Povidone-Iodine and Hydrogen Peroxide Combination Improves the Anti-Biofilm Activity of the Individual Agents on Staphylococcus aureus
Abstract
:1. Introduction
2. Results
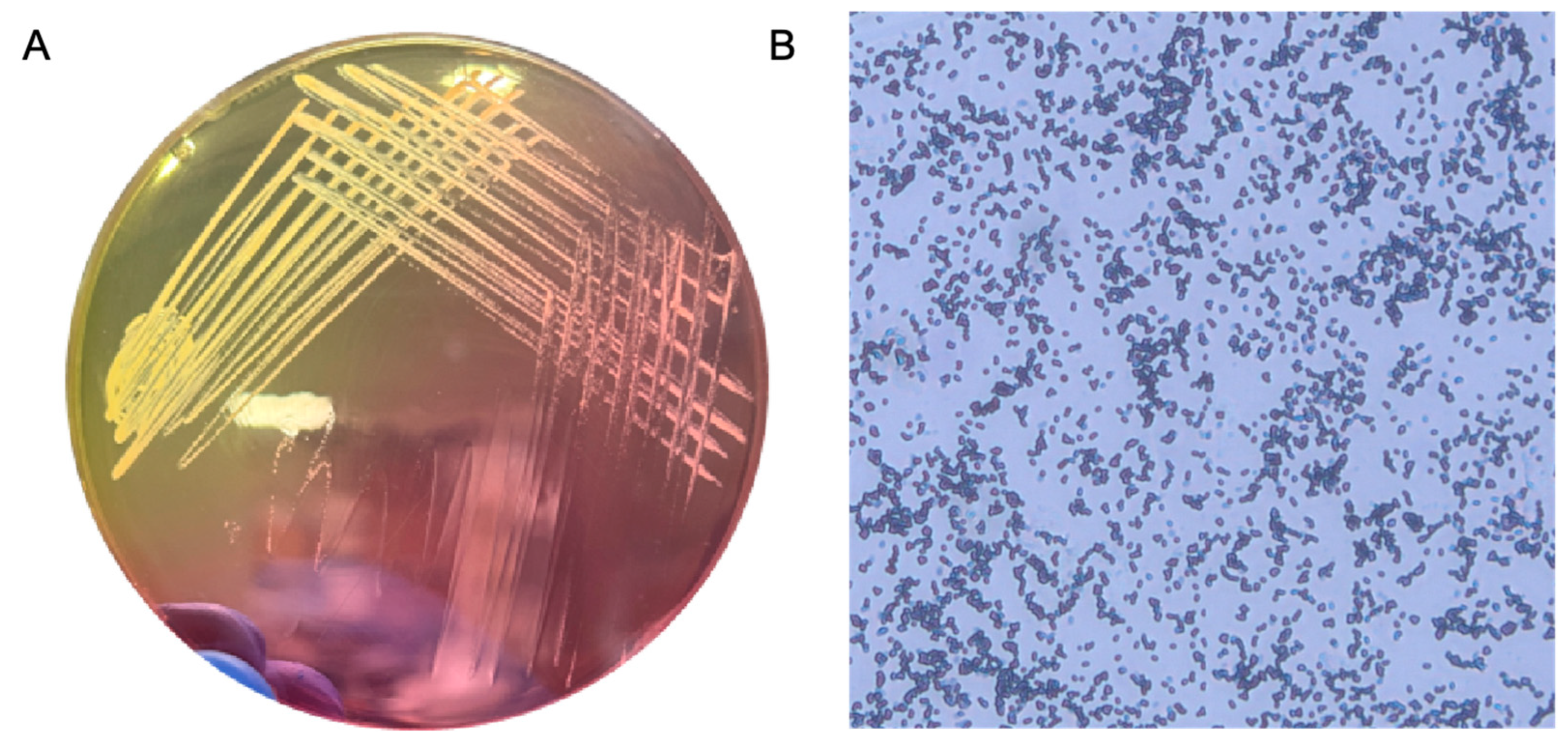
2.1. Confirmation of S. aureus Identity Using Morphological and Culture-Based Characteristics
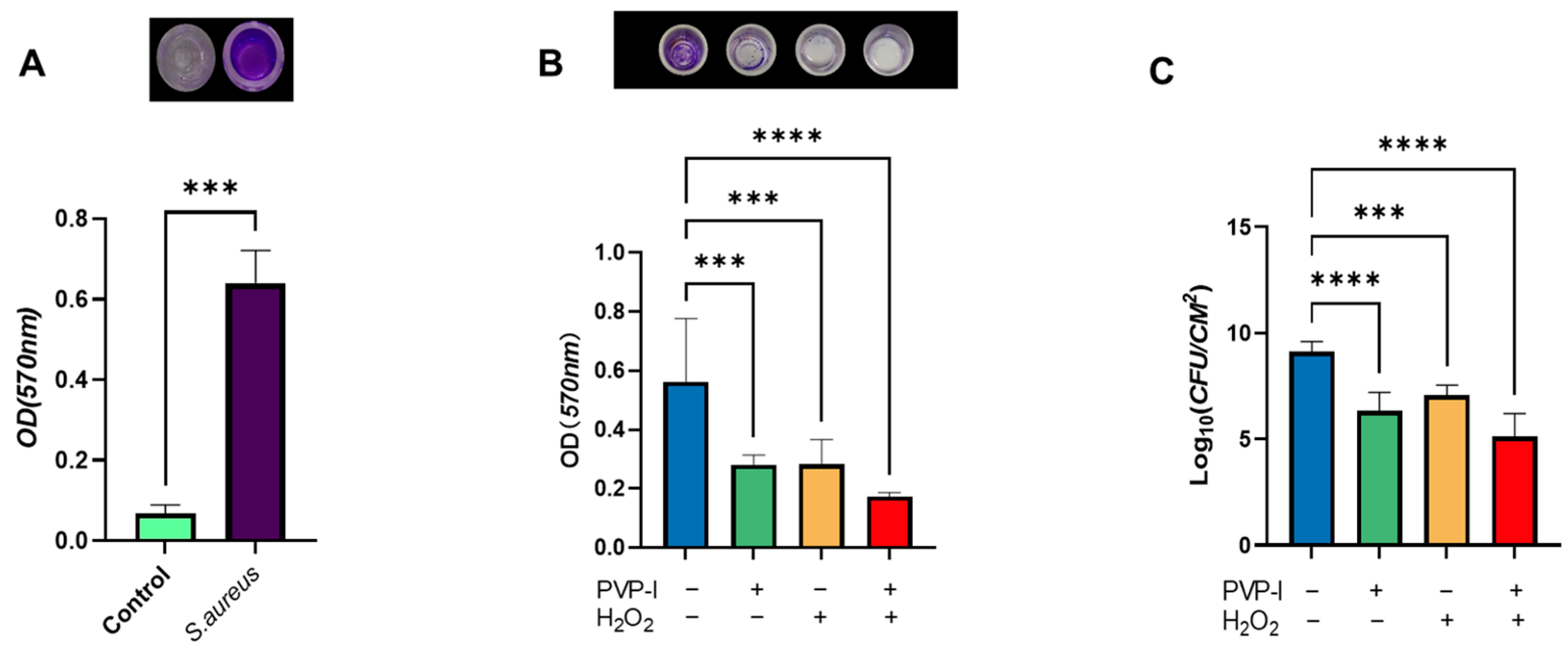
2.2. Quantitative Evaluation of Biofilm Disruption by PVP-I and H2O2
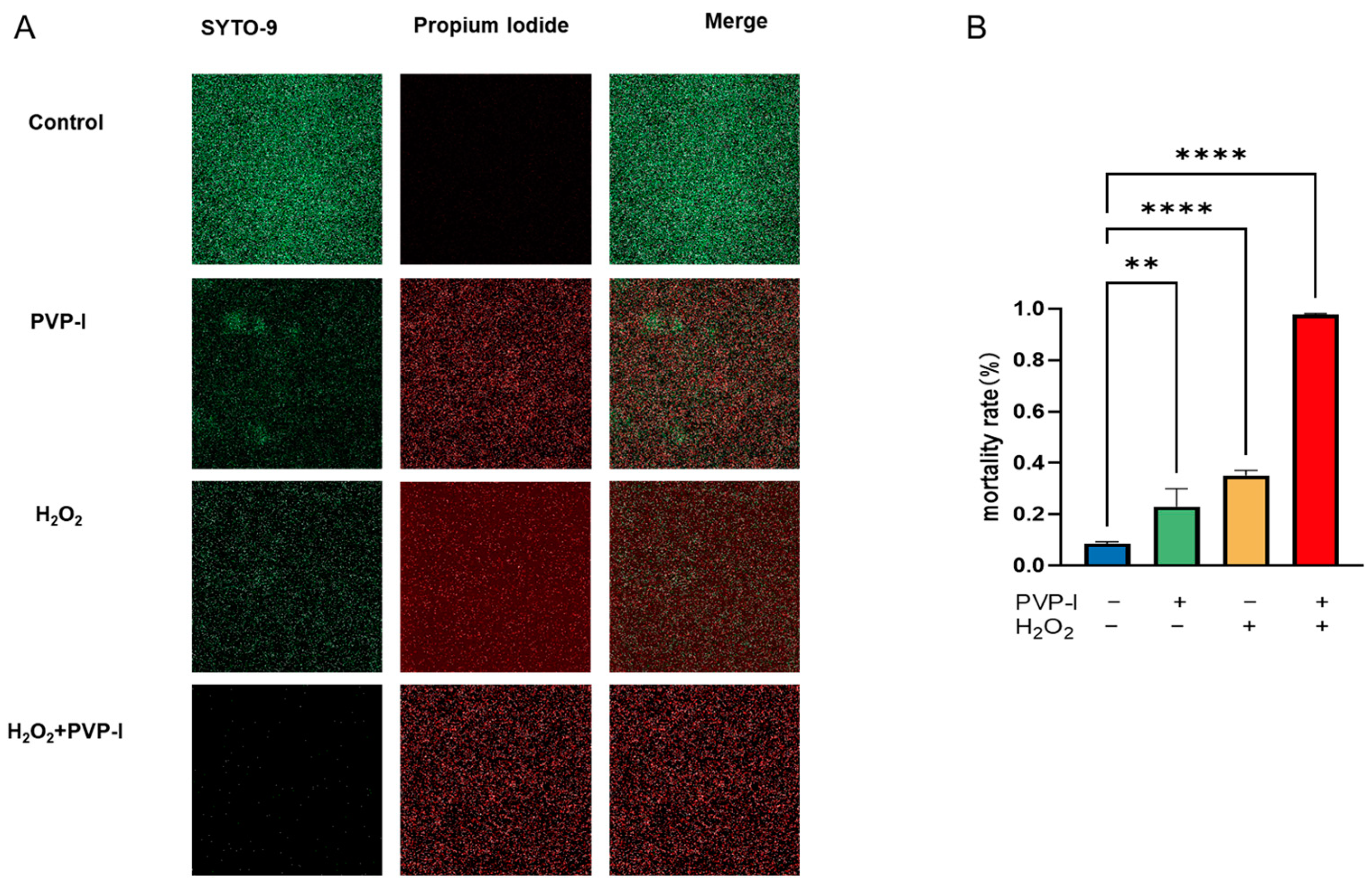
2.3. Live/Dead Assay of Biofilms Treated with PVP-I and H2O2
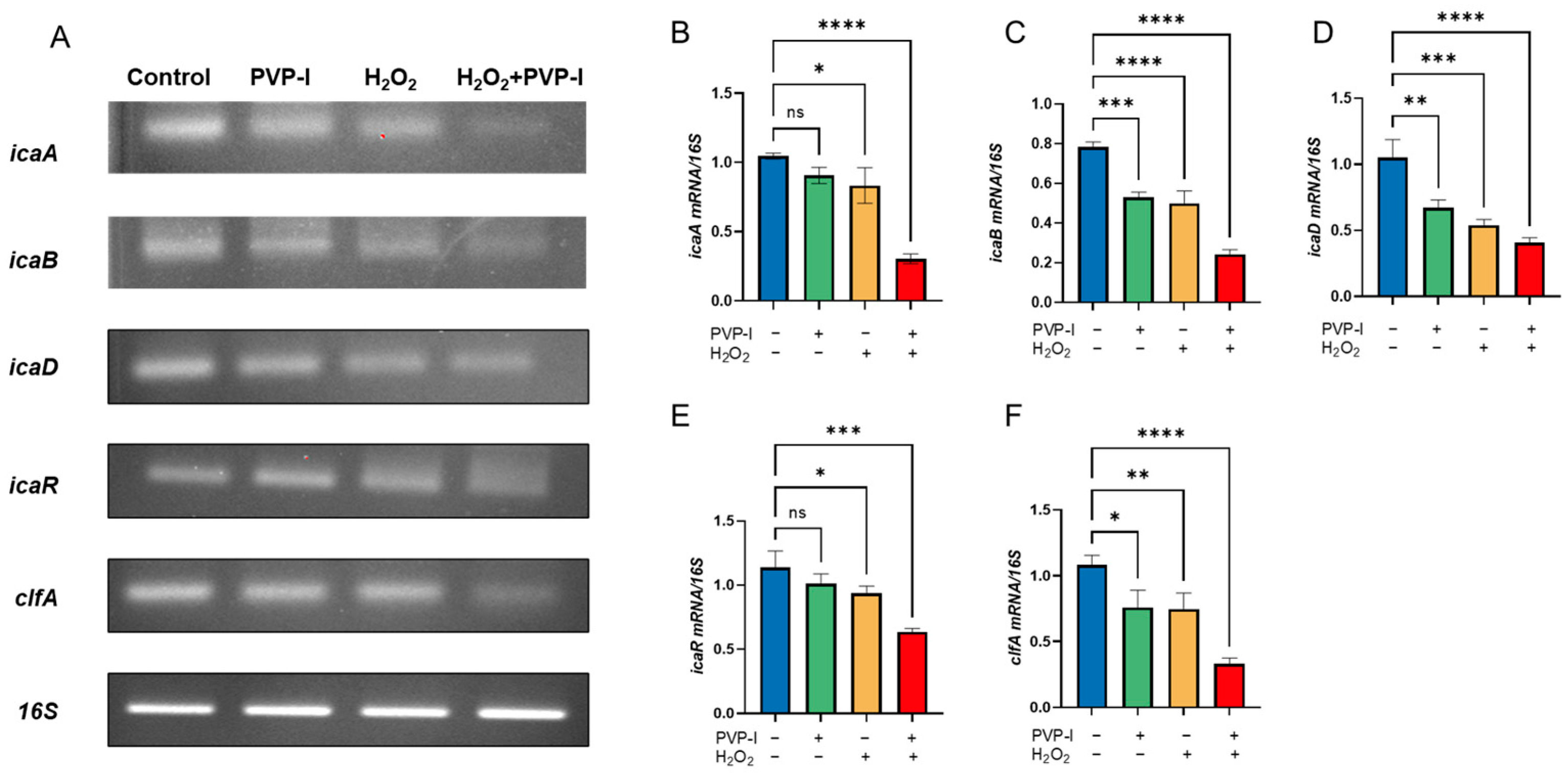
2.4. Expression of Biofilm-Associated Genes Following PVP-I and H2O2 Treatment
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Phenotypic Confirmation of S. aureus
4.2. Biofilm Formation Assay
4.2.1. Preliminary Biofilm Formation (96-Well Plate)
4.2.2. Biofilm Formation on Titanium Discs
4.3. Application of Treatments
4.4. CFU Enumeration
4.5. Biofilm Detection
4.6. Live/Dead Assay
4.7. Gene Expression Analysis (RT-PCR)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.E.; Della Valle, C.J.; Rex, J.C.; Traven, S.A.; Durante, E.C. Fungal Periprosthetic Joint Infection: A Review of Demographics and Management. J. Arthroplast. 2021, 36, 1758–1764. [Google Scholar] [CrossRef]
- Pietrocola, G.; Campoccia, D.; Motta, C.; Montanaro, L.; Arciola, C.R.; Speziale, P. Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants. Int. J. Mol. Sci. 2022, 23, 5958. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Kim, C.J.; Kim, H.B.; Oh, M.D.; Kim, Y.; Kim, A.; Oh, S.H.; Song, K.H.; Kim, E.; Cho, Y.; Choi, Y.; et al. The burden of nosocomial staphylococcus aureus bloodstream infection in South Korea: A prospective hospital-based nationwide study. BMC Infect. Dis. 2014, 14, 590. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, Y.; Wang, W.; Chen, J.; Zhu, J. The effect and mechanism of iodophors on the adhesion and virulence of Staphylococcus aureus biofilms attached to artificial joint materials. J. Orthop. Surg. Res. 2023, 18, 756. [Google Scholar] [CrossRef]
- Ruder, J.A.; Springer, B.D. Treatment of Periprosthetic Joint Infection Using Antimicrobials: Dilute Povidone-Iodine Lavage. J. Bone Jt. Infect. 2017, 2, 10–14. [Google Scholar] [CrossRef]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Raval, Y.S.; Mohamed, A.; Song, J.; Greenwood-Quaintance, K.E.; Beyenal, H.; Patel, R. Hydrogen Peroxide-Generating Electrochemical Scaffold Activity against Trispecies Biofilms. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Stewart, P.S.; Owkes, M. Simulation of catalase-dependent tolerance of microbial biofilm to hydrogen peroxide with a biofilm computer model. NPJ Biofilms Microbiomes 2023, 9, 60. [Google Scholar] [CrossRef]
- Wang, M.; Gu, K.; Wan, M.; Gan, L.; Chen, J.; Zhao, W.; Shi, H.; Li, J. Hydrogen peroxide enhanced photoinactivation of Candida albicans by a novel boron-dipyrromethene (BODIPY) derivative. Photochem. Photobiol. Sci. 2023, 22, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Lee, D.W.; Park, H.J.; Kwak, M.H.; Park, J.M.; Choi, M.G. Hydrogen Peroxide Enhances the Antibacterial Effect of Methylene Blue-based Photodynamic Therapy on Biofilm-forming Bacteria. Photochem. Photobiol. 2019, 95, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.P. Therapeutic Strategies against Biofilm Infections. Life 2023, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Hoogenkamp, M.A.; Mazurel, D.; Deutekom-Mulder, E.; de Soet, J.J. The consistent application of hydrogen peroxide controls biofilm growth and removes Vermamoeba vermiformis from multi-kingdom in-vitro dental unit water biofilms. Biofilm 2023, 5, 100132. [Google Scholar] [CrossRef]
- Shoji, M.M.; Chen, A.F. Biofilms in Periprosthetic Joint Infections: A Review of Diagnostic Modalities, Current Treatments, and Future Directions. J. Knee Surg. 2020, 33, 119–131. [Google Scholar] [CrossRef]
- Wildemann, B.; Jandt, K.D. Infections @ Trauma/Orthopedic Implants: Recent Advances on Materials, Methods, and Microbes-A Mini-Review. Materials 2021, 14, 5834. [Google Scholar] [CrossRef]
- Migliorini, F.; Weber, C.D.; Bell, A.; Betsch, M.; Maffulli, N.; Poth, V.; Hofmann, U.K.; Hildebrand, F.; Driessen, A. Bacterial pathogens and in-hospital mortality in revision surgery for periprosthetic joint infection of the hip and knee: Analysis of 346 patients. Eur. J. Med. Res. 2023, 28, 177. [Google Scholar] [CrossRef]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Lee, S.W.; Carnicelli, J.; Getya, D.; Gitsov, I.; Phillips, K.S.; Ren, D. Biofilm Removal by Reversible Shape Recovery of the Substrate. ACS Appl. Mater. Interfaces 2021, 13, 17174–17182. [Google Scholar] [CrossRef]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9, 17666. [Google Scholar] [CrossRef] [PubMed]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect Control. 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Paulitsch-Fuchs, A.H.; Bödendorfer, B.; Wolrab, L.; Eck, N.; Dyer, N.P.; Lohberger, B. Effect of Cobalt-Chromium-Molybdenum Implant Surface Modifications on Biofilm Development of S. aureus and S. epidermidis. Front. Cell. Infect. Microbiol. 2022, 12, 837124. [Google Scholar] [CrossRef]
- Grossman, A.B.; Burgin, D.J.; Rice, K.C. Quantification of Staphylococcus aureus Biofilm Formation by Crystal Violet and Confocal Microscopy. Methods Mol. Biol. 2021, 2341, 69–78. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Muhilvannan, S.; Arun Vignesh, M. Hesperidin inhibits biofilm formation, virulence and staphyloxanthin synthesis in methicillin resistant Staphylococcus aureus by targeting SarA and CrtM: An in vitro and in silico approach. World J. Microbiol. Biotechnol. 2022, 38, 44. [Google Scholar] [CrossRef]
- Gajewska, J.; Chajęcka-Wierzchowska, W. Biofilm Formation Ability and Presence of Adhesion Genes among Coagulase-Negative and Coagulase-Positive Staphylococci Isolates from Raw Cow’s Milk. Pathogens 2020, 9, 654. [Google Scholar] [CrossRef]
- Oduwole, K.O.; Glynn, A.A.; Molony, D.C.; Murray, D.; Rowe, S.; Holland, L.M.; McCormack, D.J.; O’Gara, J.P. Anti-biofilm activity of sub-inhibitory povidone-iodine concentrations against Staphylococcus epidermidis and Staphylococcus aureus. J. Orthop. Res. 2010, 28, 1252–1256. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Namvar, A.E. Detection of genes involved in biofilm formation in Staphylococcus aureus isolates. GMS Hyg. Infect. Control 2016, 11, Doc07. [Google Scholar] [CrossRef]
- Atshan, S.S.; Shamsudin, M.N.; Karunanidhi, A.; van Belkum, A.; Lung, L.T.; Sekawi, Z.; Nathan, J.J.; Ling, K.H.; Seng, J.S.; Ali, A.M.; et al. Quantitative PCR analysis of genes expressed during biofilm development of methicillin resistant Staphylococcus aureus (MRSA). Infect. Genet. Evol. 2013, 18, 106–112. [Google Scholar] [CrossRef]
- Deivanayagam, C.C.; Wann, E.R.; Chen, W.; Carson, M.; Rajashankar, K.R.; Höök, M.; Narayana, S.V. A novel variant of the immunoglobulin fold in surface adhesins of Staphylococcus aureus: Crystal structure of the fibrinogen-binding MSCRAMM, clumping factor A. Embo J. 2002, 21, 6660–6672. [Google Scholar] [CrossRef]
| Treatment Group | Agent(s) Used | Concentration | Exposure Time | Treatment Order |
|---|---|---|---|---|
| Control | PBS | — | — | — |
| PVP-I | Povidone-iodine | 1% | 5 min | Single treatment |
| H2O2 | Hydrogen peroxide | 3.5% | 5 min | Single treatment |
| Combination | H2O2 + PVP-I | 3.5% + 1% | 5 min + 5 min | H2O2 first, then PVP-I |
| Gene | Sequence (5′–3′) | Annealing Temp (°C) |
|---|---|---|
| icaA | F: CTATTTCGGGTGTCTTCACTC | 53.7 |
| R: GGCAAGCGGTTCATACTTA | ||
| icaB | F: TTGCCTGTAAGCACACTGGATGGTC | 57.5 |
| R: TACACGGTGATAATTTAATGCCAGAGC | ||
| icaD | F: ATGGACAAGTCCAGACAGAGGAAAA | 59.1 |
| R: GTCACTCATCGTAACTGCTTCAACG | ||
| icaR | F: TCAGAGAAGGGGTATGACGGTACAA | 58.2 |
| R: TCCTCAGGCGTATTAGATAATTGAACG | ||
| clfA | F: CAAGTAGCGTTAGTGCTGC | 51.8 |
| R: TGATTGAGTTGTTGCCG | ||
| 16S | F: CGTGCTACAATGGACAATACAAA | 57.2 |
| R: ATCTACGATTACTAGCGATTCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, L.; Sankaranarayanan, J.; Lee, C.-Y.; Zhou, H.; Yoon, T.-R.; Seon, J.-K.; Park, K.-S. Povidone-Iodine and Hydrogen Peroxide Combination Improves the Anti-Biofilm Activity of the Individual Agents on Staphylococcus aureus. Int. J. Mol. Sci. 2025, 26, 4390. https://doi.org/10.3390/ijms26094390
Wan L, Sankaranarayanan J, Lee C-Y, Zhou H, Yoon T-R, Seon J-K, Park K-S. Povidone-Iodine and Hydrogen Peroxide Combination Improves the Anti-Biofilm Activity of the Individual Agents on Staphylococcus aureus. International Journal of Molecular Sciences. 2025; 26(9):4390. https://doi.org/10.3390/ijms26094390
Chicago/Turabian StyleWan, Le, Jaishree Sankaranarayanan, Chan-Young Lee, Hongyan Zhou, Taek-Rim Yoon, Jong-Keun Seon, and Kyung-Soon Park. 2025. "Povidone-Iodine and Hydrogen Peroxide Combination Improves the Anti-Biofilm Activity of the Individual Agents on Staphylococcus aureus" International Journal of Molecular Sciences 26, no. 9: 4390. https://doi.org/10.3390/ijms26094390
APA StyleWan, L., Sankaranarayanan, J., Lee, C.-Y., Zhou, H., Yoon, T.-R., Seon, J.-K., & Park, K.-S. (2025). Povidone-Iodine and Hydrogen Peroxide Combination Improves the Anti-Biofilm Activity of the Individual Agents on Staphylococcus aureus. International Journal of Molecular Sciences, 26(9), 4390. https://doi.org/10.3390/ijms26094390







