Design and Pharmacological Characterization of a Novel Antithrombotic P2Y1 Receptor-Based Vaccine
Abstract
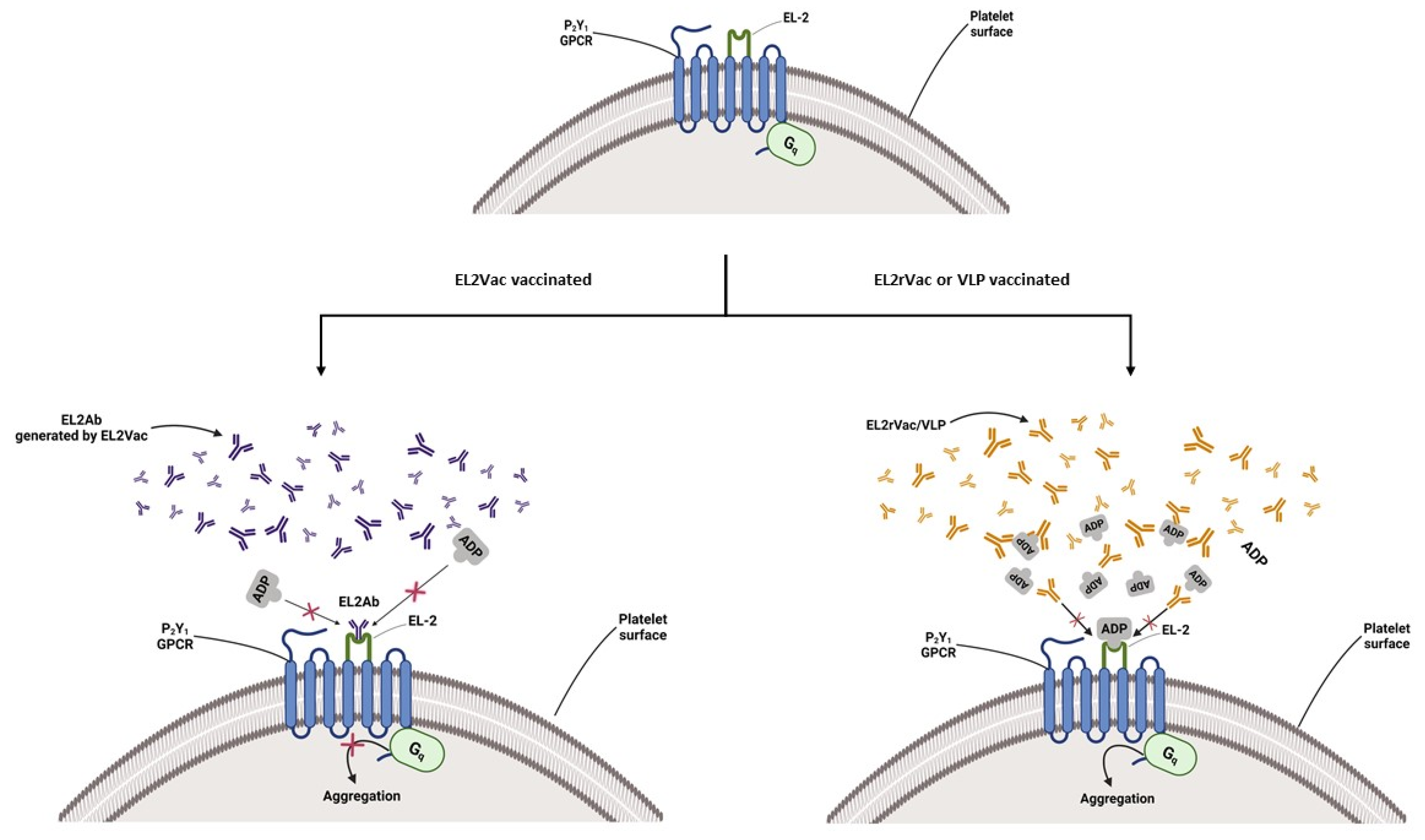
:1. Introduction
2. Results
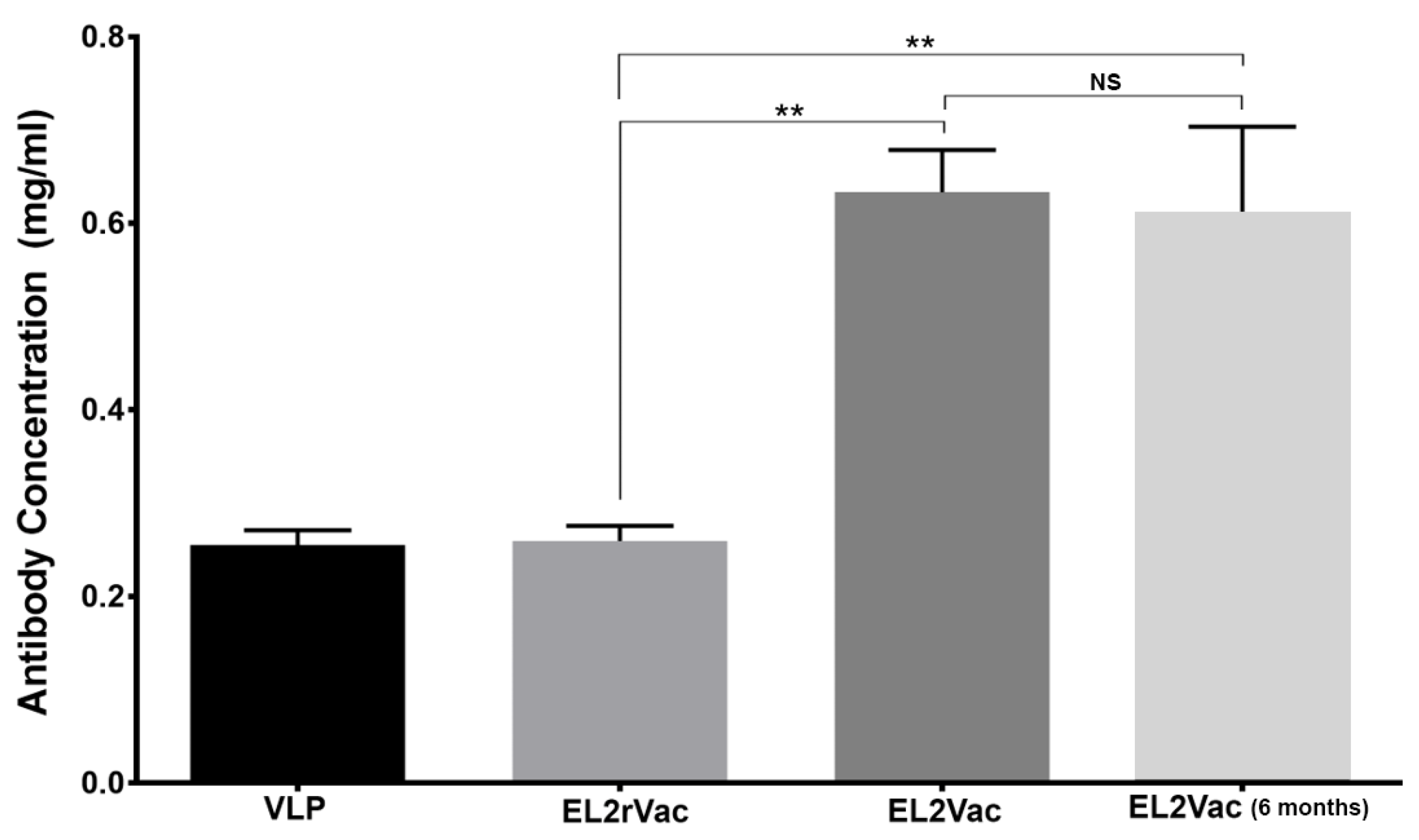
2.1. Quantification of Antibody Production in Vaccinated Mice
2.2. Platelet Counts in Vaccinated Mice
2.3. EL2Vac Inhibits ADP-Induced Platelet Aggregation
2.4. EL2Vac Inhibits ADP-Induced Alpha Granule Secretion
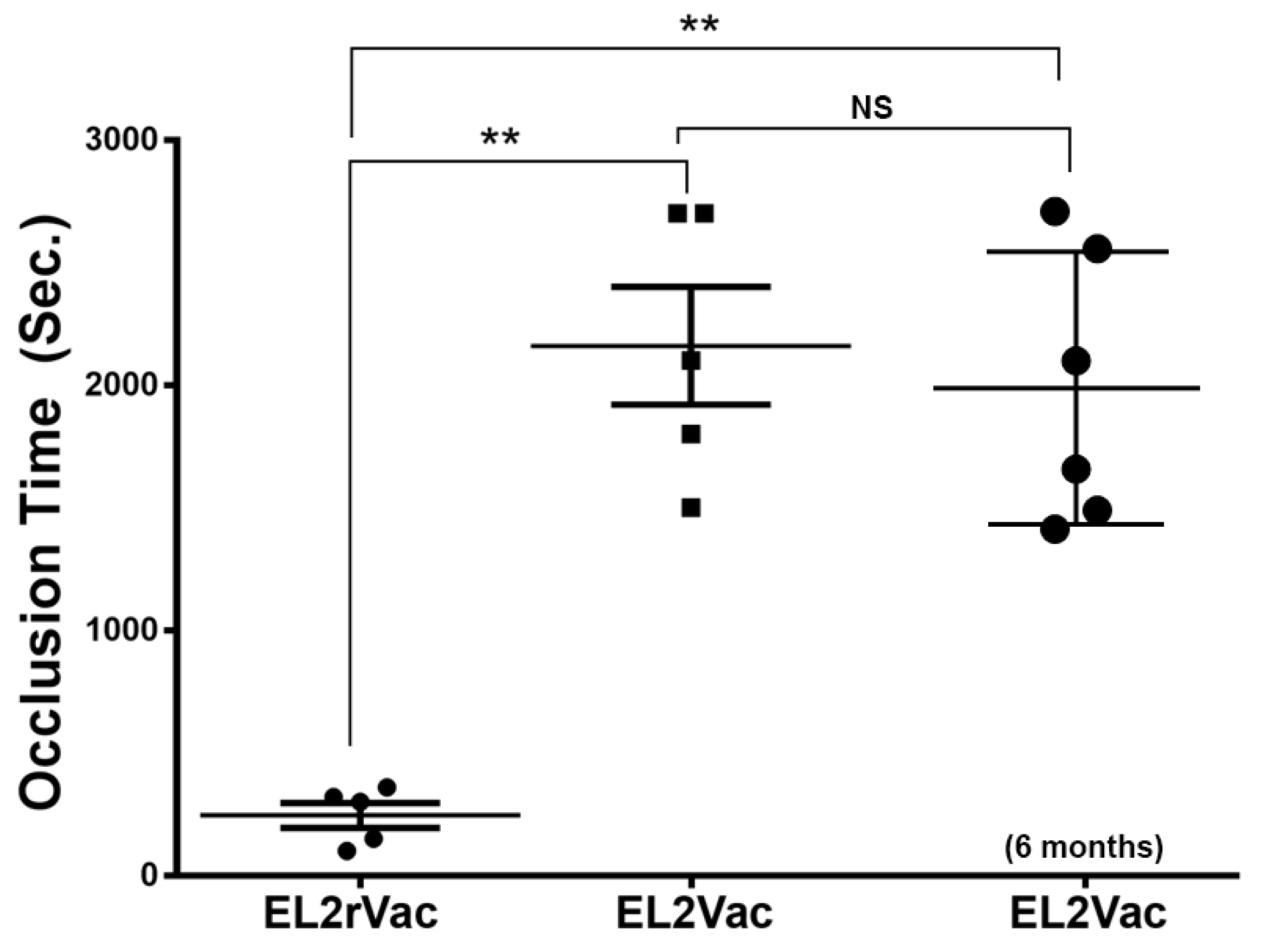
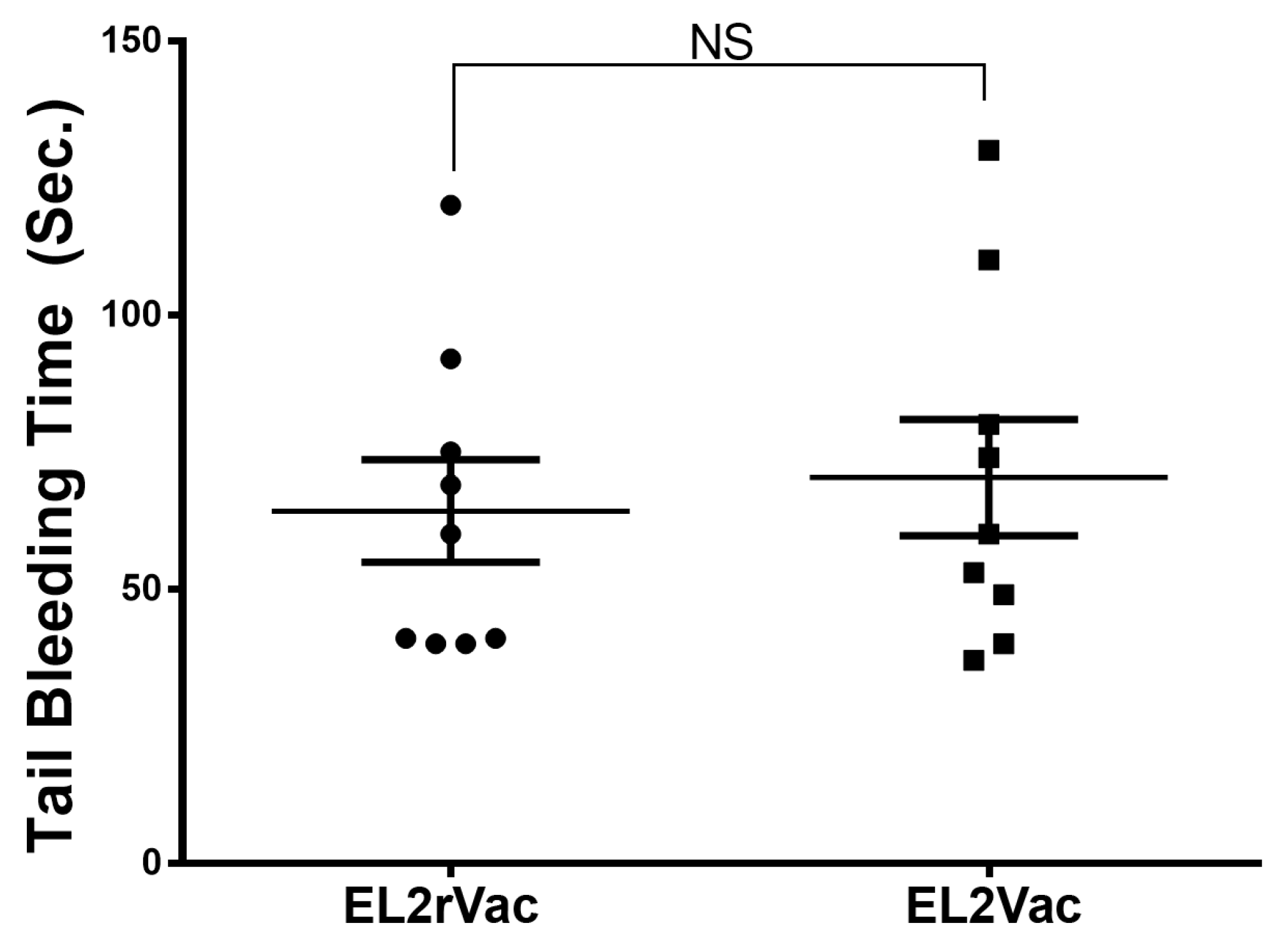
2.5. EL2Vac Prolongs the Occlusion Time Without Affecting the Tail Bleeding Time
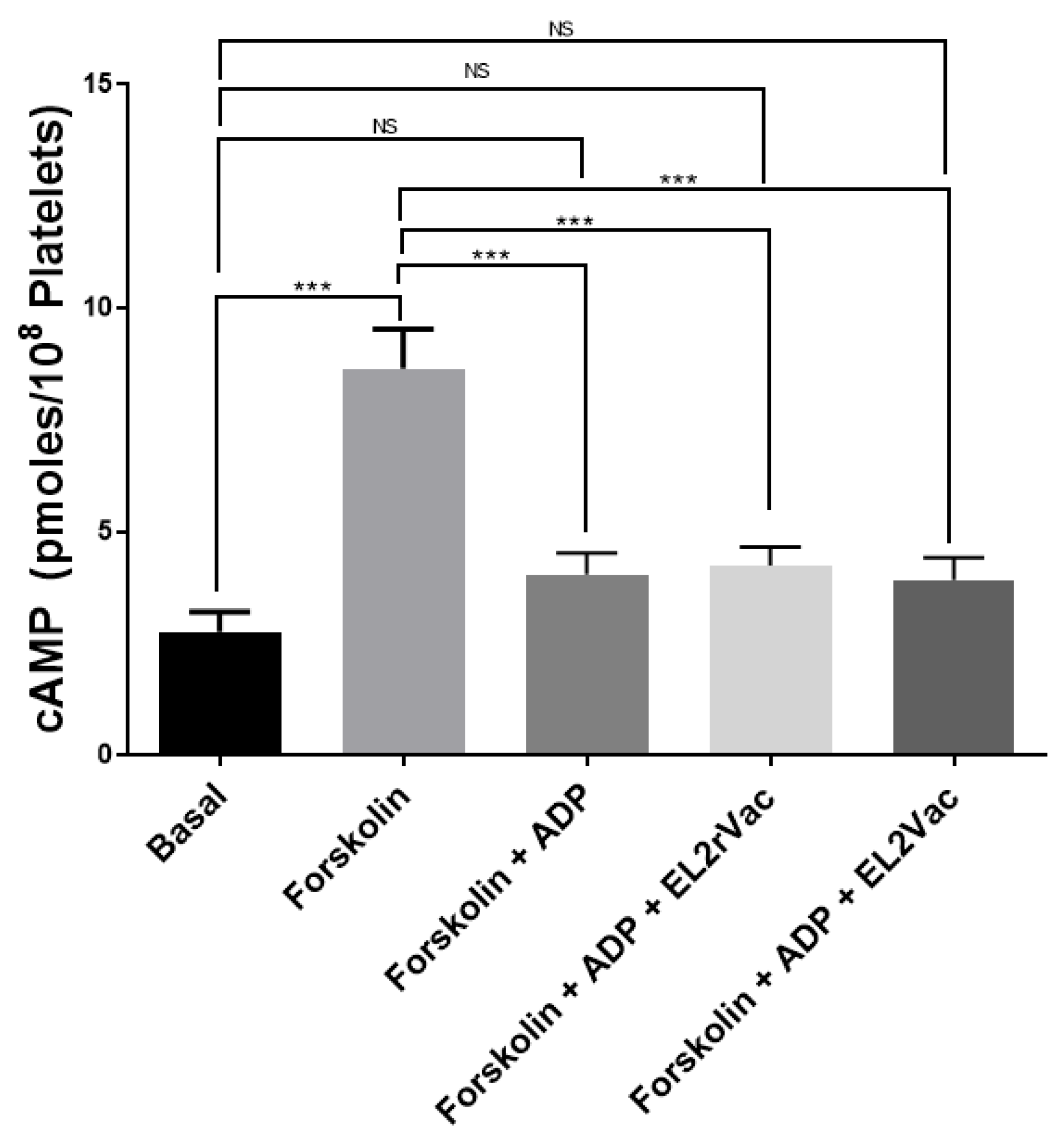
2.6. EL2Vac Does Not Inhibit the P2Y12-R
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Methods
4.2.1. EL2 and EL2Ab Peptide Based Vaccination Protocol
4.2.2. Quantification of Antibody Production in the Vaccinated Mice
4.2.3. Effect of the Peptide-Based Vaccines on Platelet Aggregation
4.2.4. Effect of the Peptide-Based Vaccines on Surface Expression of P-Selectin
4.2.5. Effect of the Peptide-Based Vaccines on FeCl3-Induced Thrombosis
4.2.6. Effect of the Peptide-Based Vaccines on the Tail Bleeding
4.2.7. Effect of the Peptide-Based Vaccine on cAMP Levels
4.2.8. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E., Jr.; Patel-Hett, S.; Hartwig, J.H. Mechanics of proplatelet elaboration. J. Thromb. Haemost. 2007, 5, 18–23. [Google Scholar] [CrossRef]
- Cattaneo, M.; Gachet, C. ADP Receptors and Clinical Bleeding Disorders. Arter. Thromb. Vasc. Biol. 1999, 19, 2281–2285. [Google Scholar] [CrossRef]
- Communi, D.; Gonzalez, N.S.; Detheux, M.; Brézillon, S.; Lannoy, V.; Parmentier, M.; Boeynaems, J.-M. Identification of a Novel Human ADP Receptor Coupled to Gi. J. Biol. Chem. 2001, 276, 41479–41485. [Google Scholar] [CrossRef]
- Anderson, R.; Theron, A.J.; Steel, H.C.; Nel, J.G.; Tintinger, G.R. ADP-Mediated Upregulation of Expression of CD62P on Human Platelets Is Critically Dependent on Co-Activation of P2Y1 and P2Y12 Receptors. Pharmaceuticals 2020, 13, 420. [Google Scholar] [CrossRef]
- Baurand, A.; Gachet, C. The P2Y1 receptor as a target for new antithrombotic drugs: A review of the P2Y1 antagonist MRS-2179. Cardiovasc. Drug Rev. 2003, 21, 67–76. [Google Scholar] [CrossRef]
- Daniel, J.L.; Dangelmaier, C.; Jin, J.; Ashby, B.; Smith, J.B.; Kunapuli, S.P. Molecular basis for ADP-induced platelet activation: I. Evidence for three distinct ADP receptors on human platelets. J. Biol. Chem. 1998, 273, 2024–2029. [Google Scholar] [CrossRef]
- Jin, J.; Daniel, J.L.; Kunapuli, S.P. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 1998, 273, 2030–2034. [Google Scholar] [CrossRef]
- Jin, J.G.; Kunapuli, S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. USA 1998, 95, 8070–8074. [Google Scholar] [CrossRef]
- Coller, B.S. Platelets and thrombolytic therapy. N. Engl. J. Med. 1990, 322, 33–42. [Google Scholar] [CrossRef]
- Weiss, H.J. Antiplatelet drugs—A new pharmacologic approach to the prevention of thrombosis. Am. Heart J. 1976, 92, 86–102. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Delaney, S.M.; LaRocca, T.; Vincent, D.; DeGuzman, F.; Jurek, M.; Koller, B.; Phillips, D.R.; Conley, P.B. P2Y 12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J. Clin. Investig. 2003, 112, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Fabre, J.-E.; Nguyen, M.; Latour, A.; Keifer, J.A.; Audoly, L.P.; Coffman, T.M.; Koller, B.H. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat. Med. 1999, 5, 1199–1202. [Google Scholar] [CrossRef] [PubMed]
- Storey, R.F.; Newby, L.J.; Heptinstall, S. Effects of P2Y 1 and P2Y 12 receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Platelets 2001, 12, 443–447. [Google Scholar] [CrossRef]
- Storey, R.F. The P2Y 12 receptor as a therapeutic target in cardiovascular disease. Platelets 2001, 12, 197–209. [Google Scholar] [CrossRef]
- Foster, C.J.; Prosser, D.M.; Agans, J.M.; Zhai, Y.; Smith, M.D.; Lachowicz, J.E.; Zhang, F.L.; Gustafson, E.; Monsma, J.F.J.; Wiekowski, M.T.; et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J. Clin. Investig. 2001, 107, 1591–1598. [Google Scholar] [CrossRef]
- Savi, P.; Herbert, J.-M. Clopidogrel and Ticlopidine: P2Y12Adenosine Diphosphate-Receptor Antagonists for the Prevention of Atherothrombosis. Semin. Thromb. Hemost. 2005, 31, 174–183. [Google Scholar] [CrossRef]
- Lenain, N.; Freund, M.; Léon, C.; Cazenave, J.-P.; Gachet, C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J. Thromb. Haemost. 2003, 1, 1144–1149. [Google Scholar] [CrossRef]
- Léon, C.; Hechler, B.; Freund, M.; Eckly, A.; Vial, C.; Ohlmann, P.; Dierich, A.; LeMeur, M.; Cazenave, J.-P.; Gachet, C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor–null mice. J. Clin. Investig. 1999, 104, 1731–1737. [Google Scholar] [CrossRef]
- Hechler, B.; Eckly, A.; Ohlmann, P.; Cazenave, J.P.; Gachet, C. The P2Y1 receptor, necessary but not sufficient to support full ADP-induced platelet aggregation, is not the target of the drug clopidogrel. Br. J. Haematol. 1998, 103, 858–866. [Google Scholar] [CrossRef]
- Wijeyeratne, Y.D.; Heptinstall, S. Anti-platelet therapy: ADP receptor antagonists. Br. J. Clin. Pharmacol. 2011, 72, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Léon, C.; Vial, C.; Vigne, P.; Frelin, C.; Cazenave, J.P.; Gachet, C. The P2Y1 Receptor Is Necessary for Adenosine 5′-Diphosphate–Induced Platelet Aggregation. Blood J. Am. Soc. Hematol. 1998, 92, 152–159. [Google Scholar] [CrossRef]
- Boyer, J.L.; Romero-Avila, T.; Schachter, J.B.; Harden, T.K. Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996, 50, 1323–1329. [Google Scholar] [CrossRef]
- Maloney, S.F.; Brass, L.F.; Diamond, S.L. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr. Biol. 2010, 2, 183–192. [Google Scholar] [CrossRef]
- Baurand, A.; Raboisson, P.; Freund, M.; Léon, C.; Cazenave, J.-P.; Bourguignon, J.-J.; Gachet, C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur. J. Pharmacol. 2001, 412, 213–221. [Google Scholar] [CrossRef]
- Waldo, G.L.; Corbitt, J.; Boyer, J.L.; Ravi, G.; Kim, H.S.; Ji, X.-D.; Lacy, J.; Jacobson, K.A.; Harden, T.K. Quantitation of the P2Y1 Receptor with a High Affinity Radiolabeled Antagonist. Mol. Pharmacol. 2002, 62, 1249–1257. [Google Scholar] [CrossRef]
- Kim, H.S.; Ohno, M.; Xu, B.; Kim, H.O.; Choi, Y.; Ji, X.D.; Maddileti, S.; Marquez, V.E.; Harden, T.K. 2-Substitution of adenine nucleotide analogues containing a bicyclo [3.1. 0] hexane ring system locked in a northern conformation: Enhanced potency as P2Y1 receptor antagonists. J. Med. Chem. 2003, 46, 4974–4987. [Google Scholar] [CrossRef]
- Cattaneo, M.; Lecchi, A.; Ohno, M.; Joshi, B.V.; Besada, P.; Tchilibon, S.; Lombardi, R.; Bischofberger, N.; Harden, T.K.; Jacobson, K.A. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem. Pharmacol. 2004, 68, 1995–2002. [Google Scholar] [CrossRef]
- Pfefferkorn, J.A.; Choi, C.; Winters, T.; Kennedy, R.; Chi, L.; Perrin, L.A.; Lu, G.; Ping, Y.-W.; McClanahan, T.; Schroeder, R.; et al. P2Y1 receptor antagonists as novel antithrombotic agents. Bioorg. Med. Chem. Lett. 2008, 18, 3338–3343. [Google Scholar] [CrossRef]
- Thalji, R.K.; Aiyar, N.; Davenport, E.A.; Erhardt, J.A.; Kallal, L.A.; Morrow, D.M.; Senadhi, S.; Burns-Kurtis, C.L.; Marino, J.P. Benzofuran-substituted urea derivatives as novel P2Y1 receptor antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 4104–4107. [Google Scholar] [CrossRef]
- Karim, Z.A.; Vemana, H.P.; Alshbool, F.Z.; Lin, O.A.; Alshehri, A.M.; Javaherizadeh, P.; Espinosa, E.V.P.; Khasawneh, F.T. Characterization of a Novel Function-Blocking Antibody Targeted Against the Platelet P2Y 1 Receptor. Arter. Thromb. Vasc. Biol. 2015, 35, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; McNevin, J.; Zhao, H.; Tebit, D.M.; Troyer, R.M.; McSweyn, M.; Ghosh, A.K.; Shriner, D.; Arts, E.J.; McElrath, M.J.; et al. Evolution of Human Immunodeficiency Virus Type 1 Cytotoxic T-Lymphocyte Epitopes: Fitness-Balanced Escape. J. Virol. 2007, 81, 12179–12188. [Google Scholar] [CrossRef]
- Bernhardt, S.L.; Gjertsen, M.K.; Trachsel, S.; Møller, M.; Eriksen, J.A.; Meo, M.; Buanes, T.; Gaudernack, G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br. J. Cancer 2006, 95, 1474–1482. [Google Scholar] [CrossRef]
- Solares, A.M.; Baladron, I.; Ramos, T.; Valenzuela, C.; Borbon, Z.; Fanjull, S.; Gonzalez, L.; Castillo, D.; Esmir, J.; Granadillo, M.; et al. Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women with High-Grade Cervical Intraepithelial Neoplasia: First-in-Human, Proof-of-Concept Trial. ISRN Obstet. Gynecol. 2011, 2011, 292951. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Lin, S.Y.-H.; Cheng, C.-W.; Su, E.C.-Y. Prediction of B-cell epitopes using evolutionary information and propensity scales. BMC Bioinform. 2013, 14, S10. [Google Scholar] [CrossRef]
- Bijker, M.S.; Melief, C.J.; Offringa, R.; van der Burg, S.H. Design and development of synthetic peptide vaccines: Past, present and future. Expert. Rev. Vaccines 2007, 6, 591–603. [Google Scholar] [CrossRef]
- Oski, F.A.; Naiman, J.L. Effect of Live Measles Vaccine on the Platelet Count. N. Engl. J. Med. 1966, 275, 352–356. [Google Scholar] [CrossRef]
- Kaicker, S.; Martinko, K.; Bussel, J.B. Effects of COVID-19 vaccination on platelet counts and bleeding in children, adolescents, and young adults with immune thrombocytopenia. Pediatr. Blood Cancer 2023, 70, e30051. [Google Scholar] [CrossRef]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef]
- Nieuwenhuis, H.K.; Sakariassen, K.S.; Houdijk, W.P.; Nievelstein, P.F.; Sixma, J.J. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: A defect in platelet spreading. Blood 1986, 68, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Staatz, W.D.; Walsh, J.J.; Pexton, T.; Santoro, S.A. The alpha 2 beta 1 integrin cell surface collagen receptor binds to the alpha 1 (I)-CB3 peptide of collagen. J. Biol. Chem. 1990, 265, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Kroll, M.H.; Harris, T.S.; Moake, J.L.; Handin, R.I.; Schafer, A.I. von Willebrand factor binding to platelet GpIb initiates signals for platelet activation. J. Clin. Investig. 1991, 88, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Feinman, R.D.; Detwiler, T.C. Interrelations of Platelet Aggregation and Secretion. J. Clin. Investig. 1977, 60, 866–873. [Google Scholar] [CrossRef]
- Shattil, S.J.; Brass, L.F. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J. Biol. Chem. 1987, 262, 992–1000. [Google Scholar] [CrossRef]
- Du, X.; Ginsberg, M.H. Integrin αIIbβ3 and platelet function. Thromb. Haemost. 1997, 78, 96–100. [Google Scholar] [CrossRef]
- Baurand, A.; Eckly, A.; Hechler, B.; Kauffenstein, G.; Galzi, J.-L.; Cazenave, J.-P.; Léon, C.; Gachet, C. Differential Regulation and Relocalization of the Platelet P2Y Receptors after Activation: A Way to Avoid Loss of Hemostatic Properties? Mol. Pharmacol. 2005, 67, 721–733. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Bergmeier, W.; Eckly, A.; Ohlmann, P.; Leon, C.; Cazenave, J.P.; Nieswandt, B.; Gachet, C. The P2Y12 receptor induces platelet aggregation through weak activation of the αIIbβ3 integrin–a phosphoinositide 3-kinase-dependent mechanism. FEBS Lett. 2001, 505, 281–290. [Google Scholar] [CrossRef]
- Hechler, B.N.C.; Roh, E.J.; Cattaneo, M.; Cazenave, J.P.; Lanza, F.; Jacobson, K.A.; Gachet, C. MRS2500 [2-iodo-N6-methyl-(N)- methanocarba-2 -deoxyadenosine-3,5 -bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J. Pharmacol. Exp. Ther. 2006, 316, 556–563. [Google Scholar] [CrossRef]
- Alshbool, F.Z.; Karim, Z.A.; Espinosa, E.V.P.; Lin, O.A.; Khasawneh, F.T. Investigation of a thromboxane A2 receptor–based vaccine for managing thrombogenesis. J. Am. Heart Assoc. 2018, 7, e009139. [Google Scholar] [CrossRef]
- Alarabi, A.B.; Khasawneh, F.T.; Alshbool, F.Z. Managing thrombus formation with EL2-5HTVac: A selective vaccination-based approach targeting the platelet serotonin 5-HT(2A)R. J. Pharmacol. Exp. Ther. 2025, 392, 103399. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.W.; Ramirez, J.; Ortuno, D.; Shi, Y.; Thomsen, W.; Richman, J.G.; Morgan, M.; Dosa, P.; Teegarden, B.R. Anti-thrombotic and vascular effects of AR246686, a novel 5-HT2A receptor antagonist. Eur. J. Pharmacol. 2008, 586, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Gunderson, E.W.; Jiang, H.; Collins, E.D.; Foltin, R.W. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol. Psychiatry 2010, 67, 59–65. [Google Scholar] [CrossRef]
- Brown, M.J.; Coltart, J.; Gunewardena, K.; Ritter, J.M.; Auton, T.R.; Glover, J.F. Randomized double-blind placebo-controlled study of an angiotensin immunotherapeutic vaccine (PMD3117) in hypertensive subjects. Clin. Sci. 2004, 107, 167–173. [Google Scholar] [CrossRef]
- Manolopoulos, P.; Glenn, J.R.; Fox, S.C.; May, J.A.; Dovlatova, N.L.; Tang, S.W.; Thomas, N.R.; Ralevic, V.; Heptinstall, S. Acyl derivatives of coenzyme A inhibit platelet function via antagonism at P2Y1 and P2Y12 receptors: A new finding that may influence the design of anti-thrombotic agents. Platelets 2008, 19, 134–145. [Google Scholar] [CrossRef]
- Gearing, K.L.; Barnes, A.; Barnett, J.; Brown, A.; Cousens, D.; Dowell, S.; Green, A.; Patel, K.; Thomas, P.; Volpe, F.; et al. Complex chimeras to map ligand binding sites of GPCRs. Protein Eng. 2003, 16, 365–372. [Google Scholar] [CrossRef]
- Alarabi, A.B.; Karim, Z.A.; Ramirez, J.E.M.; Hernandez, K.R.; Lozano, P.A.; Rivera, J.O.; Alshbool, F.Z.; Khasawneh, F.T. Short-term exposure to waterpipe/hookah smoke triggers a hyperactive platelet activation state and increases the risk of thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 335–349. [Google Scholar] [CrossRef]
- Norambuena, A.; Poblete, M.I.; Donoso, M.V.; Espinoza, C.S.; Gonzalez, A.; Huidobro-Toro, J.P. P2Y1 receptor activation elicits its partition out of membrane rafts and its rapid internalization from human blood vessels: Implications for receptor signaling. Mol. Pharmacol. 2008, 74, 1666–1677. [Google Scholar] [CrossRef]
- Srinivasan, S.; Mir, F.; Huang, J.S.; Khasawneh, F.T.; Lam, S.C.T.; Le Breton, G.C. The P2Y12 antagonists, 2-methylthioadenosine 5′-monophosphate triethylammonium salt and cangrelor (ARC69931MX), can inhibit human platelet aggregation through a Gi-independent increase in cAMP levels. J. Biol. Chem. 2009, 284, 16108–16117. [Google Scholar] [CrossRef]


| P2Y1-R Antagonist | Pharmacological Action | Limitations | Reference |
|---|---|---|---|
| A2P5P and A3P5P | Complete inhibition of platelet aggregation | Not selective | [22,23] |
| MRS2179 | Inhibition of platelet function and delay in thrombosis formation | Transient action requiring high doses | [4,6,18,24,25] |
| MRS2500 | Enhanced inhibition of ADP-induced aggregation, antithrombotic activity, higher targeting ability than MRS2179 | Potential for irreversible inhibition when used with clopidogrel | [26,27,28] |
| Di-aryl urea | Inhibited ADP/P2Y1 receptor-mediated platelet aggregation in vitro | Unknown in vivo effectiveness, short biological half-life, toxicity | [29,30] |
| Benzofuran-substituted urea derivatives | Inhibited P-selectin expression | Poor potency, oral bioavailability issues | [29,30] |
| Treatment | Mean Platelet Count ± SEM (X × 10 −3/µL) |
|---|---|
| Basal | 825 ± 87 |
| Vehicle (5 days) | 829 ±94 |
| Vehicle (14 days) | 818 ± 75 |
| EL2Vac 20 µg (≈8 weeks) | 841 ± 72 |
| VLP (≈8 weeks) | 835 ± 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Meanazel, O.; Alshbool, F.Z.; Khasawneh, F.T. Design and Pharmacological Characterization of a Novel Antithrombotic P2Y1 Receptor-Based Vaccine. Int. J. Mol. Sci. 2025, 26, 4383. https://doi.org/10.3390/ijms26094383
Al Meanazel O, Alshbool FZ, Khasawneh FT. Design and Pharmacological Characterization of a Novel Antithrombotic P2Y1 Receptor-Based Vaccine. International Journal of Molecular Sciences. 2025; 26(9):4383. https://doi.org/10.3390/ijms26094383
Chicago/Turabian StyleAl Meanazel, Osaid, Fatima Z. Alshbool, and Fadi T. Khasawneh. 2025. "Design and Pharmacological Characterization of a Novel Antithrombotic P2Y1 Receptor-Based Vaccine" International Journal of Molecular Sciences 26, no. 9: 4383. https://doi.org/10.3390/ijms26094383
APA StyleAl Meanazel, O., Alshbool, F. Z., & Khasawneh, F. T. (2025). Design and Pharmacological Characterization of a Novel Antithrombotic P2Y1 Receptor-Based Vaccine. International Journal of Molecular Sciences, 26(9), 4383. https://doi.org/10.3390/ijms26094383





