Big Data-Driven Evolution of a Diagnostic Multiplex IgE-Test: Enhancing Accuracy and Efficacy in Allergy Diagnostics
Abstract
:1. Introduction
2. Results
2.1. Extracts and Allergen Molecules of the Same Source Contained on ALEX2
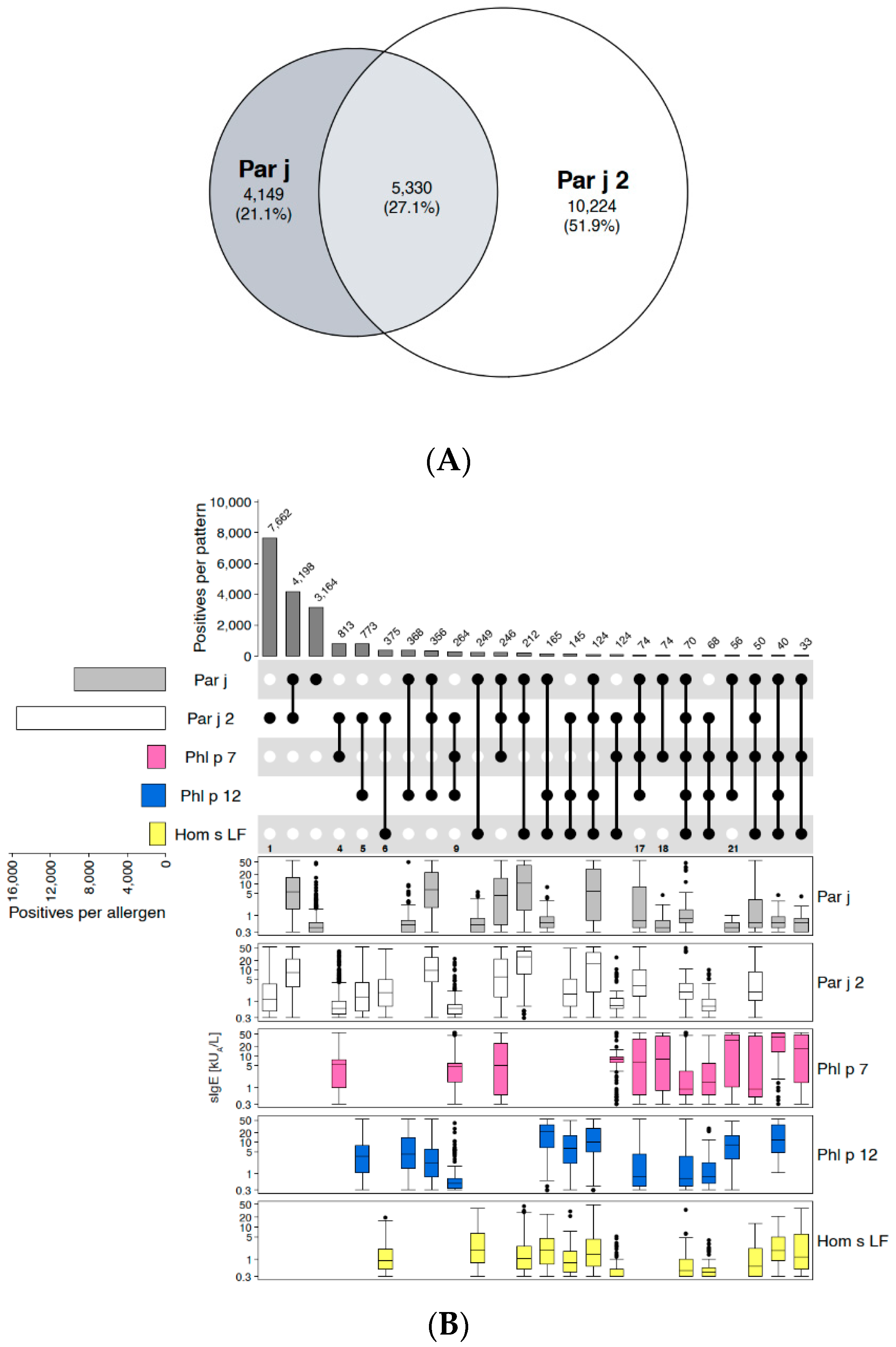
2.1.1. Example of Retaining the Extract: Spreading Pellitory (Parietaria judaica)
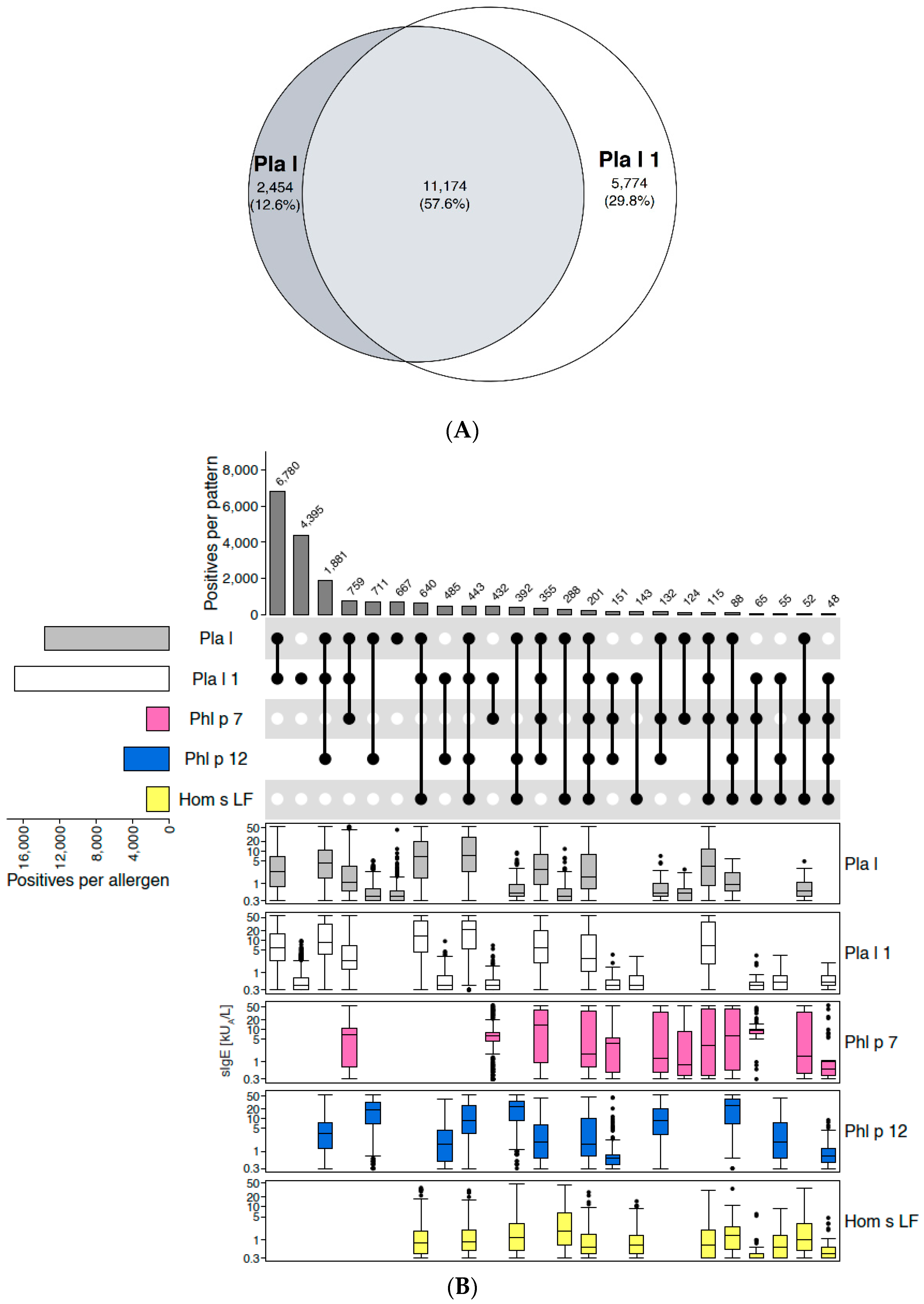
2.1.2. Example of Discontinuation of the Extract: Ribwort Plantain (Plantago lanceolata)
2.2. Allergen Families
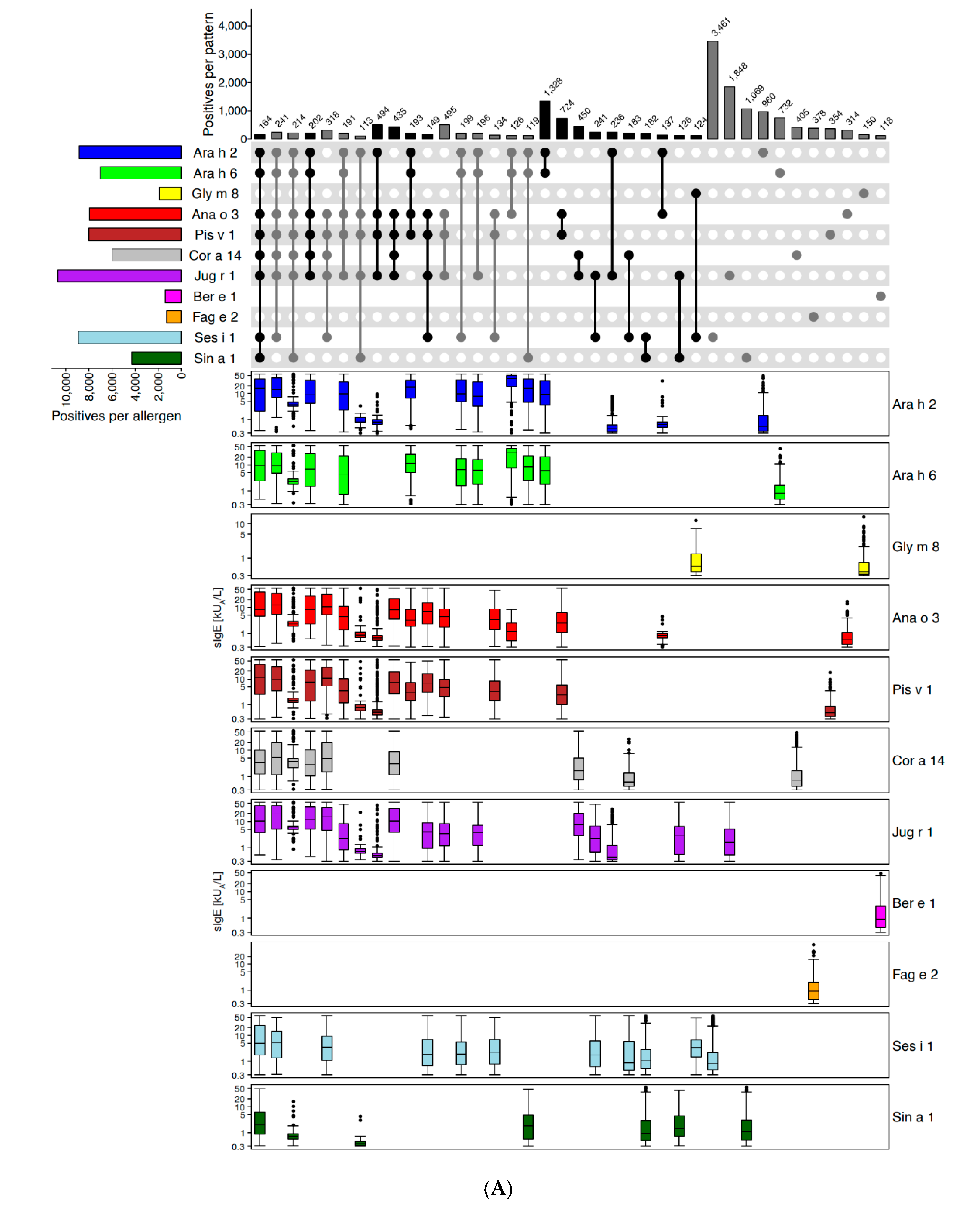
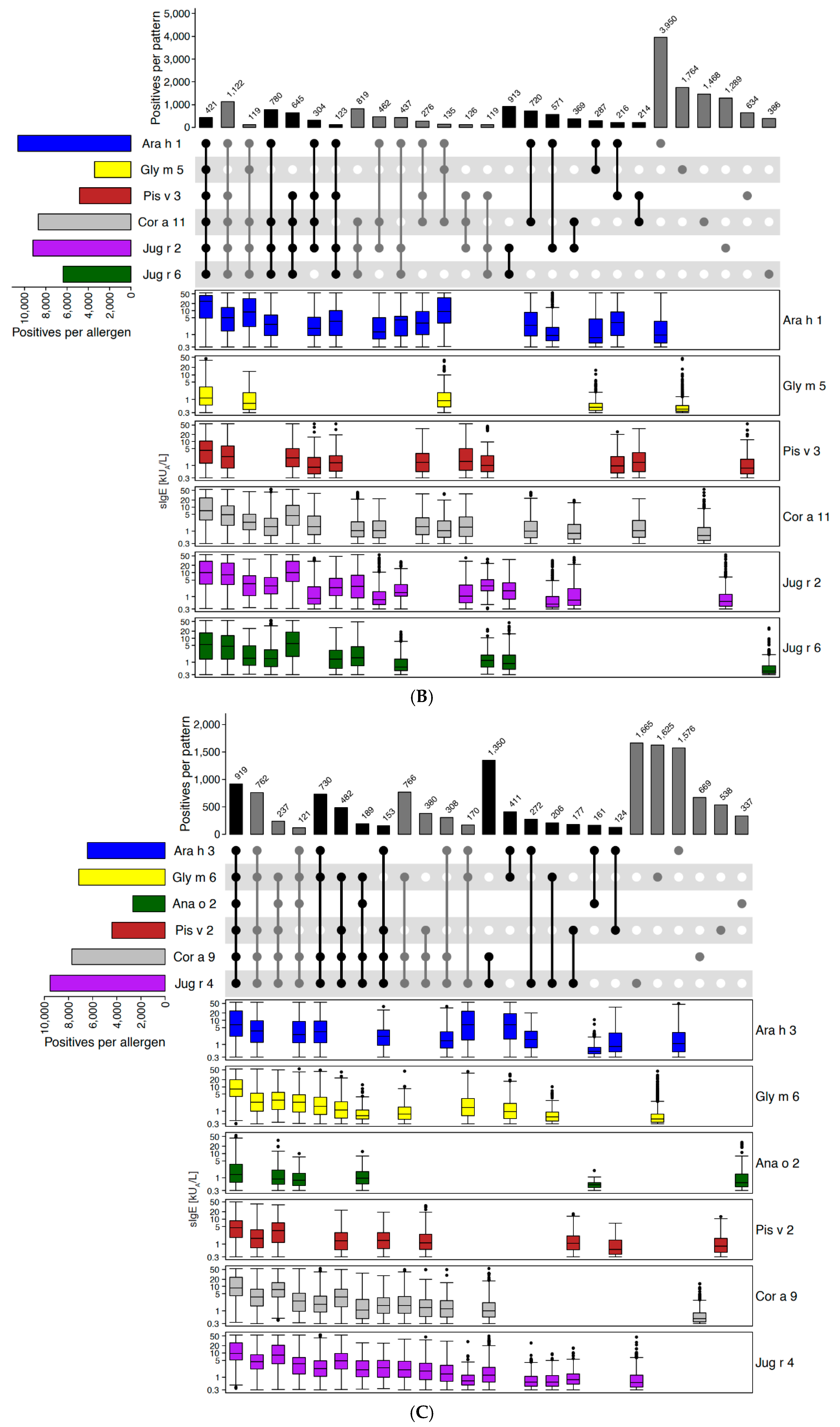
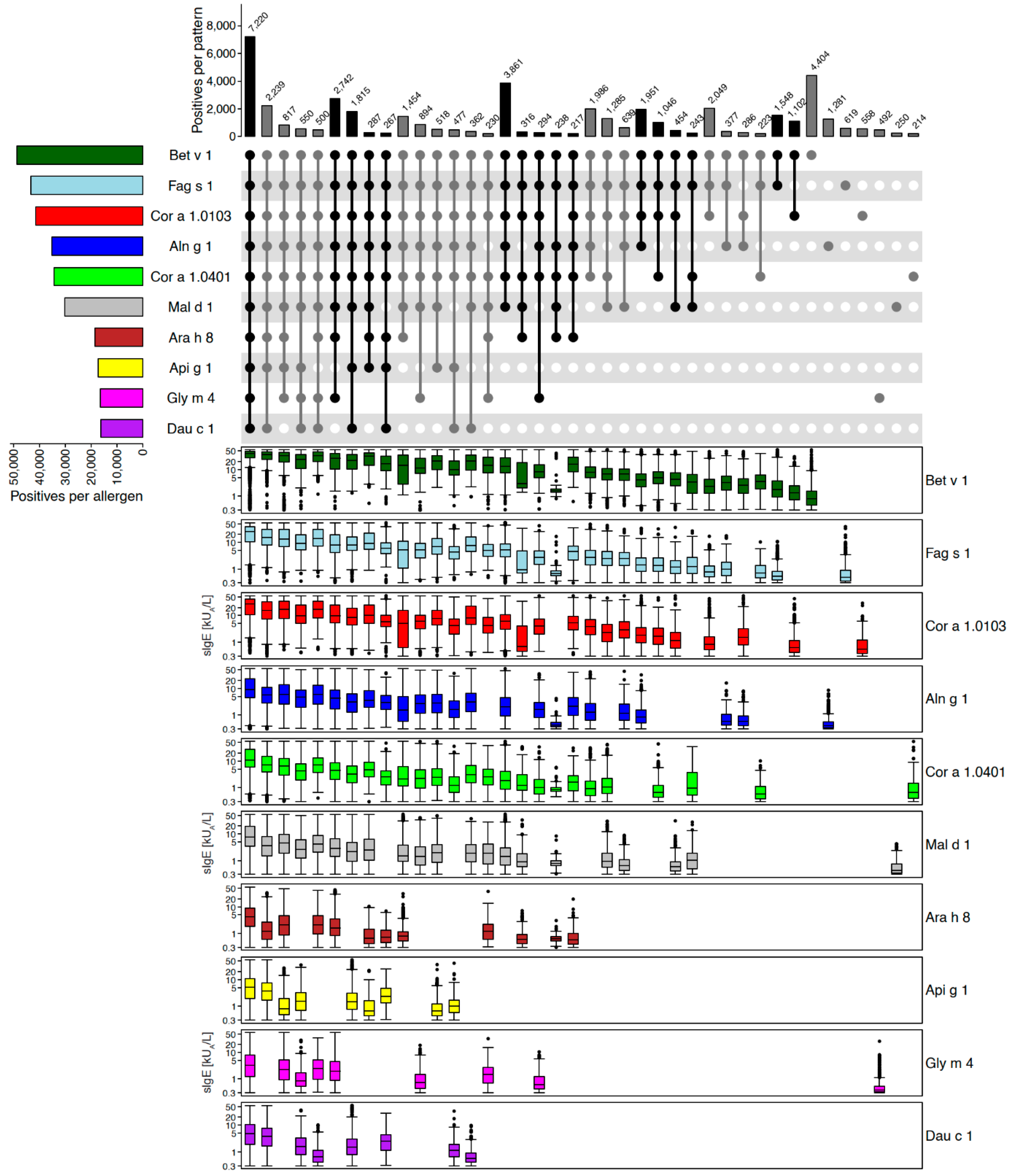
2.2.1. Example of Retaining and Adding Allergen Molecules: Storage Proteins (2S Albumins, 7S Globulins [Vicilins], 11S Globulins [Legumins])
2.2.2. Example of Removing and Adding Allergen Molecules: PR-10 Proteins
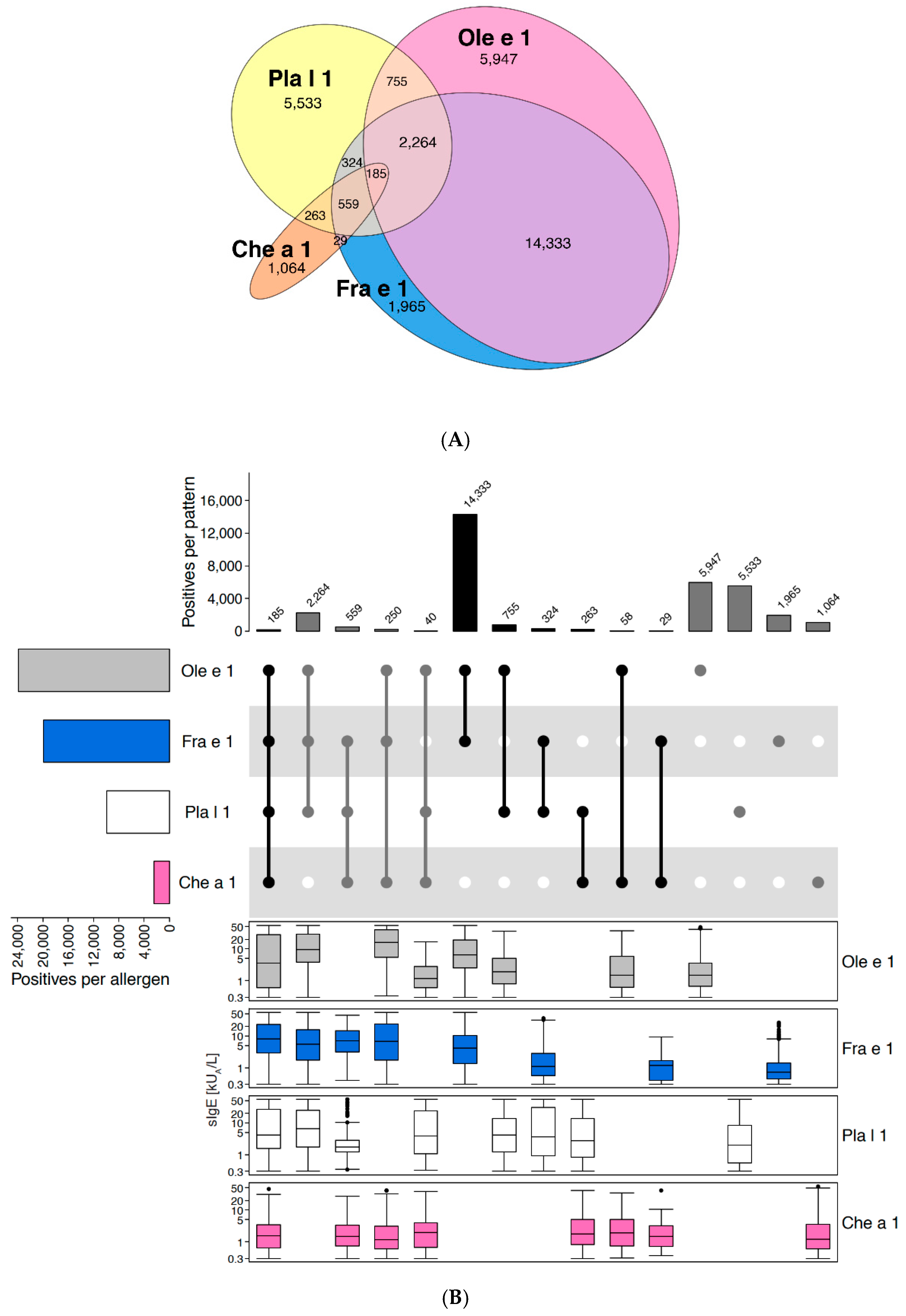
2.2.3. Example of Non-Redundant Molecules of the Same Family: Ole e 1-like Proteins
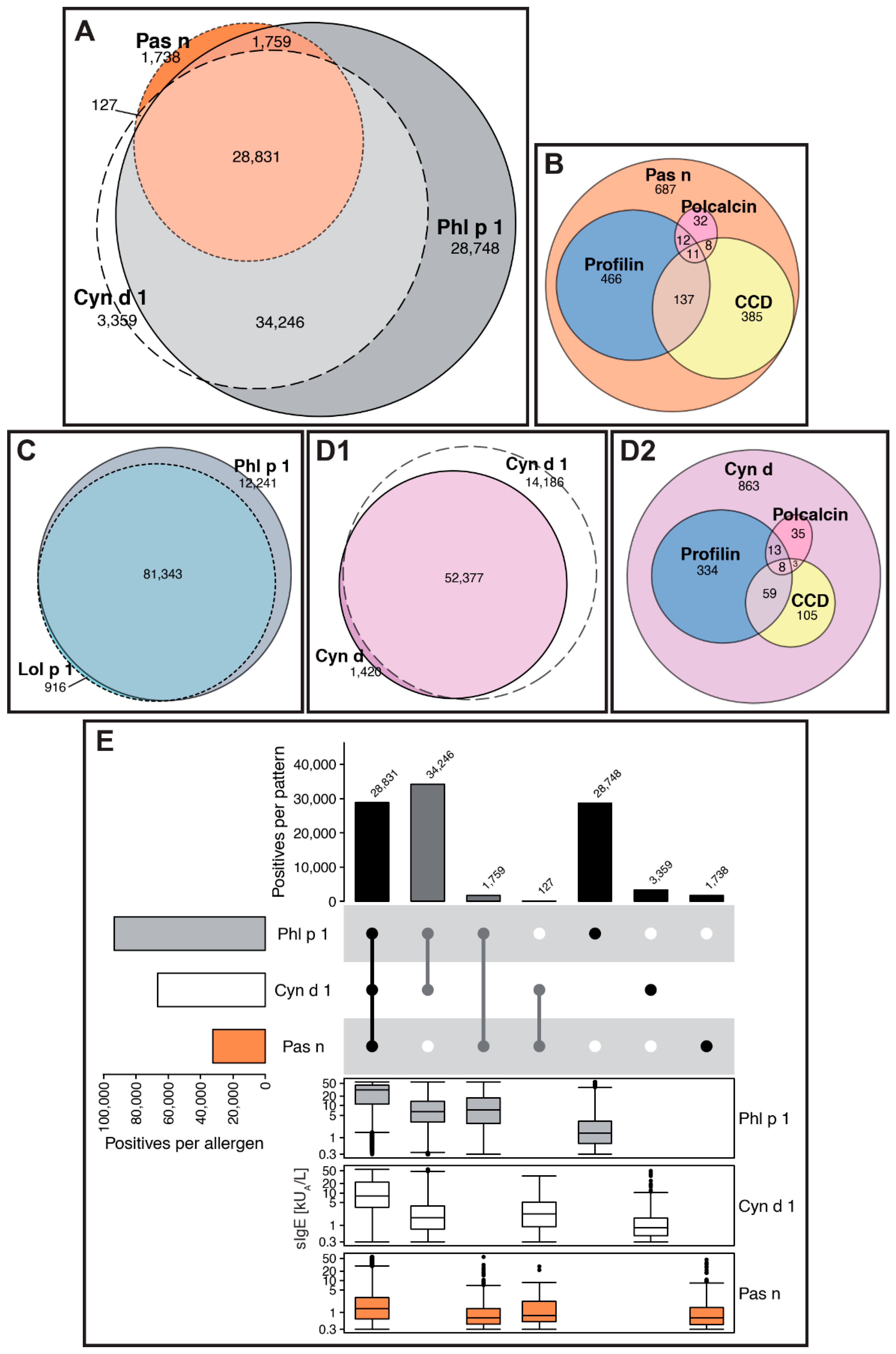
2.2.4. Example of Redundant Molecules of the Same Family: Profilins
2.3. Extracts Versus Allergen Families
Example: Grasses
2.4. Extracts and Allergens with Low Prevalence of Sensitisation
2.5. New Allergens on ALEX3
2.6. Summary of Changes from ALEX2 to ALEX3
3. Discussion
4. Materials and Methods
4.1. ALEX2 Macroarray
4.2. ALEX2 Dataset
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gomez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Curin, M.; Reininger, R.; Swoboda, I.; Focke, M.; Valenta, R.; Spitzauer, S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int. Arch. Allergy Immunol. 2011, 154, 258–263. [Google Scholar]
- Casset, A.; Mari, A.; Purohit, A.; Resch, Y.; Weghofer, M.; Ferrara, R.; Thomas, W.R.; Alessandri, C.; Chen, K.W.; de Blay, F.; et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int. Arch. Allergy Immunol. 2012, 159, 253–262. [Google Scholar] [CrossRef]
- Focke, M.; Marth, K.; Valenta, R. Molecular composition and biological activity of commercial birch pollen allergen extracts. Eur. J. Clin. Investig. 2009, 39, 429–436. [Google Scholar] [CrossRef]
- Glesner, J.; Filep, S.; Vailes, L.D.; Wunschmann, S.; Chapman, M.D.; Birrueta, G.; Frazier, A.; Jeong, K.Y.; Schal, C.; Bacharier, L.; et al. Allergen content in German cockroach extracts and sensitization profiles to a new expanded set of cockroach allergens determine in vitro extract potency for IgE reactivity. J. Allergy Clin. Immunol. 2019, 143, 1474–1481 e1478. [Google Scholar]
- Ruethers, T.; Taki, A.C.; Nugraha, R.; Cao, T.T.; Koeberl, M.; Kamath, S.D.; Williamson, N.A.; O’Callaghan, S.; Nie, S.; Mehr, S.S.; et al. Variability of allergens in commercial fish extracts for skin prick testing. Allergy 2019, 74, 1352–1363. [Google Scholar] [CrossRef]
- Thomsen, G.F.; Schlunssen, V.; Skadhauge, L.R.; Malling, T.H.; Sherson, D.L.; Omland, O.; Sigsgaard, T. Are allergen batch differences and the use of double skin prick test important? BMC Pulm. Med. 2015, 15, 33. [Google Scholar]
- Codina, R.; Lockey, R.F. Pollen used to produce allergen extracts. Ann. Allergy Asthma Immunol. 2017, 118, 148–153. [Google Scholar]
- Zimmer, J.; Bridgewater, J.; Ferreira, F.; van Ree, R.; Rabin, R.L.; Vieths, S. The History, Present and Future of Allergen Standardization in the United States and Europe. Front. Immunol. 2021, 12, 725831. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Shirazi, L.; Gasche, C.H.; Natter, S.; Gangl, A.; Smolen, J.; Spitzauer, S.; Valent, P.; Kraft, D.; Valenta, R. IgA autoreactivity: A feature common to inflammatory bowel and connective tissue diseases. Clin. Exp. Immunol. 2002, 128, 102–109. [Google Scholar] [PubMed]
- Sastre, J.; Landivar, M.E.; Ruiz-Garcia, M.; Andregnette-Rosigno, M.V.; Mahillo, I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy 2012, 67, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Peveri, S.; Pattini, S.; Costantino, M.T.; Incorvaia, C.; Montagni, M.; Roncallo, C.; Villalta, D.; Savi, E. Molecular diagnostics improves diagnosis and treatment of respiratory allergy and food allergy with economic optimization and cost saving. Allergol. Immunopathol. 2019, 47, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hiller, R.; Laffer, S.; Harwanegg, C.; Huber, M.; Schmidt, W.M.; Twardosz, A.; Barletta, B.; Becker, W.M.; Blaser, K.; Breiteneder, H.; et al. Microarrayed allergen molecules: Diagnostic gatekeepers for allergy treatment. FASEB J. 2002, 16, 414–416. [Google Scholar] [CrossRef]
- Hamilton, R.G.; Croote, D.; Lupinek, C.; Matsson, P. Evolution Toward Chip-Based Arrays in the Laboratory Diagnosis of Human Allergic Disease. J. Allergy Clin. Immunol. Pract. 2023, 11, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.; Lopes, C.; Pereira, A.M.; Araujo, L.; Moreira, A.; Delgado, L. Microarray based IgE detection in poly-sensitized allergic patients with suspected food allergy—An approach in four clinical cases. Allergol. Immunopathol. 2012, 40, 172–180. [Google Scholar] [CrossRef]
- Guillien, A.; Cadiou, S.; Slama, R.; Siroux, V. The Exposome Approach to Decipher the Role of Multiple Environmental and Lifestyle Determinants in Asthma. Int. J. Environ. Res. Public Health 2021, 18, 1138. [Google Scholar] [CrossRef] [PubMed]
- Kiewiet, M.B.G.; Lupinek, C.; Vrtala, S.; Wieser, S.; Baar, A.; Kiss, R.; Kull, I.; Melen, E.; Wickman, M.; Porta, D.; et al. A molecular sensitization map of European children reveals exposome- and climate-dependent sensitization profiles. Allergy 2023, 78, 2007–2018. [Google Scholar] [CrossRef]
- Gonzalez-Perez, R.; Galvan-Calle, C.A.; Galan, T.; Poza-Guedes, P.; Sanchez-Machin, I.; Enrique-Calderon, O.M.; Pineda, F. Molecular Signatures of Aeroallergen Sensitization in Respiratory Allergy: A Comparative Study Across Climate-Matched Populations. Int. J. Mol. Sci. 2024, 26, 284. [Google Scholar] [CrossRef]
- Chan, S.K.; Pomes, A.; Hilger, C.; Davies, J.M.; Mueller, G.; Kuehn, A.; Lopata, A.L.; Gadermaier, G.; van Hage, M.; Raulf, M.; et al. Keeping Allergen Names Clear and Defined. Front. Immunol. 2019, 10, 2600. [Google Scholar] [CrossRef]
- Ciprandi, G.; Puccinelli, P.; Incorvaia, C.; Masieri, S. Parietaria Allergy: An Intriguing Challenge for the Allergist. Medicina 2018, 54, 106. [Google Scholar] [CrossRef]
- Asturias, J.A.; Gomez-Bayon, N.; Eseverri, J.L.; Martinez, A. Par j 1 and Par j 2, the major allergens from Parietaria judaica pollen, have similar immunoglobulin E epitopes. Clin. Exp. Allergy 2003, 33, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Altmann, F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J. Int. 2016, 25, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.; Lidholm, J. Characteristics and immunobiology of grass pollen allergens. Int. Arch. Allergy Immunol. 2003, 130, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Van Ree, R.; Driessen, M.N.; Van Leeuwen, W.A.; Stapel, S.O.; Aalberse, R.C. Variability of crossreactivity of IgE antibodies to group I and V allergens in eight grass pollen species. Clin. Exp. Allergy 1992, 22, 611–617. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.; Schuyler, A.J.; Tripathi, A.; Commins, S.P. Anaphylaxis to the carbohydrate side chain alpha-gal. Immunol. Allergy Clin. N. Am. 2015, 35, 247–260. [Google Scholar] [CrossRef]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de Las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34 (Suppl. 28), e13854. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Zhang, X.; Zheng, Z.; Yao, D.; Yang, S.; Chen, T.; Zhang, Y.; Aweya, J.J. The evolutionary adaptation of shrimp hemocyanin subtypes and the consequences on their structure and functions. Fish. Shellfish. Immunol. 2024, 145, 109347. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2024. [Google Scholar]
- Klug, C.; Hemmer, W.; Roman-Carrasco, P.; Focke-Tejkl, M.; Quirce, S.; Boyano-Martinez, T.; Gaubitzer, E.; Wank, H.; Swoboda, I. Gal d 7-a major allergen in primary chicken meat allergy. J. Allergy Clin. Immunol. 2020, 146, 169–179.e165. [Google Scholar] [CrossRef]
- Feng, H.; Luo, N.; Xiong, X.; Wu, Y. Prevalence of food allergy in the Chinese population: A systematic review and meta-analysis of population-based studies. Allergy Asthma Proc. 2023, 44, 315–325. [Google Scholar] [CrossRef]
- Wu, T.C.; Tsai, T.C.; Huang, C.F.; Chang, F.Y.; Lin, C.C.; Huang, I.F.; Chu, C.H.; Lau, B.H.; Wu, L.; Peng, H.J.; et al. Prevalence of food allergy in Taiwan: A questionnaire-based survey. Intern. Med. J. 2012, 42, 1310–1315. [Google Scholar] [CrossRef]
- Dey, D.; Ghosh, N.; Pandey, N.; Gupta Bhattacharya, S. A hospital-based survey on food allergy in the population of Kolkata, India. Int. Arch. Allergy Immunol. 2014, 164, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Julanon, N.; Thiravetyan, B.; Unhapipatpong, C.; Xanthavanij, N.; Krikeerati, T.; Thongngarm, T.; Wongsa, C.; Songnuan, W.; Naiyanetr, P.; Sompornrattanaphan, M. Not Just a Banana: The Extent of Fruit Cross-Reactivity and Reaction Severity in Adults with Banana Allergy. Foods 2023, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Cardona, E.E.G.; Heathcote, K.; Teran, L.M.; Righetti, P.G.; Boschetti, E.; D’Amato, A. Novel low-abundance allergens from mango via combinatorial peptide libraries treatment: A proteomics study. Food Chem. 2018, 269, 652–660. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Baar, A.; Pahr, S.; Constantin, C.; Giavi, S.; Manoussaki, A.; Papadopoulos, N.G.; Ebner, C.; Mari, A.; Vrtala, S.; Valenta, R. Specific IgE reactivity to Tri a 36 in children with wheat food allergy. J. Allergy Clin. Immunol. 2014, 133, 585–587. [Google Scholar] [CrossRef]
- Cianferoni, A. Wheat allergy: Diagnosis and management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef]
- Pahr, S.; Constantin, C.; Papadopoulos, N.G.; Giavi, S.; Makela, M.; Pelkonen, A.; Ebner, C.; Mari, A.; Scheiblhofer, S.; Thalhamer, J.; et al. Alpha-Purothionin, a new wheat allergen associated with severe allergy. J. Allergy Clin. Immunol. 2013, 132, 1000–1003.e1-4. [Google Scholar] [CrossRef]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef]
- Potapova, E.; Sénéchal, H.; Scala, E.; Matricardi, P.M.; Poncet, P. Cyclophilins and gibberellin-regulated proteins in IgE-mediated allergic diseases. Allergo J. Int. 2023, 32, 280–288. [Google Scholar] [CrossRef]
- Limacher, A.; Glaser, A.G.; Meier, C.; Schmid-Grendelmeier, P.; Zeller, S.; Scapozza, L.; Crameri, R. Cross-reactivity and 1.4-A crystal structure of Malassezia sympodialis thioredoxin (Mala s 13), a member of a new pan-allergen family. J. Immunol. 2007, 178, 389–396. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, M.; Jeong, K.Y.; Sim, D.W.; Park, J.H.; Park, K.H.; Lee, J.H.; Park, J.W. Characterization of a Major Allergen from Mongolian Oak, Quercus mongolica, a Dominant Species of Oak in Korea. Int. Arch. Allergy Immunol. 2017, 174, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Bazon, M.L.; Grosch, J.; Schmidt-Weber, C.B.; Brochetto-Braga, M.R.; Bilò, M.B.; Jakob, T. Antigen 5 Allergens of Hymenoptera Venoms and Their Role in Diagnosis and Therapy of Venom Allergy. Curr. Allergy Asthma Rep. 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Kabasser, S.; Pratap, K.; Kamath, S.; Taki, A.C.; Dang, T.; Koplin, J.; Perrett, K.; Hummel, K.; Radauer, C.; Breiteneder, H.; et al. Identification of vicilin, legumin and antimicrobial peptide 2a as macadamia nut allergens. Food Chem. 2022, 370, 131028. [Google Scholar] [CrossRef] [PubMed]
- Forchert, L.; Potapova, E.; Panetta, V.; Dramburg, S.; Perna, S.; Posa, D.; Resch-Marat, Y.; Lupinek, C.; Rohrbach, A.; Grabenhenrich, L.; et al. Der p 23-specific IgE response throughout childhood and its association with allergic disease: A birth cohort study. Pediatr. Allergy Immunol. 2022, 33, e13829. [Google Scholar] [CrossRef]
- Matricardi, P.M.; van Hage, M.; Custovic, A.; Korosec, P.; Santos, A.F.; Valenta, R. Molecular allergy diagnosis enabling personalized medicine. J. Allergy Clin. Immunol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Barber, D.; Diaz-Perales, A.; Escribese, M.M.; Kleine-Tebbe, J.; Matricardi, P.M.; Ollert, M.; Santos, A.F.; Sastre, J. Molecular allergology and its impact in specific allergy diagnosis and therapy. Allergy 2021, 76, 3642–3658. [Google Scholar] [CrossRef]
- Huss-Marp, J.; Raulf, M.; Jakob, T. Spiking with recombinant allergens to improve allergen extracts: Benefits and limitations for the use in routine diagnostics: Part 19 of the Series Molecular Allergology. Allergo J. Int. 2015, 24, 236–243. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Lake, I.R.; Jones, N.R.; Agnew, M.; Goodess, C.M.; Giorgi, F.; Hamaoui-Laguel, L.; Semenov, M.A.; Solomon, F.; Storkey, J.; Vautard, R. Climate change and future pollen allergy in Europe. Environ. Health Perspect. 2017, 125, 385–391. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Munoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Besancenot, J.P.; Thibaudon, M. [Climate change and pollination]. Rev. Mal. Respir. 2012, 29, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Beggs, P.J. Anthropogenic climate change and allergen exposure: The role of plant biology. J. Allergy Clin. Immunol. 2012, 129, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.; Jochner, S.; Gilles, S.; McIntyre, M.; Buters, J.T.; Schmidt-Weber, C.; Behrendt, H.; Ring, J.; Menzel, A.; Traidl-Hoffmann, C. High environmental ozone levels lead to enhanced allergenicity of birch pollen. PLoS ONE 2013, 8, e80147. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Oh, J.W.; Woo, S.Y.; Seo, Y.A.; Choi, Y.J.; Kim, H.S.; Lee, W.Y.; Kim, B.J. Does the increase in ambient CO(2) concentration elevate allergy risks posed by oak pollen? Int. J. Biometeorol. 2018, 62, 1587–1594. [Google Scholar] [CrossRef]
- D’Amato, G.; Vitale, C.; Lanza, M.; Molino, A.; D’Amato, M. Climate change, air pollution, and allergic respiratory diseases: An update. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 434–440. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Heffler, E.; Puggioni, F.; Peveri, S.; Montagni, M.; Canonica, G.W.; Melioli, G. Extended IgE profile based on an allergen macroarray: A novel tool for precision medicine in allergy diagnosis. World Allergy Organ. J. 2018, 11, 7. [Google Scholar] [CrossRef]
- Campana, R.; Ringauf, A.; Wieser, S.; Mittermann, I.; Sinner, J.; Konstantoulas, C.; Forstenlechner, P. ALEX Allergy Xplorer in allergy diagnosis: A systematic review of diagnostic test accuracy. 2025, manuscript in preparation. 2025; manuscript in preparation. [Google Scholar]
- Allaire, J.J. RStudio: Integrated Development Environment for R.; RStudio: Boston, MA, USA, 2012. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupinek, C.; Forstenlechner, P.; Ringauf, A.; Campana, R.; Salihu, A.; Aumayr, M.; Mittermann, I. Big Data-Driven Evolution of a Diagnostic Multiplex IgE-Test: Enhancing Accuracy and Efficacy in Allergy Diagnostics. Int. J. Mol. Sci. 2025, 26, 4249. https://doi.org/10.3390/ijms26094249
Lupinek C, Forstenlechner P, Ringauf A, Campana R, Salihu A, Aumayr M, Mittermann I. Big Data-Driven Evolution of a Diagnostic Multiplex IgE-Test: Enhancing Accuracy and Efficacy in Allergy Diagnostics. International Journal of Molecular Sciences. 2025; 26(9):4249. https://doi.org/10.3390/ijms26094249
Chicago/Turabian StyleLupinek, Christian, Peter Forstenlechner, Anna Ringauf, Raffaela Campana, Artan Salihu, Martina Aumayr, and Irene Mittermann. 2025. "Big Data-Driven Evolution of a Diagnostic Multiplex IgE-Test: Enhancing Accuracy and Efficacy in Allergy Diagnostics" International Journal of Molecular Sciences 26, no. 9: 4249. https://doi.org/10.3390/ijms26094249
APA StyleLupinek, C., Forstenlechner, P., Ringauf, A., Campana, R., Salihu, A., Aumayr, M., & Mittermann, I. (2025). Big Data-Driven Evolution of a Diagnostic Multiplex IgE-Test: Enhancing Accuracy and Efficacy in Allergy Diagnostics. International Journal of Molecular Sciences, 26(9), 4249. https://doi.org/10.3390/ijms26094249




