Non-Oxidised Parathyroid Hormone and a Panel of Markers of Calcium–Phosphate Metabolism for Analysis of Secondary Hyperparathyroidism in Selected Patient Groups—A Quality Assurance Project
Abstract
1. Introduction
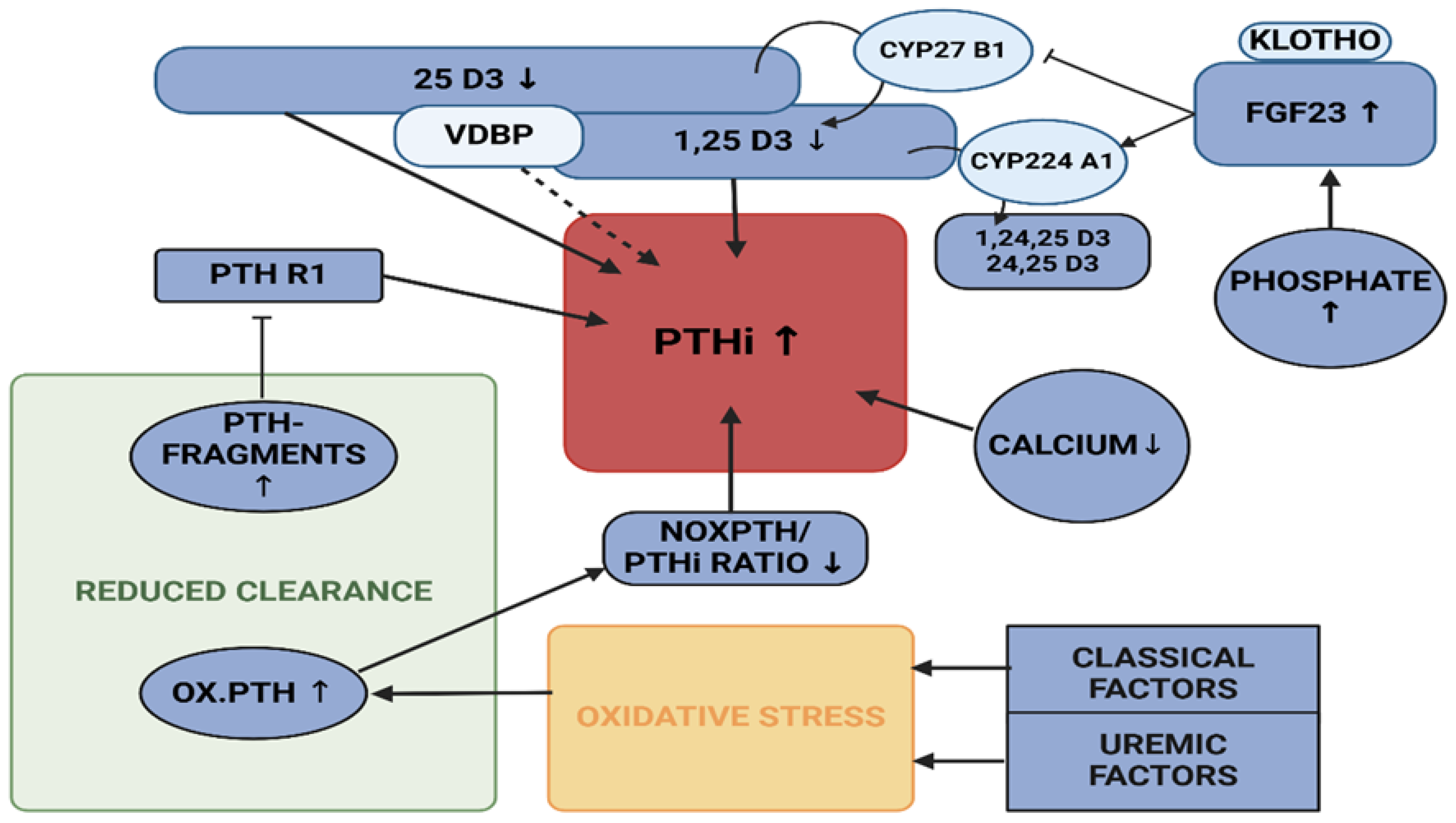
2. Results
3. Discussion
- (i)
- Individuals without any apparent disease, recruited from the P10,000 cohort, presenting with increased VDBP, associated with rather low 25 D3 levels, but no statistical effect on PTHi or noxPTH.
- (ii)
- Patients presenting with elevated CRP levels with a reduced proportion of noxPTH, while renal and mineral metabolism seem undisturbed. This may be due to an inflammatory process and, presumably, oxidative stress.
- (iii)
- Patients, whose end stage renal disease is likely to upregulate the level of PTHi and noxPTH via several mechanisms: (a) Impaired clearance of non-functional or only partially functional, oxidised PTH [20], (b) decreased clearance of receptor modulating PTH fragments [21,23,24,53], (c) The regulatory cascade of renal function decline, starting with deteriorating phosphate clearance, ensuing increase in FGF23 levels, decrease of 1,25 D3 and hypocalcaemia, resulting in secondary renal hyperparathyroidism [47].

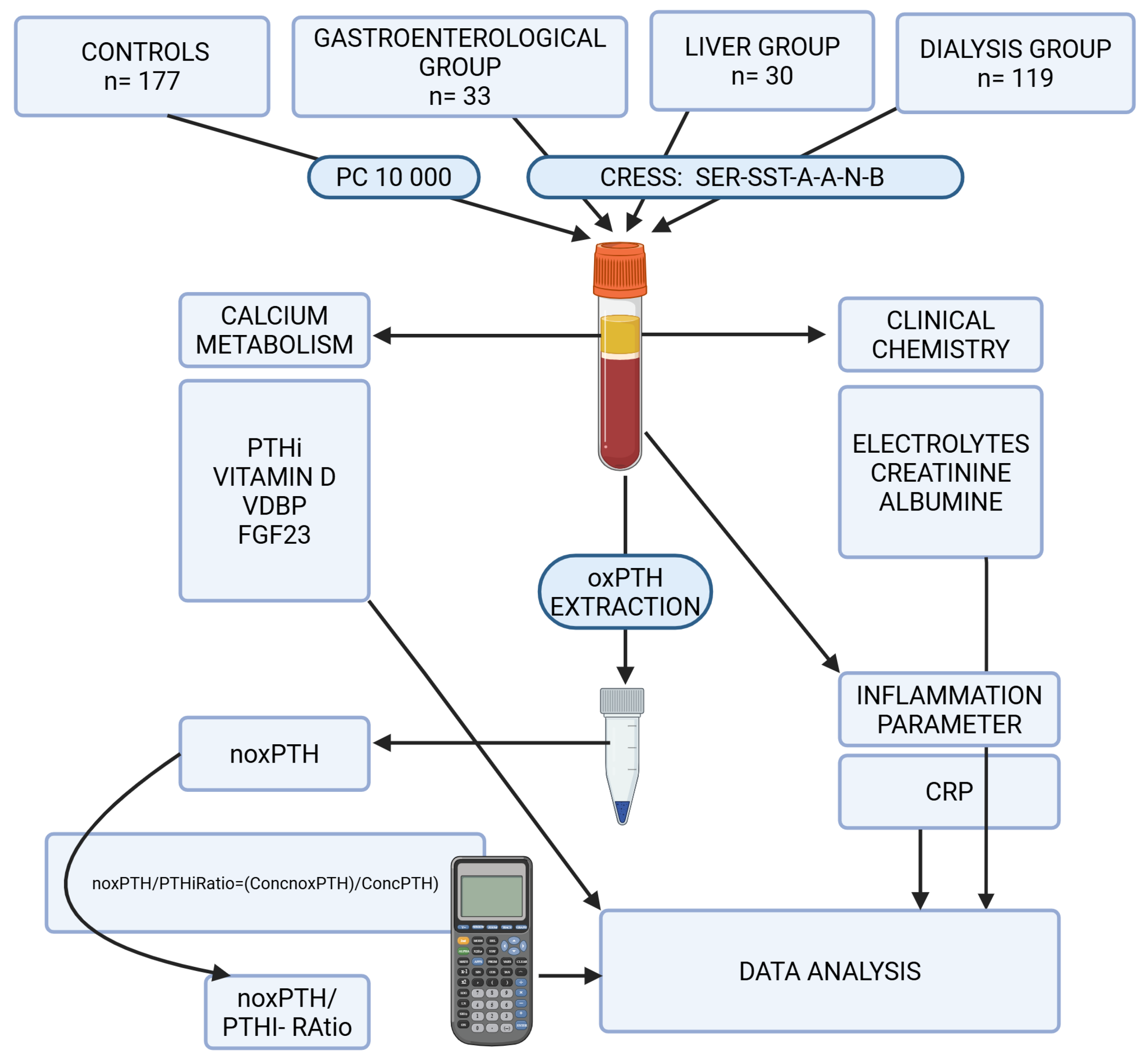
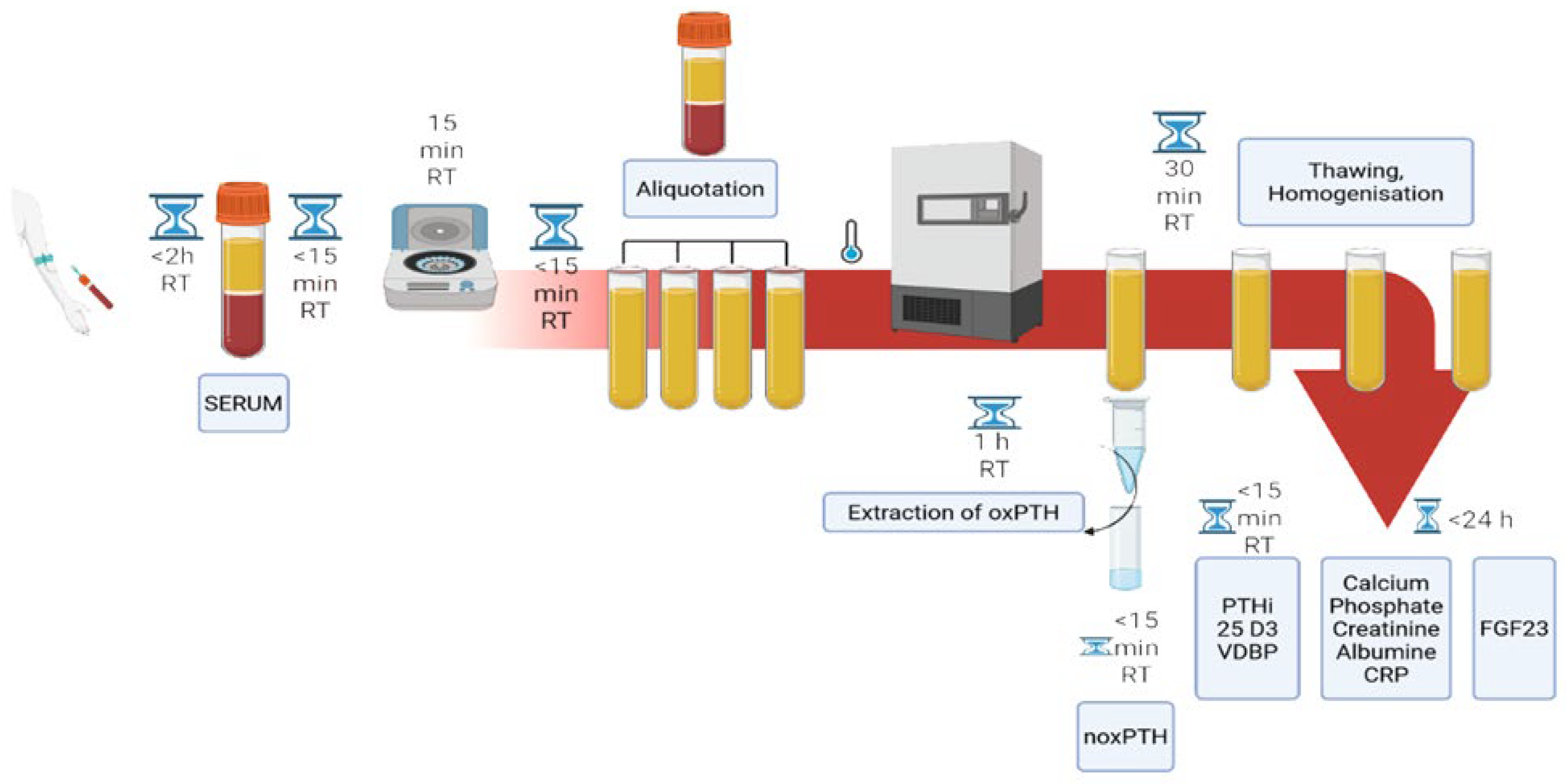
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25 D3 | 25-hydroxy-vitamin D3 |
| 1,25 D3 | 1,25-dihydroxy-vitamin D3 |
| CKD | chronic kidney disease |
| CMIA | chemoluminescent microparticle immunoassay |
| CV | variation coefficient |
| conc. | concentration |
| CRP | C-reactive protein |
| ECLIA | electrochemiluminescence immunoassay |
| ELISA | enzyme linked immunosorbant assay |
| FGF23 | fibroblast growth factor 23 |
| LC-MS | liquid chromatography-mass spectrometry |
| noxPTH | non-oxidised parathyroid hormone |
| noxPTH/PTHi Ratio | non-oxidised parathyroid hormone/intact parathyroid hormone ratio |
| oxPTH | oxidised parathyroid hormone |
| PTH R1 | parathyroid hormone receptor 1 |
| VDBP | vitamin D binding protein |
References
- Scharla, S.H.; Minne, H.W.; Sattar, P.; Mende, U.; Blind, E.; Schmidt-Gayk, H.; Wüster, C.; Ho, T.; Ziegler, R. Treatment of tumor hypercalcemia with clodronate. Effect on parathormone and calcitriol. Dtsch. Med. Wochenschr. 1987, 112, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P. Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2020, 105, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Habener, J.F.; Amherdt, M.; Ravazzola, M.; Orci, L. Parathyroid hormone biosynthesis. Correlation of conversion of biosynthetic precursors with intracellular protein migration as determined by electron microscope autoradiography. J. Cell Biol. 1979, 80, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.P.; Schaefer, F.; Bruch, A.; Veldhuis, J.D.; Schmidt-Gayk, H.; Stein, G.; Ritz, E.; Mehls, O. Control of pulsatile and tonic parathyroid hormone secretion by ionized calcium. J. Clin. Endocrinol. Metab. 1996, 81, 4236–4243. [Google Scholar]
- Schmitt, C.P.; Schaefer, F.; Bruch, A.; Veldhuis, J.D.; Schmidt-Gayk, H.; Stein, G.; Ritz, E.; Mehls, O. 1,25(OH)2-vitamin D3 reduces spontaneous and hypocalcemia-stimulated pulsatile component of parathyroid hormone secretion. J. Am. Soc. Nephrol. 1998, 9, 54–62. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D Metabolites: Analytical Challenges and Clinical Relevance. Calcif. Tissue Int. 2023, 112, 158–177. [Google Scholar] [CrossRef]
- Seiler-Mussler, S.; Limbach, A.S.; Emrich, I.E.; Pickering, J.W.; Roth, H.J.; Fliser, J.; Heine, G.H. Association of Nonoxidized Parathyroid Hormone with Cardiovascular and Kidney Disease Outcomes in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 569–576. [Google Scholar] [CrossRef]
- Ureña-Torres, P.A.; Vervloet, M.; Mazzaferro, S.; Oury, F.; Brandenburg, V.; Bover, J.; Cavalier, E.; Cohen-Solal, M.; Covic, A.; Drüeke, T.B.; et al. Novel insights into parathyroid hormone: Report of The Parathyroid Day in Chronic Kidney Disease. Clin. Kidney J. 2019, 12, 269–280. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Sprague, S.; Almond, A.; Cavalier, E.; Fraser, W.D.; Algeciras-Schimnich, A.; Singh, R.; Souberbielle, J.C.; Vesper, H.W. Perspective and priorities for improvement of parathyroid hormone (PTH) measurement—A view from the IFCC Working Group for PTH. Clin. Chim. Acta 2017, 467, 42–47. [Google Scholar] [CrossRef]
- Cheng, J.; Mu, D.; Wang, D.; Qiu, L.; Cheng, X. Preanalytical considerations in parathyroid hormone measurement. Clin. Chim. Acta 2023, 539, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Armbruster, F.P.; Stoeva, S.; Reichetzeder, C.; Grön, H.J.; Lieker, I.; Khadzhynov, D.; Slowinski, T.; Roth, H.J. Measuring parathyroid hormone (PTH) in patients with oxidative stress—Do we need a fourth generation parathyroid hormone assay? PLoS ONE 2012, 7, e40242. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Oberthür, D.; Slowinski, T.; Querfeld, U.; Schaefer, F.; Doyon, A.; Tepel, M.; Roth, H.J.; Grön, H.J.; Reichetzeder, C.; et al. Modeling of oxidized PTH (oxPTH) and non-oxidized PTH (n-oxPTH) receptor binding and relationship of oxidized to non-oxidized PTH in children with chronic renal failure, adult patients on hemodialysis and kidney transplant recipients. Kidney Blood Press. Res 2013, 37, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, C.Z.; Kritmetapak, K.; Singh, R.J.; Vesper, H.W.; Kumar, R. High-Resolution Mass Spectrometry for the Measurement of PTH and PTH Fragments: Insights into PTH Physiology and Bioactivity. J. Am. Soc. Nephrol. 2022, 33, 1448–1458. [Google Scholar] [CrossRef]
- Li, M.; Hasan, A.A.; Chu, C.; Hocher, J.G.; Liu, Y.; Zhang, X.; Chen, X.; Yard, B.; Krämer, B.K.; Hocher, B. Only bioactive forms of PTH (n-oxPTH and Met18(ox)-PTH) inhibit synthesis of sclerostin—Evidence from in vitro and human studies. Pflüg. Arch. Eur. J. Physiol. 2024, 476, 889–899. [Google Scholar] [CrossRef]
- Hocher, B.; Zeng, S. Clear the Fog around Parathyroid Hormone Assays: What Do iPTH Assays Really Measure? Clin. J. Am. Soc. Nephrol. 2018, 13, 524–526. [Google Scholar] [CrossRef]
- D’Amour, P. Circulating PTH molecular forms: What we know and what we don’t. Kidney Int. 2006, 70, S29–S33. [Google Scholar] [CrossRef]
- Rouleau, M.F.; Warshawsky, H.; Goltzman, D. Parathyroid hormone binding in vivo to renal, hepatic, and skeletal tissues of the rat using a radioautographic approach. Endocrinology 1986, 118, 919–931. [Google Scholar] [CrossRef]
- Martin, K.J.; Hruska, K.A.; Lewis, J.; Anderson, C.; Slatopolsky, E. The renal handling of parathyroid hormone: Role of peritubular uptake and glomerular filtration. J. Clin. Investig. 1977, 60, 808–814. [Google Scholar] [CrossRef]
- Martin, K.J.; Finch, L.J.; Hruska, K.A.; Slatopolsky, E. Effect of biological activity of PTH on its peripheral metabolism in the rat. Kidney Int. 1987, 31, 937–940. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; Rhee, E.P.; Elmariah, S.; de Boer, I.H.; Kestenbaum, B. Renal Clearance of Mineral Metabolism Biomarkers. J. Am. Soc. Nephrol. 2016, 27, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Korkor, K.; Martin, K.J.; Slatopolsky, E. Peripheral metabolism of intact parathyroid hormone: Role of liver and kidney and the effect of chronic renal failure. J. Clin. Investig. 1981, 67, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kritmetapak, K.; Losbanos, L.; Berent, T.E.; Ashrafzadeh-Kian, S.L.; Algeciras-Schimnich, A.; Hines, J.M.; Singh, R.J.; Kumar, R. Hyperphosphatemia with elevated serum PTH and FGF23, reduced 1,25(OH)(2)D and normal FGF7 concentrations characterize patients with CKD. BMC Nephrol. 2021, 22, 114. [Google Scholar] [CrossRef]
- Kritmetapak, K.; Losbanos, L.; Hines, J.M.; O’Grady, K.L.; Ulmer, C.Z.; Vesper, H.W.; Enders, F.T.; Singh, R.J.; Kumar, R. Chemical Characterization and Quantification of Circulating Intact PTH and PTH Fragments by High-Resolution Mass Spectrometry in Chronic Renal Failure. Clin. Chem. 2021, 67, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Hruska, K.; Greenwalt, A.; Klahr, S.; Slatopolsky, E. Selective uptake of intact parathyroid hormone by the liver: Differences between hepatic and renal uptake. J. Clin. Investig. 1976, 58, 781–788. [Google Scholar] [CrossRef]
- Bringhurst, F.R.; Stern, A.M.; Yotts, M.; Mizrahi, N.; Segre, G.V.; Potts, J.T. Peripheral metabolism of PTH: Fate of biologically active amino terminus in vivo. Am. J. Physiol. 1988, 255 Pt 1, E886–E893. [Google Scholar] [CrossRef]
- Bringhurst, F.R.; Stern, A.M.; Yotts, M.; Mizrahi, N.; Segre, G.V.; Potts, J.T. Peripheral metabolism of [35S] parathyroid hormone in vivo: Influence of alterations in calcium availability and parathyroid status. J. Endocrinol. 1989, 122, 237–245. [Google Scholar] [CrossRef]
- Divieti, P.; John, M.R.; Jüppner, H.; Bringhurst, F.R. Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology 2002, 143, 171–176. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Boutten, A.; Carlier, M.C.; Chevenne, D.; Coumaros, G.; Lawson-Body, E.; Massart, C.; Monge, M.; Myar, J.; Parent, X.; et al. Inter-method variability in PTH measurement: Implication for the care of CKD patients. Kidney Int. 2006, 70, 345–350. [Google Scholar] [CrossRef]
- Cavalier, E.; Daly, A.F.; Betea, D.; Pruteanu-Apetrii, P.N.; Delanaye, P.; Stubbs, P.; Bradwell, A.R.; Chapelle, J.P.; Beckers, A. The ratio of parathyroid hormone as measured by third- and second-generation assays as a marker for parathyroid carcinoma. J. Clin. Endocrinol. Metab. 2010, 95, 3745–3749. [Google Scholar] [CrossRef]
- D’Amour, P.; Brossard, J.H.; Rousseau, L.; Nguyen-Yamamoto, L.; Nassif, E.; Lazure, C.; Gauthier, D.; Lavigne, J.R.; Zahradnik, R.J. Structure of non-(1-84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney Int. 2005, 68, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Hines, J.M.; Lopez, M.F.; Krastins, B.; Hoofnagle, A.N. Mass spectrometric immunoassay raises doubt for the existence of parathyroid hormone fragment 7-84. Clin. Chem. 2015, 61, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.F.; Rezai, T.; Sarracino, D.A.; Prakash, A.; Krastins, B.; Athanas, M.; Singh, R.J.; Barnidge, D.R.; Oran, P.; Borges, C.; et al. Selected reaction monitoring-mass spectrometric immunoassay responsive to parathyroid hormone and related variants. Clin. Chem. 2010, 56, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Hasan, A.A.; Hocher, C.F.; Kalk, P.; Kleuser, B.; Krämer, B.K.; Hocher, B. Inverse correlation of intact PTH, oxidized PTH as well as non-oxidized PTH with 25-hydroxyvitamin D3 in kidney transplant recipients. Front. Endocrinol. 2023, 14, 1178166. [Google Scholar] [CrossRef]
- Tepel, M.; Armbruster, F.P.; Grön, H.J.; Scholze, A.; Reichetzeder, C.; Roth, H.J.; Hocher, B. Nonoxidized, biologically active parathyroid hormone determines mortality in hemodialysis patients. J. Clin. Endocrinol. Metab. 2013, 98, 4744–4751. [Google Scholar] [CrossRef]
- European Parliament: Consolidated Text: Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU (Text with EEA Relevance) Text with EEA Relevance. 2022. Available online: https://eur-lex.europa.eu (accessed on 19 February 2025).
- European Parliament: Guidance on Classification Rules for In Vitro Diagnostic Medical Devices under Regulation (EU). 2023. Available online: https://health.ec.europa.eu/latest-updates/update-mdcg-2020-16-rev2-guidance-classification-rules-vitro-diagnostic-medical-devices-under-2023-02-10_en (accessed on 19 February 2025).
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef]
- van de Loo, I.; Hartbeck, B. Fachwissen Endokrinologie; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Holick, M.F.; Siris, E.S.; Binkley, N.; Beard, M.K.; Khan, A.; Katzer, J.T.; Petruschke, R.A.; Chen, E.; de Papp, A.E. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J. Clin. Endocrinol. Metab. 2005, 90, 3215–3224. [Google Scholar] [CrossRef]
- Zhu, A.; Kuznia, S.; Boakye, D.; Schöttker, B.; Brenner, H. Vitamin D-Binding Protein, Bioavailable, and Free 25(OH)D, and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3894. [Google Scholar] [CrossRef]
- Locatelli, F.; Canaud, B.; Eckardt, K.U.; Stenvinke, l.P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transplant. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Yamada, S.; Tokumoto, M.; Tatsumoto, N.; Taniguchi, M.; Noguchi, H.; Nakano, T.; Masutani, K.; Ooboshi, H.; Tsuruya, K.; Takanari, K. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J. Physiol. Ren. Physiol. 2014, 306, F1418–F1428. [Google Scholar] [CrossRef]
- Kremer, R.; Goltzman, D. Parathyroid hormone stimulates mammalian renal 25-hydroxyvitamin D3-1 alpha-hydroxylase in vitro. Endocrinology 1982, 110, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, T.; Drezner, M.K.; Lobaugh, B. Abnormal parathyroid hormone stimulation of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the hypophosphatemic mouse. Evidence for a generalized defect of vitamin D metabolism. J. Clin. Investig. 1986, 77, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Controlling Chronic Diseases and Acute Infections with Vitamin D Sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Ix, J.H.; Sprague, S.M.; Raphael, K.L.; Fried, L.; Gassman, J.J.; Raj, D.; Cheung, A.K.; Kusek, J.W.; Flessner, M.F.; et al. Rationale and Approaches to Phosphate and Fibroblast Growth Factor 23 Reduction in CKD. J. Am. Soc. Nephrol. 2015, 26, 2328–2339. [Google Scholar] [CrossRef]
- Muntner, P.; Jones, T.M.; Hyre, A.D.; Melamed, M.L.; Alper, A.; Raggi, P.; Leonard, M.B. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin. J. Am. Soc. Nephrol. 2009, 4, 186–194. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Roth, H.; Fouque, D.P. Parathyroid hormone measurement in CKD. Kidney Int. 2010, 77, 93–100. [Google Scholar] [CrossRef]
- Cavalier, E.; Delanaye, P.; Vranken, L.; Bekaert, A.C.; Carlisi, A.; Chapelle, J.P.; Souberbielle, J.C. Interpretation of serum PTH concentrations with different kits in dialysis patients according to the KDIGO guidelines: Importance of the reference (normal) values. Nephrol. Dial. Transplant. 2012, 27, 1950–1956. [Google Scholar] [CrossRef]
- Cavalier, E.; Vasikaran, S.; Bhattoa, H.P.; Heijboer, H.C.; Makris, K.; Ulmer, C.Z. The path to the standardization of PTH: Is this a realistic possibility? A position paper of the IFCC C-BM. Clin. Chim. Acta 2021, 515, 44–51. [Google Scholar] [CrossRef]
- Dhillon-Jhattu, S.; McGill, R.L.; Ennis, J.L.; Worcester, E.M.; Zisman, A.L.; Coe, F.L. Vitamin D and Parathyroid Hormone Levels in CKD. Am. J. Kidney Dis. 2023, 81, 122–124. [Google Scholar] [CrossRef]
- Nguyen-Yamamoto, L.; Rousseau, L.; Brossard, H.J.; Lepage, R.; Gao, P.; Cantor, T.; D’Amour, P. Origin of parathyroid hormone (PTH) fragments detected by intact-PTH assays. Eur. J. Endocrinol. 2002, 147, 123–131. [Google Scholar] [CrossRef]
- Lehmann, S.; Guadagni, F.; Moore, H.; Ashton, G.; Barnes, M.; Benson, E.; Clements, J.; Koppandi, I.; Coppola, D.; Demiroglu, S.Y.; et al. Standard preanalytical coding for biospecimens: Review and implementation of the Sample PREanalytical Code (SPREC). Biopreserv. Biobank. 2012, 10, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Frey, V.N.; Langthaler, P.; Raphaelis, E.; Ring-Dimitriou, S.; Kedenko, L.; Aigner, E.; Martinz, J.; Paulweber, B.; Gostner, I.; Bathke, A.C.; et al. Paracelsus 10,000: An Observational Cohort Study About the Health Status of the Population of Salzburg, Austria. Rationale, Objectives and Study Design. Paracelsus Proc. Exp. Med. 2023, 2, 1–17. [Google Scholar]
- Austrian-Standards, ÖNORM EN ISO 9001; Österreichisches Normungsinstitut (ÖNORM): Vienna, Austria, 2015.
- Austrian-Standards, ÖNORM EN ISO 15189; Medical Laboratories—Requirements for Quality and Competence (ISO 15189:2012). Austrian Standards Institute/Österreichisches Normungsinstitut (ÖNORM): Vienna, Austria, 2013. (In German)
- Hanon, E.A.; Sturgeon, C.M.; Lamb, E.J. Sampling and storage conditions influencing the measurement of parathyroid hormone in blood samples: A systematic review. Clin. Chem. Lab. Med. 2013, 51, 1925–1941. [Google Scholar] [CrossRef]
- El-Maouche, D.; Dumitrescu, C.E.; Andreopoulou, P.; Gafni, G.I.; Brillante, B.A.; Bhattacharyya, N.; Fedarko, N.S.; Collins, N.T. Stability and degradation of fibroblast growth factor 23 (FGF23): The effect of time and temperature and assay type. Osteoporos. Int. 2016, 27, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2013, 310, 2191–2194. [Google Scholar]
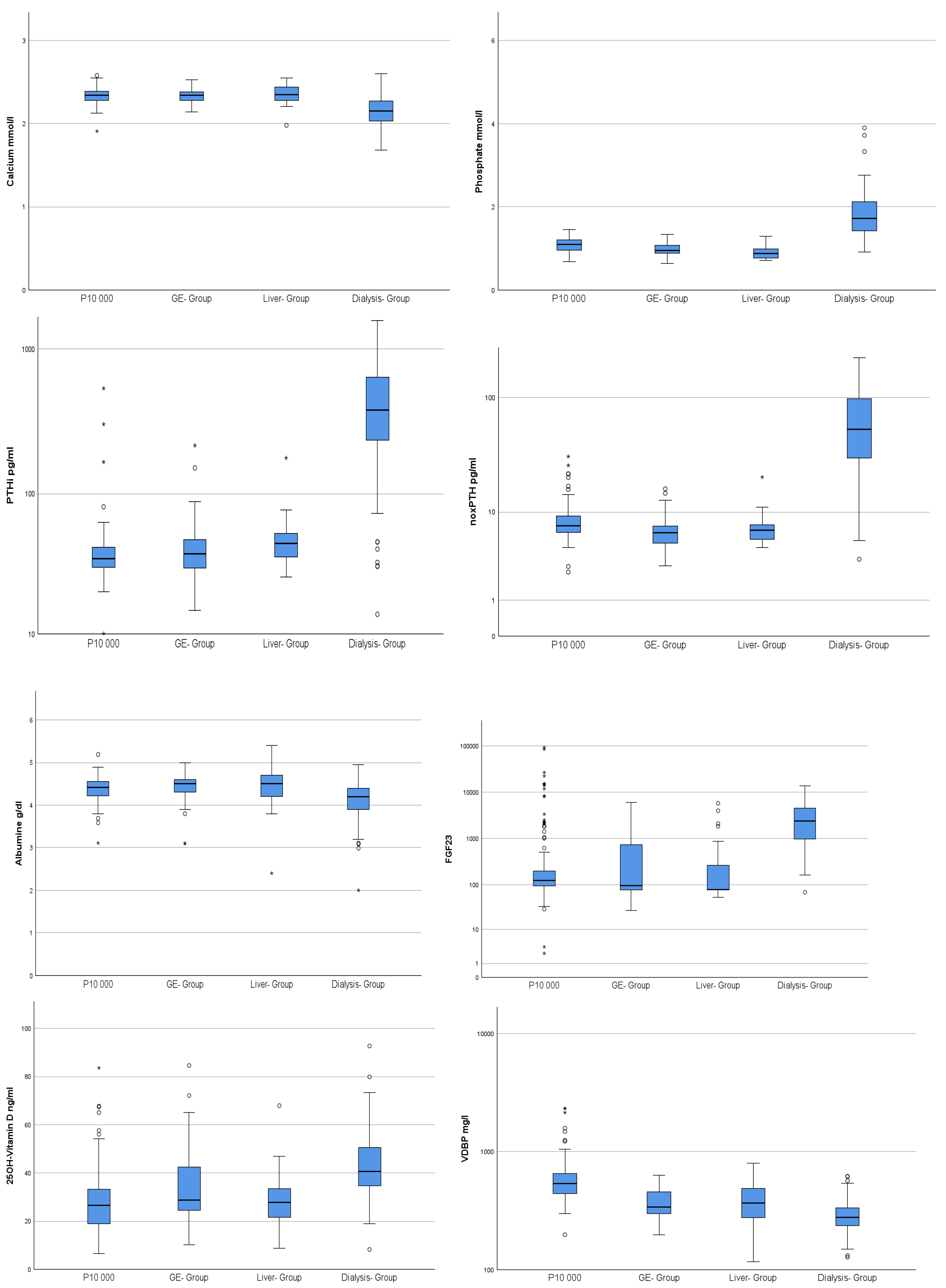
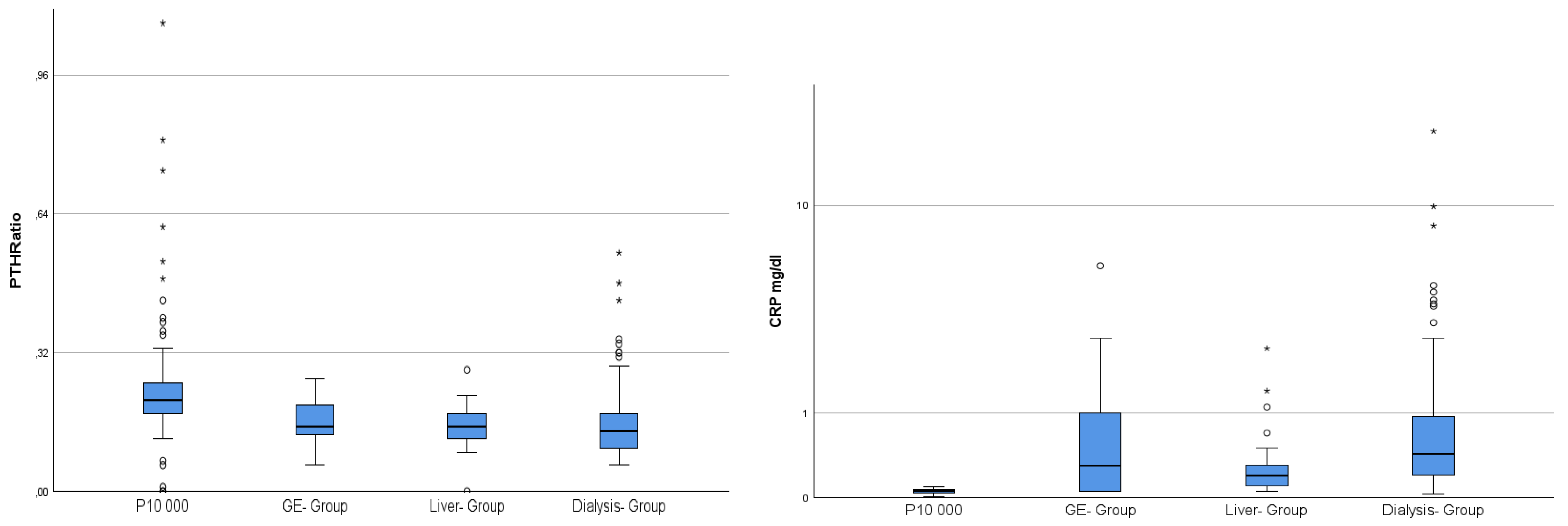
| P10,000 n = 177 | GE-Group n = 33 | Liver-Group n = 29 | Dialysis n = 119 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age years | 53.95 | 2.64 | 48.9 | 16.52 | 56.37 | 16.78 | 64.13 | 17.36 |
| Calcium mmol/L | 2.33 | 0.09 | 2.34 | 0.09 | 2.35 | 0.12 | 2.15 | 0.18 |
| Phosphate mmol/L | 1.09 | 0.16 | 0.97 | 0.17 | 0.91 | 0.17 | 1.85 | 0.72 |
| Creatinine mg/dL | 0.80 | 0.13 | 1.12 | 0.96 | 0.98 | 0.29 | 8.71 | 2.88 |
| CRP mg/dL | 0.06 | 0.02 | 0.69 | 1.13 | 0.35 | 0.50 | 1.06 | 2.21 |
| Albumine g/dL | 4.39 | 0.27 | 4.37 | 0.45 | 4.41 | 0.54 | 4.12 | 0.45 |
| PTHi pg/mL | 41.93 | 44.72 | 47.70 | 38.91 | 49.48 | 27.41 | 478.80 | 343.35 |
| noxPTH pg/mL | 8.32 | 3.64 | 6.75 | 3.00 | 7.14 | 3.03 | 68.99 | 51.82 |
| noxPTH/PTHi Ratio | 0.23 | 0.12 | 0.16 | 0.04 | 0.15 | 0.05 | 0.16 | 0.08 |
| 25 D3 ng/mL | 26.92 | 12.76 | 34.45 | 16.26 | 28.70 | 12.56 | 42.66 | 12.61 |
| VDBP ng/mL | 1144.94 | 5111.95 | 385.60 | 120.70 | 405.16 | 168.99 | 294.62 | 95.77 |
| FGF23 pg/mL | 2151.06 | 10,156.76 | 898.77 | 1524.48 | 642.76 | 1354.57 | 3268.43 | 3053.23 |
| Analysis | Manufacturer | Method | Sensitivity | Specificity/Cross Reactivity | CV% |
|---|---|---|---|---|---|
| Albumine | Roche | Extinction | 2 g/L | no interference | <0.8 |
| Calcium | Roche | Extinction | 0.2 mmol/L | no interference | <1.5 |
| FGF 23 | BioTechne | ELISA | 78.1 pg/mL | no cross-reactivity | n.d. |
| CRP | Roche | Extinction | na | no interference | <3.3 |
| Creatinine | Roche | Extinction | 0.2 mg/dL | no interference | 1.4 |
| Phosphate | Roche | Extinction | 0.1 mmol/L | no interference | <1.4 |
| PTHi | Roche | ECLIA | 1.2 pg/mL | cross-reactivity of 93% with the PTH fragment 7-84 | <3.4 |
| Paratrin Prolife | ImmunDiagnostik | Immunologic | n.d. | cross-reactivity of 93% with the PTH fragment 7-84 | <5.8% including subsequent analysis by PTHi/Roche [35] |
| VDBP | ImmunDiagnostik | ELISA | 0.944 ng/mL | no cross-reactivity | <13.9 |
| 25 D3 | Abbott | CMIA | 3.5 ng/mL | cross reactivity of >80.5% with OH D2 >70.4% with 24.25 OH D3 and 24.25 D2 | <8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber-Schoenauer, U.; Cadamuro, J.; Kipman, U.; Stoellinger, E.; Lichtenauer, M.; Paar, V.; Kedenko, L.; Guggenbichler, K.; Paulweber, B.; Pirich, C.; et al. Non-Oxidised Parathyroid Hormone and a Panel of Markers of Calcium–Phosphate Metabolism for Analysis of Secondary Hyperparathyroidism in Selected Patient Groups—A Quality Assurance Project. Int. J. Mol. Sci. 2025, 26, 4279. https://doi.org/10.3390/ijms26094279
Huber-Schoenauer U, Cadamuro J, Kipman U, Stoellinger E, Lichtenauer M, Paar V, Kedenko L, Guggenbichler K, Paulweber B, Pirich C, et al. Non-Oxidised Parathyroid Hormone and a Panel of Markers of Calcium–Phosphate Metabolism for Analysis of Secondary Hyperparathyroidism in Selected Patient Groups—A Quality Assurance Project. International Journal of Molecular Sciences. 2025; 26(9):4279. https://doi.org/10.3390/ijms26094279
Chicago/Turabian StyleHuber-Schoenauer, Ursula, Janne Cadamuro, Ulrike Kipman, Emma Stoellinger, Michael Lichtenauer, Vera Paar, Ludmilla Kedenko, Kathrin Guggenbichler, Bernhard Paulweber, Christian Pirich, and et al. 2025. "Non-Oxidised Parathyroid Hormone and a Panel of Markers of Calcium–Phosphate Metabolism for Analysis of Secondary Hyperparathyroidism in Selected Patient Groups—A Quality Assurance Project" International Journal of Molecular Sciences 26, no. 9: 4279. https://doi.org/10.3390/ijms26094279
APA StyleHuber-Schoenauer, U., Cadamuro, J., Kipman, U., Stoellinger, E., Lichtenauer, M., Paar, V., Kedenko, L., Guggenbichler, K., Paulweber, B., Pirich, C., & Salmhofer, H. (2025). Non-Oxidised Parathyroid Hormone and a Panel of Markers of Calcium–Phosphate Metabolism for Analysis of Secondary Hyperparathyroidism in Selected Patient Groups—A Quality Assurance Project. International Journal of Molecular Sciences, 26(9), 4279. https://doi.org/10.3390/ijms26094279








