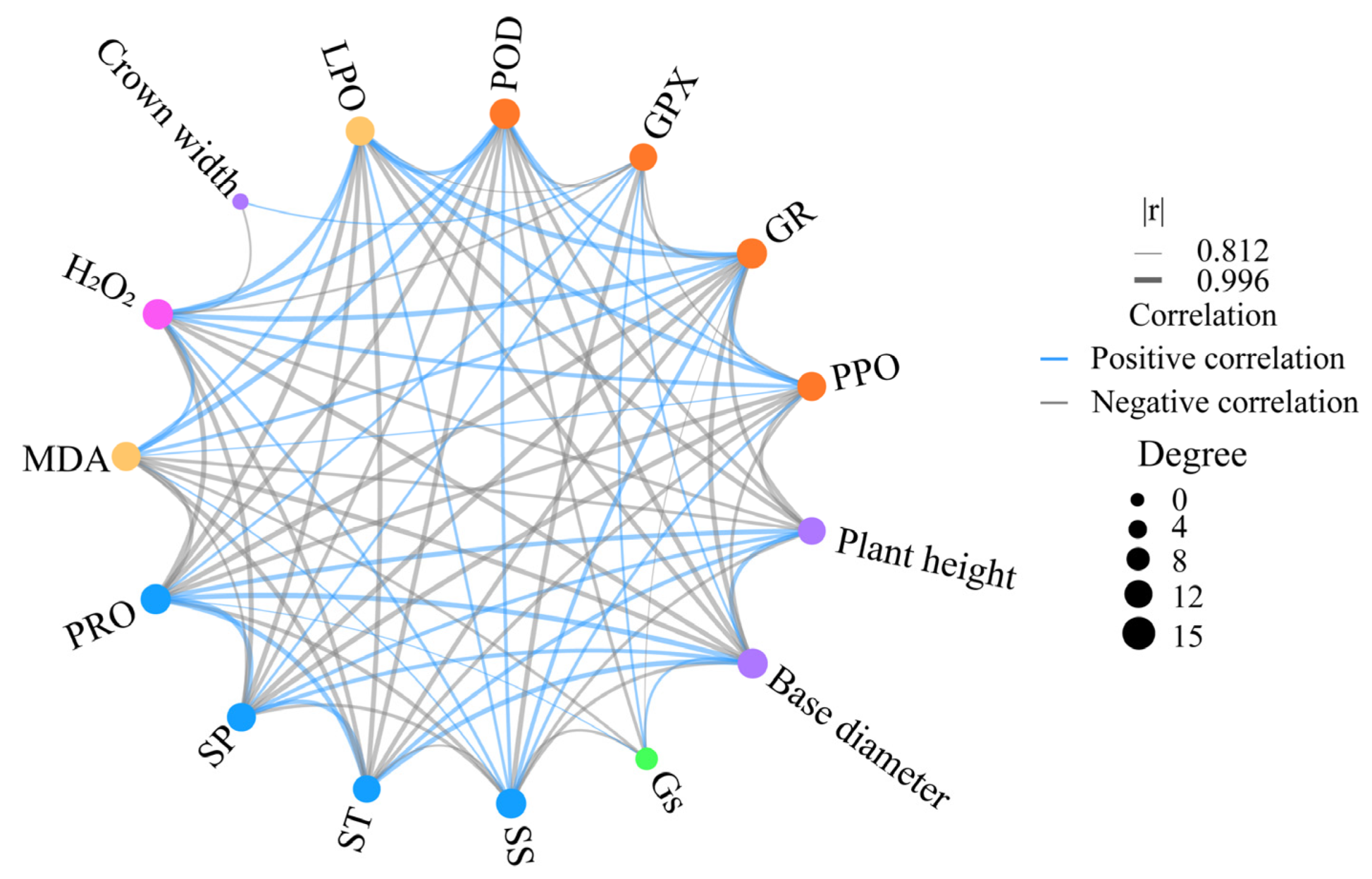
2.3. The Trade-Off Between Growth and Physiology in C. leucocladum
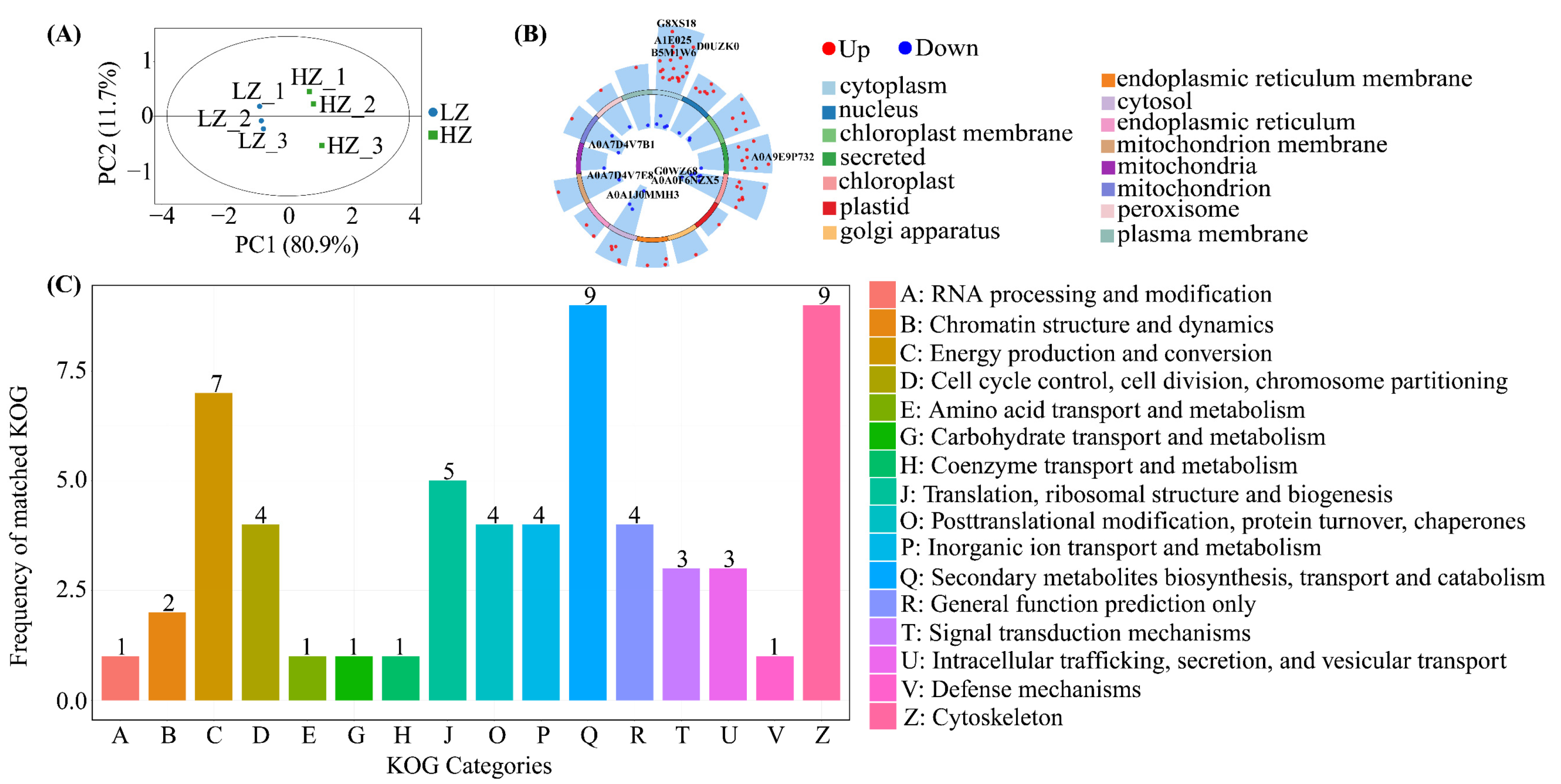
2.4. Protein Expression in C. leucocladum in a Drought Environment
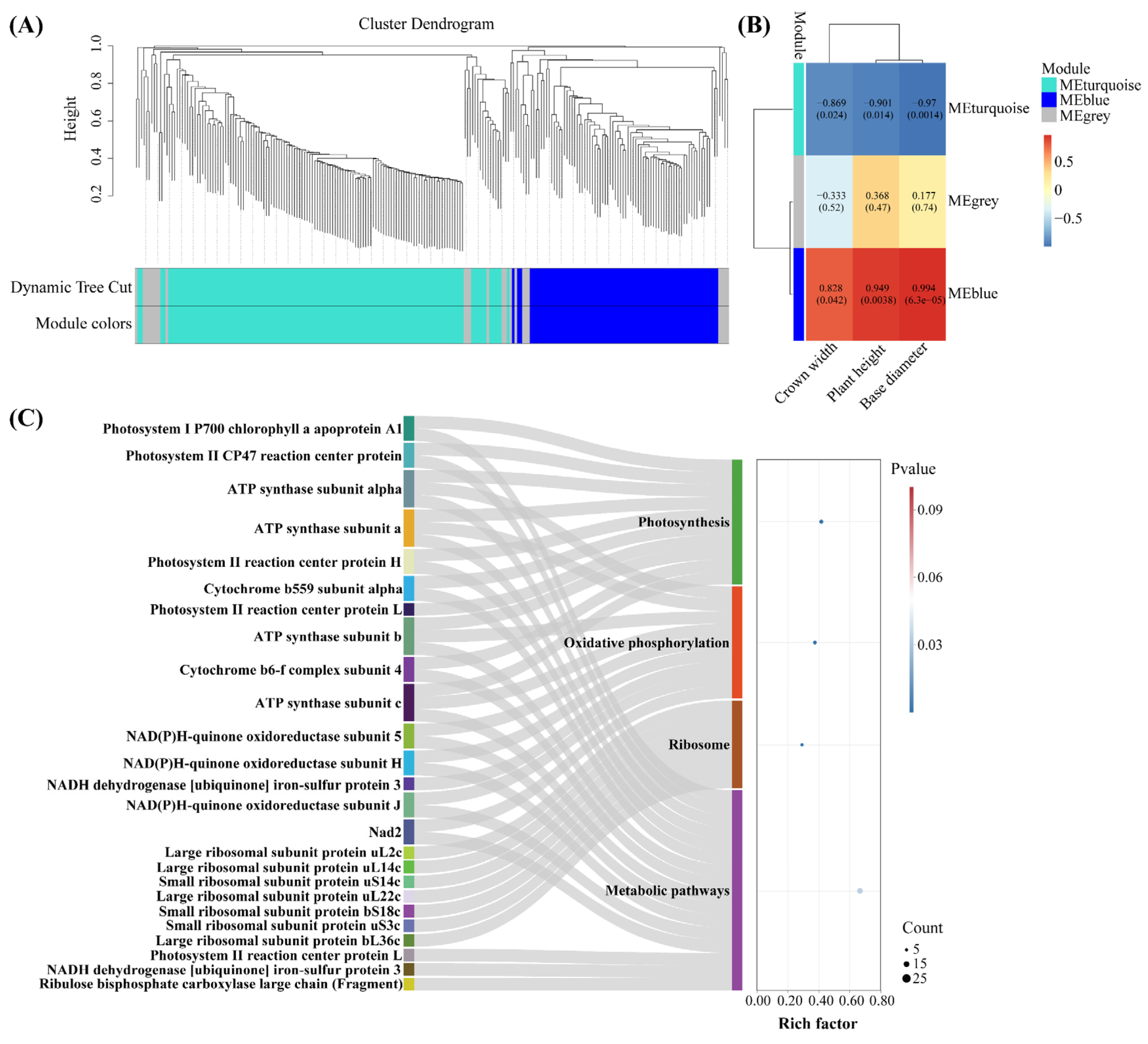
2.5. Screening of Key Drought-Tolerant Proteins Using WGCNA
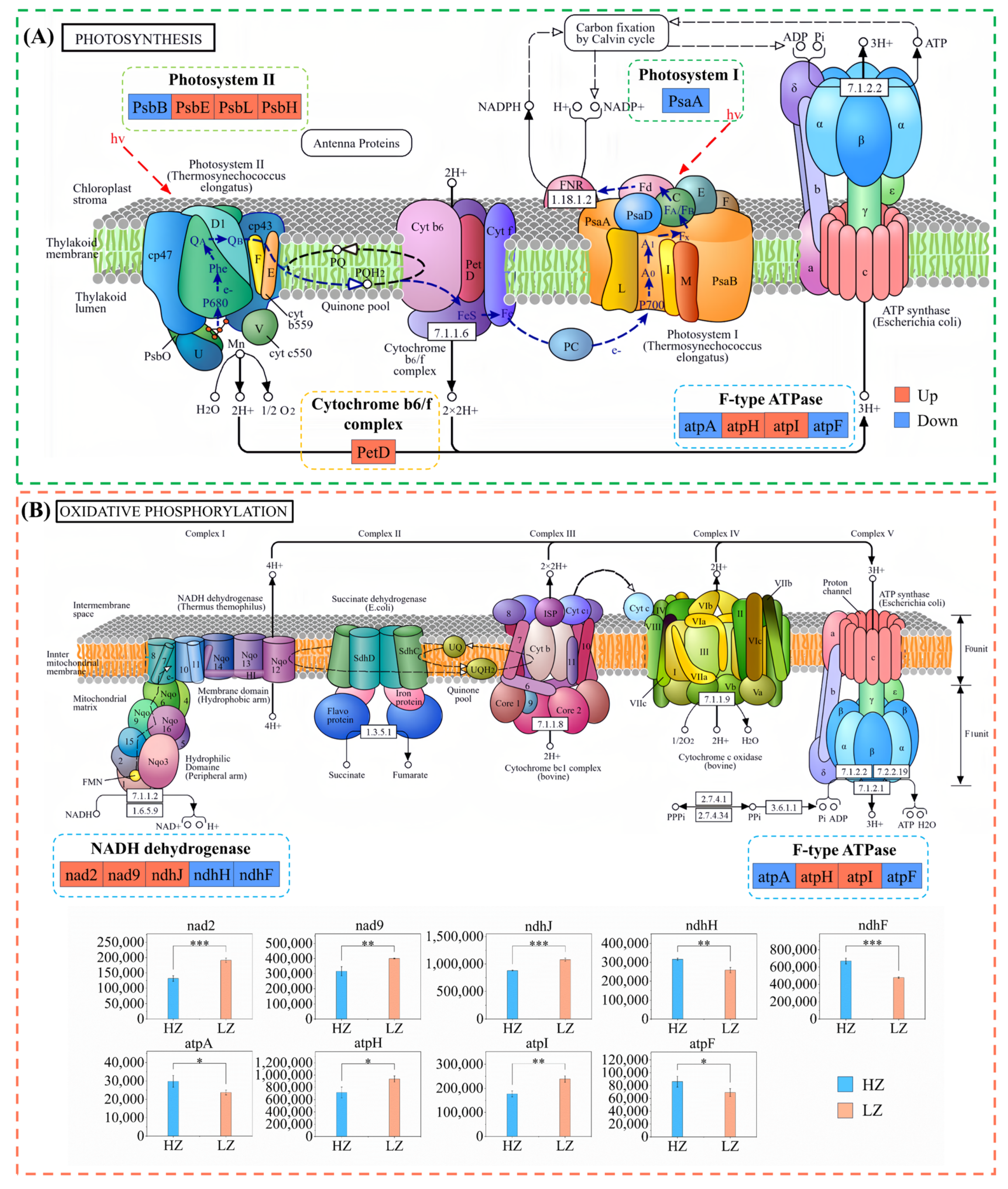
2.6. DEPs Involved in Photosynthesis and Oxidative Phosphorylation Under Drought Stress
3. Discussion
3.1. Physiological Mechanisms Behind Response to Drought Environments in C. leucocladum
3.2. Trade-Offs Between Growth and Physiology in Response to Drought in C. leucocladum
3.3. Drought Stress Promotes Expression of Photosynthetic and Oxidative Phosphorylation-Related Proteins in C. leucocladum
4. Materials and Methods
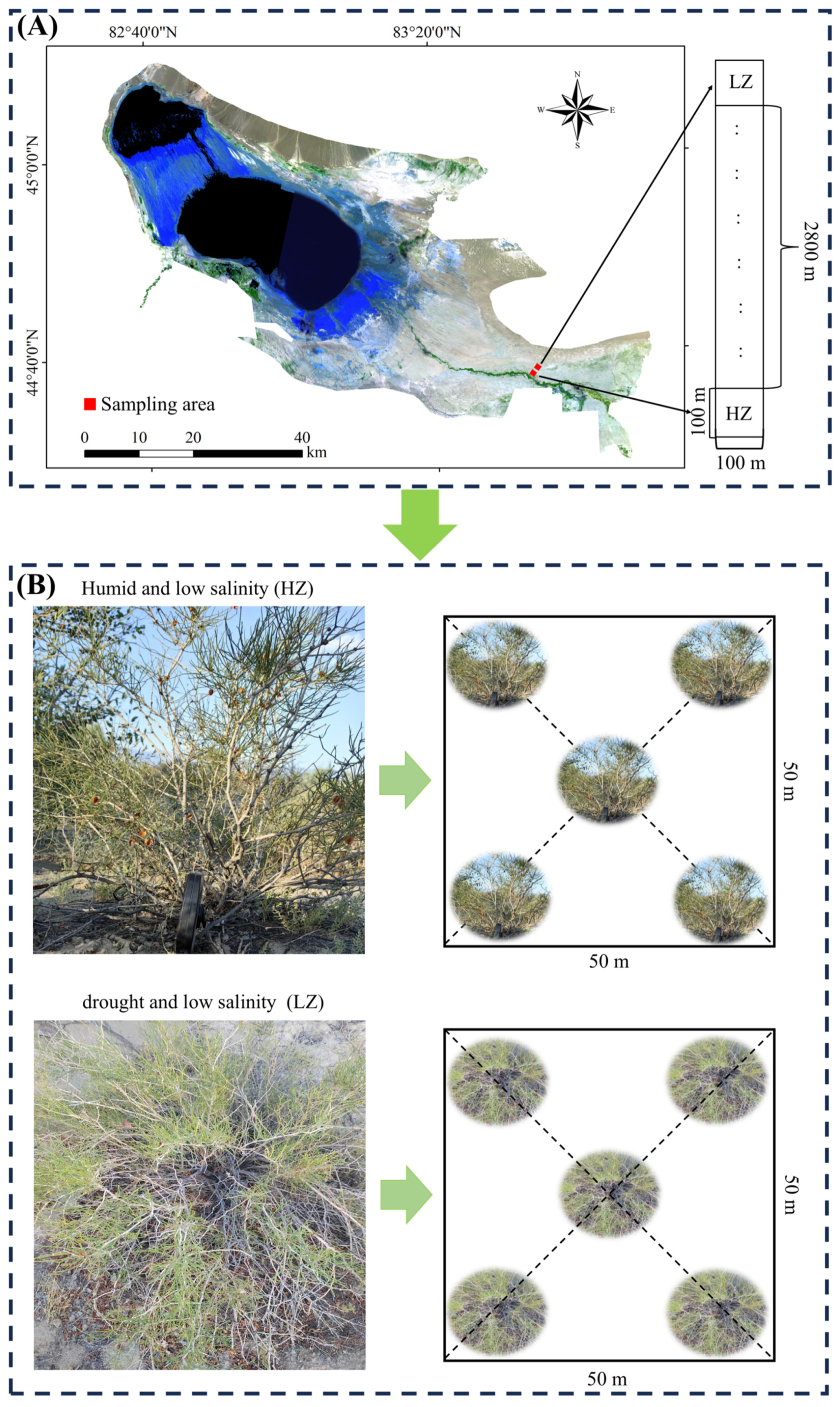
4.1. Research Area
4.2. Site Layout and Plant Selection
4.3. Determining Plant Morphology
4.4. Determining Photosynthetic Parameters
4.5. Physiological Parameters
4.6. TMT (Tandem Mass Tag) Quantitative Proteomics Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, J.D.; Kaspari, S.; Gabrielli, P.; Wegner, A.; Beaudon, E.; Sierra-Hernández, M.R.; Thompson, L. Drought-induced biomass burning as a source of black carbon to the central himalaya since 1781 CE as reconstructed from the dasuopu ice core. Atmos. Chem. Phys. 2021, 21, 5615–5633. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Wang, Y. Physiological and proteomic changes of Castanopsis fissa in response to drought stress. Sci. Rep. 2023, 13, 12567. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.L.; Hu, Q.N.; Liu, J.Q.; He, X.L. Relationship of root dark septate endophytes and soil factors to plant species and seasonal variation in extremely arid desert in Northwest China. Appl. Soil Ecol. 2022, 175, 104454. [Google Scholar] [CrossRef]
- Time, A.; Garrido, M.; Acevedo, E. Water relations and growth response to drought stress of Prosopis tamarugo Phil. A review. J. Soil Sci. Plant Nutr. 2018, 18, 329–343. [Google Scholar] [CrossRef]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Vieira, S.A.; Ponte, L.F.A.; Silveira, J.A.G. Coordinate changes in photosynthesis, sugar accumulation and antioxidative enzymes improve the performance of Jatropha curcas plants under drought stress. Biomass Bioenerg. 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Alkhedir, G.M.; Taniguchi, T. Morphological and physiological adaptation of a desert shrub, Encelia farinosa, under drought stress. Acta Oecologica 2024, 122, 103976. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, L.E.; Coelho, V.T.; Lopes, G.; Souza, T.; Porto, A.C.; Lira, J.; Massote, R.; Rocha, C.; Gomes, M.P. Performance of Hevea brasiliensis under drought conditions on osmoregulation and antioxidant activity through evaluation of vacuolar invertase and reducing sugars. Plant Sci. Today 2021, 8, 312–323. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteome changes under abiotic stress contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Hu, J.; Yang, B.; Shi, L.; Huang, F.; Tsai, S.N.; Ngai, S.M.; He, Y.; Zhang, J. Proteomic characterization of Phragmites communis in ecotypes of swamp and desert dune. Proteomics 2009, 9, 3950–3967. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.B.; Liu, X.Q.; Jin, Y.D.; Wu, J.; Li, S.; Li, Y.X.; Chen, B.Q.; Zhang, Y.X.; Wei, L.X.; Li, W.; et al. Drought induces epitranscriptome and proteome changes in stem-differentiating xylem of Populus trichocarpa. Plant Physiol. 2022, 109, 459–479. [Google Scholar] [CrossRef]
- Du, W.; Ruan, C.J.; Li, J.B.; Li, H.; Ding, J.; Zhao, S.Y.; Jiang, X. Quantitative proteomic analysis of Xanthoceras sorbifolium Bunge seedlings in response to drought and heat stress. Plant Physiol. Biochem. 2021, 160, 8–17. [Google Scholar] [CrossRef]
- Chang, Y.L.; Lv, G.H. Nitraria sibirica adapts to long-term soil water deficit by reducing photosynthesis, stimulating antioxidant systems, and accumulating osmoregulators. Plant Physiol. Biochem. 2024, 206, 108265. [Google Scholar] [CrossRef]
- Ren, J.; Tao, L.; Liu, X.M. Effect of sand burial depth on seed germination and seedling emergence of Calligonum L. species. J. Arid Environ. 2002, 51, 603–611. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, X.T. Microsatellite DNA loci from the drought desert plant Calligonum mongolicum Turcz. (Polygonaceae). Conserv. Genet. 2009, 10, 1891–1893. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, J.L.; Jiang, L.M.; Lv, G.H.; Hu, D.; Wu, D.Y.; Yang, X.D. Rhizosphere effect and water constraint jointly determined the roles of microorganism in soil phosphorus cycling in arid desert regions. Catena 2023, 222, 106809. [Google Scholar] [CrossRef]
- Camisón, Á.; Ángela Martín, M.; Javier Dorado, F.; Moreno, G.; Solla, A. Changes in carbohydrates induced by drought and waterlogging in Castanea sativa. Trees 2020, 34, 579–591. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Y.; Xie, Z.; Guo, D.; Chen, C.; Fan, Q.; Deng, X.; Liu, J.H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2019, 17, 1394–1407. [Google Scholar] [CrossRef]
- Zhou, X.B.; Zhang, Y.M.; Ji, X.H.; Downing, A.; Serpe, M. Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China. Environ. Exp. Bot. 2011, 74, 1–8. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.J.; Sun, X.F.; Zhao, L.L.; Wang, P.C. Transcriptomic and metabolomic analyses reveal key metabolites, pathways and candidate genes in Sophora davidii (Franch.) skeels seedlings under drought stress. Front. Plant Sci. 2022, 13, 785702. [Google Scholar] [CrossRef]
- Itam, M.; Mega, R.; Shota Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.J.; Akashi, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, B.; Li, L.; Zeng, F.; Li, X. Negative effects of long-term exposure to salinity, drought, and combined stresses on halophyte Halogeton Glomeratus. Physiol. Plantarum 2021, 173, 2307–2322. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Zuo, Y.L.; Su, F.; He, X.L.; Li, M. Colonization by dark septate endophytes improves the growth of Hedysarum scoparium under multiple inoculum levels. Symbiosis 2020, 82, 201–214. [Google Scholar] [CrossRef]
- Loreti, E.; Poggi, A.; Novi, G.; Alpi, A.; Perata, P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol. 2005, 137, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; Amrani, A.E.I. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef]
- Cano, F.J.; López, R.; Warren, C.R. Implications of the mesophyll conductance to CO2 for photosynthesis and water-use efficiency during long-term water stress and recovery in two contrasting eucalyptus species. Plant Cell Environ. 2014, 37, 2470–2490. [Google Scholar] [CrossRef]
- Jia, H.; Shao, M.; He, Y.; Guan, R.; Chu, P.; Jiang, H.D. Proteome dynamics and physiological responses to short-term salt stress in Brassica napus Leaves. PLoS ONE 2015, 10, e0144808. [Google Scholar] [CrossRef]
- Abid, G.; Jebara, M.; Debode, F.; Vertommen, D.; Ruys, S.P.D.; Ghouili, E.; Jebara, S.H.; Ouertani, R.N.; Ayed, M.E.; Oliveira, A.C.D.; et al. Comparative physiological, biochemical and proteomic analyses reveal key proteins and crucial regulatory pathways related to drought stress tolerance in faba bean (Vicia faba L.) leaves. Curr. Plant Biol. 2024, 37, 100320. [Google Scholar] [CrossRef]
- Yan, M.; Zheng, L.; Li, B.; Shen, R.F.; Lan, P. Comparative proteomics reveals new insights into the endosperm responses to drought, salinity and submergence in germinating wheat seeds. Plant Mol. Biol. 2021, 105, 287–302. [Google Scholar] [CrossRef]
- Pandey, K.; Kumar, R.S.; Prasad, P.; Sushma Pande, V.; Trivedi, P.K.; Shirke, P.A. Synchronised interaction of carbon and nitrogen provides drought tolerance in Cyamopsis tetragonoloba. Environ. Exp. Bot. 2022, 199, 104899. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species? Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siedow, J.N.; Umbach, A.L. The mitochondrial cyanide-resistant oxidase: Structural conservation amid regulatory diversity. BBA Bioenerg. 2000, 1459, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.W.; Lv, G.H. Species diversity and dominant species’ niches of eremophyte communities of the Tugai forest in the Ebinur basin of Xinjiang, China. Biodivers. Sci. 2017, 25, 34–45. [Google Scholar] [CrossRef]
- Yang, X.D.; Qie, Y.D.; Teng, D.X.; Ali, A.; Xu, Y.; Bolan, N.; Liu, W.G.; Lv, G.H.; Ma, L.G.; Yang, S.T.; et al. Prediction of groundwater depth in an arid region based on maximum tree height. J. Hydrol. 2019, 574, 46–52. [Google Scholar] [CrossRef]
- Li, C.J.; Han, H.; Ablimiti, M.; Liu, R.; Zhang, H.; Fan, J.L. Morphological and physiological responses of desert plants to drought stress in a man-made landscape of the Taklimakan desert shelter belt. Ecol. Indic. 2022, 140, 109037. [Google Scholar] [CrossRef]
- Hoch, G.; Popp, M.; Körner, C. Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos 2002, 98, 361–374. [Google Scholar] [CrossRef]
- Campion, E.M.; Loughran, S.T.; Walls, D. Protein quantitation and analysis of purity. In Protein Chromatography; Walls, D., Loughran, S., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1485. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Yang, T.H.; Verslues, P.E. Dynamic proline metabolism: Importance and regulation in water limited environments. Front. Plant Sci. 2015, 6, 484. [Google Scholar] [CrossRef]
- Minami, M.; Yoshikawa, H. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Satterfield, C.N.; Bonnell, A.H. Interferences in titanium sulfate method for hydrogen peroxide. Anal. Chem. 1955, 27, 1174–1175. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Awad, M.A.; Al-Qurashi, A.D. Antioxidant activity, antioxidant compounds, antioxidant and hydrolytic enzymes activities of ‘Barhee’ dates at harvest and during storage as affected by pre-harvest spray of some growth regulators. Sci. Hortic. 2014, 167, 91–99. [Google Scholar] [CrossRef]
- Jiang, B.H.; Su, C.; Wang, Y.N.; Xu, X.; Li, Y.; Ma, D.F. Genome-wide identification of Glutathione peroxidase (GPX) family genes and silencing TaGPX3.2A reduced disease resistance in wheat. Plant Physiol. Bioch. 2023, 204, 108139. [Google Scholar] [CrossRef] [PubMed]
- Tyagi JMishra, A.; Kumari, S.; Singh, S.; Agarwal, H.; Pudake, R.N.; Varma, A.; Joshi, N.C. Deploying a microbial consortium of Serendipita indica, Rhizophagus intraradices, and Azotobacter chroococcum to boost drought tolerance in maize. Environ. Exp. Bot. 2023, 206, 105142. [Google Scholar] [CrossRef]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Zhang, Z.; Payne, S.H.; Zhang, B.; McDermott, J.E.; Zhou, J.Y.; Petyuk, V.A.; Chen, L.; Ray, D.; et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer. Cell 2016, 166, 755–765. [Google Scholar] [CrossRef]
- Plubell, D.L.; Wilmarth, P.A.; Zhao, Y.; Fenton, A.M.; Minnier, J.; Reddy, A.P.; Klimek, J.; Yang, X.; David, L.L.; Pamir, N. Extended multiplexing of Tandem Mass Tags (TMT) labeling reveals age and high fat diet specific proteome changes in mouse epididymal adipose tissue. Mol. Cell Proteom. 2017, 16, 873–890. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gao, L.; Liu, H.; Liu, C. iTRAQ-based proteomic analysis of neonatal kidney from offspring of protein restricted rats reveals abnormalities in intraflagellar transport proteins. Cell. Physiol. Biochem. 2018, 44, 185–199. [Google Scholar] [CrossRef]
- Ashkenazi, M.; Bader, G.D.; Kuchinsky, A.; Moshelion, M.; States, D.J. Cytoscape ESP: Simple search of complex biological networks. Bioinformatics 2008, 24, 1465–1466. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Lv, G. Responses of Calligonum leucocladum to Prolonged Drought Stress Through Antioxidant System Activation, Soluble Sugar Accumulation, and Maintaining Photosynthetic Homeostasis. Int. J. Mol. Sci. 2025, 26, 4403. https://doi.org/10.3390/ijms26094403
Yang F, Lv G. Responses of Calligonum leucocladum to Prolonged Drought Stress Through Antioxidant System Activation, Soluble Sugar Accumulation, and Maintaining Photosynthetic Homeostasis. International Journal of Molecular Sciences. 2025; 26(9):4403. https://doi.org/10.3390/ijms26094403
Chicago/Turabian StyleYang, Fang, and Guanghui Lv. 2025. "Responses of Calligonum leucocladum to Prolonged Drought Stress Through Antioxidant System Activation, Soluble Sugar Accumulation, and Maintaining Photosynthetic Homeostasis" International Journal of Molecular Sciences 26, no. 9: 4403. https://doi.org/10.3390/ijms26094403
APA StyleYang, F., & Lv, G. (2025). Responses of Calligonum leucocladum to Prolonged Drought Stress Through Antioxidant System Activation, Soluble Sugar Accumulation, and Maintaining Photosynthetic Homeostasis. International Journal of Molecular Sciences, 26(9), 4403. https://doi.org/10.3390/ijms26094403





