Advancements in Plant-Derived sRNAs Therapeutics: Classification, Delivery Strategies, and Therapeutic Applications
Abstract
:1. Introduction
2. The Classification of Plant-Derived sRNAs
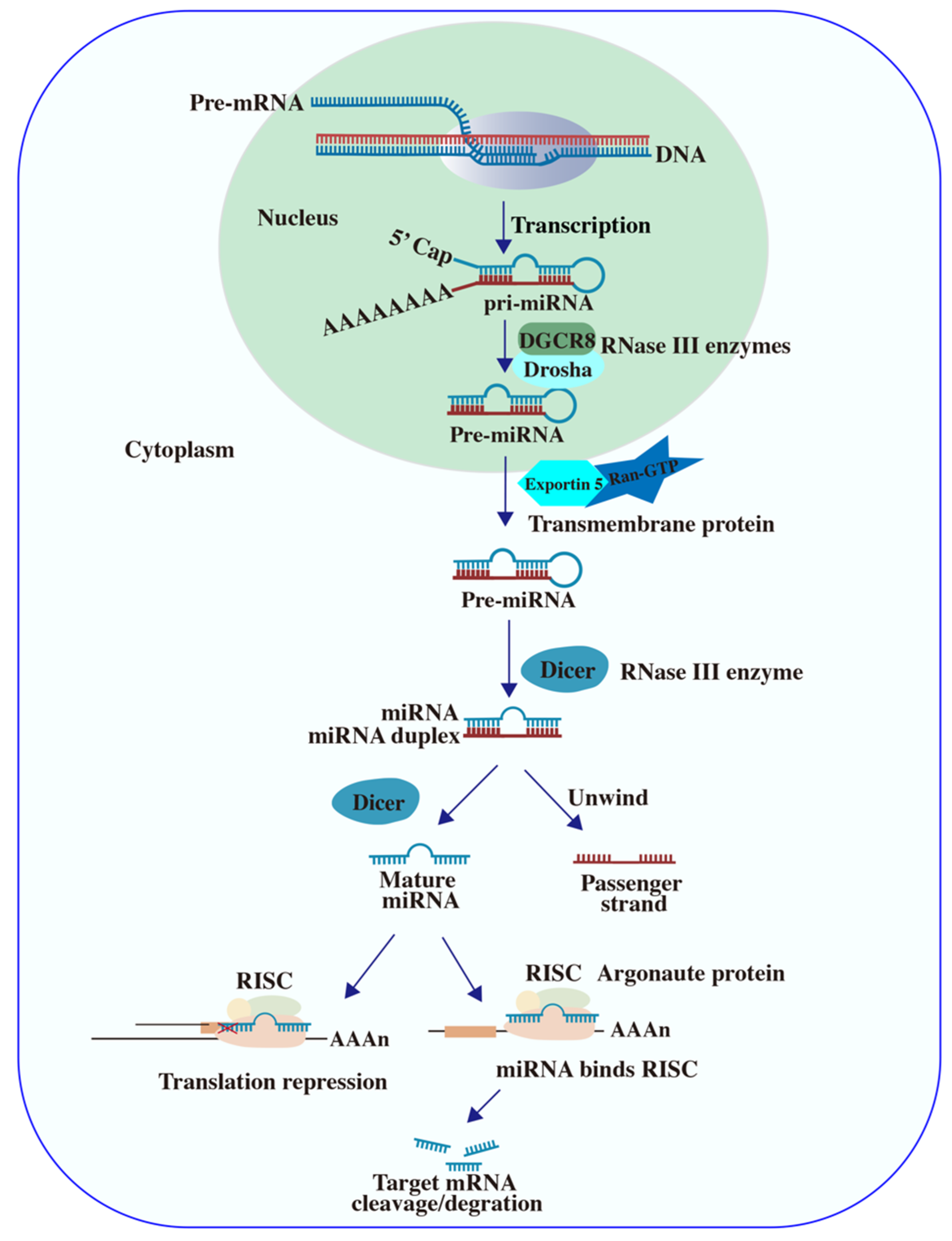
2.1. Plant miRNA
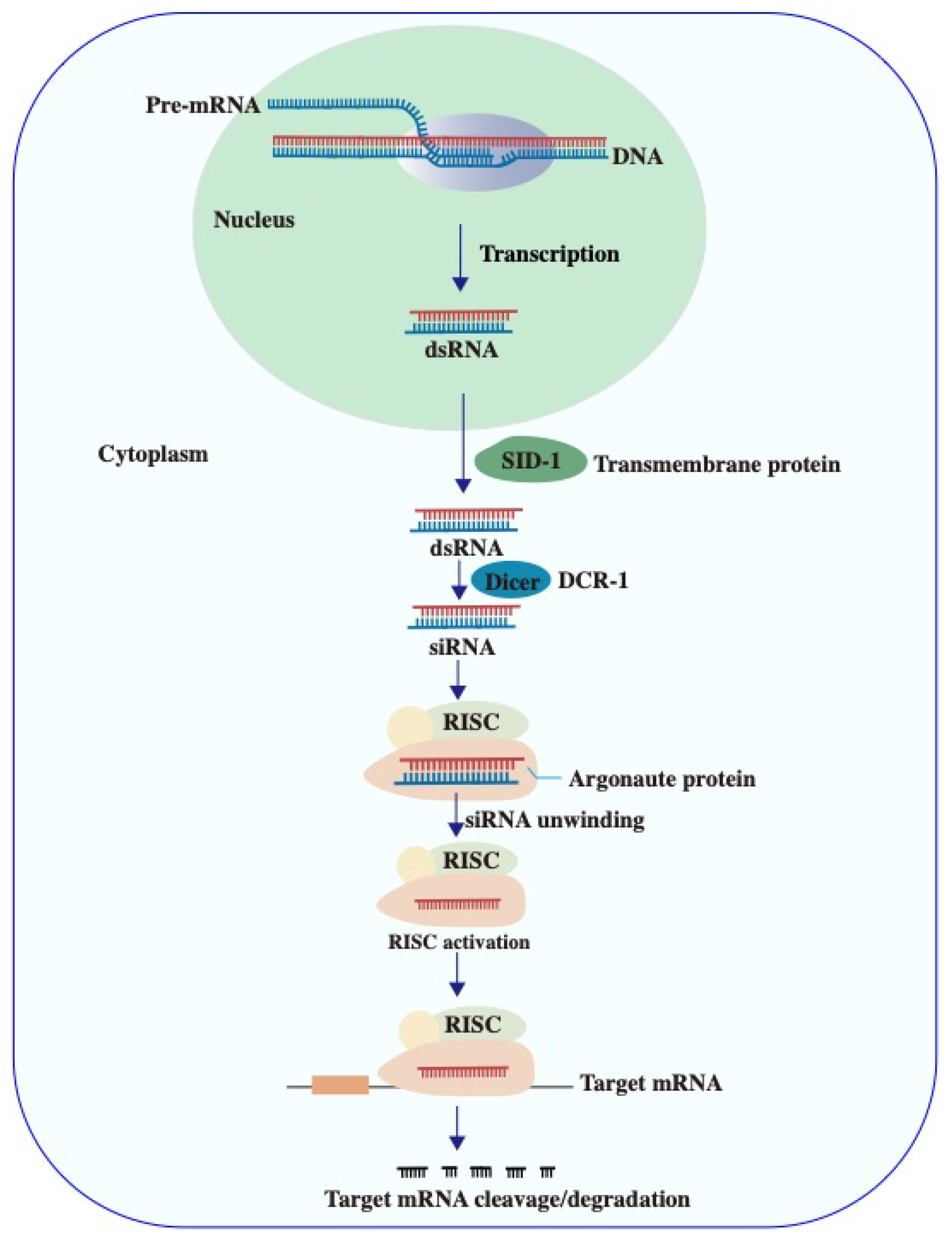
2.2. Plant siRNA
2.3. Plant Oligonucleotide
3. Delivery Systems of Plant-Derived sRNAs
3.1. Plant-Derived Exosomes
3.1.1. Plant Exosome-like Nanoparticles (PENs)
3.1.2. Plant Exosome-like Nanovesicles (PELNVs)
3.2. Herbal Decoctosome
3.3. Bencaosome
3.4. Adeno-Associated Viruses (AAV)
4. The Pharmacological Activities of Plant-Derived sRNAs
4.1. Pulmonary Protective Activity
4.2. Antiviral Activity
4.3. Anti-Hypertension
4.4. Anti-Hyperglycemia Activity
4.5. Anti-Osteoporosis Activity
4.6. Anti-Skin Aging Activity
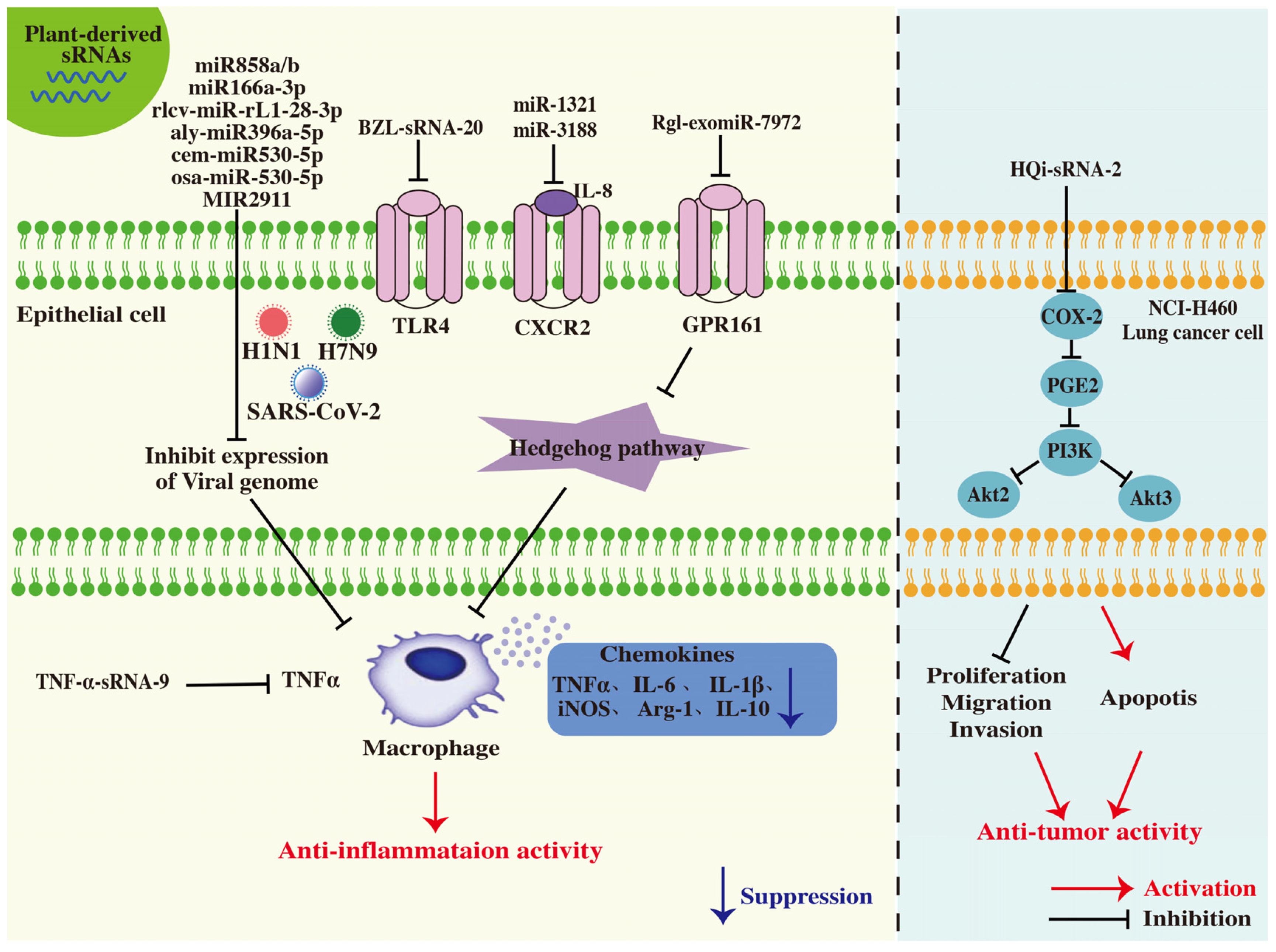
4.7. Anti-Inflammatory Activity
4.8. Anti-Tumor Activity
5. The Advantages of Plant-Derived sRNA Therapy
5.1. Minor Adverse Effect
5.2. Better Patient Compliance
5.3. Enhanced Targeting and Specificity
6. Limitations and Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, D.; Ma, X.; Zuo, Z.; Shao, W.; Wang, H.; Meng, Y. Bioinformatics resources for deciphering the biogenesis and action pathways of plant small RNAs. Rice 2017, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Meyers, B.C. Plant Small RNAs: Their Biogenesis, Regulatory Roles, and Functions. Annu. Rev. Plant Biol. 2023, 74, 21–51. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, M.; Lu, L.; Zhang, X. Diverse Functions of Small RNAs in Different Plant-Pathogen Communications. Front. Microbiol. 2016, 7, 1552. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Reichel, M.; Li, Y.; Millar, A.A. The functional scope of plant microRNA-mediated silencing. Trends Plant Sci. 2014, 19, 750–756. [Google Scholar] [CrossRef]
- Martins, L.M.; Law, J.A. Moving targets: Mechanisms regulating siRNA production and DNA methylation during plant development. Curr. Opin. Plant Biol. 2023, 75, 102435. [Google Scholar] [CrossRef]
- Zhao, D.; Qin, Y.; Liu, J.; Tang, K.; Lu, S.; Liu, Z.; Lin, Y.; Zhang, C.; Huang, F.; Chang, J.; et al. Orally administered BZL-sRNA-20 oligonucleotide targeting TLR4 effectively ameliorates acute lung injury in mice. Sci. China Life Sci. 2023, 66, 1589–1599. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, N.; Liu, D.; Yang, X.; Dong, Y.; Jiang, C. COX-2/PTGS2-targeted herbal-derived oligonucleotide drug HQi-sRNA-2 was effective in spontaneous mouse lung cancer model. IUBMB Life 2024, 76, 937–950. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef]
- Tang, K.; Wang, X.; Zhao, Y.; Li, X.; Jiang, Z.; Mei, S.; Chen, M.; Ma, Y.; Du, X.; Qiao, X.; et al. Oral administration of the herbal oligonucleotide XKC-sRNA-h3 prevents angiotensin II-induced hypertension in mice. Sci. China Life Sci. 2023, 66, 2370–2379. [Google Scholar] [CrossRef]
- Tang, K.; Wang, X.; Jiang, Z.; Chen, M.; Deng, X.; Mei, S.; Ma, Y.; Du, X.; Guo, S.; Lin, Y.; et al. Oral administration of herbal oligonucleotide drug JGL-sRNA-h7 ameliorates hyperglycemia in db/db mice and beagle dogs. IUBMB Life 2024, 76, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Wang, H.; Tie, H.; Liao, J.; Luo, Y.; Huang, W.; Yu, R.; Song, L.; Zhu, J. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: Preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J. Nanobiotechnol. 2023, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, F.; Dong, L.; Wu, H.; Xu, J.; Li, H.; Wang, J.; Zhou, Z.; Liu, C.; Wang, Y.; et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. 2021, 31, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Huang-Doran, I.; Zhang, C.Y.; Vidal-Puig, A. Extracellular vesicles: Novel mediators of cell communication in metabolic disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef]
- Ranganathan, K.; Sivasankar, V. MicroRNAs—Biology and clinical applications. J. Oral Maxillofac. Pathol. 2014, 18, 229–234. [Google Scholar] [CrossRef]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Liu, H.; Lei, C.; He, Q.; Pan, Z.; Xiao, D.; Tao, Y. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol. Cancer 2018, 17, 64. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Y.; Richards, A.M. Effective tools for RNA-derived therapeutics: siRNA interference or miRNA mimicry. Theranostics 2021, 11, 8771–8796. [Google Scholar] [CrossRef]
- Majlessi, M.; Nelson, N.C.; Becker, M.M. Advantages of 2′-O-methyl oligoribonucleotide probes for detecting RNA targets. Nucleic Acids Res. 1998, 26, 2224–2229. [Google Scholar] [CrossRef]
- Ji, L.; Chen, X. Regulation of small RNA stability: Methylation and beyond. Cell Res. 2012, 22, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yang, Z.; Li, J.; Minakhina, S.; Yang, M.; Padgett, R.W.; Steward, R.; Chen, X. Methylation as a crucial step in plant microRNA biogenesis. Science 2005, 307, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Chen, X.; Li, J.; Li, L.; Bian, Z.; Sun, F.; Lu, J.; Yin, Y.; Cai, X.; et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef]
- Hammond, S.M.; Bernstein, E.; Beach, D.; Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000, 404, 293–296. [Google Scholar] [CrossRef]
- Gregory, R.I.; Chendrimada, T.P.; Cooch, N.; Shiekhattar, R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005, 123, 631–640. [Google Scholar] [CrossRef]
- Saurabh, S.; Vidyarthi, A.S.; Prasad, D. RNA interference: Concept to reality in crop improvement. Planta 2014, 239, 543–564. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Tang, Q.; Khvorova, A. RNAi-based drug design: Considerations and future directions. Nat. Rev. Drug Discov. 2024, 23, 341–364. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.H.; Sailor, M.J. Rekindling RNAi therapy: Materials design requirements for in vivo siRNA delivery. Adv. Mater. 2019, 31, e1903637. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release 2016, 235, 236–244. [Google Scholar] [CrossRef]
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef]
- Schalk, C.; Cognat, V.; Graindorge, S.; Vincent, T.; Voinnet, O.; Molinier, J. Small RNA-mediated repair of UV-induced DNA lesions by the DNA DAMAGE-BINDING PROTEIN 2 and ARGONAUTE 1. Proc. Natl. Acad. Sci. USA 2017, 114, E2965–E2974. [Google Scholar] [CrossRef]
- Donaire, L.; Barajas, D.; Martínez-García, B.; Martínez-Priego, L.; Pagán, I.; Llave, C. Structural and genetic requirements for the biogenesis of tobacco rattle virus-derived small interfering RNAs. J. Virol. 2008, 82, 5167–5177. [Google Scholar] [CrossRef]
- Tretter, E.M.; Alvarez, J.P.; Eshed, Y.; Bowman, J.L. Activity range of Arabidopsis small RNAs derived from different biogenesis pathways. Plant Physiol. 2008, 147, 58–62. [Google Scholar] [CrossRef]
- Wu, F.; Huang, Y.; Jiang, W.; Jin, W. Genome-wide identification and validation of tomato-encoded sRNA as the cross-species antifungal factors targeting the virulence genes of Botrytis cinerea. Front. Plant Sci. 2023, 14, 1072181. [Google Scholar] [CrossRef]
- Sauer, N.J.; Mozoruk, J.; Miller, R.B.; Warburg, Z.J.; Walker, K.A.; Beetham, P.R.; Schöpke, C.R.; Gocal, G.F. Oligonucleotide-directed mutagenesis for precision gene editing. Plant Biotechnol. J. 2016, 14, 496–502. [Google Scholar] [CrossRef]
- Chen, X.; Rechavi, O. Plant and animal small RNA communications between cells and organisms. Nat. Rev. Mol. Cell Biol. 2022, 23, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liang, Z.; Xu, J.; Zhao, Y.; Li, X.; Zhang, Y.; Zhao, D.; Chen, R.; Liu, Y.; Joshi, T.; et al. Plant-derived phosphocholine facilitates cellular uptake of anti-pulmonary fibrotic HJT-sRNA-m7. Sci. China Life Sci. 2019, 62, 309–320. [Google Scholar] [CrossRef]
- Qian, Q.; Liu, X.; He, W.; An, Y.; Chen, Q.; Wu, J.; Deng, Y.; Guo, L.; Zhang, Y.; Wang, T. TG accumulation inhibitory effects of Jinqi formula by AMPK signaling pathway. J. Ethnopharmacol. 2012, 143, 41–48. [Google Scholar] [CrossRef]
- Qiao, X.; Huang, F.; Shi, X.; Deng, X.; Zhang, C.; Mei, S.; Wang, Z.; Zhou, C.; Jiang, C.; Tan, X. Herbal small RNAs in patients with COVID-19 linked to reduced DEG expression. Sci. China Life Sci. 2023, 66, 1280–1289. [Google Scholar] [CrossRef]
- Zhang, C.J.; Qu, X.Y.; Yu, Z.Y.; Yang, J.; Zhu, B.; Zhong, L.Y.; Sun, J.; He, J.H.; Zhu, Y.X.; Dong, L.; et al. Research of the dynamic regulatory mechanism of Compound Danshen Dripping Pills on myocardial infarction based on metabolic trajectory analysis. Phytomedicine 2024, 130, 155626. [Google Scholar] [CrossRef]
- Worrall, D.; Liang, Y.K.; Alvarez, S.; Holroyd, G.H.; Spiegel, S.; Panagopulos, M.; Gray, J.E.; Hetherington, A.M. Involvement of sphingosine kinase in plant cell signalling. Plant J. 2008, 56, 64–72. [Google Scholar] [CrossRef]
- Liu, N.J.; Zhang, T.; Liu, Z.H.; Chen, X.; Guo, H.S.; Ju, B.H.; Zhang, Y.Y.; Li, G.Z.; Zhou, Q.H.; Qin, Y.M.; et al. Phytosphinganine Affects Plasmodesmata Permeability via Facilitating PDLP5-Stimulated Callose Accumulation in Arabidopsis. Mol. Plant 2020, 13, 128–143. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, Y.; Sun, N.; Du, X.; Dong, Y.; Mei, S.; Deng, X.; Li, X.; Guo, S.; Tang, K.; et al. A comprehensive analysis of the Bencao (herbal) small RNA Atlas reveals novel RNA therapeutics for treating human diseases. Sci. China Life Sci. 2023, 66, 2380–2398. [Google Scholar] [CrossRef]
- Li, X.; Liang, Z.; Du, J.; Wang, Z.; Mei, S.; Li, Z.; Zhao, Y.; Zhao, D.; Ma, Y.; Ye, J.; et al. Herbal decoctosome is a novel form of medicine. Sci. China Life Sci. 2019, 62, 333–348. [Google Scholar] [CrossRef]
- Ji, C.; Kriaucionis, S.; Kessler, B.M.; Jiang, C. From herbal small RNAs to one medicine. Sci. China Life Sci. 2019, 62, 285–287. [Google Scholar] [CrossRef]
- Liu, Y.D.; Chen, H.R.; Zhang, Y.; Yan, G.; Yan, H.J.; Zhu, Q.; Peng, L.H. Progress and challenges of plant-derived nucleic acids as therapeutics in macrophage-mediated RNA therapy. Front. Immunol. 2023, 14, 1255668. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Lu, L.; Li, Z.; Guo, Q.; Ou, L.; Wang, R.; Tian, X. Plant-derived exosome-like nanoparticles for microRNA delivery in cancer treatment. Drug Deliv. Transl. Res. 2025, 15, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, J.; Zhang, X.; Li, Y.; Qin, Q.; Song, H.; Yuan, C.; Huang, Z. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed. Pharmacother. 2024, 174, 116543. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Duroux, M.; Moos, T.; Andresen, T.L.; Simonsen, J.B. On the use of liposome controls in studies investigating the clinical potential of extracellular vesicle-based drug delivery systems—A commentary. J. Control. Release 2018, 269, 10–14. [Google Scholar] [CrossRef]
- Chu, K.; Liu, J.; Zhang, X.; Wang, M.; Yu, W.; Chen, Y.; Xu, L.; Yang, G.; Zhang, N.; Zhao, T. Herbal Medicine-Derived Exosome-Like Nanovesicles: A Rising Star in Cancer Therapy. Int. J. Nanomed. 2024, 19, 7585–7603. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the microRNA profile of Ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal Caco-2 cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Zhu, H.; Chang, M.; Wang, Q.; Chen, J.; Liu, D.; He, W. Identifying the potential of miRNAs in Houttuynia cordata-derived exosome-like nanoparticles against respiratory RNA viruses. Int. J. Nanomed. 2023, 18, 5983–6000. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-derived Edible Nanoparticle-based lipid Nano-drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.; et al. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Huang, J.; Cao, X.; Wu, W.; Han, L.; Wang, F. Investigating the proliferative inhibition of HepG2 cells by exosome-like nanovesicles derived from Centella asiatica extract through metabolomics. Biomed. Pharmacother. 2024, 176, 116855. [Google Scholar] [CrossRef]
- Jin, Z.; Na, J.; Lin, X.; Jiao, R.; Liu, X.; Huang, Y. Plant-derived exosome-like nanovesicles: A novel nanotool for disease therapy. Heliyon 2024, 10, e30630. [Google Scholar] [CrossRef]
- Li, X.; Bao, H.; Wang, Z.; Wang, M.; Fan, B.; Zhu, C.; Chen, Z. Biogenesis and function of Multivesicular bodies in plant immunity. Front. Plant Sci. 2018, 9, 979. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for ligand display on Ginger exosome-like nanovesicles to systemic deliver siRNA for Cancer suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Ma, C.; Liu, K.; Wang, F.; Fei, X.; Niu, C.; Li, T.; Liu, L. Neutrophil membrane-engineered Panax ginseng root-derived exosomes loaded miRNA 182-5p targets NOX4/Drp-1/NLRP3 signal pathway to alleviate acute lung injury in sepsis: Experimental studies. Int. J. Surg. 2024, 110, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Guo, Z.; Chen, X.; Yan, R.; Ma, W.; Yang, X.; Lin, Y. Targeting modulation of intestinal flora through oral route by an antimicrobial nucleic acid-loaded exosome-like nanovesicles to improve Parkinson’s disease. Sci. Bull. 2024, 69, 3925–3935. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, Z.; Feng, Z.; Huang, J.; Shi, J.; Wang, K.; Jiang, X.; Yang, J.; Ning, Y.; Lu, F.; et al. Platycodon grandiflorum exosome-like nanoparticles: The material basis of fresh platycodon grandiflorum optimality and its mechanism in regulating acute lung injury. J. Nanobiotechnol. 2025, 23, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef]
- Zhang, S.; Hong, Z. Mobile RNAs—The magical elf traveling between plant and the associated organisms. ExRNA 2019, 1, 8. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zheng, Y.; Pan, L.; Li, J.; Di, L.; Cao, P.; Wang, R. Considerations regarding the application of Chinese herbal medicine-derived extracellular vesicle-like particles in a drug delivery system. Acupunct. Herb. Med. 2024, 4, 423–426. [Google Scholar] [CrossRef]
- Du, X.; Guo, S.; Mu, X.; Mei, S.; Yang, R.; Zhang, H.; Jiang, C.; Zhang, J. Bencaosome [16:0 Lyso PA+XLGB28-sRNA] improves osteoporosis by simultaneously promoting osteogenesis and inhibiting osteoclastogenesis in mice. IUBMB Life 2024, 76, 832–844. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, X.; Tang, K.; Chen, K.; Jiang, C. Baked licorice-derived sRNA alleviates lung injury mild ARDS mouse models possibly through targeting TNF-α. Basic Clin. Med. 2023, 43, 1030–1039. [Google Scholar]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Huang, L.; Xu, Q.; Ling, X.; Liu, J. Cordyceps Militaris alleviates severity of murine acute lung injury through miRNAs-mediated CXCR2 inhibition. Cell. Physiol. Biochem. 2015, 36, 2003–2011. [Google Scholar] [CrossRef]
- Li, X.; Gong, C.; Naeem, A.; Liu, J.; Yang, M.; Zhang, J.; Shang, H. Priming immunity via herbal components and their nanomedicines for the treatment of cancer. Acupunct. Herb. Med. 2024, 4, 436–462. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Y.; Jiang, X.M.; Wang, Y.; Chen, X.; Xiao, G.; Zhang, C.Y.; Yi, Y.; Zhang, L.K.; Li, L. Decreased HD-MIR2911 absorption in human subjects with the SIDT1 polymorphism fails to inhibit SARS-CoV-2 replication. Cell Discov. 2020, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Snow, J.W. Formidable challenges to the notion of biologically important roles for dietary small RNAs in ingesting mammals. Genes Nutr. 2017, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Xu, J.; Fan, S.; Zhu, N.; Meng, Q.; Dai, S.; Yuan, X. Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants 2024, 13, 2712. [Google Scholar] [CrossRef]
- Qiu, F.S.; Wang, J.F.; Guo, M.Y.; Li, X.J.; Shi, C.Y.; Wu, F.; Zhang, H.H.; Ying, H.Z.; Yu, C.H. Rgl-exomiR-7972, a novel plant exosomal microRNA derived from fresh Rehmanniae Radix, ameliorated lipopolysaccharide-induced acute lung injury and gut dysbiosis. Biomed. Pharmacother. 2023, 165, 115007. [Google Scholar] [CrossRef]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Wang, R.; Luo, J.Y.; He, J.J.; Ye, R.S.; Xie, M.Y.; Xi, Q.Y.; Jiang, Q.Y.; Sun, J.J.; et al. Plant MIR156 regulates intestinal growth in mammals by targeting the Wnt/β-catenin pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C434–C448. [Google Scholar] [CrossRef]
- Hou, D.; He, F.; Ma, L.; Cao, M.; Zhou, Z.; Wei, Z.; Xue, Y.; Sang, X.; Chong, H.; Tian, C.; et al. The potential atheroprotective role of plant MIR156a as a repressor of monocyte recruitment on inflamed human endothelial cells. J. Nutr. Biochem. 2018, 57, 197–205. [Google Scholar] [CrossRef]
- Zhou, L.K.; Zhou, Z.; Jiang, X.M.; Zheng, Y.; Chen, X.; Fu, Z.; Xiao, G.; Zhang, C.Y.; Zhang, L.K.; Yi, Y. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020, 6, 54. [Google Scholar] [CrossRef]
- Li, W.; Ding, J.; Chen, S.; Chen, J.; Wang, C.; Li, J.; Shi, H.; Yin, X.; Wang, J.; Liu, J.; et al. Alleviation of colitis by honeysuckle MIR2911 via direct regulation of gut microbiota. J. Control. Release 2024, 376, 123–137. [Google Scholar] [CrossRef]
- Liu, C.; Xu, M.; Yan, L.; Wang, Y.; Zhou, Z.; Wang, S.; Sun, Y.; Zhang, J.; Dong, L. Honeysuckle-derived microRNA2911 inhibits tumor growth by targeting TGF-β1. Chin. Med. 2021, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhou, H.; Xu, X.; Jiang, T.; Li, S.; Wang, D.; Nie, Z.; Sheng, Q. Identification and Investigation of miRNAs from Gastrodia elata Blume and Their Potential Function. Front. Pharmacol. 2020, 11, 542405. [Google Scholar] [CrossRef] [PubMed]
- Mangukia, N.; Rao, P.; Patel, K.; Pandya, H.; Rawal, R.M. Identifying potential human and medicinal plant microRNAs against SARS-CoV-2 3′UTR region: A computational genomics assessment. Comput. Biol. Med. 2021, 136, 104662. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef]
- Mangukia, N.; Rao, P.; Patel, K.; Pandya, H.; Rawal, R.M. Unveiling the nature’s fruit basket to computationally identify Citrus sinensis csi-mir169-3p as a probable plant miRNA against Reference and Omicron SARS-CoV-2 genome. Comput. Biol. Med. 2022, 146, 105502. [Google Scholar] [CrossRef]
- Yang, R.; Lin, F.; Wang, W.; Dai, G.; Ke, X.; Wu, G. Investigating the therapeutic effects and mechanisms of Carthamus tinctorius L.-derived nanovesicles in atherosclerosis treatment. Cell Commun. Signal. 2024, 22, 178. [Google Scholar] [CrossRef]
- Qin, Y.; Zheng, B.; Yang, G.S.; Yang, H.J.; Zhou, J.; Yang, Z.; Zhang, X.H.; Zhao, H.Y.; Shi, J.H.; Wen, J.K. Salvia miltiorrhiza-Derived Sal-miR-58 Induces Autophagy and Attenuates Inflammation in Vascular Smooth Muscle Cells. Mol. Ther. Nucleic Acids 2020, 21, 492–511. [Google Scholar] [CrossRef]
- Yang, G.S.; Zheng, B.; Qin, Y.; Zhou, J.; Yang, Z.; Zhang, X.H.; Zhao, H.Y.; Yang, H.J.; Wen, J.K. Salvia miltiorrhiza-derived miRNAs suppress vascular remodeling through regulating OTUD7B/KLF4/NMHC IIA axis. Theranostics 2020, 10, 7787–7811. [Google Scholar] [CrossRef]
- Han, J.; Wu, T.; Jin, J.; Li, Z.; Cheng, W.; Dai, X.; Yang, K.; Zhang, H.; Zhang, Z.; Zhang, H.; et al. Exosome-like nanovesicles derived from Phellinus linteus inhibit Mical2 expression through cross-kingdom regulation and inhibit ultraviolet-induced skin aging. J. Nanobiotechnol. 2022, 20, 455. [Google Scholar] [CrossRef]
- Chin, A.R.; Fong, M.Y.; Somlo, G.; Wu, J.; Swiderski, P.; Wu, X.; Wang, S.E. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016, 26, 217–228. [Google Scholar] [CrossRef]
- Minutolo, A.; Potestà, M.; Roglia, V.; Cirilli, M.; Iacovelli, F.; Cerva, C.; Fokam, J.; Desideri, A.; Andreoni, M.; Grelli, S.; et al. Plant microRNAs from Moringa oleifera regulate immune response and HIV Infection. Front. Pharmacol. 2020, 11, 620038. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Batchinsky, A.I.; Roberts, T.R.; Antebi, B.; Necsoiu, C.; Choi, J.H.; Herzig, M.; Cap, A.P.; McDaniel, J.S.; Rathbone, C.R.; Chung, K.K.; et al. Intravenous autologous bone marrow-derived mesenchymal stromal cells delay acute Respiratory Distress Syndrome in swine. Am. J. Respir. Crit. Care Med. 2023, 208, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Q.; Liu, S.; Yang, X.; Zhang, Y.; Huang, F. Sini decoction alleviates E. coli induced acute lung injury in mice via equilibrating ACE-AngII-AT1R and ACE2-Ang-(1-7)-Mas axis. Life Sci. 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, J.; Wang, W.; Liu, S.; Yang, X.; Chen, M.; Cheng, L.; Lu, J.; Guo, T.; Huang, F. Sini decoction ameliorates sepsis-induced acute lung injury via regulating ACE2-Ang (1-7)-Mas axis and inhibiting the MAPK signaling pathway. Biomed. Pharmacother. 2019, 115, 108971. [Google Scholar] [CrossRef]
- Kang, X.; Jin, D.; Jiang, L.; Zhang, Y.; Zhang, Y.; An, X.; Duan, L.; Yang, C.; Zhou, R.; Duan, Y.; et al. Efficacy and mechanisms of traditional Chinese medicine for COVID-19: A systematic review. Chin. Med. 2022, 17, 30. [Google Scholar] [CrossRef]
- Luo, H.; Gao, Y.; Zou, J.; Zhang, S.; Chen, H.; Liu, Q.; Tan, D.; Han, Y.; Zhao, Y.; Wang, S. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chin. Med. 2020, 15, 94. [Google Scholar] [CrossRef]
- Sun, C.R.; Xu, D.; Yang, F.; Hou, Z.; Luo, Y.; Zhang, C.Y.; Shan, G.; Huang, G.; Yao, X.; Chen, Y.; et al. Human SIDT1 mediates dsRNA uptake via its phospholipase activity. Cell Res. 2024, 34, 84–87. [Google Scholar] [CrossRef]
- Mohammed, S.A.D.; Liu, H.; Baldi, S.; Chen, P.; Lu, F.; Liu, S. GJD Modulates Cardiac/Vascular Inflammation and decreases Blood Pressure in Hypertensive Rats. Mediat. Inflamm. 2022, 2022, 7345116. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S. Comprehensive management of Cardiovascular risk factors for adults with Type 2 Diabetes: A scientific statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Huang, W.; Suo, J.; Chen, X.; Ding, K.; Sun, Q.; Zhang, H. Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. Int. J. Biol. Macromol. 2020, 145, 484–491. [Google Scholar] [CrossRef]
- Ensrud, K.; Crandall, C. Osteoporosis. Ann. Intern. Med. 2017, 167, ITC17–ITC32. [Google Scholar] [CrossRef]
- Zeng, J.; Li, C.; Gu, Z. A network pharmacological study to unveil the mechanisms of xianlinggubao capsule in the treatment of osteoarthritis and osteoporosis. Arch. Med. Sci. 2024, 20, 557–566. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with Ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Choi, W.; Cho, J.H.; Park, S.H.; Kim, D.S.; Lee, H.P.; Kim, D.; Kim, H.S.; Kim, J.H.; Cho, J.Y. Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species. J. Ginseng Res. 2024, 48, 211–219. [Google Scholar] [CrossRef]
- Sarkar, A.; Saquib, M.; Chakraborty, D.; Mann, S.; Malik, S.; Agnihotri, P.; Joshi, L.; Malhotra, R.; Biswas, S. Clo-miR-14: A medicinally valued spice-derived miRNA with therapeutic implications in rheumatoid arthritis. Biosci. Rep. 2024, 44, BSR20240311. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sahu, S.; Kumari, P.; Gopi, S.R.; Malhotra, R.; Biswas, S. Genome-wide identification and functional annotation of miRNAs in anti-inflammatory plant and their cross-kingdom regulation in Homo sapiens. J. Biomol. Struct. Dyn. 2017, 35, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [PubMed]
- Malynn, B.A.; Ma, A. A20 takes on tumors: Tumor suppression by an ubiquitin-editing enzyme. J. Exp. Med. 2009, 206, 977–980. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.; Zhang, Q.; Wu, Y.; Xia, G.; Xia, H.; Wang, L.; Shang, H.; Lin, S. The traditional Chinese medicines treat chronic heart failure and their main bioactive constituents and mechanisms. Acta Pharm. Sin. B 2023, 13, 1919–1955. [Google Scholar] [CrossRef]
- Ye, L.; Fan, S.; Zhao, P.; Wu, C.; Liu, M.; Hu, S.; Wang, P.; Wang, H.; Bi, H. Potential herb–drug interactions between anti-COVID-19 drugs and traditional Chinese medicine. Acta Pharm. Sin. B 2023, 13, 3598–3637. [Google Scholar] [CrossRef]
- Wan, S.; Xie, X.; Yang, G.; Feng, F. Discovery of the toxicity-related quality markers and mechanisms of Zhi-Zi-Hou-Po decoction based on Chinmedomics combined with differentially absorbed components and network pharmacology. J. Ethnopharmacol. 2024, 320, 117408. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Liu, S.; Kuang, B.; Tian, Z.; Zhang, L.; Yang, S.; Lin, M.; Chen, N.; Liu, X.; et al. Progress of exosomal microRNAs and Traditional Chinese Medicine monomers in Neurodegenerative Diseases. Phytother. Res. 2024, 38, 5323–5349. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Sun, Y.-L.; Li, X.-M. The role of Chinese medicine in COVID-19 pneumonia: A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 38, 2153–2159. [Google Scholar] [CrossRef]
- Hashemi, N.; Odze, R.D.; McGowan, M.P.; Santos, R.D.; Stroes, E.S.G.; Cohen, D.E. Liver histology during Mipomersen therapy for severe hypercholesterolemia. J. Clin. Lipidol. 2014, 8, 606–611. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, K.; Fang, C.; Zhou, X.; Ding, X.; Zhang, Q.Y. Dietary regulation of mouse intestinal P450 expression and drug metabolism. Drug Metab. Dispos. 2013, 41, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Szoka, F.C. Nucleic acid delivery: The missing pieces of the puzzle? Acc. Chem. Res. 2012, 45, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, X.; Zhou, X.; Ur-Rehman, U.; Yu, M.; Liang, H.; Guo, H.; Guo, X.; Kong, Y.; Su, Y.; et al. In vivo self-assembled small RNAs as a new generation of RNAi therapeutics. Cell Res. 2021, 31, 631–648. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.G.; Qiao, H. Technology insight: Plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The limitless future of RNA therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef]
- Miao, Y.; Fu, C.; Yu, Z.; Yu, L.; Tang, Y.; Wei, M. Current status and trends in small nucleic acid drug development: Leading the future. Acta Pharm. Sin. B 2024, 14, 3802–3817. [Google Scholar] [CrossRef]
- Corvigno, S.; Liu, Y.; Bayraktar, E.; Stur, E.; Bayram, N.N.; Ahumada, A.L.; Nagaraju, S.; Rodriguez-Aguayo, C.; Chen, H.; Vu, T.C.; et al. Enhanced plant-derived vesicles for nucleotide delivery for cancer therapy. NPJ Precis. Oncol. 2024, 8, 86. [Google Scholar] [CrossRef]

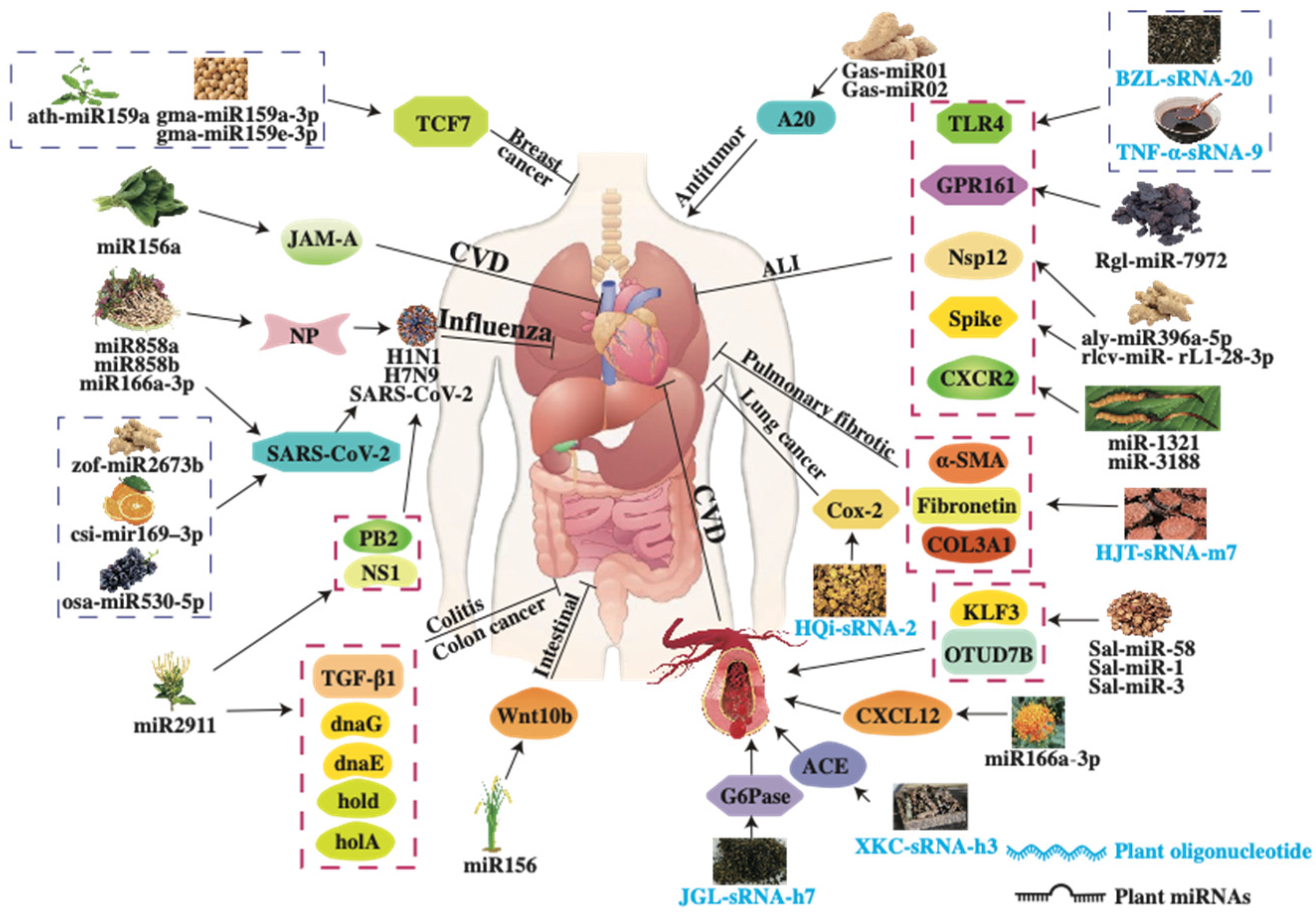
| sRNAs | Origin | Delivery System | Therapeutic Disease | Target Gene | Model | Refs. |
|---|---|---|---|---|---|---|
| BZL-sRNA-20 | Scutellaria barbata | Bencaosome | Acute lung injury | Toll-like receptor 4 (TLR4) | In vitro: LPS-induced THP-1 cell model, lipoteichoic acid (LTA)-induced U937 cell model and polyinosinic-polycytidylic acid (poly(I:C))-induced A549 cells; H5N1 virus-infected A549 cell model, SARS-CoV-2 infected Vero E6 cell model. In vivo: LPS and SARS-CoV-2-induced acute lung injury C57BL/6J mice models. | [6] |
| HJT-sRNA-m7 | Rhodiola crenulata | Liposome | Pulmonary fibrotic | α-SMA, fibronetin, and COL3A1 | In vivo: bleomycin-induced pulmonary fibrosis model in C57BL/6J mice. | [42] |
| PGY-sRNA-6 | Taraxacum mongolicum | Bencaosome and decoctosome | Inflammatory | RELA | In vitro: poly(I:C)-induced A549 cell; In vivo: poly(I:C)-induced inflammatory model in C57BL/6J mice. | [49] |
| Rgl-exomiR-7972 | Rehmanniae Radix | Exosome | Acute lung injury (ALI) | G protein-coupled receptor 161 (GPR161) | In vitro: LPS-induced RAW264.7 cell; In vivo: LPS-induced acute lung inflammation model in BALB/c mice. | [85] |
| TNF-α-sRNA-9 | Sini decoction | Bencaosome | Acute lung injury | TLR4 | In vitro: LTA-induced U937 cell, poly(I:C)-induced A549 cell, and Vero E6 infected with SARS-CoV-2; In vivo: LPS-induced acute lung injury model in C57BL/6J mice. | [78] |
| HQi-sRNA-2 | Scutellaria baicalensis | Bencaosome | Lung cancer | Cyclooxygenase-2 (COX-2) | In vivo: KrasLSL-G12Dp53fl/fl lung cancer mice model. | [8] |
| aly-miR396a-5p | Ginger | Exosome-like nanoparticle | Lung inflammation | Nonstructural protein 12 (Nsp12) | In vivo: transfected with SARS-CoV-2 plasmids administered to C57BL/6 mice; In vitro: Vero E2 cells infected with SARS-CoV-2. | [86] |
| rlcv-miR- rL1-28-3p | Ginger | Exosome-like nanoparticle | Lung inflammation | Spike | In vitro: Vero E2 cells infected with SARS-CoV-2. | [86] |
| miR156 | Crops | - | Intestinal cell proliferation | Wnt10b | In vitro: procine jejunum epithelial (IPEC-J2) cell | [87] |
| MIR156a | Cabbage, spinach, lettuce | Exosome | Cardiovascular disease (CVD) | Junction adhesion molecule-A (JAM-A) | In vitro: HAEC cell | [88] |
| MIR168a | Rice | Microvesicle | - | LDLRAP1 | In vivo: C57BL/6J mice. | [7] |
| miR2911 | Honeysuckle | Cell-derived microvesicle | Influenza A viruses | H1N1-encoded PB2 and NS1 genes | In vitro: H1N1 virus in Madin-Darby Cannine Kidney (MDCK) cells In vivo: H1N1, H5N1 and H7N9 influenza viruses inoculated BALB/c mice models. | [9] |
| MIR2911 | Honeysuckle | Exosome | SARS-CoV-2 | SARS-CoV-2 genome | In vitro: SARS-CoV-2 propagated in Vero E6 cells In vivo: moderate type patient infected by SARS-CoV-2 virus. | [82,89] |
| MIR2911 | Honeysuckle | Small extracellular vesicles | colitis | holA, holD, dnaE, dnaG and ligB | In vivo: DSS-induced colitis model in C57BL/6J mice | [90] |
| miR2911 | Honeysuckle | - | Colon cancer | Transforming Growth Factor-β1 (TGF-β1) | In vivo: tumor-bearing Sidt1+/+ and Sidt1-/- mice models. | [91] |
| Gas-miR01/Gas-miR02 | Gastrodia elata Blume | - | Tumor | A20 | In vivo: 293T cells | [92] |
| Novel_40/cca-miR156b/vvi-miR396a/ath-miR159a/gma-miR396h | Ginger | Exosome-like nanoparticles | Inflammatory | - | In vitro: LPS-induced inflammation model in Caco-2 cell. | [57] |
| zof-miR2673b | Zingiber officinale | - | - | SARS-CoV-2 genome | - | [93] |
| Cme/osa-miR530-5p | Ginger and grapefruit | Edible nanoparticles (ENPs) | SARS-CoV-2 | SARS- CoV-2 genome | - | [94] |
| csi-mir169–3p | Citrus sinensis | - | - | SARS-CoV-2 genome | - | [95] |
| miR166a-3p | Carthamus tinctorius L. | Nanovesicles | Atherosclerosis | Chemokine ligand 12 (CXCL12) | In vitro: ox-LDL-induced inflammation model in HUVECs In vivo: ApoE-/- mice | [96] |
| Sal-miR-58 | Salvia miltiorrhiza | - | Abdominal aortic aneurysm (AAA) | Kruppel-like factor 3 (KLF3) | In vitro: Ang II-induced mouse vascular smooth muscle cells (VSMCs); In vivo: Ang II-induced AAA model in ApoE-/- mice. | [97] |
| Sal-miR-1/Sal-miR-3 | Salvia miltiorrhiza | F-127 pluronic gel | Vascular remodel induced by vascular injury | OTU deubiquitinase 7B (OTUD7B) | In vitro: thrombin-induced the migration of VSMCs and monocyte adhesion to VSMCs; In vivo: intimal hyperplasia induced by carotid artery ligation in C57BL/6 mice. | [98] |
| miR-1321/miR-3188 | Cordyceps militaris | Adeno-associated viruses (AAV) | Acute lung injury (ALI) | CXC-chemokine receptor 2 (CXCR2) | In vivo: bleomycin-induced ALI model in Balb/c mice | [80] |
| miR858a/miR858b | Houttuynia cordata | Exosome-Like Nanoparticles | RNA Viruses | NP gene in H1N1 | - | [59] |
| miR166a-3p | Houttuynia cordata | Exosome-Like Nanoparticles | RNA Viruses | ORF1ab gene in SARS-CoV-2 | - | [59] |
| XKC-sRNA-h3 | Prunella vulgaris L. | Becaosome | Hypertension | ACE | In vivo: Ang II-induced hypertensive cardiac damage C57BL/6J mice model. | [10] |
| JGL-sRNA-h7 | Gynostemma pentaphyllum [Thunb.] Makino | Bencaosome | Hyperglycemia | glucose-6-phosphatase (G6Pase) | In vivo: hyperglycemia in db/db mice model and beagle dogs’ model. | [11] |
| XLGB28-sRNA | Xianlinggubao (XLGB) formula | Bencaosome | Osteoporosis | tumor necrosis factor superfamily member 11 (TNFSF11) | In vitro: ascorbic acid and β-glycerol phosphate induced osteogenic MC3T3-E1 cells model. In vivo: estrogen deficiency-induced osteoporosis in C57BL/6J mice model. | [77] |
| miR-CM1 | Phellinus linteus | Exosome-like nanovesicle | Ultraviolet-induced skin aging | Microtubule-Associated Protein-Lysine N-Methyltransferase 2 (Mical2), Dual-specificity phosphatase 18 (DUSP18), GRB2-related adaptor protein (GRAP) and RNA polymerase I transcription factor (RRN3) | In vitro: UV-induced HaCaT cell model. In vivo: UV-induced skin photoaging Kunming mice model. | [99] |
| ath-miR159a | Arabidopsis thaliana | - | Breast cancer | Transcription factor 7 (TCF7) | In vivo: MDA-MB-231 xenograft tumor induced NOD-scid IL2Rgnull (NSG) mice model. | [100] |
| gma-miR159a-3p/gma-miR159e-3p | Glycine max | - | Breast cancer | TCF7 | In vivo: MDA-MB-231 xenograft tumor induced NOD-scid IL2Rgnull (NSG) mice model. | [100] |
| p-miR858b | Moringa oleifera | - | HIV infection | Vav1 oncogene (VAV1) | In vitro: HIV-infected PBMCs cell | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Q.; Hua, H.; Zhao, J. Advancements in Plant-Derived sRNAs Therapeutics: Classification, Delivery Strategies, and Therapeutic Applications. Int. J. Mol. Sci. 2025, 26, 4277. https://doi.org/10.3390/ijms26094277
Rao Q, Hua H, Zhao J. Advancements in Plant-Derived sRNAs Therapeutics: Classification, Delivery Strategies, and Therapeutic Applications. International Journal of Molecular Sciences. 2025; 26(9):4277. https://doi.org/10.3390/ijms26094277
Chicago/Turabian StyleRao, Qianru, Hua Hua, and Junning Zhao. 2025. "Advancements in Plant-Derived sRNAs Therapeutics: Classification, Delivery Strategies, and Therapeutic Applications" International Journal of Molecular Sciences 26, no. 9: 4277. https://doi.org/10.3390/ijms26094277
APA StyleRao, Q., Hua, H., & Zhao, J. (2025). Advancements in Plant-Derived sRNAs Therapeutics: Classification, Delivery Strategies, and Therapeutic Applications. International Journal of Molecular Sciences, 26(9), 4277. https://doi.org/10.3390/ijms26094277






