Association Between Periodontal Pathogens and Inflammation in Patients with Acute Coronary Syndromes
Abstract
:1. Introduction
2. Results
2.1. Basic Characteristics of the Study Population
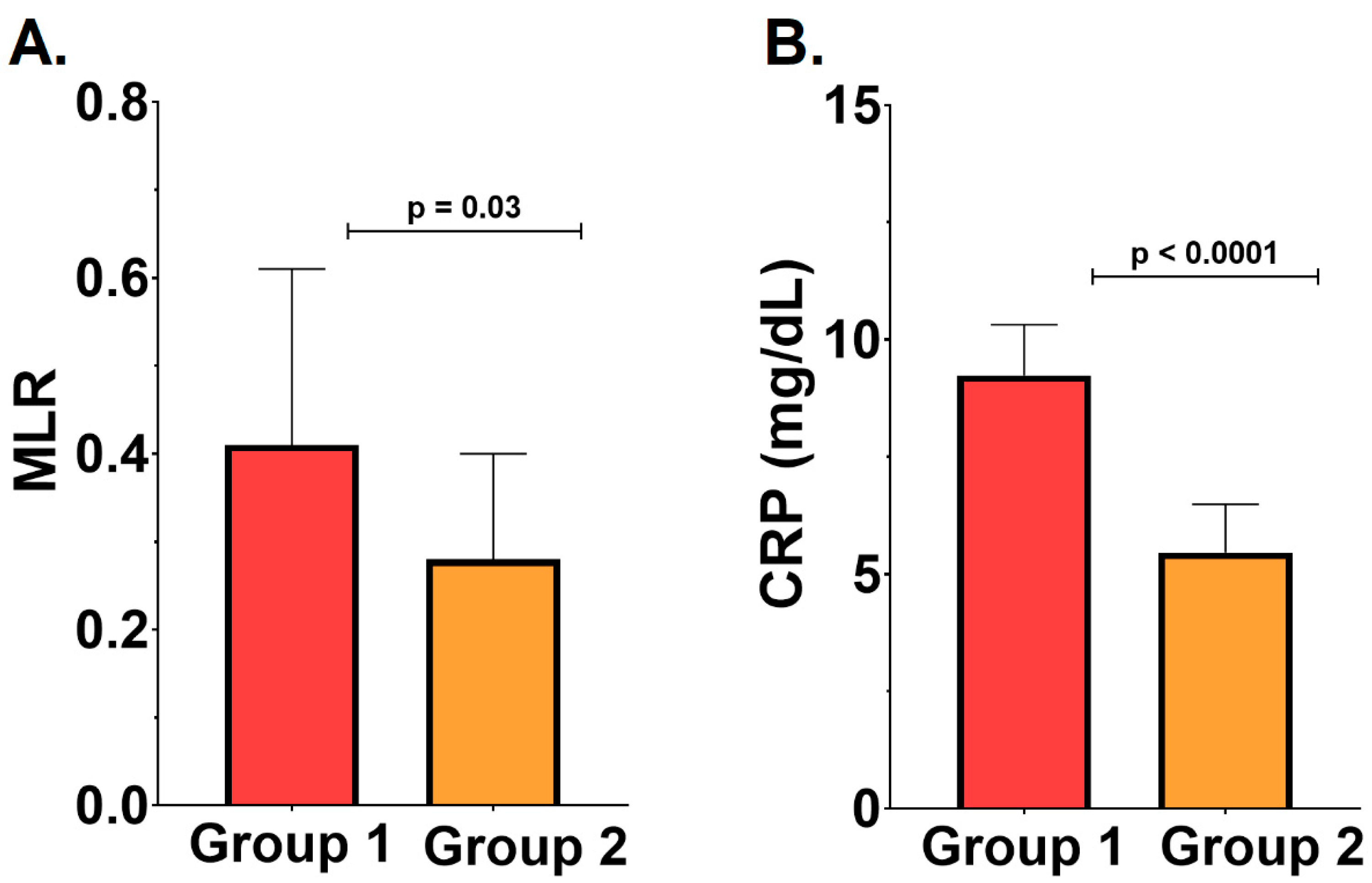
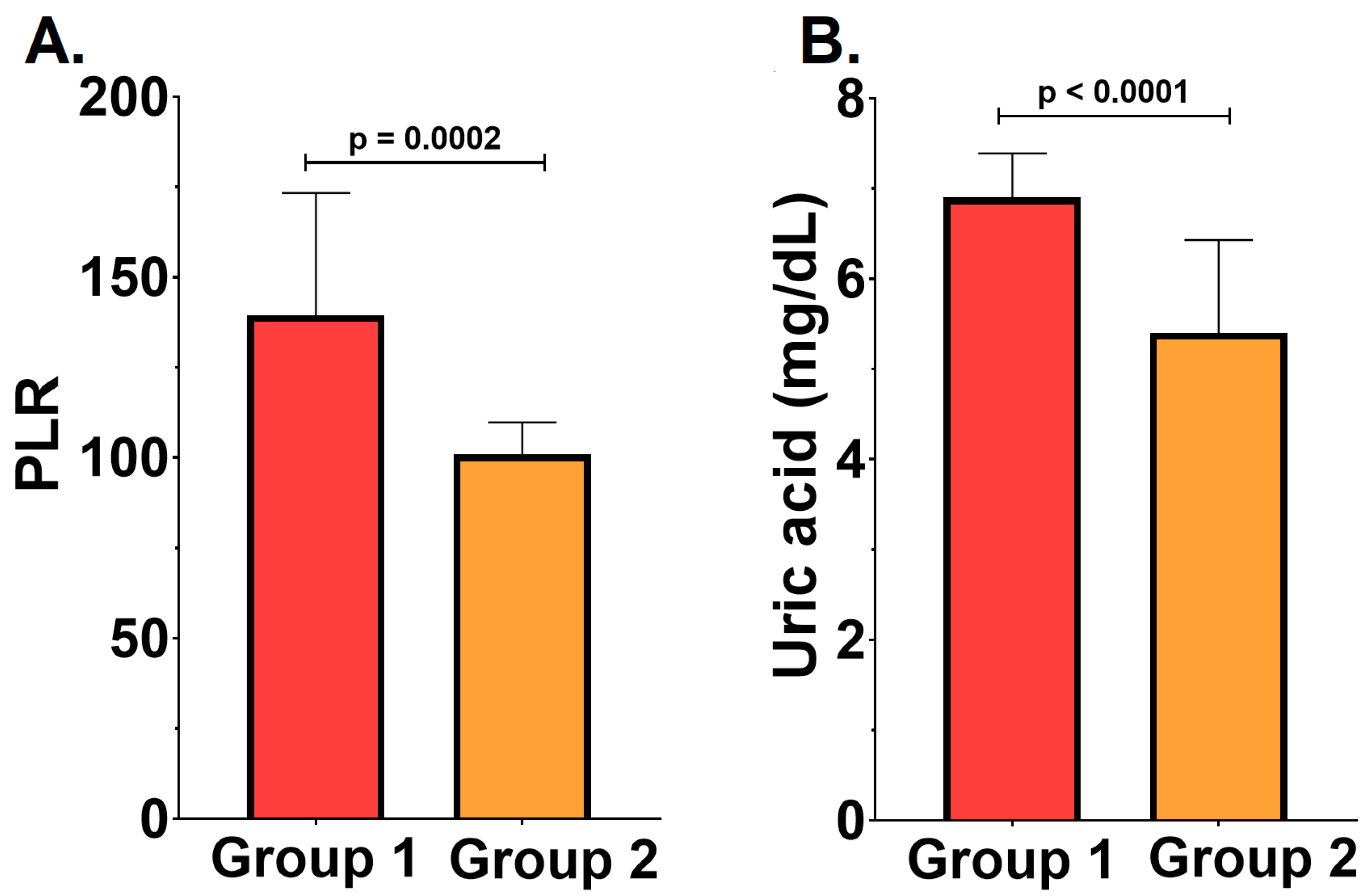
2.2. Severity of the Periodontal Disease, Inflammatory Status, and Cardiovascular Outcomes
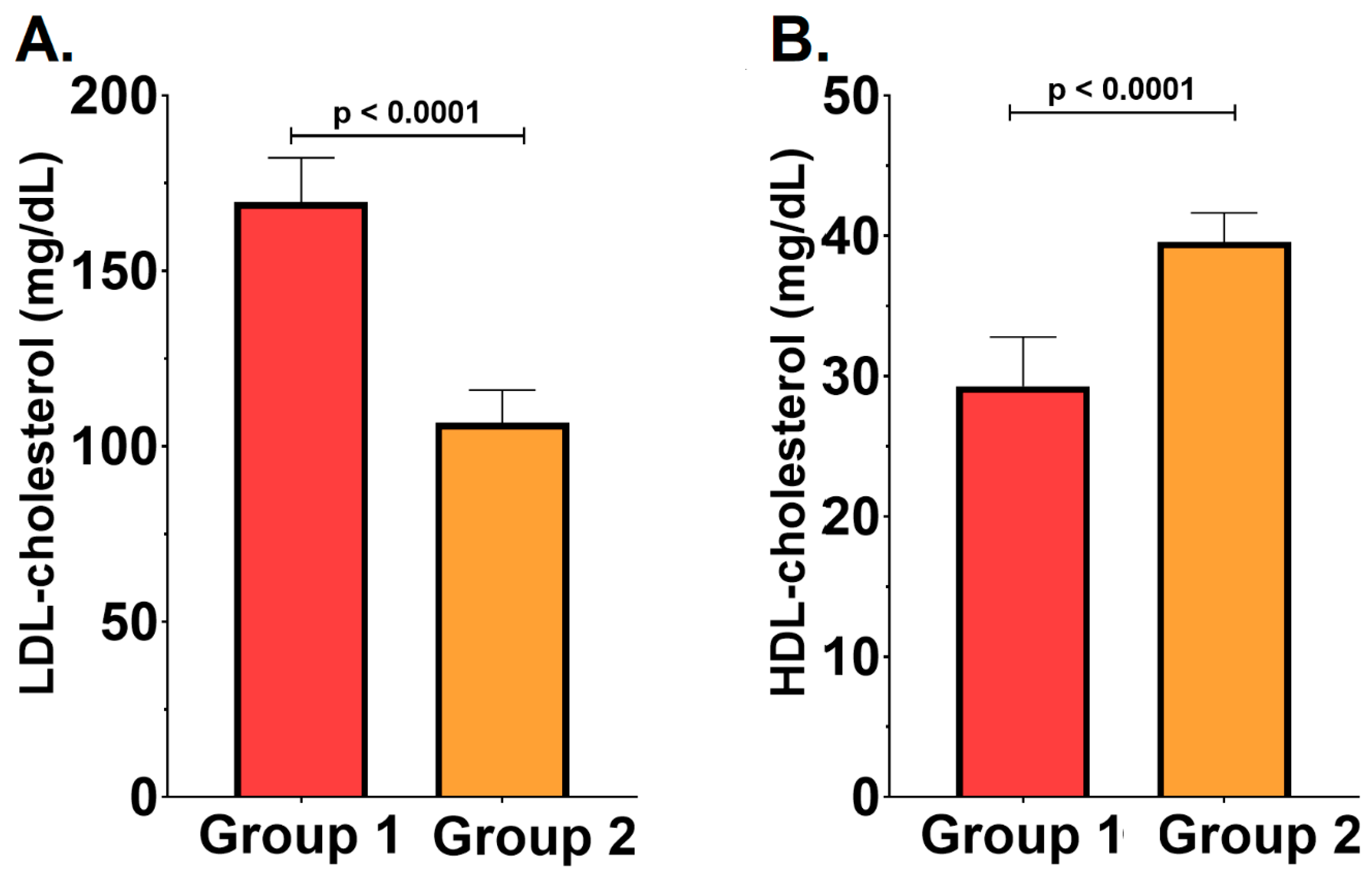
2.3. Severity of the Periodontal Disease and the Lipid Profile
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Serum Biomarkers
4.3. Cardiovascular Risk Factors
4.4. Dental Examination
4.5. Statistical Analysis
4.6. Ethical Consideration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, J.D.; Philips, K.; Moss, K.; Sen, S.; Morelli, T.; Preisser, J.; Pankow, J. Periodontal disease classifications and incident coronary heart disease in the Atherosclerosis Risk in Communities study. J. Periodontol. 2020, 91, 1409–1418. [Google Scholar] [CrossRef]
- Gwon, J.G.; Choi, J.; Kim, S.H.; Kim, S.H.; Ryu, J.J.; Cho, D.H.; Song, I.S. Risk of acute and chronic coronary syndrome in a population with periodontitis: A cohort study. Oral Dis. 2022, 28 (Suppl. 2), 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Buicu, F.; Rodean, I.P.; Halațiu, V.B.; Chițu, I.M.; Benedek, T. The Link Between Periostin Serum Levels and Inflammation in Patients with Acute Coronary Syndrome. J. Cardiovasc. Emerg. 2024, 10, 151–158. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Rodean, I.P.; Biriș, C.I.; Halațiu, V.B.; Modiga, A.; Lazăr, L.; Benedek, I.; Benedek, T. Is There a Link between COVID-19 Infection, Periodontal Disease and Acute Myocardial Infarction? Life 2021, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Arabi, G.; Zeller, T.; Seedorf, H.; Reissmann, D.R.; Heydecke, G.; Schaefer, A.S.; Seedorf, U. Genetic susceptibility contributing to periodontal and cardiovascular disease. J. Dent. Res. 2017, 96, 610–617. [Google Scholar] [CrossRef]
- Liljestrand, J.M.; Paju, S.; Pietiäinen, M.; Buhlin, K.; Persson, G.R.; Nieminen, M.S.; Sinisalo, J.; Mäntylä, P.; Pussinen, P.J. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis 2018, 268, 177–184. [Google Scholar] [CrossRef]
- Beukers, N.G.F.M.; van der Heijden, G.J.M.G.; van Wijk, A.J.; Loss, B.G. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular disease among 60174 participants in a large dental school in the Netherlands. J. Epidemiol. Community Health 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Gamboa, F.; Muñoz, C.C.; Numpaque, G.; Sequeda-Castañeda, L.G.; Gutierrez, S.J.; Tellez, N. Antimicrobial Activity of Piper marginatum Jacq and Ilex guayusa Loes on Microorganisms Associated with Periodontal Disease. Int. J. Microbiol. 2018, 2018, 4147383. [Google Scholar] [CrossRef]
- Matsha, T.E.; Prince, Y.; Davids, S.; Chikte, U.; Erasmus, R.T.; Kengne, A.P.; Davison, G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020, 99, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Puletic, M.; Popovic, B.; Jankovic, S.; Brajovic, G. Detection Rates of Periodontal Bacteria and Herpesviruses in Different Forms of Periodontal Disease. Microbiol. Immunol. 2020, 64, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of Virulence Factors for the Persistence of Oral Bacteria in the Inflamed Gingival Crevice and in the Pathogenesis of Periodontal Disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef]
- Torres, P.J.; Thompson, J.; McLean, J.S.; Kelley, S.T.; Edlund, A. Discovery of a Novel Periodontal Disease-Associated Bacterium. Microb. Ecol. 2019, 77, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Lo, E.C.M.; McGrath, C.; Mei, M.L.; Dai, R. Using next-generation sequencing to detect oral microbiome change following periodontal interventions: A systematic review. Oral Dis. 2021, 27, 1073–1089. [Google Scholar] [CrossRef]
- Mohammed, S.M.; Hasan, A.S.; Al-Hindy, H.A.A.M.; Mousa, M.J. C-Reactive Protein is Associated with the Severity of Periodontal Disease—An Observational Study Among Acute Myocardial Infarction Patients. Syst. Rev. Pharm. 2020, 11, 252–257. [Google Scholar] [CrossRef]
- Rodean, I.-P.; Lazăr, L.; Halațiu, V.-B.; Biriș, C.; Benedek, I.; Benedek, T. Periodontal Disease Is Associated with Increased Vulnerability of Coronary Atheromatous Plaques in Patients Undergoing Coronary Computed Tomography Angiography—Results from the Atherodent Study. J. Clin. Med. 2021, 10, 1290. [Google Scholar] [CrossRef]
- Halațiu, V.-B.; Benedek, I.; Rodean, I.-P.; Cojocariu, L.-O.; Mihăilă, T.; Blîndu, E.; Roșca, A.; Mátyás, B.-B.; Gerculy, R.; Buicu, F.; et al. Coronary Computed Tomography Angiography-Derived Modified Duke Index Is Associated with Peri-Coronary Fat Attenuation Index and Predicts Severity of Coronary Inflammation. Medicina 2024, 60, 765. [Google Scholar] [CrossRef]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Dhungana, G.; Srisai, D.; Sampath, C.; Soliman, J.; Kelly, R.M.; Saleh, H.Y.; Sedik, A.; Raynes, E.; Ferguson, A.; Alluri, L.S.C.; et al. Unveiling the Molecular Crosstalk Between Periodontal and Cardiovascular Diseases: A Systematic Review. Dent. J. 2025, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Rus, M.; Negruțiu, B.M.; Sava, C.N.; Pasca, G.; Andronie-Cioara, F.L.; Crisan, S.; Popescu, M.I.; Staniș, C.E.; Judea Pusta, C. The Association Between Periodontal Disease and Acute Coronary Syndrome—A Clinical Analysis. J. Clin. Med. 2025, 14, 2447. [Google Scholar] [CrossRef] [PubMed]
- Gotsman, I.; Lotan, C.; Soskolne, W.A.; Rassovsky, S.; Pugatsch, T.; Lapidus, L.; Novikov, Y.; Masrawa, S.; Stabholz, A. Periodontal Destruction Is Associated with Coronary Artery Disease and Periodontal Infection with Acute Coronary Syndrome. J. Periodontol. 2007, 78, 849–858. [Google Scholar] [CrossRef]
- Persson, R.E.; Hollender, L.G.; Powell, L.V.; MacEntee, M.I.; Wyatt, C.C.; Kiyak, H.A.; Persson, G.R. Assessment of periodontal conditions and systemic disease in older subjects. I. Focus on osteoporosis. J. Clin. Periodontol. 2002, 29, 796–802. [Google Scholar] [CrossRef]
- Bagavad Gita, J.; George, A.V.; Pavithra, N.; Chandrasekaran, S.C.; Latchumanadhas, K.; Gnanamani, A. Dysregulation of miR-146a by periodontal pathogens: A risk for acute coronary syndrome. J. Periodontol. 2019, 90, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Nordendahl, E.; Gustafsson, A.; Norhammar, A.; Näsman, P.; Rydén, L.; Kjellström, B.; PAROKRANK Steering Committee. Severe Periodontitis Is Associated with Myocardial Infarction in Females. J. Dent. Res. 2018, 97, 1114–1121. [Google Scholar] [CrossRef]
- Łysek, R.P.; Szafraniec, K.; Polak, M.; Jankowski, P.; Micek, A.; Wolfshaut-Wolak, R.; Czarnecka, D.; Potempa, J.; Pająk, A. Relationship between past myocardial infarction, periodontal disease and Porphyromonas gingivalis serum antibodies: A case-control study. Cardiol. J. 2018, 25, 386–392. [Google Scholar] [CrossRef]
- Olavegogeascoechea, P.A.; Allevato, J.A.; Olavegogeascoechea, F.N.; Contreras, P.; Thalasselis, D.C.; Valenzuela, G.; Urdiales, P.L.; Brusca, M.I. Relationship between periodontal disease (periodontitis) and acute vascular events a case-control study enperiva study. J. Dent. Health Oral Disord. Ther. 2017, 7, 350–354. [Google Scholar] [CrossRef]
- Lahdentausta, L.S.J.; Paju, S.; Mäntylä, P.; Buhlin, K.; Tervahartiala, T.; Pietiäinen, M.; Alfthan, H.; Nieminen, M.S.; Sinisalo, J.; Sorsa, T.; et al. Saliva and serum biomarkers in periodontitis and coronary artery disease. J. Clin. Periodontol. 2018, 45, 1045–1055. [Google Scholar] [CrossRef]
- Torrungruang, K.; Jitpakdeebordin, S.; Charatkulangkun, O.; Gleebbua, Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia Co-Infection Are Associated with Severe Periodontitis in a Thai Population. PLoS ONE 2015, 10, e0136646. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Pettersson, T.; Ohlsson, O.; Persson, G.R. Bacterial profile and burden of periodontal infection in subjects with a diagnosis of acute coronary syndrome. J. Periodontol. 2006, 77, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ma, X.; Wang, W. Inflammation and Coronary Heart Disease Risk in Patients with Depression in China Mainland: A Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2020, 16, 81–86. [Google Scholar] [CrossRef]
- Kadhim, S.S.; Al-Windy, S.A.; Al-Nami, M.S.; Al-kuraishy, H.M.; Al-Gareeb, A.I. Possible Role of Statins on the Inflammatory Biomarkers in Patients With Periodontal Disease: A Cross-Sectional Study. Dent. Hypotheses 2019, 10, 70–75. [Google Scholar] [CrossRef]
- Gu, Y.; Golub, L.M.; Lee, H.M.; Hou, W.; Ryan, M.E. Periodontal disease in acute coronary syndrome patients. Am. J. Dent. 2021, 34, 97–100. [Google Scholar]
- Silva, C.; Abrantes, A.C.; Fontes, A.C.; Dias, I.; Domingues, R.; Peixoto, F.; Viegas, C. Evaluation of Haematological Ratios at: Different Stages of Canine Periodontal Disease. Vet. Sci. 2024, 11, 581. [Google Scholar] [CrossRef]
- Caloian, C.S.; Șurlin, P.; Ciurea, A.; Pop, D.; Caloian, B.; Leucuța, D.C.; Țigu, A.B.; Rasperini, G.; Micu, I.C.; Stanomir, A.; et al. Exploring Periodontal Conditions, Salivary Markers, and Systemic Inflammation in Patients with Cardiovascular Diseases. Biomedicines 2024, 12, 1341. [Google Scholar] [CrossRef]
- Almășan, O.; Leucuța, D.-C.; Hedeșiu, M. Blood Cell Count Inflammatory Markers as Prognostic Indicators of Periodontitis: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 992. [Google Scholar] [CrossRef]
- Al-Mumin, A.; Al-Hindy, H.A. Hyperuricemia has a deleterious role in patients with acute coronary syndrome presented with poor oral hygiene. Int. J. Pharm. Res. 2020, 12, 1196–1202. [Google Scholar] [CrossRef]
- Al-Shamma, Y.M.H.; Alkhafaji, A.A.A.; AL-Hindy, A.A. Caries burden is associated with serum uric acid and CRP in patients treated for acute coronary syndorme. HIV Nurs. 2023, 23, 50–56. [Google Scholar]
- Chabuk, M.I.; Sharba, A.M.; Alsafar, A.R.A. Association of Periodontal Disease with Serum Uric Acid and CRP in Patients Treated for Acute Coronary Syndrome: A Comparative Study. NeuroQuantology 2021, 19, 7–12. [Google Scholar] [CrossRef]
- Al-Joda, B.M.S. Biochemical Influence of Uric Acid and Some Inflammatory Biomarkers on the Association of Oral Hygiene with Cardiovascular Diseases. Int. J. Curr. Sci. Res. Rev. 2022, 5, 2216–2223. [Google Scholar]
- Flores, M.F.; Montenegro, M.M.; Furtado, M.V.; Polanczyk, C.A.; Rösing, C.K.; Haas, A.N. Periodontal status affects C-reactive protein and lipids in patients with stable heart disease from a tertiary care cardiovascular clinic. J. Periodontol. 2014, 85, 545–553. [Google Scholar] [CrossRef]
- Zehra, A.; Tanwir, F.; Ali, S.B.; Ali, H.; Khan, A.N.; Bakhsh, A. Comparative Evaluation of Lipid Profile and C-Reactive Protein in Chronic Periodontitis and Coronary Heart Disease: Lipid Profile and C-Reactive Protein in Periodontitis and Heart Disease. Pak. J. Health Sci. 2024, 5, 91–96. [Google Scholar] [CrossRef]
- Kim, S.-R.; Nam, S.-H. Association between Periodontal Disease and Levels of Triglyceride and Total Cholesterol among Korean Adults. Healthcare 2020, 8, 337. [Google Scholar] [CrossRef]
- Sandi, R.M.; Pol, K.G.; Basavaraj, P.; Khuller, N.; Singh, S. Association of Serum Cholesterol, Triglyceride, High- and Low-Density Lipoprotein (HDL and LDL) Levels in Chronic Periodontitis Subjects with Risk for Cardiovascular Disease (CVD): A Cross Sectional Study. J. Clin. Diagn. Res. 2014, 8, 214–216. [Google Scholar] [CrossRef]
- Nibali, L.; Stephen, A.S.; Allaker, R.P.; Di Pino, A.; Terranova, V.; Pisano, M.; Di Marca, S.; Ferrara, V.; Scicali, R.; Purrello, F.; et al. Associations between Host Genetic Variants and Subgingival Microbiota in Patients with the Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 16649. [Google Scholar] [CrossRef]
- Nibali, L.; Stephen, A.; Hagi-Pavli, E.; Allaker, R.; Pino, A.D.; Terranova, V.; Pisano, M.; Marca, S.D.; Ferrara, V.; Scicali, R.; et al. Analysis of gingival crevicular fluid biomarkers in patients with metabolic syndrome. J. Dent. 2022, 118, 104065. [Google Scholar] [CrossRef]
- Salvi, G.E.; Roccuzzo, A.; Imber, J.C.; Stähli, A.; Klinge, B.; Lang, N.P. Clinical periodontal diagnosis. Periodontol. 2000, 2023; advance online publication. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 42) | Group 1—RC (n = 29) | Group 2—ROC (n = 13) | p Value | |
|---|---|---|---|---|
| Age (years) | 58.34 ± 10.7 | 59 ± 10.64 | 57.69 ± 10.76 | 0.71 |
| BMI (kg/m2) | 30.41 ± 4.97 | 30.65 ± 4.995 | 30.17 ± 4.95 | 0.78 |
| Male gender (n, %) | 20 (48%) | 17 (59%) | 3 (23%) | 0.04 |
| DM (n, %) | 16 (38%) | 13 (45%) | 3 (23%) | 0.30 |
| AF (n, %) | 2 (5%) | 1 (3%) | 1 (8%) | 0.53 |
| CHF (n, %) | 28 (97%) | 19 (66%) | 9 (69%) | 0.9 |
| Hypertension (n, %) | 37 (88%) | 25 (86%) | 12 (92%) | 0.9 |
| Smoker status (n, %) | 13 (31%) | 9 (31%) | 4 (31%) | 0.9 |
| CKD (n, %) | 12 (29%) | 9 (31%) | 3 (23%) | 0.72 |
| PAD (n, %) | 6 (14%) | 3 (10%) | 3 (23%) | 0.35 |
| Stroke (n, %) | 2 (5%) | 1 (3%) | 1 (8%) | 0.53 |
| Previous MI (n, %) | 14 (33%) | 11 (38%) | 3 (23%) | 0.49 |
| Family history of CVD (n, %) | 22 (52%) | 20 (69%) | 2 (15%) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodean, I.-P.; Halațiu, V.-B.; Popa, T.M.; Blîndu, E.; Mihăilă, T.; Țolescu, C.; Modiga, A.; Benedek, I.; Benedek, T. Association Between Periodontal Pathogens and Inflammation in Patients with Acute Coronary Syndromes. Int. J. Mol. Sci. 2025, 26, 4360. https://doi.org/10.3390/ijms26094360
Rodean I-P, Halațiu V-B, Popa TM, Blîndu E, Mihăilă T, Țolescu C, Modiga A, Benedek I, Benedek T. Association Between Periodontal Pathogens and Inflammation in Patients with Acute Coronary Syndromes. International Journal of Molecular Sciences. 2025; 26(9):4360. https://doi.org/10.3390/ijms26094360
Chicago/Turabian StyleRodean, Ioana-Patricia, Vasile-Bogdan Halațiu, Teodora Maria Popa, Emanuel Blîndu, Theofana Mihăilă, Constantin Țolescu, Andrei Modiga, Imre Benedek, and Theodora Benedek. 2025. "Association Between Periodontal Pathogens and Inflammation in Patients with Acute Coronary Syndromes" International Journal of Molecular Sciences 26, no. 9: 4360. https://doi.org/10.3390/ijms26094360
APA StyleRodean, I.-P., Halațiu, V.-B., Popa, T. M., Blîndu, E., Mihăilă, T., Țolescu, C., Modiga, A., Benedek, I., & Benedek, T. (2025). Association Between Periodontal Pathogens and Inflammation in Patients with Acute Coronary Syndromes. International Journal of Molecular Sciences, 26(9), 4360. https://doi.org/10.3390/ijms26094360








