The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture
Abstract
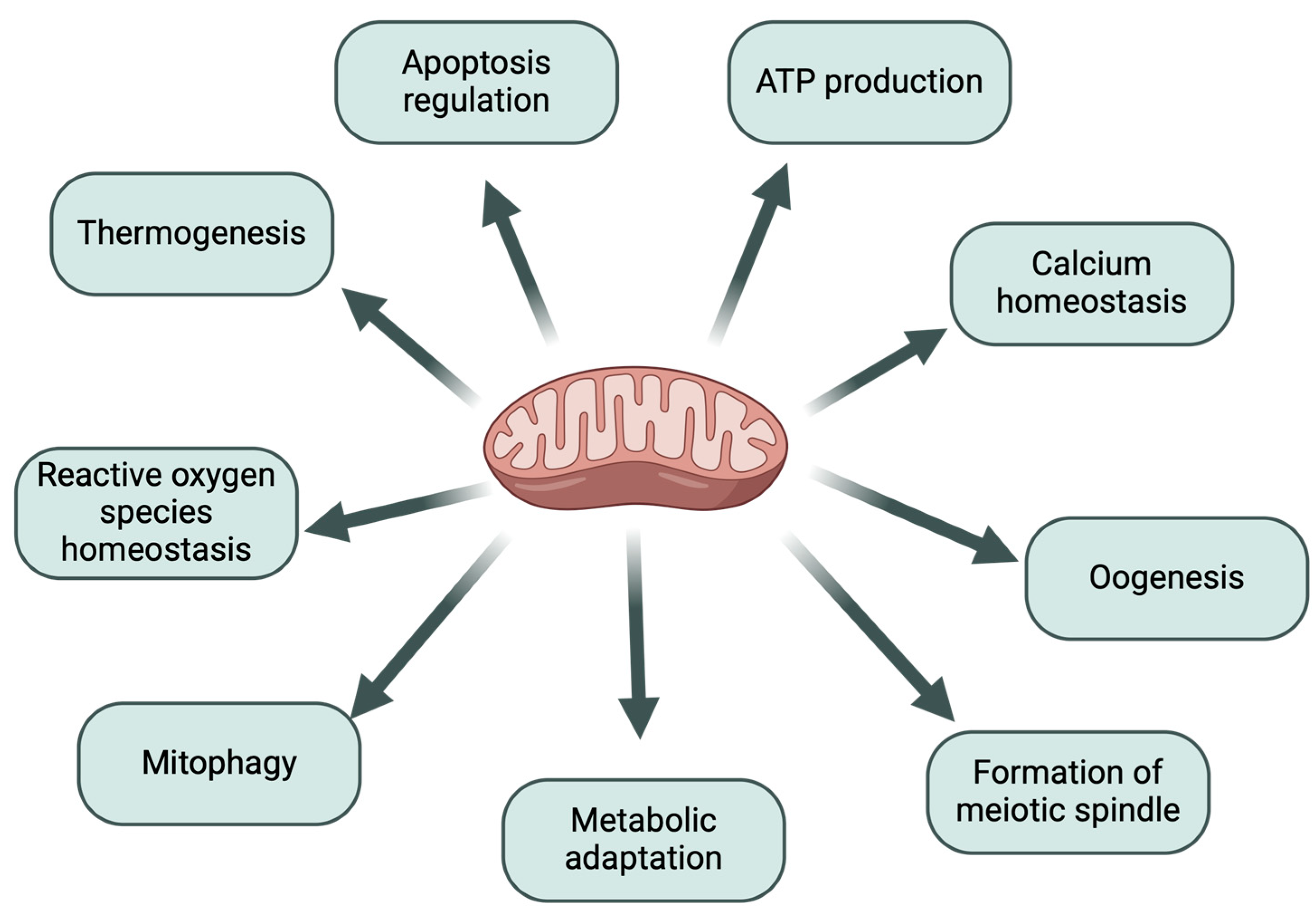
:1. Introduction
1.1. The Importance of Mitochondria in the Process of Oogenesis
1.2. The Relationship Between Mitochondrial Function and Oocyte Quality
1.3. The Relationship Between Mitochondrial Function and Embryo Quality
2. Metabolic Processes in Mitochondria
2.1. Oxygen Metabolism
2.2. Lipid Metabolism
2.3. Ca2+ Metabolism
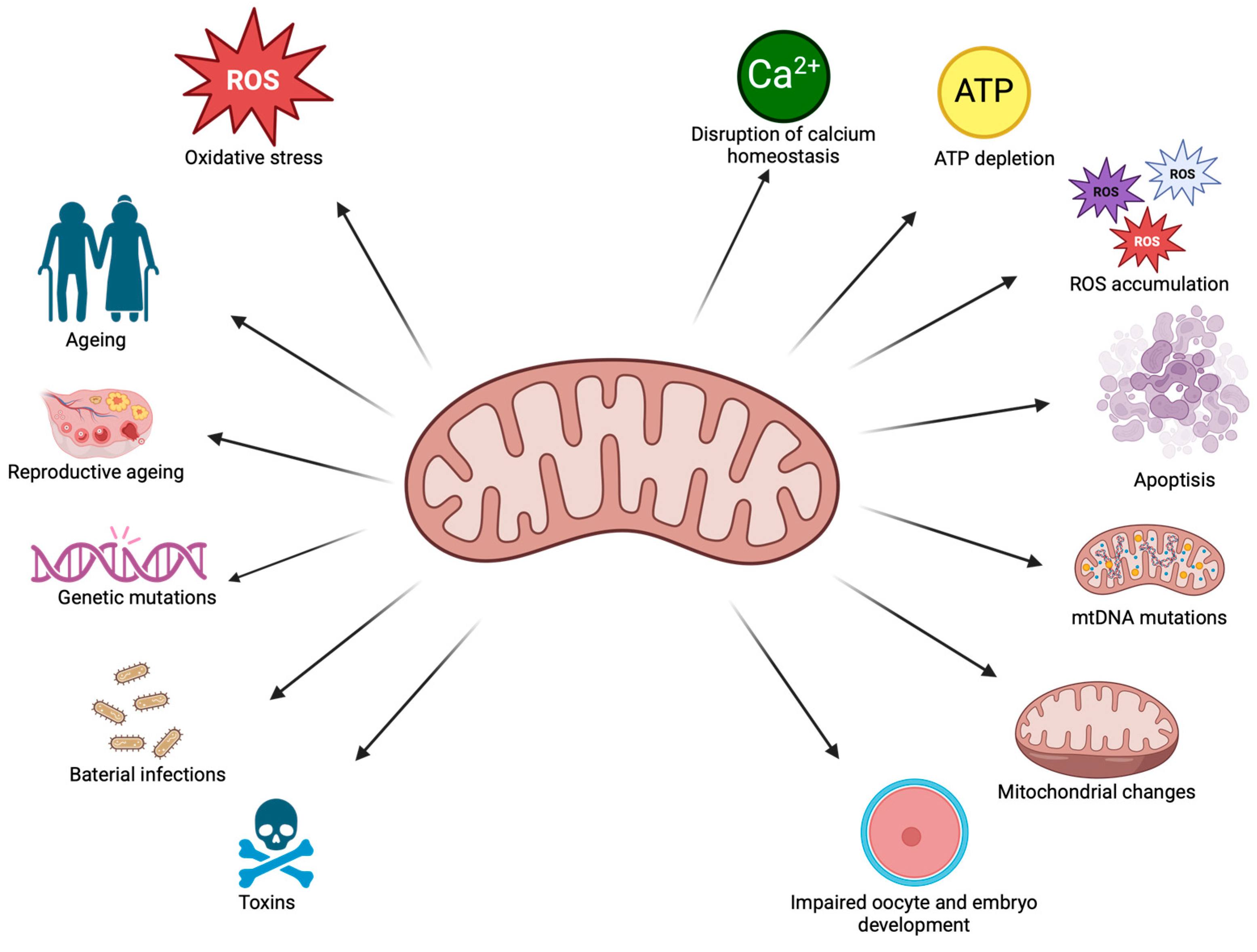
3. Mitochondrial Changes
3.1. Ageing Processes in Mitochondria
3.2. Thermal Stress
3.3. Exposure to Toxic Substances
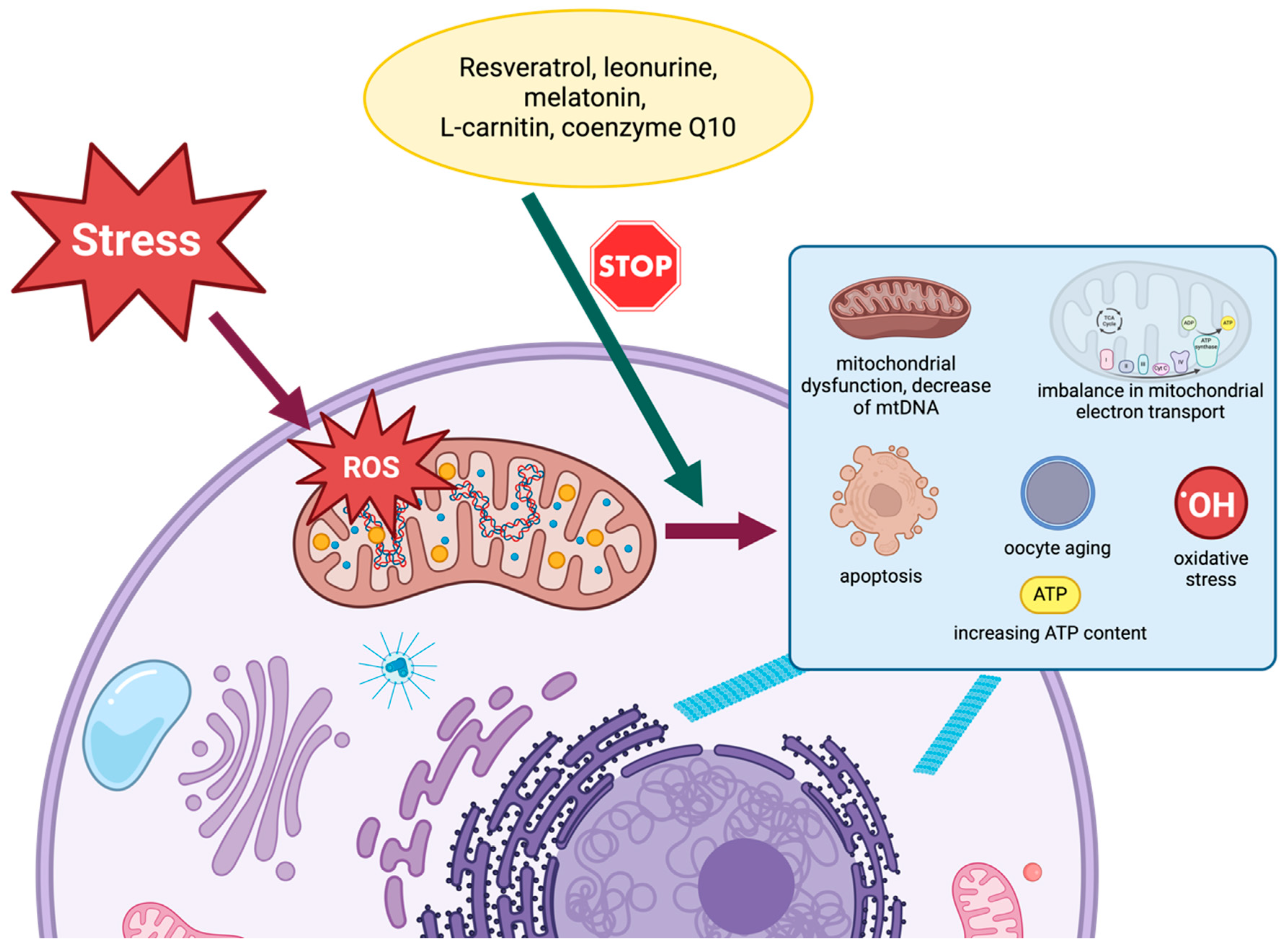
4. Methods of Regenerating Mitochondria
4.1. Mitochondrial Replacement Therapy (MRT)
4.2. Resveratrol
4.3. Leonurine
4.4. Melatonin
4.5. L-Carnitine
4.6. Coenzyme Q10
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-APB | 2-aminoethoxydiphenyl borate |
| Acetyl-CoA | Acetyl Coenzyme A |
| ALC | acetyl-L-carnitine |
| AMPK | AMP-activated protein kinase |
| ART | assisted reproductive technologies |
| ATP | adenosine triphosphate |
| C | cytochrome C |
| CABL1 | critical calcium-binding protein |
| Calb1 | calbindin 1 gene |
| CL | cardiolipin |
| CoCl2 | cobalt chloride |
| COCs | cumulus-oocyte complexes |
| CoQ | Coenzyme Q10 |
| CoQ10 | coenzyme Q10 |
| COX | cytochrome c oxidase |
| CTX | cyclophosphamide |
| DCA | dichloroacetate |
| DOR | diminished ovarian reserve |
| ER | endoplasmic reticulum |
| ETC | electron transport chain |
| FA | fatty acid |
| FASN | fatty acid synthase |
| FoxO | forkhead box |
| G6PDH | glucose-6-phosphate dehydrogenase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GLUT | glucose transporter |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GV | germinal vesicle |
| H2O2 | hydrogen peroxide |
| HIF-2α | hypoxia-inducible factor 2 alpha |
| HIF1α | hypoxia-inducible factor 1α |
| HS | heat stress |
| HSP | heat shock protein |
| IA | iodoacetate |
| IMM | inner mitochondrial membrane |
| IMS | mitochondrial intermembrane space |
| IP3R | inositol trisphosphate receptor |
| IVF | in vitro fertilisation |
| IVM | in vitro maturation |
| LC | L- carnitine |
| MCT | monocarboxylate transporter |
| MCU | mitochondrial calcium uniporter |
| Mfn 1 | Mitofusin 1 |
| Mfn 2 | Mitofusin 2 |
| MPC | mitochondrial pyruvate carrier |
| MRT | mitochondrial replacement therapy |
| mtDNA | mitochondrial DNA |
| mTOR | mechanistic target of rapamycin |
| NAD+ | nicotinamide adenine dinucleotide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NAM | nicotinamide |
| NBS1 | nibrin |
| NDs | neurodegenerative diseases |
| NF-κB | nuclear factor-κB |
| OAA | oxaloacetate |
| OMM | outer mitochondrial membrane |
| OXPHOS | oxidative phosphorylation |
| PAHs | polycyclic aromatic hydrocarbons |
| PBE | polar body extrusion |
| PDH | Pyruvate dehydrogenase |
| PDK | pyruvate dehydrogenase kinase |
| PGC-1α | proliferator-activated receptor-gamma coactivator |
| PKCα | protein kinase C alpha |
| POA | premature ovarian ageing |
| POI | premature ovarian insufficiency |
| PPP | pentose phosphate pathway |
| Q10 | coenzyme Q10 |
| ROS | reactive oxygen species |
| RSV | resveratrol |
| RyR | ryanodine receptor |
| SCM-198, LEO | leonurine |
| SIRT | sirtuin, silent information regulator |
| SOD | superoxide dismutase |
| SOD1 | superoxide dismutase 3 |
| SOD2 | superoxide dismutase 2 |
| TAG | triacylglyceride |
| TCA | tricarboxylic acid cycle |
| TG | thapsigargin |
| TZP | transzonal projections |
| VDAC | voltage-dependent anion channel |
References
- Moretti-Horten, D.N.; Peselj, C.; Taskin, A.A.; Myketin, L.; Schulte, U.; Einsle, O.; Drepper, F.; Luzarowski, M.; Vögtle, F.-N. Synchronized assembly of the oxidative phosphorylation system controls mitochondrial respiration in yeast. Dev. Cell 2024, 59, 1043–1057.e8. [Google Scholar] [CrossRef] [PubMed]
- Masuda, D.; Nakanishi, I.; Ohkubo, K.; Ito, H.; Matsumoto, K.; Ichikawa, H.; Chatatikun, M.; Klangbud, W.K.; Kotepui, M.; Imai, M.; et al. Mitochondria Play Essential Roles in Intracellular Protection against Oxidative Stress—Which Molecules among the ROS Generated in the Mitochondria Can Escape the Mitochondria and Contribute to Signal Activation in Cytosol? Biomolecules 2024, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Schiaffarino, O.; Valdivieso González, D.; García-Pérez, I.M.; Peñalva, D.A.; Almendro-Vedia, V.G.; Natale, P.; López-Montero, I. Mitochondrial membrane models built from native lipid extracts: Interfacial and transport properties. Front. Mol. Biosci. 2022, 9, 910936. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Ye, F.; Tang, J.; Liu, B.; Lin, J.; Zhou, P.-H.; Lu, B.; Wu, M.; Lu, J.-H.; et al. Mic19 depletion impairs endoplasmic reticulum-mitochondrial contacts and mitochondrial lipid metabolism and triggers liver disease. Nat. Commun. 2024, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.T.; Funai, K. Mitochondrial membrane lipids in the regulation of bioenergetic flux. Cell Metab. 2024, 36, 1963–1978. [Google Scholar] [CrossRef]
- Koma, R.; Shibaguchi, T.; Pérez López, C.; Oka, T.; Jue, T.; Takakura, H.; Masuda, K. Localization of myoglobin in mitochondria: Implication in regulation of mitochondrial respiration in rat skeletal muscle. Physiol. Rep. 2021, 9, e14769. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; He, Y.; Feng, C.; Yuan, J.; Guo, Y.; Guo, Z.; Wang, X. Cellular Discrepancy of Platinum Complexes in Interfering with Mitochondrial DNA. ACS Cent. Sci. 2025, 11, 393–403. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Seli, E. The role of mitochondrial dynamics in oocyte and early embryo development. Semin. Cell Dev. Biol. 2024, 159–160, 52–61. [Google Scholar] [CrossRef]
- Li, L.-F.; Yu, J.; Li, R.; Li, S.-S.; Huang, J.-Y.; Wang, M.-D.; Jiang, L.-N.; Xu, J.-H.; Wang, Z. Apoptosis, Mitochondrial Autophagy, Fission, and Fusion Maintain Mitochondrial Homeostasis in Mouse Liver Under Tail Suspension Conditions. Int. J. Mol. Sci. 2024, 25, 11196. [Google Scholar] [CrossRef]
- Dong, F.; Zhu, M.; Zheng, F.; Fu, C. Mitochondrial fusion and fission are required for proper mitochondrial function and cell proliferation in fission yeast. FEBS J. 2022, 289, 262–278. [Google Scholar] [CrossRef]
- Dubois, M.; Boulghobra, D.; Rochebloine, G.; Pallot, F.; Yehya, M.; Bornard, I.; Gayrard, S.; Coste, F.; Walther, G.; Meyer, G.; et al. Hyperglycemia triggers RyR2-dependent alterations of mitochondrial calcium homeostasis in response to cardiac ischemia-reperfusion: Key role of DRP1 activation. Redox Biol. 2024, 70, 103044. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Altmeppen, G.; So, C.; Welp, L.M.; Penir, S.; Ruhwedel, T.; Menelaou, K.; Harasimov, K.; Stützer, A.; Blayney, M.; et al. Mammalian oocytes store mRNAs in a mitochondria-associated membraneless compartment. Science 2022, 378, eabq4835. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-H.; Chou, H.-H.; Kuan, C.-S.; Liu, S.-C.; Kao, C.-W.; Wu, Y.-H.; Lai, H.-H.; Hsieh, C.-L.; Liang, Y.-T.; Chen, C.-Y.; et al. Dependency of mitochondrial quantity on blastocyst timeline obscures its actual effect to pregnancy outcomes. Front. Endocrinol. 2024, 15, 1415865. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhao, J.; Rodriguez-Wallberg, K.A. Comprehensive atlas of mitochondrial distribution and dynamics during oocyte maturation in mouse models. Biomark. Res. 2024, 12, 125. [Google Scholar] [CrossRef]
- Contreras-Solís, I.; Catalá, M.; Soto-Heras, S.; Roura, M.; Paramio, M.T.; Izquierdo, D. Effect of follicle size on hormonal status of follicular fluid, oocyte ATP content, and in vitro embryo production in prepubertal sheep. Domest. Anim. Endocrinol. 2021, 75, 106582. [Google Scholar] [CrossRef]
- Sobek, A.; Tkadlec, E.; Klaskova, E.; Prochazka, M. Cytoplasmic Transfer Improves Human Egg Fertilization and Embryo Quality: An Evaluation of Sibling Oocytes in Women with Low Oocyte Quality. Reprod. Sci. 2021, 28, 1362–1369. [Google Scholar] [CrossRef]
- Ge, H.; Tollner, T.L.; Hu, Z.; Dai, M.; Li, X.; Guan, H.; Shan, D.; Zhang, X.; Lv, J.; Huang, C.; et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol. Reprod. Dev. 2012, 79, 392–401. [Google Scholar] [CrossRef]
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of Maternal Age on Oocyte and Embryo Competence. Front. Endocrinol. 2018, 9, 327. [Google Scholar] [CrossRef]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef]
- Dumollard, R.; Duchen, M.; Carroll, J. The Role of Mitochondrial Function in the Oocyte and Embryo. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 77, pp. 21–49. ISBN 978-0-12-373662-8. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Seli, E. Mitochondria as therapeutic targets in assisted reproduction. Human Reprod. 2024, 39, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhu, L.; Zhu, Y.; Huang, M.; Lin, Y.; Li, H.; Hu, P.; Zhang, J.; Shen, B.; Xu, Z. A PCR-independent approach for mtDNA enrichment and next-generation sequencing: Comprehensive evaluation and clinical application. J. Transl. Med. 2024, 22, 386. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Fazzini, F.; Lamina, C.; Rantner, B.; Kollerits, B.; Stadler, M.; Klein-Weigel, P.; Fraedrich, G.; Kronenberg, F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. J. Intern. Med. 2020, 287, 569–579. [Google Scholar] [CrossRef]
- Colnaghi, M.; Pomiankowski, A.; Lane, N. The need for high-quality oocyte mitochondria at extreme ploidy dictates mammalian germline development. eLife 2021, 10, e69344. [Google Scholar] [CrossRef]
- Khan, S.A.; Reed, L.; Schoolcraft, W.B.; Yuan, Y.; Krisher, R.L. Control of mitochondrial integrity influences oocyte quality during reproductive aging. Mol. Human Reprod. 2023, 29, gaad028. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Xu, J.; Cao, X.; Zhang, D.; Liu, M.; Liu, S.; Dong, X.; Shi, H. Mitochondrial dysfunction in cumulus cells is related to decreased reproductive capacity in advanced-age women. Fertil. Steril. 2022, 118, 393–404. [Google Scholar] [CrossRef]
- Lu, L.; Wang, T.; Liu, A.; Ye, H. A Single-Cell Atlas of Crab Ovary Provides New Insights into Oogenesis in Crustaceans. Adv. Sci. 2025, 12, 2409688. [Google Scholar] [CrossRef] [PubMed]
- Chiaratti, M.R. Uncovering the important role of mitochondrial dynamics in oogenesis: Impact on fertility and metabolic disorder transmission. Biophys. Rev. 2021, 13, 967–981. [Google Scholar] [CrossRef]
- Shan, L.-Y.; Tian, Y.; Liu, W.-X.; Fan, H.-T.; Li, F.-G.; Liu, W.-J.; Li, A.; Shen, W.; Sun, Q.-Y.; Liu, Y.-B.; et al. LSM14B controls oocyte mRNA storage and stability to ensure female fertility. Cell. Mol. Life Sci. 2023, 80, 247. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Yang, C.; Su, R.; Long, M.; Liu, J.; Shi, L.; Xue, Y.; Su, Y. LSM14B is an Oocyte-Specific RNA-Binding Protein Indispensable for Maternal mRNA Metabolism and Oocyte Development in Mice. Adv. Sci. 2023, 10, 2300043. [Google Scholar] [CrossRef]
- Guo, X.; Jiao, L.; Yi, Y.; Zhang, H.; Liu, Y.; Wang, Z.; Sun, S. NAMPT regulates mitochondria function and lipid metabolism during porcine oocyte maturation. J. Cell. Physiol. 2024, 239, e31156. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-W.; Adhikari, D.; Carroll, J. Miro1 depletion disrupts spatial distribution of mitochondria and leads to oocyte maturation defects. Front. Cell Dev. Biol. 2022, 10, 986454. [Google Scholar] [CrossRef]
- Deng, K.; Du, D.; Fan, D.; Pei, Z.; Zhang, S.; Xu, C. Growth Hormone Promotes Oocyte Maturation In Vitro by Protecting Mitochondrial Function and Reducing Apoptosis. Reprod. Sci. 2023, 30, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Satouh, Y.; Sato, K. Reorganization, specialization, and degradation of oocyte maternal components for early development. Reprod. Med. Biol. 2023, 22, e12505. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Mohey-Elsaeed, O.; Pintelon, I.; Leroy, J.L.M.R. Risks of using mitoquinone during in vitro maturation and its potential protective effects against lipotoxicity-induced oocyte mitochondrial stress. J. Assist. Reprod. Genet. 2024, 41, 371–383. [Google Scholar] [CrossRef]
- Ju, W.; Zhao, Y.; Yu, Y.; Zhao, S.; Xiang, S.; Lian, F. Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions. Front. Endocrinol. 2024, 15, 1361289. [Google Scholar] [CrossRef]
- Li, X.-Q.; Wang, Y.; Yang, S.-J.; Liu, Y.; Ma, X.; Liu, L.; Li, S.-H.; Niu, D.; Duan, X. Melatonin protects against maternal diabetes-associated meiotic defects by maintaining mitochondrial function. Free Radic. Biol. Med. 2022, 188, 386–394. [Google Scholar] [CrossRef]
- Wang, Q.; Ratchford, A.M.; Chi, M.M.-Y.; Schoeller, E.; Frolova, A.; Schedl, T.; Moley, K.H. Maternal Diabetes Causes Mitochondrial Dysfunction and Meiotic Defects in Murine Oocytes. Mol. Endocrinol. 2009, 23, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhu, S.; Zhang, H.; Li, C.; Qiu, D.; Ge, J.; Guo, X.; Wang, Q. Mitofusin1 in oocyte is essential for female fertility. Redox Biol. 2019, 21, 101110. [Google Scholar] [CrossRef]
- Li, X.; Straub, J.; Medeiros, T.C.; Mehra, C.; Den Brave, F.; Peker, E.; Atanassov, I.; Stillger, K.; Michaelis, J.B.; Burbridge, E.; et al. Mitochondria shed their outer membrane in response to infection-induced stress. Science 2022, 375, eabi4343. [Google Scholar] [CrossRef]
- Inagaki, S.; Suzuki, Y.; Kawasaki, K.; Kondo, R.; Imaizumi, Y.; Yamamura, H. Mitofusin 1 and 2 differentially regulate mitochondrial function underlying Ca2+ signaling and proliferation in rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2023, 645, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Cai, Y.; Dai, Y.; Li, F.; Mo, S.; Werz, O.; Chen, X. Mitochondrial Fusion Mediated by Mitofusin 1 Regulates Macrophage Mycobactericidal Activity by Enhancing Autophagy. Infect. Immun. 2021, 89, e00306-21. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martínez-Zamora, M.A.; Böke, E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 2022, 607, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Onukwufor, J.O.; Farooqi, M.A.; Vodičková, A.; Koren, S.A.; Baldzizhar, A.; Berry, B.J.; Beutner, G.; Porter, G.A.; Belousov, V.; Grossfield, A.; et al. A reversible mitochondrial complex I thiol switch mediates hypoxic avoidance behavior in C. elegans. Nat. Commun. 2022, 13, 2403. [Google Scholar] [CrossRef]
- Elías-López, A.L.; Vázquez-Mena, O.; Sferruzzi-Perri, A.N. Mitochondrial dysfunction in the offspring of obese mothers and it’s transmission through damaged oocyte mitochondria: Integration of mechanisms. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166802. [Google Scholar] [CrossRef]
- Wang, F.; Meng, T.-G.; Li, J.; Hou, Y.; Luo, S.-M.; Schatten, H.; Sun, Q.-Y.; Ou, X.-H. Mitochondrial Ca2+ Is Related to Mitochondrial Activity and Dynamic Events in Mouse Oocytes. Front. Cell Dev. Biol. 2020, 8, 585932. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, X.; Luo, C.; Mao, X.; Qin, J.; Chi, Y.; He, B.; Hao, Y.; Niu, X.; Huang, B.; et al. Effects of intracellular Ca2+ on developmental potential and ultrastructure of cryopreserved-warmed oocyte in mouse. Cryobiology 2024, 114, 104834. [Google Scholar] [CrossRef] [PubMed]
- Weiser, A.; Hermant, A.; Bermont, F.; Sizzano, F.; Karaz, S.; Alvarez-Illera, P.; Santo-Domingo, J.; Sorrentino, V.; Feige, J.N.; De Marchi, U. The mitochondrial calcium uniporter (MCU) activates mitochondrial respiration and enhances mobility by regulating mitochondrial redox state. Redox Biol. 2023, 64, 102759. [Google Scholar] [CrossRef]
- Morimoto, Y.; Gamage, U.S.K.; Yamochi, T.; Saeki, N.; Morimoto, N.; Yamanaka, M.; Koike, A.; Miyamoto, Y.; Tanaka, K.; Fukuda, A.; et al. Mitochondrial Transfer into Human Oocytes Improved Embryo Quality and Clinical Outcomes in Recurrent Pregnancy Failure Cases. Int. J. Mol. Sci. 2023, 24, 2738. [Google Scholar] [CrossRef]
- Winstanley, Y.E.; Liu, J.; Adhikari, D.; Gonzalez, M.B.; Russell, D.L.; Carroll, J.; Robker, R.L. Dynamics of Mitochondrial DNA Copy Number and Membrane Potential in Mouse Pre-Implantation Embryos: Responses to Diverse Types of Oxidative Stress. Genes 2024, 15, 367. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, K.; Sui, L.; Li, P.; Du, Y.; Hu, J.; Li, M.; Yang, X.; Liang, X. Low-level pyruvate inhibits early embryonic development and maternal mRNA clearance in mice. Theriogenology 2021, 166, 104–111. [Google Scholar] [CrossRef]
- Li, P.; Zhang, H.; Yan, K.; Sui, L.; Du, Y.; Hu, J.; Xu, H.; Yang, X.; Liang, X. Insufficient pyruvate in culture medium arrests mouse embryos at the first cleavage stage associated with abnormal epigenetic modifications. Theriogenology 2022, 181, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, Y.; Han, R.; Kuang, T.; Min, C.; Wang, H.; Zhao, Y.; Wang, J.; Yang, L.; Che, D. Effects of pyruvate on early embryonic development and zygotic genome activation in pigs. Theriogenology 2022, 189, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Tong, H.; Wang, X.; Lv, X.; He, L.; Yang, X.; Wang, Y.; Xu, K.; Liang, Q.; Feng, Q.; et al. Embryonic diapause due to high glucose is related to changes in glycolysis and oxidative phosphorylation, as well as abnormalities in the TCA cycle and amino acid metabolism. Front. Endocrinol. 2023, 14, 1135837. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-Y.; Zhu, H.-Y.; Shi, R.-J.; Wu, Y.-F.; Fan, Y.; Jin, L. Regulation of glucose metabolism: Effects on oocyte, preimplantation embryo, assisted reproductive technology and embryonic stem cell. Heliyon 2024, 10, e38551. [Google Scholar] [CrossRef]
- Da Fonseca Junior, A.M.; Ispada, J.; Dos Santos, E.C.; De Lima, C.B.; Da Silva, J.V.A.; Paulson, E.; Goszczynski, D.E.; Goissis, M.D.; Ross, P.J.; Milazzotto, M.P. Adaptative response to changes in pyruvate metabolism on the epigenetic landscapes and transcriptomics of bovine embryos. Sci. Rep. 2023, 13, 11504. [Google Scholar] [CrossRef]
- Conaghan, J.; Handyside, A.H.; Winston, R.M.L.; Leese, H.J. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. Reproduction 1993, 99, 87–95. [Google Scholar] [CrossRef]
- Pawlak, P.; Lipinska, P.; Sell-Kubiak, E.; Kajdasz, A.; Derebecka, N.; Warzych, E. Energy metabolism disorders during in vitro maturation of bovine cumulus-oocyte complexes interfere with blastocyst quality and metabolism. Dev. Biol. 2024, 509, 51–58. [Google Scholar] [CrossRef]
- Bao, S.; Yin, T.; Liu, S. Ovarian aging: Energy metabolism of oocytes. J. Ovarian Res. 2024, 17, 118. [Google Scholar] [CrossRef]
- Imanaka, S.; Shigetomi, H.; Kobayashi, H. Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance. Reprod. Sci. 2022, 29, 653–667. [Google Scholar] [CrossRef]
- Okonkwo, E.; Saha, B.; Sahu, G.; Bera, A.; Sharma, P. Blood-Based Lateral-Flow Immunoassays Dipstick Test for Damaged Mitochondrial Electron Transport Chain in Pyruvate Treated Rats with Combined Blast Exposure and Hemorrhagic Shock. J. Clin. Med. 2025, 14, 754. [Google Scholar] [CrossRef] [PubMed]
- German, H.M.; Ciapaite, J.; Verhoeven-Duif, N.M.; Jans, J.J.M. Anaplerosis by medium-chain fatty acids through complex interplay with glucose and glutamine metabolism. J. Biol. Chem. 2025, 301, 108307. [Google Scholar] [CrossRef] [PubMed]
- AL-Zubaidi, U.; Liu, J.; Cinar, O.; Robker, R.L.; Adhikari, D.; Carroll, J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol. Human Reprod. 2019, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Serrano-García, N.; Castillo-Rodríguez, R.A.; Robledo-Cadena, D.X.; Jimenez-Farfan, D.; Marín-Hernández, Á.; Silva-Adaya, D.; Rodríguez-Pérez, C.E.; Gallardo-Pérez, J.C. Metabolic dysregulation of tricarboxylic acid cycle and oxidative phosphorylation in glioblastoma. Rev. Neurosci. 2024, 35, 813–838. [Google Scholar] [CrossRef]
- Casuso, R.A. Mitochondrial puzzle in muscle: Linking the electron transport system to overweight. Obes. Rev. 2024, 25, e13794. [Google Scholar] [CrossRef]
- Chenna, S.; Koopman, W.J.H.; Prehn, J.H.M.; Connolly, N.M.C. Mechanisms and mathematical modeling of ROS production by the mitochondrial electron transport chain. Am. J. Physiol.-Cell Physiol. 2022, 323, C69–C83. [Google Scholar] [CrossRef]
- Bahety, D.; Böke, E.; Rodríguez-Nuevo, A. Mitochondrial morphology, distribution and activity during oocyte development. Trends Endocrinol. Metab. 2024, 35, 902–917. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Van Der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef]
- Günther, R.; Pal, A.; Williams, C.; Zimyanin, V.L.; Liehr, M.; Von Neubeck, C.; Krause, M.; Parab, M.G.; Petri, S.; Kalmbach, N.; et al. Alteration of Mitochondrial Integrity as Upstream Event in the Pathophysiology of SOD1-ALS. Cells 2022, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-S.; Mezera, V.; Dighe, P.; Melov, S.; Gerencser, A.A.; Sweis, R.F.; Pliushchev, M.; Wang, Z.; Esbenshade, T.; McKibben, B.; et al. Superoxide produced by mitochondrial site IQ inactivates cardiac succinate dehydrogenase and induces hepatic steatosis in Sod2 knockout mice. Free Radic. Biol. Med. 2021, 164, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.; Lewis, S.C. Bridging lipid metabolism and mitochondrial genome maintenance. J. Biol. Chem. 2024, 300, 107498. [Google Scholar] [CrossRef]
- Giedt, M.S.; Thomalla, J.M.; White, R.P.; Johnson, M.R.; Lai, Z.W.; Tootle, T.L.; Welte, M.A. Adipose triglyceride lipase promotes prostaglandin-dependent actin remodeling by regulating substrate release from lipid droplets. Development 2023, 150, dev201516. [Google Scholar] [CrossRef] [PubMed]
- (Han) Van Der Kolk, J.H.; Gross, J.J.; Gerber, V.; Bruckmaier, R.M. Disturbed bovine mitochondrial lipid metabolism: A review. Vet. Q. 2017, 37, 262–273. [Google Scholar] [CrossRef]
- Ruidas, B. Mitochondrial lipid metabolism in metastatic breast cancer. Mitochondrial Commun. 2024, 2, 58–66. [Google Scholar] [CrossRef]
- Kannan, M.; Lahiri, S.; Liu, L.-K.; Choudhary, V.; Prinz, W.A. Phosphatidylserine synthesis at membrane contact sites promotes its transport out of the ER. J. Lipid Res. 2017, 58, 553–562. [Google Scholar] [CrossRef]
- Panov, A.V.; Mayorov, V.I.; Dikalova, A.E.; Dikalov, S.I. Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria. Int. J. Mol. Sci. 2022, 24, 379. [Google Scholar] [CrossRef]
- Wang, Y.; Palmfeldt, J.; Gregersen, N.; Makhov, A.M.; Conway, J.F.; Wang, M.; McCalley, S.P.; Basu, S.; Alharbi, H.; St. Croix, C.; et al. Mitochondrial fatty acid oxidation and the electron transport chain comprise a multifunctional mitochondrial protein complex. J. Biol. Chem. 2019, 294, 12380–12391. [Google Scholar] [CrossRef]
- Lee, A.K.; Tse, A. Dominant Role of Mitochondria in Calcium Homeostasis of Single Rat Pituitary Corticotropes. Endocrinology 2005, 146, 4985–4993. [Google Scholar] [CrossRef]
- Pérez-Fuentes, N.; Alvariño, R.; Alfonso, A.; González-Jartín, J.; Vieytes, M.R.; Botana, L.M. The Mode of Action of Enniatins A and B is Mediated by Interaction with SOC Reservoirs (A) and Mitochondrial Permeability Transition Pore (B). Expo. Health 2024, 16, 1115–1126. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jia, M.; Zhu, S.; Ren, H.; Wang, G.; Xin, G.; Sun, M.; Wang, X.; Lin, Q.; Jiang, Q.; et al. Mitotic ER-mitochondria contact enhances mitochondrial Ca2+ influx to promote cell division. Cell Rep. 2024, 43, 114794. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.-P.; Welkenhuyzen, K.; Luyten, T.; Ponsaerts, R.; Dewaele, M.; Molgó, J.; Agostinis, P.; Missiaen, L.; De Smedt, H.; Parys, J.B.; et al. Ins(1,4,5) P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy 2011, 7, 1472–1489. [Google Scholar] [CrossRef]
- Duxfield, A.; Munkley, J.; Briggs, M.D.; Dennis, E.P. CRELD2 is a novel modulator of calcium release and calcineurin-NFAT signalling during osteoclast differentiation. Sci. Rep. 2022, 12, 13884. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Vindrieux, D.; Goehrig, D.; Jaber, S.; Collin, G.; Griveau, A.; Wiel, C.; Bendridi, N.; Djebali, S.; Farfariello, V.; et al. Calcium channel ITPR2 and mitochondria–ER contacts promote cellular senescence and aging. Nat. Commun. 2021, 12, 720. [Google Scholar] [CrossRef]
- Zhou, Y.; Jing, S.; Liu, S.; Shen, X.; Cai, L.; Zhu, C.; Zhao, Y.; Pang, M. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. J. Nanobiotechnol 2022, 20, 188. [Google Scholar] [CrossRef]
- Zhang, I.X.; Herrmann, A.; Leon, J.; Jeyarajan, S.; Arunagiri, A.; Arvan, P.; Gilon, P.; Satin, L.S. ER stress increases expression of intracellular calcium channel RyR1 to modify Ca2+ homeostasis in pancreatic beta cells. J. Biol. Chem. 2023, 299, 105065. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Shen, Q.; Yang, X.; Yu, C.; Cai, C.; Cai, G.; Meng, X.; Zou, F. Mitochondrial Ca2+ uniporter is critical for store-operated Ca2+ entry-dependent breast cancer cell migration. Biochem. Biophys. Res. Commun. 2015, 458, 186–193. [Google Scholar] [CrossRef]
- De Ridder, I.; Kerkhofs, M.; Lemos, F.O.; Loncke, J.; Bultynck, G.; Parys, J.B. The ER-mitochondria interface, where Ca2+ and cell death meet. Cell Calcium 2023, 112, 102743. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Qin, Z.; Li, J.; Sun, B.; Liu, L. Regulation of calcium homeostasis in endoplasmic reticulum–mitochondria crosstalk: Implications for skeletal muscle atrophy. Cell Commun. Signal 2025, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Q.; Li, P.; Dong, R.; Lei, Y.; Hu, Y.; Yan, Y.; Song, G. The ROS Mediates MCUb in Mitochondria-Regulated Apoptosis of TM4 Cells Induced by Titanium Dioxide Nanoparticles. Biol. Trace Elem. Res. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Katona, M.; Bartók, Á.; Nichtova, Z.; Csordás, G.; Berezhnaya, E.; Weaver, D.; Ghosh, A.; Várnai, P.; Yule, D.I.; Hajnóczky, G. Capture at the ER-mitochondrial contacts licenses IP3 receptors to stimulate local Ca2+ transfer and oxidative metabolism. Nat. Commun. 2022, 13, 6779. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef]
- Parmar, J.; Von Jonquieres, G.; Gorlamandala, N.; Chung, B.; Craig, A.J.; Pinyon, J.L.; Birnbaumer, L.; Klugmann, M.; Moorhouse, A.J.; Power, J.M.; et al. TRPC Channels Activated by G Protein-Coupled Receptors Drive Ca2+ Dysregulation Leading to Secondary Brain Injury in the Mouse Model. Transl. Stroke Res. 2024, 15, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Li, D.; Wang, Z.; Liu, Y.; Yang, J.; Li, C.; Li, X.; Ma, J.; Zhang, M.; Lu, Y.; et al. Interleukin-17 upregulation participates in the pathogenesis of heart failure in mice via NF-κB-dependent suppression of SERCA2a and Cav1.2 expression. Acta Pharmacol. Sin. 2021, 42, 1780–1789. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Almeida, A.; Delgado-Esteban, M.; Bolaños, J.P.; Medina, J.M. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J. Neurochem. 2002, 81, 207–217. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Ng, Y.S.; Turnbull, D.M. Mitochondrial disease: Genetics and management. J. Neurol. 2016, 263, 179–191. [Google Scholar] [CrossRef]
- Carvalho, F.; Spier, A.; Chaze, T.; Matondo, M.; Cossart, P.; Stavru, F. Listeria monocytogenes Exploits Mitochondrial Contact Site and Cristae Organizing System Complex Subunit Mic10 to Promote Mitochondrial Fragmentation and Cellular Infection. mBio 2020, 11, e03171-19. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.-M.; Choi, J.; Kang, S.; So, S.; Kim, D.; Ahn, J.-Y.; Jung, H.-Y.; Jeong, J.-Y.; Kang, E. Mitochondrial DNA Haplogroup Related to the Prevalence of Helicobacter pylori. Cells 2021, 10, 2482. [Google Scholar] [CrossRef] [PubMed]
- Nevoit, G.; Jarusevicius, G.; Potyazhenko, M.; Mintser, O.; Bumblyte, I.A.; Vainoras, A. Mitochondrial Dysfunction and Risk Factors for Noncommunicable Diseases: From Basic Concepts to Future Prospective. Diseases 2024, 12, 277. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Schulz, J.; Mukherjee, A.; Park, K.-W.; Armijo, E.; Soto, C. Extensive accumulation of misfolded protein aggregates during natural aging and senescence. Front. Aging Neurosci. 2023, 14, 1090109. [Google Scholar] [CrossRef]
- Li, S.-Y.; Gong, X.-Y.; Ndikuryayo, F.; Yang, W.-C. The emerging role of oxygen redox in pathological progression of disorders. Ageing Res. Rev. 2025, 104, 102660. [Google Scholar] [CrossRef]
- Zhou, Q.; Ren, C.; Li, J.; Wang, L.; Duan, Y.; Yao, R.; Tian, Y.; Yao, Y. The crosstalk between mitochondrial quality control and metal-dependent cell death. Cell Death Dis. 2024, 15, 299. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, K.; Galganski, L.; Budzinska, A.; Jarmuszkiewicz, W. Coenzyme Q deficiency in endothelial mitochondria caused by hypoxia; remodeling of the respiratory chain and sensitivity to anoxia/reoxygenation. Free Radic. Biol. Med. 2024, 214, 158–170. [Google Scholar] [CrossRef]
- Ma, H.; Guo, X.; Cui, S.; Wu, Y.; Zhang, Y.; Shen, X.; Xie, C.; Li, J. Dephosphorylation of AMP-activated protein kinase exacerbates ischemia/reperfusion-induced acute kidney injury via mitochondrial dysfunction. Kidney Int. 2022, 101, 315–330. [Google Scholar] [CrossRef]
- Hu, L.-L.; Liao, M.-H.; Liu, Y.-X.; Xing, C.-H.; Nong, L.-L.; Yang, F.-L.; Sun, S.-C. Loss of AMPK activity induces organelle dysfunction and oxidative stress during oocyte aging. Biol. Direct 2024, 19, 29. [Google Scholar] [CrossRef]
- Sadria, M.; Layton, A.T. Interactions among mTORC, AMPK and SIRT: A computational model for cell energy balance and metabolism. Cell Commun. Signal 2021, 19, 57. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S. Exploring potential pathways from oxidative stress to ovarian aging. J. Obstet. Gynaecol. 2025, 51, e16166. [Google Scholar] [CrossRef]
- Kasapoğlu, I.; Seli, E. Mitochondrial Dysfunction and Ovarian Aging. Endocrinology 2020, 161, bqaa001. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Du, Z.; Wu, H.; Zhao, R.; Liu, J.; Gao, S.; Zeng, S. CALB1 and RPL23 Are Essential for Maintaining Oocyte Quality and Function During Aging. Aging Cell 2025, e14466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Tian, Y.; Xu, X.; Wang, B.; Huang, Z.; Lou, S.; Kang, J.; Zhang, N.; Weng, J.; et al. Iron accumulation in ovarian microenvironment damages the local redox balance and oocyte quality in aging mice. Redox Biol. 2024, 73, 103195. [Google Scholar] [CrossRef] [PubMed]
- Vo, K.C.T.; Sato, Y.; Kawamura, K. Improvement of oocyte quality through the SIRT signaling pathway. Reprod. Med. Biol. 2023, 22, e12510. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S. Mitochondrial DNA Damage and Its Repair Mechanisms in Aging Oocytes. Int. J. Mol. Sci. 2024, 25, 13144. [Google Scholar] [CrossRef]
- An, Z.; Xie, C.; Lu, H.; Wang, S.; Zhang, X.; Yu, W.; Guo, X.; Liu, Z.; Shang, D.; Wang, X. Mitochondrial Morphology and Function Abnormality in Ovarian Granulosa Cells of Patients with Diminished Ovarian Reserve. Reprod. Sci. 2024, 31, 2009–2020. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.-H.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef]
- Borsky, P.; Holmannova, D.; Andrys, C.; Kremlacek, J.; Fiala, Z.; Parova, H.; Rehacek, V.; Svadlakova, T.; Byma, S.; Kucera, O.; et al. Evaluation of potential aging biomarkers in healthy individuals: Telomerase, AGEs, GDF11/15, sirtuin 1, NAD+, NLRP3, DNA/RNA damage, and klotho. Biogerontology 2023, 24, 937–955. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, J.; Wu, T.; Zhang, J.; Wang, Y.; Su, J.; Zhang, Y. SIRT1 reduces epigenetic and non-epigenetic changes to maintain the quality of postovulatory aged oocytes in mice. Exp. Cell Res. 2021, 399, 112421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, Y.; Li, L.; Wang, H.-H.; Ma, X.-S.; Qian, W.-P.; Shen, W.; Schatten, H.; Sun, Q.-Y. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging 2016, 8, 685–694. [Google Scholar] [CrossRef]
- Pasquariello, R.; Ermisch, A.F.; Silva, E.; McCormick, S.; Logsdon, D.; Barfield, J.P.; Schoolcraft, W.B.; Krisher, R.L. Alterations in oocyte mitochondrial number and function are related to spindle defects and occur with maternal aging in mice and humans†. Biol. Reprod. 2019, 100, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Adrian, A.E.; Liu, T.T.; Pascal, L.E.; Bauer, S.R.; DeFranco, D.B.; Ricke, W.A. Aging-Related Mitochondrial Dysfunction Is Associated with Fibrosis in Benign Prostatic Hyperplasia. J. Gerontol. Ser. A 2024, 79, glad222. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Yu, D.; Zhao, P.; Liu, Y. Heat stress inhibits the proliferation and differentiation of myoblasts and is associated with damage to mitochondria. Front. Cell Dev. Biol. 2023, 11, 1171506. [Google Scholar] [CrossRef]
- Chen, L.; Thorup, V.M.; Kudahl, A.B.; Østergaard, S. Effects of heat stress on feed intake, milk yield, milk composition, and feed efficiency in dairy cows: A meta-analysis. J. Dairy Sci. 2024, 107, 3207–3218. [Google Scholar] [CrossRef]
- Jing, J.; Wang, J.; Xiang, X.; Yin, S.; Tang, J.; Wang, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; et al. Selenomethionine alleviates chronic heat stress-induced breast muscle injury and poor meat quality in broilers via relieving mitochondrial dysfunction and endoplasmic reticulum stress. Anim. Nutr. 2024, 16, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.-H.; Nejad, J.G.; Kim, H.-R.; Lee, H.-G. Effect of seven days heat stress on feed and water intake, milk characteristics, blood parameters, physiological indicators, and gene expression in Holstein dairy cows. J. Therm. Biol. 2024, 123, 103929. [Google Scholar] [CrossRef]
- Wrzecińska, M.; Kowalczyk, A.; Kordan, W.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorder of Biological Quality and Autophagy Process in Bovine Oocytes Exposed to Heat Stress and the Effectiveness of In Vitro Fertilization. Int. J. Mol. Sci. 2023, 24, 11164. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of Heat Stress-Induced Oxidative Stress on the Milk Protein Biosynthesis of Dairy Cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef]
- Shi, J.; Ji, Z.; Yao, X.; Yao, Y.; Li, C.; Liang, Q.; Zhang, X. HSP90 Enhances Mitophagy to Improve the Resistance of Car-Diomyocytes to Heat Stress in Wenchang Chickens. Int. J. Mol. Sci. 2024, 25, 11695. [Google Scholar] [CrossRef]
- Havalová, H.; Ondrovičová, G.; Keresztesová, B.; Bauer, J.A.; Pevala, V.; Kutejová, E.; Kunová, N. Mitochondrial HSP70 Chaperone System—The Influence of Post-Translational Modifications and Involvement in Human Diseases. Int. J. Mol. Sci. 2021, 22, 8077. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Dovolou, E.; Kalemkeridou, M.; Nanas, I.; Dadouli, K.; Moutou, K.; Mamuris, Z.; Amiridis, G.S. Heat Shock Protein 70 Improves In Vitro Embryo Yield and Quality from Heat Stressed Bovine Oocytes. Animals 2021, 11, 1794. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.; Tolba, S.; Mahrose, K.; Felemban, S.G.; Khafaga, A.F.; Khalifa, N.E.; Jaremko, M.; Moustafa, M.; Alshaharni, M.O.; Algopish, U.; et al. Heat shock proteins as a key defense mechanism in poultry production under heat stress conditions. Poult. Sci. 2024, 103, 103537. [Google Scholar] [CrossRef]
- Suzuki, N. Fine Tuning of ROS, Redox and Energy Regulatory Systems Associated with the Functions of Chloroplasts and Mitochondria in Plants under Heat Stress. Int. J. Mol. Sci. 2023, 24, 1356. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.I.; Wang, Z.; Liu, B.; Chang-qing, S.H.E.N.; Jing-yi, T.U.; Shi-cheng, W.A.N.G.; Rui-ling, L.E.I.; Si-qi, P.E.N.G.; Xiong, X.I.A.O.; Yong-ju, Z.H.A.O.; et al. The effects and mechanisms of heat stress on mammalian oocyte and embryo development. J. Therm. Biol. 2024, 124, 103927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-L.; Li, W.-D.; Zhu, K.-X.; Zhou, X.; Li, L.; Lee, T.-L.; Shen, W. Aging-related aneuploidy is associated with mitochondrial imbalance and failure of spindle assembly. Cell Death Discov. 2023, 9, 235. [Google Scholar] [CrossRef]
- Diao, R.Y.; Gustafsson, Å.B. Mitochondrial quality surveillance: Mitophagy in cardiovascular health and disease. Am. J. Physiol.-Cell Physiol. 2022, 322, C218–C230. [Google Scholar] [CrossRef]
- Khan, I.; Mesalam, A.; Heo, Y.S.; Lee, S.-H.; Nabi, G.; Kong, I.-K. Heat Stress as a Barrier to Successful Reproduction and Potential Alleviation Strategies in Cattle. Animals 2023, 13, 2359. [Google Scholar] [CrossRef]
- Reddam, A.; McLarnan, S.; Kupsco, A. Environmental Chemical Exposures and Mitochondrial Dysfunction: A Review of Recent Literature. Curr. Environ. Health Rep. 2022, 9, 631–649. [Google Scholar] [CrossRef]
- Duarte-Hospital, C.; Tête, A.; Brial, F.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Kim, M.J.; Blanc, E.B.; Coumoul, X.; Bortoli, S. Mitochondrial Dysfunction as a Hallmark of Environmental Injury. Cells 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Kim, G.-D. Particulate Matter-Induced Emerging Health Effects Associated with Oxidative Stress and Inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A. Modulators of mitochondrial ATP-sensitive potassium channel affect cytotoxicity of heavy metals: Action on isolated rat liver mitochondria and AS-30D ascites hepatoma cells. Ecotoxicol. Environ. Saf. 2023, 256, 114829. [Google Scholar] [CrossRef]
- Choi, S.-H.; Ochirpurev, B.; Jo, H.Y.; Won, J.-U.; Toriba, A.; Kim, H. Effects of polycyclic aromatic hydrocarbon exposure on mitochondrial DNA copy number. Hum. Exp. Toxicol. 2023, 42, 09603271231216968. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, W.; Li, Y.; Zhao, L.; Li, C.; Wang, L.; Fang, J.; Song, S.; Ji, Y.; Fang, T.; et al. The Levels of Polycyclic Aromatic Hydrocarbons and Their Derivatives in Plasma and Their Effect on Mitochondrial DNA Methylation in the Oilfield Workers. Toxics 2023, 11, 466. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Z.; Li, P.; Ji, Y.; Song, S.; Zheng, N.; Zhao, L.; Jia, Y.; Fang, J.; Wang, H.; et al. Association between mitochondrial DNA methylation and internal exposure to polycyclic aromatic hydrocarbons (PAHs), nitrated-PAHs (NPAHs) and oxygenated-PAHs (OPAHs) in young adults from Tianjin, China. Ecotoxicol. Environ. Saf. 2022, 241, 113799. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Yang, S.; Li, X.; Liu, L.; Ma, X.; Niu, D.; Duan, X. Exposure to phenanthrene affects oocyte meiosis by inducing mitochondrial dysfunction and endoplasmic reticulum stress. Cell Prolif. 2023, 56, e13335. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301. [Google Scholar] [CrossRef]
- Wu, H.; Feng, L.; Wu, H.; Wang, L.; Xu, H.; Fu, F. Synergistic effects of PS-NPs and Cd on ovarian toxicity in adolescent rats: Ferroptosis by induction of mitochondrial redox imbalance via the SIRT3-SOD2/Gpx4 pathway. Ecotoxicol. Environ. Saf. 2025, 290, 117622. [Google Scholar] [CrossRef]
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef]
- Xiao, C.; Lai, D. Impact of oxidative stress induced by heavy metals on ovarian function. J. Appl. Toxicol. 2025, 45, 107–116. [Google Scholar] [CrossRef]
- Wrzecińska, M.; Kowalczyk, A.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals. Biology 2021, 10, 882. [Google Scholar] [CrossRef]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy Metal Exposure: Molecular Pathways, Clinical Implications, and Protective Strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef]
- Shao, C.-S.; Zhou, X.-H.; Miao, Y.-H.; Wang, P.; Zhang, Q.-Q.; Huang, Q. In situ observation of mitochondrial biogenesis as the early event of apoptosis. iScience 2021, 24, 103038. [Google Scholar] [CrossRef]
- Kang, X.; Yan, L.; Wang, J. Spatiotemporal Distribution and Function of Mitochondria in Oocytes. Reprod. Sci. 2024, 31, 332–340. [Google Scholar] [CrossRef]
- Nicolson, G.L. Metabolic syndrome and mitochondrial function: Molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. J. Cell. Biochem. 2007, 100, 1352–1369. [Google Scholar] [CrossRef] [PubMed]
- Paull, D.; Emmanuele, V.; Weiss, K.A.; Treff, N.; Stewart, L.; Hua, H.; Zimmer, M.; Kahler, D.J.; Goland, R.S.; Noggle, S.A.; et al. Nuclear genome transfer in human oocytes eliminates mitochondrial DNA variants. Nature 2013, 493, 632–637. [Google Scholar] [CrossRef]
- Sloan, D.B.; Fields, P.D.; Havird, J.C. Mitonuclear linkage disequilibrium in human populations. Proc. R. Soc. B. 2015, 282, 20151704. [Google Scholar] [CrossRef]
- Tachibana, M.; Amato, P.; Sparman, M.; Woodward, J.; Sanchis, D.M.; Ma, H.; Gutierrez, N.M.; Tippner-Hedges, R.; Kang, E.; Lee, H.-S.; et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature 2013, 493, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Tatay, L.; Hernández-Andreu, J.; Aznar, J. Mitochondrial Modification Techniques and Ethical Issues. J. Clin. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chen, Y.; Gu, X.; Miao, M.; Hu, D.; Zhou, H.; Chen, J.; Teichmann, A.T.; Yang, Y. Review of the Potential Therapeutic Effects and Molecular Mechanisms of Resveratrol on Endometriosis. Int. J. Women’s Health 2023, 15, 741–763. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D.; Lin, C.-W. Effects of Resveratrol and 4-hexylresorcinol on Hydrogen Peroxide-induced Oxidative DNA Damage in Human Lymphocytes. Free Radic. Res. 2003, 37, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, R.; Rajkovic, J.; Gostimirovic, M.; Gojkovic-Bukarica, L.; Radunovic, N. Resveratrol and Reproductive Health. Life 2022, 12, 294. [Google Scholar] [CrossRef]
- Zaychenko, G.; Stryga, O.; Sinitsyna, O.; Doroshenko, A.; Sulaieva, O.; Falalyeyeva, T.; Kobyliak, N. Resveratrol Effects on the Reproductive System in Ovariectomized Rats: Deciphering Possible Mechanisms. Molecules 2022, 27, 4916. [Google Scholar] [CrossRef]
- Bartra, C.; Yuan, Y.; Vuraić, K.; Valdés-Quiroz, H.; Garcia-Baucells, P.; Slevin, M.; Pastorello, Y.; Suñol, C.; Sanfeliu, C. Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Antioxidants 2024, 13, 177. [Google Scholar] [CrossRef]
- Taherian, M.; Norenberg, M.D.; Panickar, K.S.; Shamaladevi, N.; Ahmad, A.; Rahman, P.; Jayakumar, A.R. Additive Effect of Resveratrol on Astrocyte Swelling Post-exposure to Ammonia, Ischemia and Trauma In Vitro. Neurochem. Res. 2020, 45, 1156–1167. [Google Scholar] [CrossRef]
- Omraninava, M.; Razi, B.; Aslani, S.; Imani, D.; Jamialahmadi, T.; Sahebkar, A. Effect of resveratrol on inflammatory cytokines: A meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2021, 908, 174380. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Chen, S.-D.; Hsu, C.-Y.; Chen, S.-F.; Chen, N.-C.; Jou, S.-B. Resveratrol Promotes Mitochondrial Biogenesis and Protects against Seizure-Induced Neuronal Cell Damage in the Hippocampus Following Status Epilepticus by Activation of the PGC-1α Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 998. [Google Scholar] [CrossRef]
- Nishigaki, A.; Tsubokura, H.; Tsuzuki-Nakao, T.; Okada, H. Hypoxia: Role of SIRT1 and the protective effect of resveratrol in ovarian function. Reprod. Med. Biol. 2022, 21, e12428. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, Y.; Gong, S.; Zi, X.; Zhang, D. Resveratrol Promotes Proliferation, Antioxidant Properties, and Progesterone Production in Yak (Bos grunniens) Granulosa Cells. Animals 2024, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Dong, Y.; Wang, Z.; Ding, H.; Wang, J.; Zhao, J.; Liu, H.; Lv, W. Melatonin Attenuates Oxidative Stress-Induced Apoptosis of Bovine Ovarian Granulosa Cells by Promoting Mitophagy via SIRT1/FoxO1 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12854. [Google Scholar] [CrossRef]
- Sugiyama, M.; Kawahara-Miki, R.; Kawana, H.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J. Reprod. Dev. 2015, 61, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, R.; Celi, P.; Zhuo, Y.; Ding, X.; Zeng, Q.; Bai, S.; Xu, S.; Yin, H.; Lv, L.; et al. Resveratrol Alleviating the Ovarian Function Under Oxidative Stress by Alternating Microbiota Related Tryptophan-Kynurenine Pathway. Front. Immunol. 2022, 13, 911381. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Sato, Y.; Kawagoe, Y.; Shimizu, T.; Kawamura, K. Short-term resveratrol treatment restored the quality of oocytes in aging mice. Aging 2022, 14, 5628–5640. [Google Scholar] [CrossRef]
- Higashida, K.; Kim, S.H.; Jung, S.R.; Asaka, M.; Holloszy, J.O.; Han, D.-H. Effects of Resveratrol and SIRT1 on PGC-1α Activity and Mitochondrial Biogenesis: A Reevaluation. PLoS Biol. 2013, 11, e1001603. [Google Scholar] [CrossRef]
- Nishigaki, A.; Kido, T.; Kida, N.; Kakita-Kobayashi, M.; Tsubokura, H.; Hisamatsu, Y.; Okada, H. Resveratrol protects mitochondrial quantity by activating SIRT1/PGC-1α expression during ovarian hypoxia. Reprod. Med. Biol. 2020, 19, 189–197. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Xia, X.; Zhang, T.; Nie, M.; Xiong, J.; Fang, X. Resveratrol protected against the development of endometriosis by promoting ferroptosis through miR-21-3p/p53/SLC7A11 signaling pathway. Biochem. Biophys. Res. Commun. 2024, 692, 149338. [Google Scholar] [CrossRef]
- Sun, X.; Fu, P.; Xie, L.; Chai, S.; Xu, Q.; Zeng, L.; Wang, X.; Jiang, N.; Sang, M. Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int. J. Mol. Med. 2020, 47, 335–345. [Google Scholar] [CrossRef]
- Della Corte, L.; Noventa, M.; Ciebiera, M.; Magliarditi, M.; Sleiman, Z.; Karaman, E.; Catena, U.; Salvaggio, C.; Falzone, G.; Garzon, S. Phytotherapy in endometriosis: An up-to-date review. J. Complement. Integr. Med. 2020, 17, 20190084. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Zhang, H.; Cheng, S.; Wang, Y.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.É.S.; Gouveia, B.B.; Barberino, R.S.; Menezes, V.G.; Macedo, T.J.S.; Cavalcante, A.Y.P.; Monte, A.P.O.; Santos, J.M.S.; Matos, M.H.T. Resveratrol promotes in vitro activation of ovine primordial follicles by reducing DNA damage and enhancing granulosa cell proliferation via phosphatidylinositol 3-kinase pathway. Reprod. Domest. Anim. 2018, 53, 1298–1305. [Google Scholar] [CrossRef]
- Li, N.; Liu, L. Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. 2018, 44, 1431–1438. [Google Scholar] [CrossRef]
- Ragonese, F.; Monarca, L.; De Luca, A.; Mancinelli, L.; Mariani, M.; Corbucci, C.; Gerli, S.; Iannitti, R.G.; Leonardi, L.; Fioretti, B. Resveratrol depolarizes the membrane potential in human granulosa cells and promotes mitochondrial biogenesis. Fertil. Steril. 2021, 115, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, E.; Kamili, G.; Ou, S.; Yang, D. Effect of resveratrol on superovulation in mice. Biomed. Pharmacother. 2022, 146, 112565. [Google Scholar] [CrossRef]
- Gutierrez-Castillo, E.; Diaz, F.A.; Talbot, S.A.; Bondioli, K.R. Effect of bovine oocyte vitrification with EGTA and post-warming recovery with resveratrol on meiotic spindle, mitochondrial function, reactive oxygen species, and developmental competence. Theriogenology 2023, 196, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Luo, Y.; Zhou, D.; Liu, H.; Zhou, G.; Meng, L.; Hou, Y.; Liu, C.; Li, J.; Fu, X. Leonurine improves bovine oocyte maturation and subsequent embryonic development by reducing oxidative stress and improving mitochondrial function. Theriogenology 2023, 199, 11–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Wen, Y.; Xiong, Q.; Liu, H.; Wu, J.; Zou, Y.; Zhu, Y. SCM-198 attenuates early atherosclerotic lesions in hypercholesterolemic rabbits via modulation of the inflammatory and oxidative stress pathways. Atherosclerosis 2012, 224, 43–50. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, X.; Wang, Q.; Wu, X.; Yang, T.; Chen, Y.; Zhu, Y.; Wang, X. SCM-198 ameliorates the quality of postovulatory and maternally aged oocytes by reducing oxidative stress. J. Ovarian Res. 2024, 17, 178. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, Y.; Sun, Y.; Jiang, J.; Li, Z.; Wang, Z.; Wang, M.; Gu, X. Leonurine Exerts Anti-Inflammatory Effects in Lipopolysaccharide (LPS)-Induced Endometritis by Modulating Mouse JAK-STAT/PI3K-Akt/PPAR Signaling Pathways. Genes 2024, 15, 857. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, J.-B.; Liu, W.; Yao, X.; Guo, H.; Jin, Z.-L.; Zhang, M.-J.; Yuan, B.; Jiang, H.; Kim, N.-H. Leonurine improves in vitro porcine embryo development competence by reducing reactive oxygen species production and protecting mitochondrial function. Theriogenology 2020, 156, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.-N.; Hai, D.-M.; Ma, L.; Cui, Y.-H.; Hu, H.-T.; Liu, N.; Juan-Du; Lan, X.-B.; Yu, J.-Q.; Yang, J.-M. Protective effects of leonurine hydrochloride on pyroptosis in premature ovarian insufficiency via regulating NLRP3/GSDMD pathway. Int. Immunopharmacol. 2023, 114, 109520. [Google Scholar] [CrossRef]
- Yang, D.; Jia, W.; Zhu, Y.Z. Leonurine, a Potential Agent of Traditional Chinese Medicine: Recent Updates and Future Perspectives. Nat. Product. Commun. 2016, 11, 1934578X1601101. [Google Scholar] [CrossRef]
- Xu, W.; Cui, J.; Zhou, F.; Bai, M.; Deng, R.; Wang, W. Leonurine protects against dexamethasone-induced cytotoxicity in pancreatic β-cells via PI3K/Akt signaling pathway. Biochem. Biophys. Res. Commun. 2020, 529, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Hu, H.; Niu, H.; Sun, X.; Li, Y. Melatonin restores the declining maturation quality and early embryonic development of oocytes in aged mice. Theriogenology 2023, 210, 110–118. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Wen, D.; Li, R.; Lu, S.; Xu, R.; Tang, Y.; Sun, Y.; Zhao, X.; Pan, M.; et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022, 49, 102215. [Google Scholar] [CrossRef]
- Cho, J.H.; Bhutani, S.; Kim, C.H.; Irwin, M.R. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav. Immun. 2021, 93, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-Q.; Jia, B.-Y.; Li, J.-J.; Fu, X.-W.; Zhou, G.-B.; Hou, Y.-P.; Zhu, S.-E. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 2011, 76, 785–793. [Google Scholar] [CrossRef]
- Li, J.-M.; Zhang, Z.; Kong, A.; Lai, W.; Xu, W.; Cao, X.; Zhao, M.; Li, J.; Shentu, J.; Guo, X.; et al. Dietary L-carnitine regulates liver lipid metabolism via simultaneously activating fatty acid β-oxidation and suppressing endoplasmic reticulum stress in large yellow croaker fed with high-fat diets. Br. J. Nutr. 2023, 129, 29–40. [Google Scholar] [CrossRef]
- Reader, K.L.; Cox, N.R.; Stanton, J.-A.L.; Juengel, J.L. Effects of acetyl-L-carnitine on lamb oocyte blastocyst rate, ultrastructure, and mitochondrial DNA copy number. Theriogenology 2015, 83, 1484–1492. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Weng, J.; Yin, T.; Yang, J.; Feng, H.L. Biological roles of L-carnitine in oocyte and early embryo development. Mol. Reprod. Dev. 2021, 88, 673–685. [Google Scholar] [CrossRef]
- Lee, Y.-C.G.; Chou, H.-C.; Chen, Y.-T.; Tung, S.-Y.; Ko, T.-L.; Buyandelger, B.; Wen, L.-L.; Juan, S.-H. l-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci. Rep. 2022, 12, 4673. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef] [PubMed]
- Modak, A.K.; Alam, M.H.; Islam, M.N.; Paul, N.; Akter, I.; Hashem, M.A.; Kabir, A.A.; Moniruzzaman, M. L-Carnitine Supports the In Vitro Growth of Buffalo Oocytes. Animals 2022, 12, 1957. [Google Scholar] [CrossRef]
- Catandi, G.D.; Cheng, M.-H.; Chicco, A.J.; Chen, T.; Carnevale, E.M. L-carnitine enhances developmental potential of bovine oocytes matured under high lipid concentrations in vitro. Anim. Reprod. Sci. 2023, 252, 107249. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, C.; De Andrés, F.; Zougagh, M.; Ríos, Á. A multi-step approach for the accurate screening and determination of Coenzyme Q10 (Nano)micelles. Anal. Chim. Acta 2025, 1348, 343820. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, X.; Li, C.; Chen, P.; Lan, Y.; Huang, Y.; Xu, W.; Zhou, J. Association of Coenzyme Q10 with Premature Ovarian Insufficiency. Reprod. Sci. 2023, 30, 1548–1554. [Google Scholar] [CrossRef]
- Cao, S.; Yan, H.; Tang, W.; Zhang, H.; Liu, J. Effects of dietary coenzyme Q10 supplementation during gestation on the embryonic survival and reproductive performance of high-parity sows. J. Anim. Sci. Biotechnol. 2023, 14, 75. [Google Scholar] [CrossRef]
- McRae, M.P. Coenzyme Q10 Supplementation in Reducing Inflammation: An Umbrella Review. J. Chiropr. Med. 2023, 22, 131–137. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Hu, J.; Liu, Z. Overexpression of enzymes in glycolysis and energy metabolic pathways to enhance coenzyme Q10 production in Rhodobacter sphaeroides VK-2-3. Front. Microbiol. 2022, 13, 931470. [Google Scholar] [CrossRef]
- Yen, H.-C.; Yeh, W.-Y.; Lee, S.-H.; Feng, Y.-H.; Yang, S.-L. Characterization of human mitochondrial PDSS and COQ proteins and their roles in maintaining coenzyme Q10 levels and each other’s stability. Biochim. Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148192. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-J.; Zhou, W.; Nie, Z.-W.; Zhou, D.; Xu, Y.-N.; Ock, S.A.; Yan, C.-G.; Cui, X.-S. Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging 2020, 12, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Liu, S.; Miao, J.-K.; Mou, Q.; Liu, X.-M.; Wang, P.-C.; Huo, L.-J.; Du, Z.-Q. CoQ10 improves meiotic maturation of pig oocytes through enhancing mitochondrial function and suppressing oxidative stress. Theriogenology 2021, 159, 77–86. [Google Scholar] [CrossRef]
- Heydarnejad, A.; Ostadhosseini, S.; Varnosfaderani, S.R.; Jafarpour, F.; Moghimi, A.; Nasr-Esfahani, M.H. Supplementation of maturation medium with CoQ10 enhances developmental competence of ovine oocytes through improvement of mitochondrial function. Mol. Reprod. Dev. 2019, 86, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Tsui, K.-H.; Li, C.-J. Mitoquinone shifts energy metabolism to reduce ROS-induced oxeiptosis in female granulosa cells and mouse oocytes. Aging 2023, 15, 246–260. [Google Scholar] [CrossRef]

| Sunstance | Source | Functions | Effects on Oocytes, and Ovarian Health |
|---|---|---|---|
| Resveratrol | Grapes, peanuts, wine, tea |
|
|
| Leonurine | Leonurus japonicus (Herb) |
|
|
| Melatonin | Pineal gland, retina, ovary |
|
|
| L-carnitine | Animal muscle tissues |
|
|
| Coenzyme Q10 | Mitochondria, biological membranes |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałęska, E.; Kowalczyk, A.; Wrzecińska, M.; García, M.C.; Czerniawska-Piątkowska, E.; Gwoździewicz, S.; Witkiewicz, W.; Dobrzański, Z. The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture. Int. J. Mol. Sci. 2025, 26, 4098. https://doi.org/10.3390/ijms26094098
Gałęska E, Kowalczyk A, Wrzecińska M, García MC, Czerniawska-Piątkowska E, Gwoździewicz S, Witkiewicz W, Dobrzański Z. The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture. International Journal of Molecular Sciences. 2025; 26(9):4098. https://doi.org/10.3390/ijms26094098
Chicago/Turabian StyleGałęska, Elżbieta, Alicja Kowalczyk, Marcjanna Wrzecińska, Mercedes Camiña García, Ewa Czerniawska-Piątkowska, Szymon Gwoździewicz, Wojciech Witkiewicz, and Zbigniew Dobrzański. 2025. "The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture" International Journal of Molecular Sciences 26, no. 9: 4098. https://doi.org/10.3390/ijms26094098
APA StyleGałęska, E., Kowalczyk, A., Wrzecińska, M., García, M. C., Czerniawska-Piątkowska, E., Gwoździewicz, S., Witkiewicz, W., & Dobrzański, Z. (2025). The Importance of Mitochondrial Processes in the Maturation and Acquisition of Competences of Oocytes and Embryo Culture. International Journal of Molecular Sciences, 26(9), 4098. https://doi.org/10.3390/ijms26094098








