Oleanolic Acid Lactones as Effective Agents in the Combat with Cancers—Cytotoxic and Antioxidant Activity, SAR Analysis, Molecular Docking and ADMETox Profile
Abstract
1. Introduction
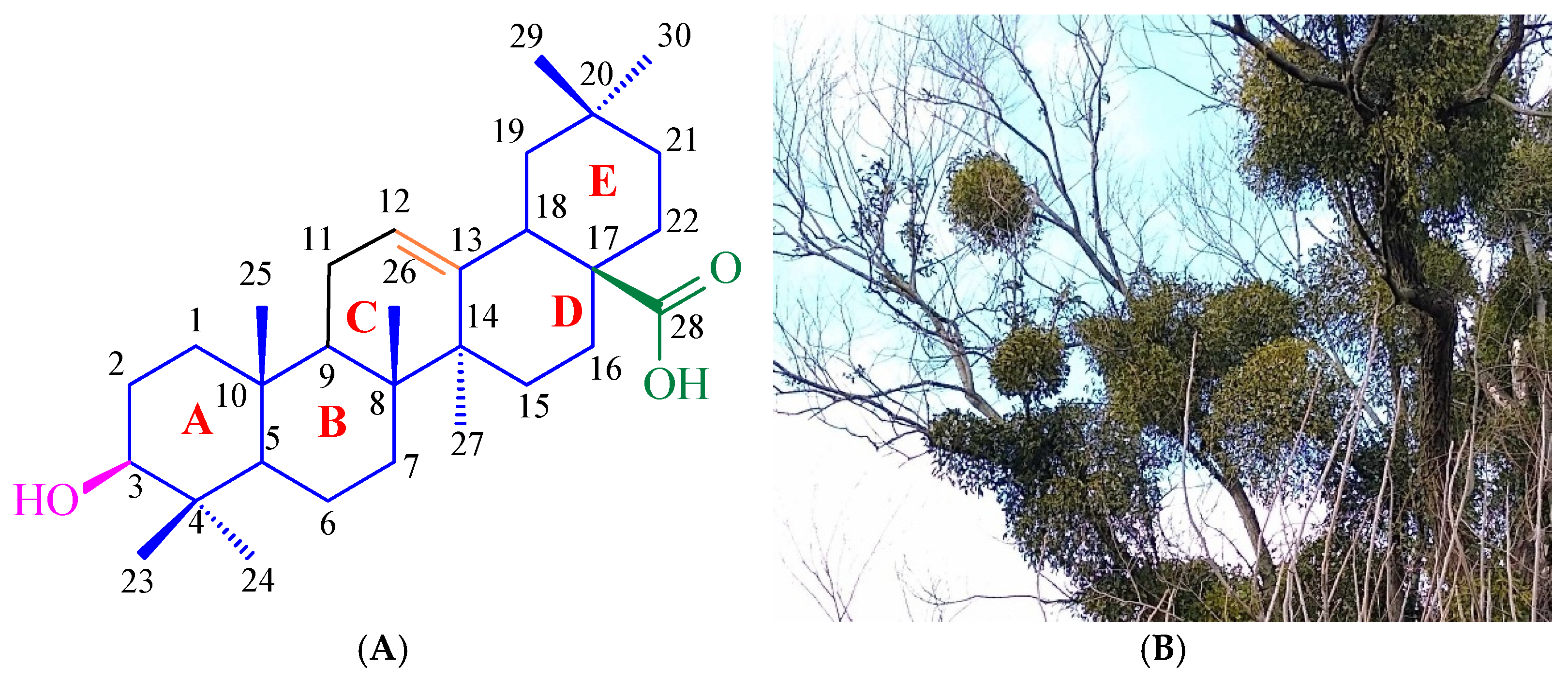
2. Results
2.1. Synthesis of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
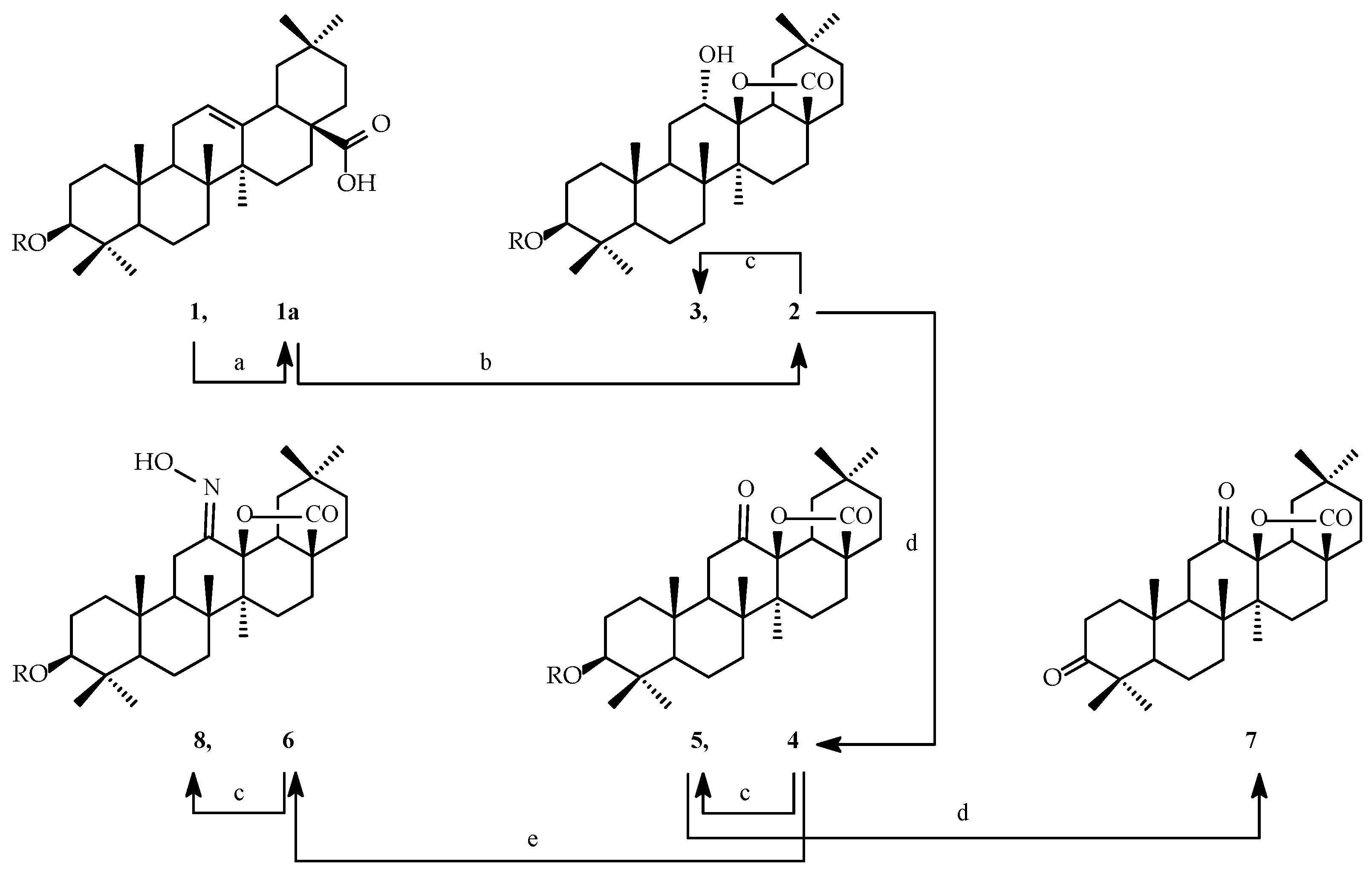
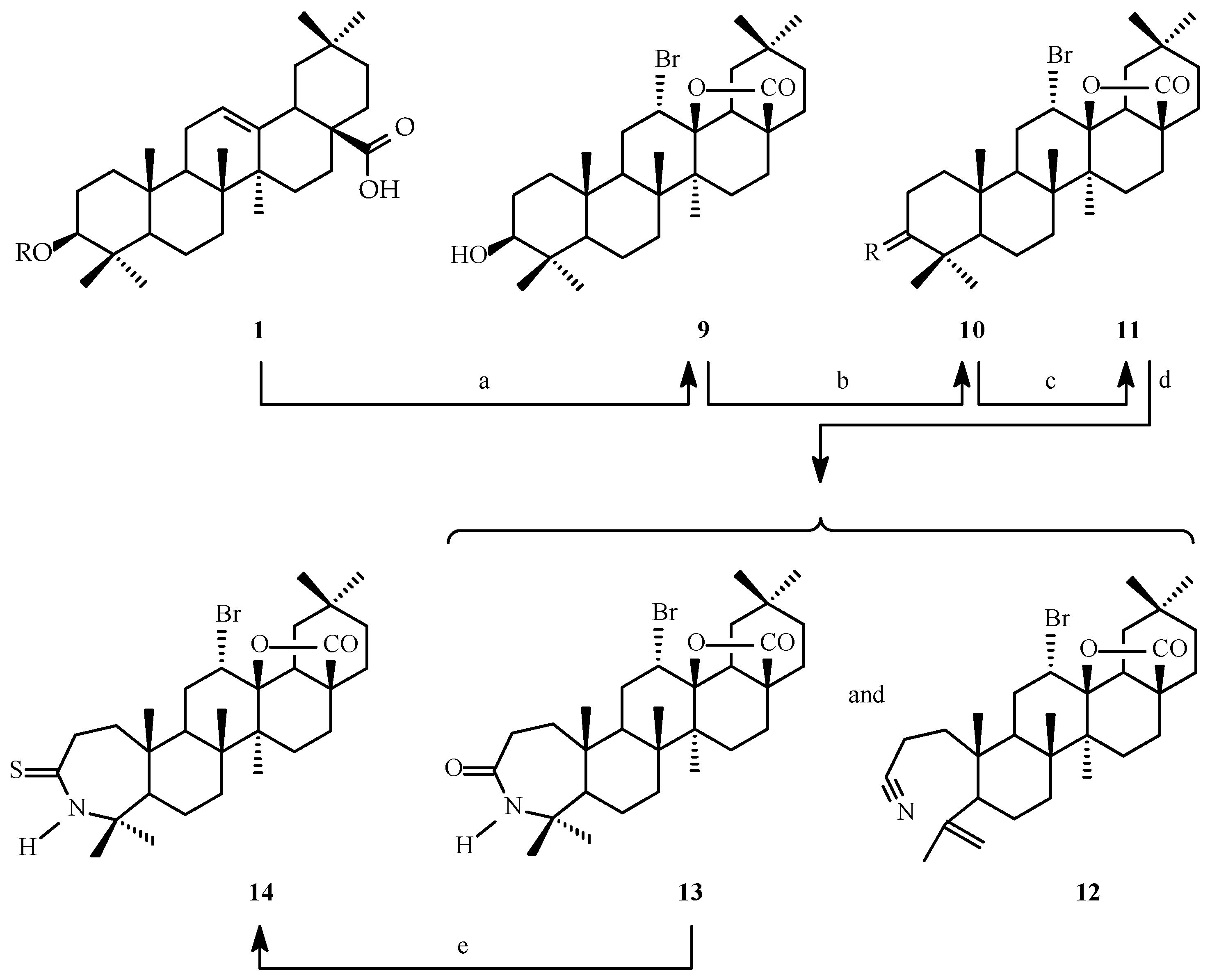
2.2. SAR Analysis of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
2.3. MTT Assay of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
2.3.1. Cytotoxic Properties
2.3.2. Selectivity Index
2.3.3. Apoptotic Index
| ≥2.50 | 2.49–2.00 | 1.99–1.50 | 1.49–1.00 | ≤0.99 |
| Comp. No. | Cell Line, SI | |||
|---|---|---|---|---|
| CHO | A-549 | PC-3 | SKOV-3 | |
| 1 | 2.71 | 2.83 | 1.33 | 1.32 |
| 2 | 1.79 | 1.60 | 2.30 | 2.54 |
| 3 | 0.81 | 1.64 | 1.75 | 1.81 |
| 4 | 0.79 | 1.70 | 1.62 | 1.84 |
| 5 | 1.35 | 1.36 | 1.67 | 1.40 |
| 6 | 0.71 | 3.08 | 2.08 | 2.26 |
| 7 | --- * | --- * | --- * | --- * |
| 8 | --- * | --- * | --- * | --- * |
| 9 | 1.21 | 1.71 | 1.95 | 1.93 |
| 10 | 1.83 | 2.51 | 3.02 | 3.24 |
| 11 | 0.52 | 0.83 | 1.15 | 1.33 |
| 12 | 0.99 | 1.15 | 1.46 | 1.47 |
| 13 | --- * | 1.00 | 1.22 | 1.18 |
| 14 | 0.97 | 1.27 | 1.28 | 1.23 |
2.4. Molecular Docking of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
2.4.1. Detecting Cavities
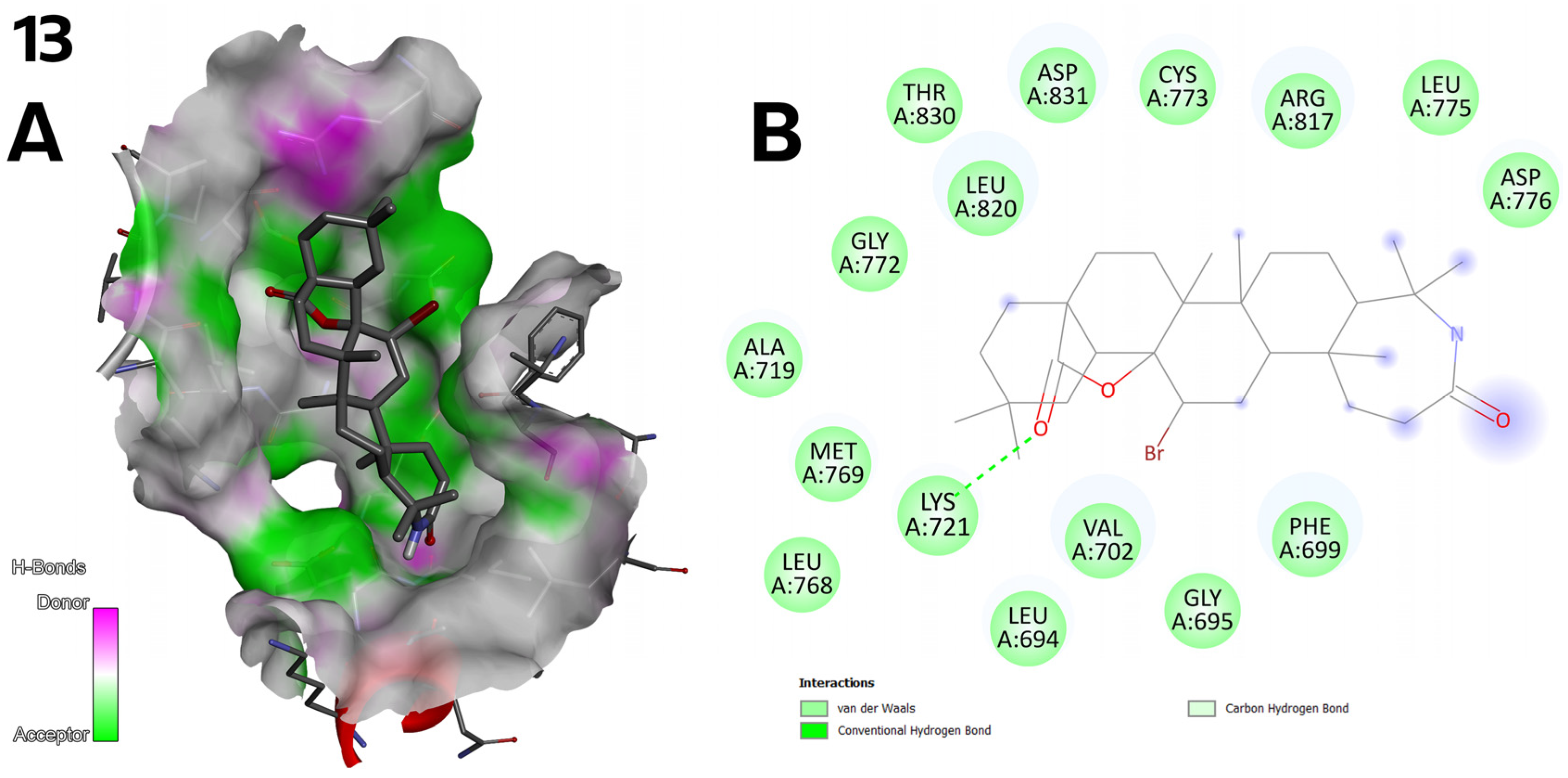
2.4.2. Molecular Docking Results
| Pocket ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | −8.0 | −8.0 | −7.8 | −8.0 | −8.5 | −9.8 | −7.9 | −7.9 | −9.7 | −8.3 | −9.0 | −6.9 | −8.6 | −8.1 |
| C2 | −8.9 | −9.4 | −8.8 | −9.2 | −8.9 | −10.3 | −9.1 | −9.2 | −8.7 | −9.7 | −9.6 | −8.1 | −9.8 | −9.9 |

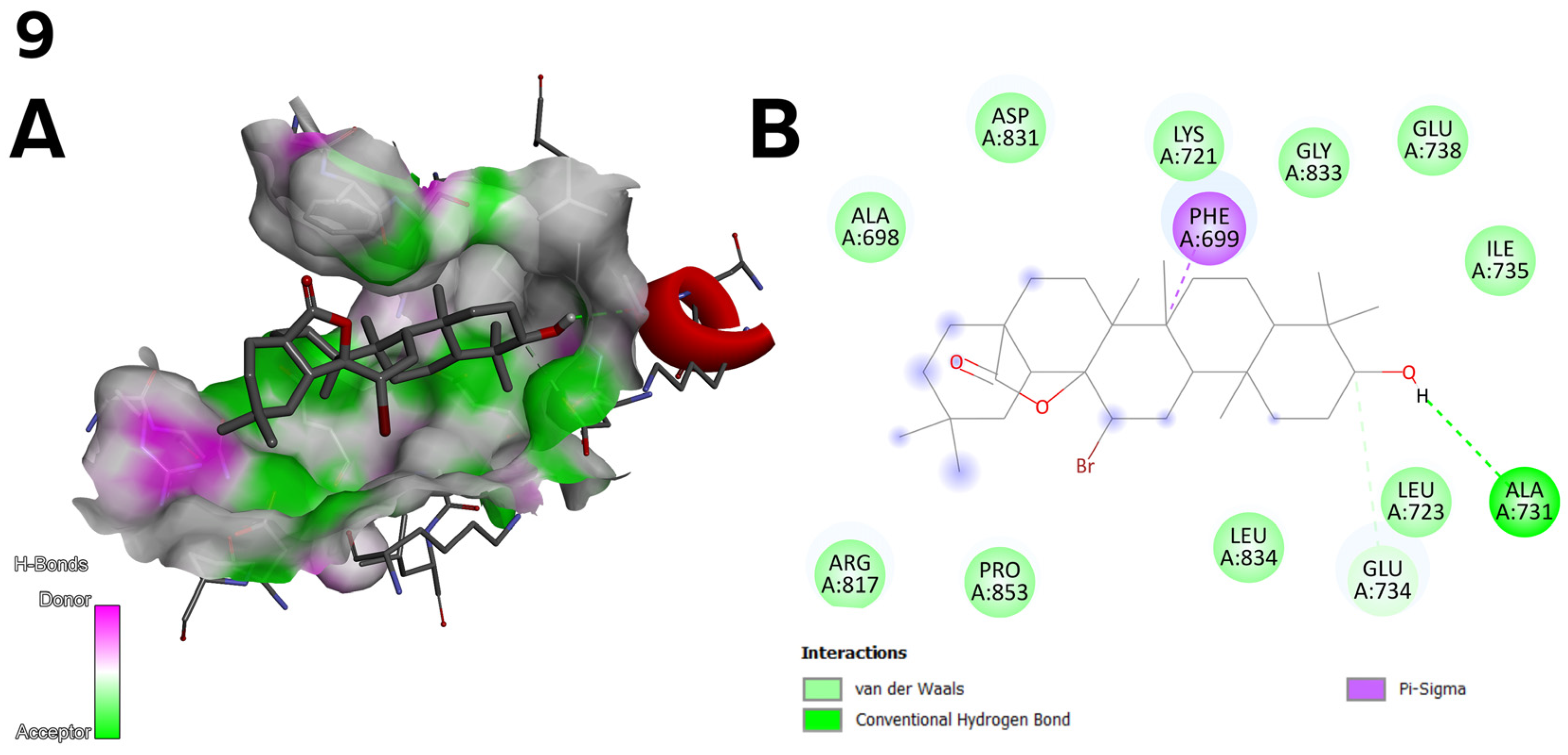
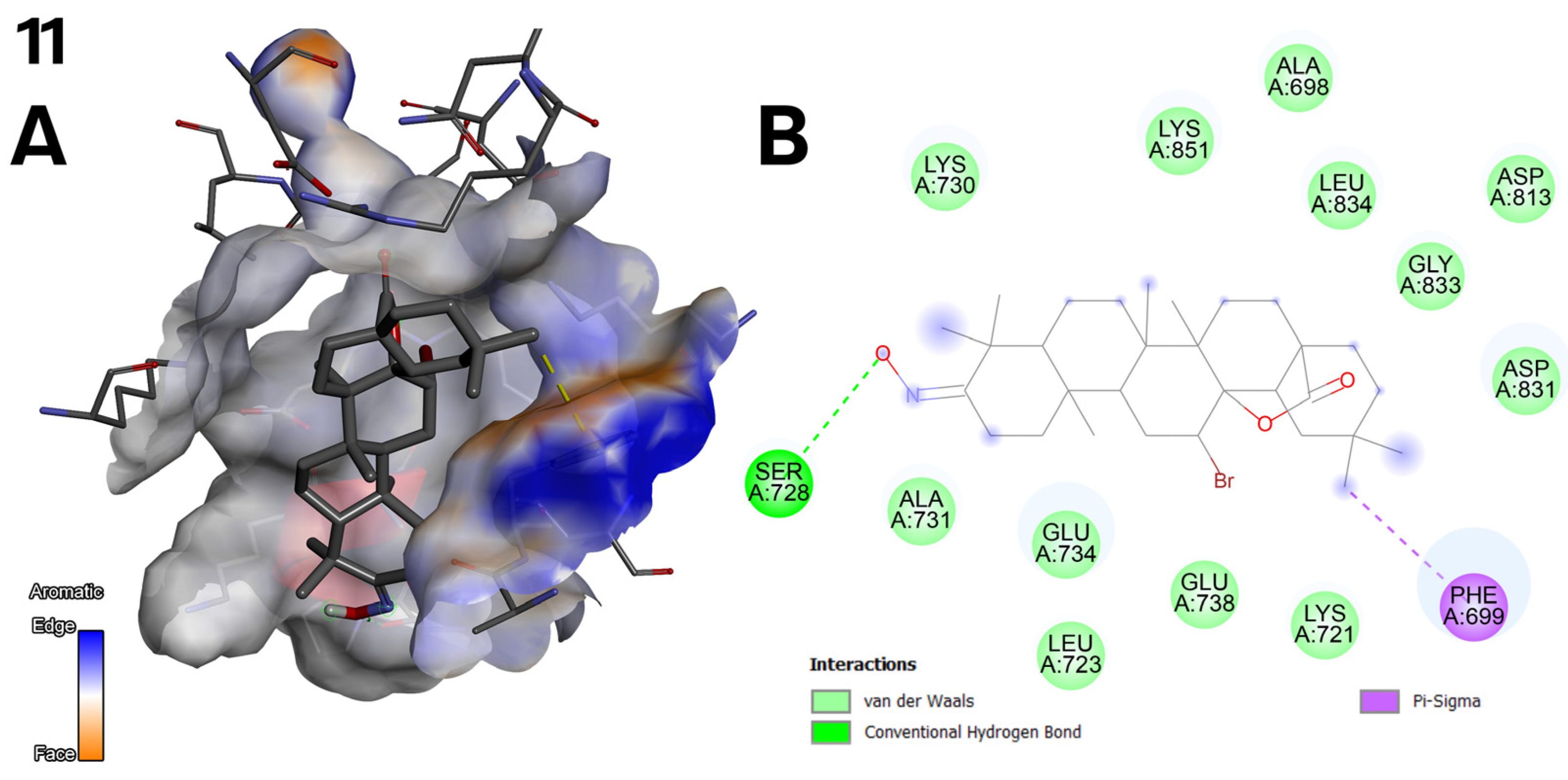
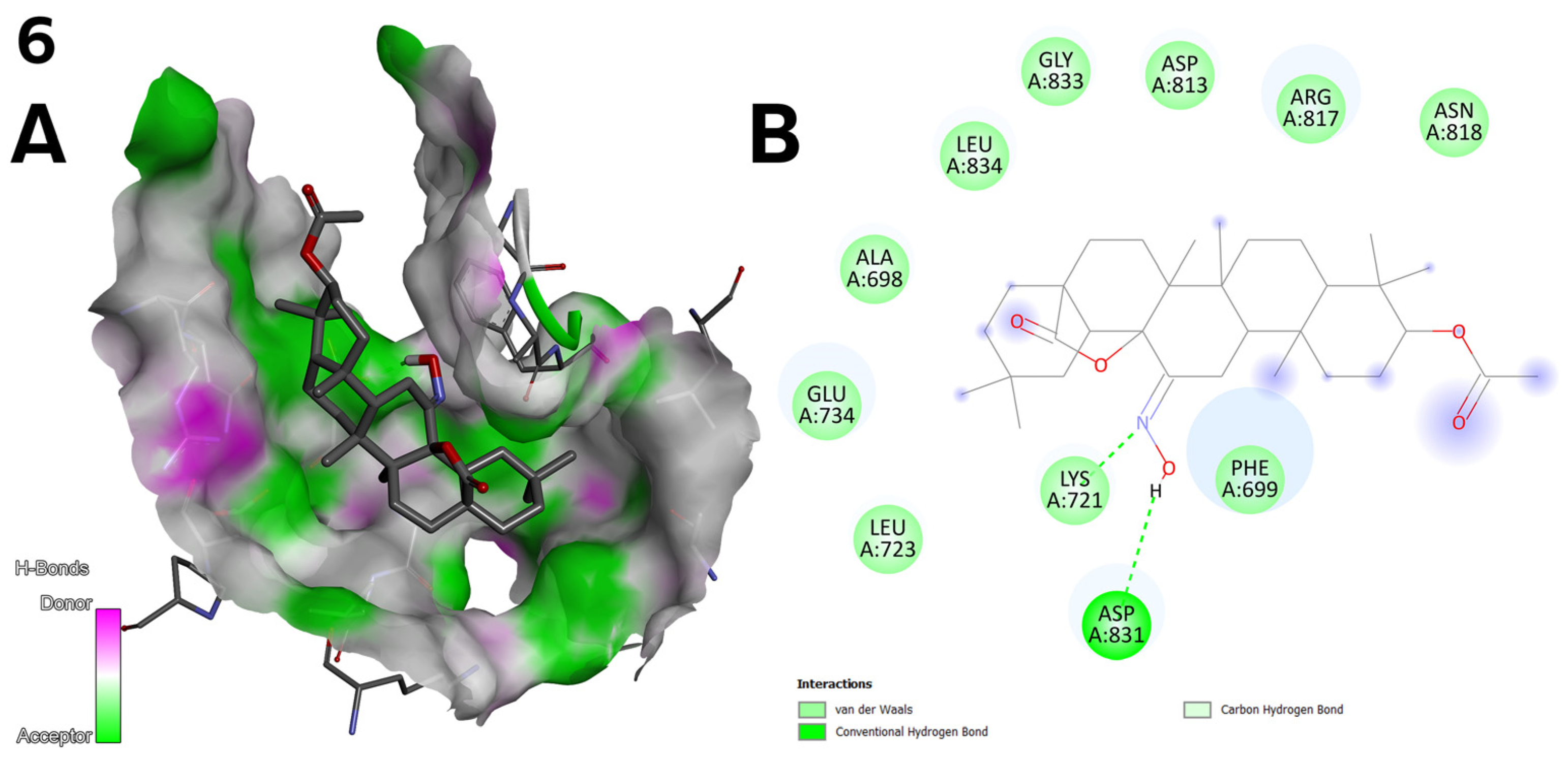
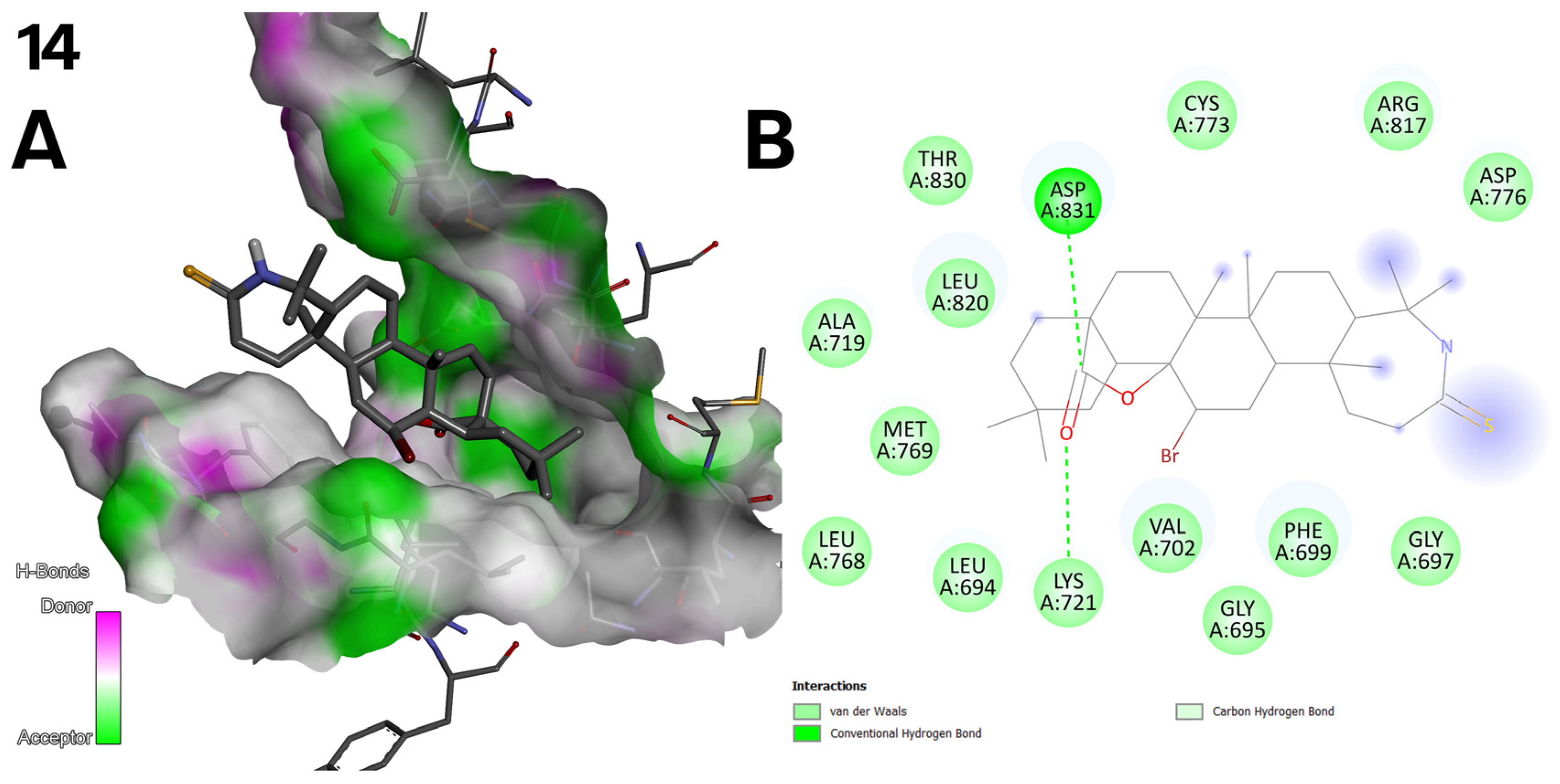
2.5. Antioxidant Activity of Oleanolic Acid, Lactones (2–8), and Bromolactones (9–14)

2.6. ADMETox Analysis of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
3. Discussion
3.1. Synthesis of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
3.2. SAR Analysis of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
- Impact of bromine atom upon lipophilicity and solubility: The bromine atom is relatively large and heavy and has moderate polarity. Its presence increases the lipophilicity of the molecule. Excessive lipophilicity may hinder the dissolution of the substance in water, which reduces its bioavailability and causes accumulation in lipid membranes, reducing accessibility to the site of action.
- Changes in the shape and volume of the molecule: The bromine atom has a large diameter, which may introduce steric difficulties in the molecule. These difficulties may disturb interactions with the receptor or enzyme (e.g., prevent the substance from adequately binding to the active site) and reduce the affinity of the substance for the biological target.
- Impact on metabolic reactions: The bromine atom may affect the metabolic stability of a substance because it may be susceptible to biotransformation reactions (e.g., dehalogenation reaction), leading to the formation of an inactive metabolite or may cause the molecule to become more susceptible to elimination from the body (e.g., by conjugation with glutathione or glucuronic acid).
- Impact on hydrogen bonds: The introduction of a bromine atom replaces functional groups that could participate in the formation of hydrogen bonds (e.g., hydroxyl or amino groups). Such a new structure may limit the substance’s ability to form interactions key to its biological activity.
3.3. MTT Assay of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
3.3.1. Cytotoxic Activity
- Genetic stability and ease of cultivation: CHO cells are well stabilized and easy to culture in vitro. They are adapted to grow both in suspension and on surfaces, which facilitates their use in various experiments.
- Capacity for genetic modification: CHO cells can be easily subjected to genetic modification, which allows them to be adapted to specific studies, such as assessing the cytotoxicity of drugs or studying the mechanisms of action of various substances.
- Non-cancerous: Because CHO cells are non-cancerous, they are a good model for studies that aim to assess the effect of substances on healthy eukaryotic cells. Using a tumour line could complicate the interpretation of the results because such cells have disturbed regulatory mechanisms, which may lead to atypical responses to the tested substances.
- Mammalian representativeness: CHO cells are of mammalian origin, making them more similar to human cells in structure, metabolism, and biochemical mechanisms. Therefore, they are a better model than bacterial or insect-origin cells.
- High sensitivity to toxins: CHO cells are sensitive to many toxic compounds, making them a good model for cytotoxicity testing. They can be used to evaluate various substances, from drugs to environmental toxins.
- Wide acceptance in regulatory research: The CHO cell line is accepted by many regulatory institutions, e.g., the FDA (Food and Drug Administration) and EMA (European Medicines Agency), as a model used in preclinical research. This acceptance is due to the well-established place of CHO cells in pharmacological and toxicological research.
- Participation in hydrogen bonds: Hydrogen bonds are crucial for the ligand–receptor recognition and stabilization of biological complexes, translating into higher biological activity.
- ○
- Hydroxyl groups (-OH) can act as hydrogen bond donors and acceptors. They facilitate the formation of specific interactions with the functional groups of target proteins (e.g., amino acid residues in the enzyme’s active site).
- ○
- The hydroxyimine group (=NOH) can act as a hydrogen bond donor and the nitrogen atom is an acceptor. This structure allows an oxime to form stable complexes with proteins or nucleic acids.
- Stabilization of enzymatic reactions: This property allows precise regulation of enzymatic functions.
- ○
- Hydroxyl groups can stabilize the transient states of enzymes during catalysis and interact with metals in the active sites of enzymes, acting as coordinating ligands (e.g., in metalloproteins).
- ○
- Hydroxyimine groups, thanks to the presence of nitrogen and oxygen atoms, can inhibit the activity of some enzymes by chelating metal ions (e.g., Zn2+ in enzymes of the metalloenzyme class).
- Impact on hydrophobic and hydrophilic interactions: Hydroxyl and hydroxyimine groups facilitate the introduction of polar sites on the surface of molecules and may increase the selectivity of interactions with biological targets that have polar surfaces (e.g., active sites of enzymes).
- Capacity for chemical reactions: Modifications may affect the biological activity or stability of the compound in the body. Hydroxyl and hydroxyimine groups can be the site of chemical reactions, e.g., phosphorylation, glycosylation or acetylation (important in biological processes), and the formation of covalent bonds with target proteins (e.g., irreversible enzyme inhibitors).
- Antioxidant properties: The hydroxyl group can neutralize reactive oxygen species (ROS) or reactive nitrogen species (RNS) by donating hydrogen. The hydroxyimine group has a similar activity thanks to a hydroxyl group constituting its fragment.
- Increasing specificity of action: Hydroxyl and hydroxyimine groups can improve the binding specificity of the ligand to the target protein, avoiding interactions with unrelated proteins.
3.3.2. Selectivity Index
3.3.3. Apoptotic Index
3.4. Molecular Docking of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
3.4.1. Discussion of Molecular Docking Results
3.4.2. Correlation of Molecular Docking Results, Biological Activity, and
Chemical Modifications
- Accuracy and repeatability of docking: Molecular docking was performed rigorously. For each compound, docking was performed three times in two significant pockets (C1 and C2) of the EGFR protein (PDB: 1M17). The results presented in the tables are the average of these replicates, ensuring their reliability. Using the CB-Dock2 server, based on machine learning algorithms, represents a modern approach to docking analysis. Unlike traditional methods such as AutoDock or Vina, CB-Dock2 automatically identifies the optimal binding pockets, which minimises subjective user decisions and increases the precision of the results.
- Correlation between docking and in vitro results: The two best examples of correlation between molecular docking results and in vitro results are as follows:
- ○
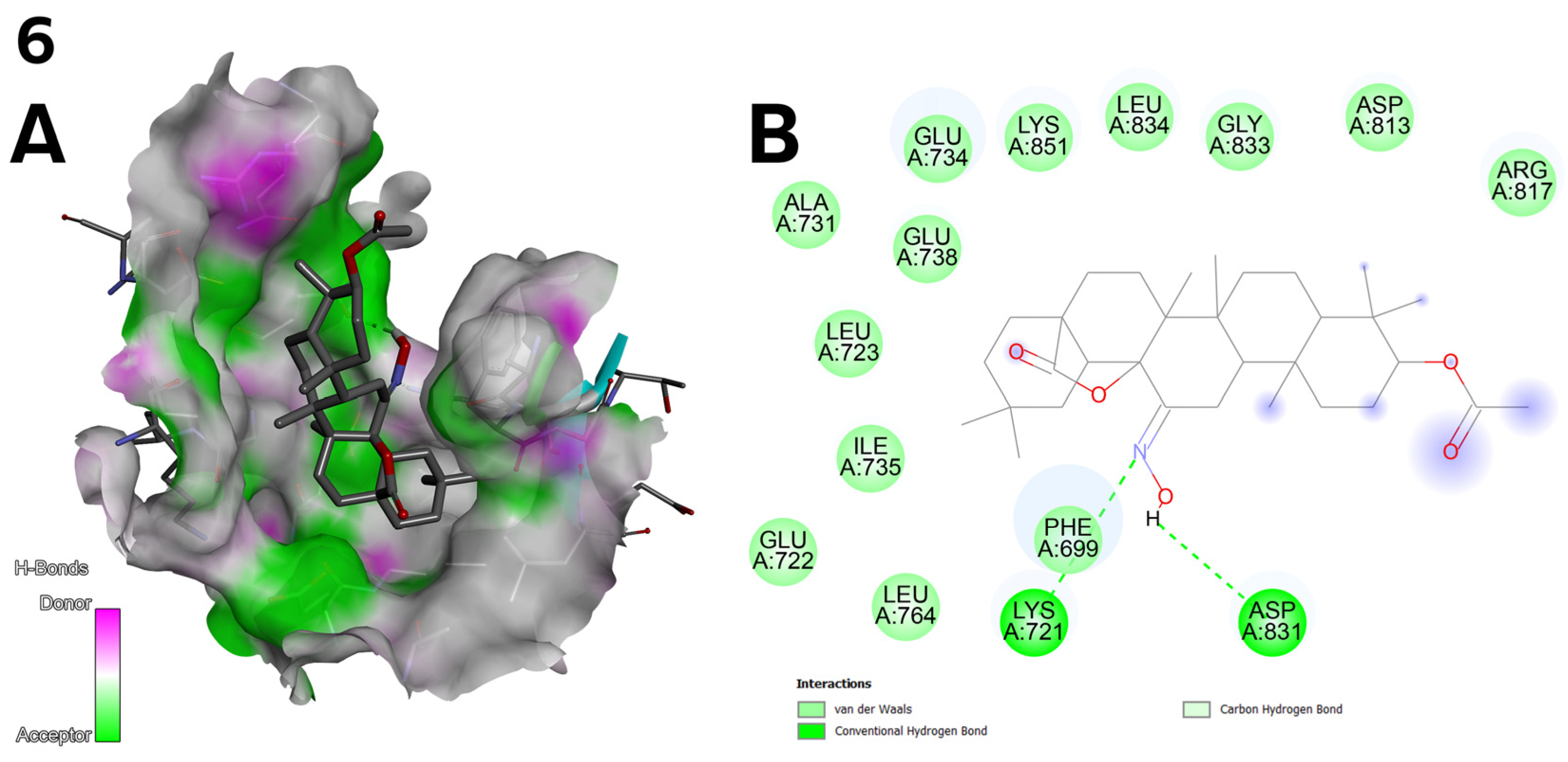
- Compound 6 (Figure 2) achieved the highest binding energy in the C1 (−9.8 kcal/mol) and C2 (−10.3 kcal/mol) pocket (Table 7) and showed the best IC50 values in the cytotoxic assay (1.27 µM for line A-549; Table 3). Strong hydrogen bonds with LYS 721 and ASP 831 and van der Waals interactions with protein residues explain its high activity.
- ○
- Correlation between docking and structural design: The structural modifications of the compounds were not accidental but resulted from rational design:
- ○
- A hydroxyimine group (C=NOH) at the C-12 position, as in compound 6 (Figure 2), was introduced to increase the number of possible hydrogen bonds with key residues of the EGFR protein. Docking results confirm that such bonds (e.g., with ASP 831) are responsible for the high activity of this compound.
- ○
- The bromine atom in the bromolactones 9–14 (Figure 3) was introduced to increase lipophilicity and allow additional halogen interactions. In the case of compound 10, the bromine enhances interactions with the C2 pocket, as reflected in the docking results.
- A critical approach to the results: As Table 3, Table 4 and Table 5 and Figure 11 present, not all compounds showed high biological activity, which is natural in finding new drugs. However, the lack of activity of some compounds (e.g., compounds 7 and 8; Figure 2) provided valuable information on the structural determinants of activity. The results indicate that the presence of two carbonyl groups in compound 7 reduces the ability to bind to EGFR. Moreover, the absence of the corresponding hydrogen bond-donating groups in compound 8 explains its low activity.
3.5. Antioxidant Activity of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
3.6. ADMETox Analysis of Oleanolic Acid Lactones (2–8) and Bromolactones (9–14)
3.7. Challenges in Translating Natural Compound Derivatives to Clinical Settings
- Complex chemical structures: the structures of derivatives of natural compounds are often characterized by a complicated structure. This factor may significantly complicate the synthesis of these derivatives on a large scale. The synthesis of such compounds often requires a complex cycle of chemical transformations, requiring expensive, hard-to-access or dangerous reagents and solvents, and the process of synthesizing, isolating and purifying these derivatives can be time-consuming, labour-intensive and energy-intensive. Obtaining high-purity compounds is time-consuming and expensive but, at the same time, crucial for clinical applications. In the case of the triterpene derivatives we presented, there is no need to use multi-stage chemical reaction systems, and the reagents used are readily available, low-cost and safe to use. Our optimization of the synthesis methods of these compounds allows us to obtain them in quantities necessary not only for micro-scale research but, if necessary, on a much larger scale.
- Low bioavailability: Many natural compound derivatives have poor solubility in water, which limits their absorption from the gastrointestinal tract. Moreover, such compounds may be unstable in a physiological environment (e.g. in the presence of enzymes or low pH). The high susceptibility to first-pass metabolism in the liver limits their availability in general circulation.
- Pharmacokinetic and pharmacodynamic problems: Many semi-synthetic derivatives have a short half-life in the body, requiring frequent administration or controlled release systems. Such derivatives may bind to plasma proteins in an unfavourable manner, which limits their biological activity. In addition, some natural compounds have low specificity of action, which may lead to side effects.
- Toxicity and side effects: Many natural compound derivatives are characterized by a narrow therapeutic index, a narrow margin of safety between the therapeutic and toxic doses. There may be unexpected toxic effects in the body that were not apparent in preclinical studies.
- Technological challenges: Extraction and purification of natural compounds from plant raw materials or microorganisms can be complicated and expensive. The production of such compounds on a large scale in industrial conditions is difficult, especially for compounds obtained from rare or difficult-to-access sources. Moreover, developing appropriate drug forms (e.g. capsules, tablets, injections) requires additional research and technology.
- Complexity of preclinical and clinical studies:
- ○
- In vitro and in vivo modelling: Difficulty in creating appropriate biological models to study the activity and toxicity of natural compounds.
- ○
- Transfer to clinical trials: Natural compounds may be potent in the laboratory, but their effectiveness in living organisms may be limited.
- ○
- Durability of clinical trials: Conducting clinical trials (phase I-III) is time-consuming and expensive, especially for compounds with uncertain therapeutic potential.
- Regulatory issues:
- ○
- Registration and approval: Registration requirements for natural medicines are stringent, especially regarding safety, effectiveness and quality.
- ○
- Standardization: Difficulties in standardizing natural compounds, especially when obtained from different sources (e.g. different plant species).
- ○
- Intellectual property: Problems related to patent protection of natural compounds that have often been known for a long time and may be considered unpatentable.
- Sustainable sourcing of raw materials: Natural compounds that are the subject of research by scientists in many fields of science often come from rare plants, microorganisms or marine organisms, the exploitation of which may be irreversible.
4. Materials and Methods
4.1. Materials
4.1.1. NMR
4.1.2. Syntheses, Column Chromatography, TLC and HP TLC and Antioxidant Activity
4.1.3. MTT Assay
4.1.4. Apoptosis
4.1.5. Antioxidant Activity
4.2. Methods
4.2.1. NMR Spectra of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.2. Synthesis of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.3. General Procedures
4.2.4. Obtaining of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.5. SAR Analysis of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.6. Cytotoxic Properties of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.7. Apoptotic Activity of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.8. Statistical Analysis
4.2.9. Molecular Docking for Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.10. Antioxidant Activity of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
4.2.11. ADMETox Analysis of Oleanolic Acid Lactones 2–8 and Bromolactones 9–14
5. Conclusions
- Limitations:
- ➢
- Some compounds (e.g., 7, 8, and 13) showed poor solubility and limited biological activity;
- ➢
- Despite using an advanced CB-Dock2 algorithm, molecular docking does not fully account for receptor flexibility.
- Strengths:
- ➢
- A high correlation between docking results and biological testing;
- ➢
- Comprehensive assessment of activities: cytotoxicity, pro-apoptotic potential, antioxidant effects, and ADMETox profile;
- ➢
- Data provide a basis for designing next-generation semisynthetic triterpenes.
- Changes at position C-12 (e.g., introducing Zn2+ chelators);
- Adding polar fragments to enhance bioavailability;
- Testing additional cancer cell lines and conducting in vivo pharmacokinetic analyses;
- Molecular dynamics (MD) simulations are planned to better understand ligand–receptor complex stability.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ±s | standard deviation |
| -OH | hydroxyl group |
| =NOH | hydroxyimine group |
| µg | microgram = 10−6 g |
| µg/mL | microgram per milliliter |
| µM | micromole = 10−6 moles |
| δ | chemical shift |
| 1H NMR | proton nuclear magnetic resonance |
| 1H, 2H, etc. | intensity of 1H NMR signal (intensity of 1 proton, 2 protons, etc.) |
| 13C NMR | carbon nuclear magnetic resonance |
| 2D | two-dimensional |
| 3D | three-dimensional |
| AcOEt | ethyl acetate |
| ADMETox | Adsorption, Distribution, Metabolisms, Excretion, and Toxicity |
| AI | Apoptotic Index |
| ATTC | American Type Culture Collection |
| Å | angstrom |
| Å3 | cubic angstrom |
| BMS | alert for mapping molecular promiscuity and identification of undesirable and reactive compounds |
| C-3, C-13, etc. | carbon atom at the 3, 12, etc. position of oleanane skeleton |
| C3-H, C12-H, etc. | hydrogen atom attached to the C-3, C-12, etc. atom of oleanane skeleton |
| C18-Hβ | hydrogen atom at β orientation attached to the C-18 atom of oleanane skeleton |
| C6H6 | benzene |
| calcd. | calculated |
| CDCl3 | deutherated chloroform |
| CH | tertiary carbon atom |
| CH2 | secondary carbon atom |
| CH3 | primary carbon atom |
| Comp. | compound |
| Cq | quaternary carbon atom |
| CUPRAC | Cupric Ion Reducing Antioxidant Capacity |
| CYP | cytochrome P |
| dd | doublet of doublets |
| decomp. | decomposition |
| DMSO-d6 | deutherated dimethylsulfoxide |
| DNA | deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl hydrate |
| DPPH· | 2,2-diphenyl-1-picrylhydrazyl hydrate radical |
| Elem. Anal. | Elemental analysis |
| ELISA | enzyme-linked immunosorbent assay; a commonly used laboratory test to detect antibodies in the blood |
| EMA | European Medicines Agency |
| EtOH | ethanol |
| F-12K | Kaighn’s Modification of Ham’s F-12 Medium |
| fChar | formal charge |
| FDA | Food and Drug Administration |
| HP TLC | High Performance Liquid Chromatography |
| HR MS | High Resolution Mass Spectrometry |
| Hz | Hertz |
| IC50 | half maximal inhibitory concentration |
| J | coupling constant |
| JSME | Japan Society of Mechanical Engineers |
| kcal/mol | a unit to measure an amount of energy per number of molecules/atoms or other perticles |
| LogD | logP at physiological pH 7.4 |
| lit. | literature, according to literature data |
| logP | logaritm of the octanol/water partition coefficient |
| logS | logaritm of the aqueous solubility |
| m-CPBA | m-chloroperbenzoic acid |
| MHz | megahertz = 106 Hertzes |
| mmol | milimol = 10−3 moles |
| MMPs | matrix metalloproteinases |
| MNA | Multilevel Neighborhoods of Atoms |
| M.p. | melting point |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear-kappa B |
| nHA | number of hydrogen bond acceptors |
| nHD | number of hydrogen bond donors |
| nHet | number of heteroatoms |
| No. | number |
| NP | Natural Product-likeness |
| nRig | number of rigid bonds |
| nRing | number of rings |
| nRot | number of rotatable bonds |
| OA | oleanolic acid |
| obtd. | obtained |
| Pa | probability of activity |
| PAINS | Pan Assay Interference Compounds |
| PASS | Prediction of Activity Spectra for Substances |
| Pgp | p-glycoprotein |
| Pi | probability of inactivity |
| ppm | part per million |
| precipt. | precipitated |
| Rf | retension factor |
| RMPI 1640 | Roswell Park Memorial Institute 1640 medium |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| r.t. | room temperature |
| s | singlet |
| SAR | Structure—Activity Relationship |
| SI | Selectivity Index |
| t | triplet |
| TLC | Thin Liquid Chromatography |
| TMS | tetramethylsilane |
| UFF | Universal Force Field |
| VIS | visible light |
| VIS | visible light |
| Zn2+ | divalent zinc cation |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Can. J. Clin. 2021, 71, 191–280. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.fr (accessed on 27 December 2024).
- Gullett, N.P.; Ruhul Amin, A.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef]
- Yeung, M.F.; Che, C.T. A review on the presence of oleanolic acid in natural products. Nat. Proda Medica 2009, 2, 77–290. [Google Scholar]
- Krukiewicz, K.; Jarosz, T.; Zak, J.K.; Łapkowski, M.; Ruszkowski, P.; Bobkiewicz-Kozłowska, T.; Bednarczyk-Cwynar, B. Advancing the delivery of anticancer drugs: Conjugated polymer/triterpenoid composite. Acta Biomater. 2015, 19, 158–165. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, K.; Pfüller, U.; Scheffler, A. Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef]
- Guinda, Á.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Li, H.B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef]
- Sen, A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef]
- Caltana, L.; Rutolo, D.; Nieto, M.L.; Brusco, A. Further evidence for the neuroprotective role of oleanolic acid in a model of focal brain hypoxia in rats. Neurochem. Int. 2014, 79, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, J.; Liu, B.B.; Luo, L.; Liu, Q.; Geng, D. BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J. Psychiatry Neurosci. 2014, 39, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, L.; Li, J.; Ji, J.; Chen, K.; Yu, Q.; Li, S.; Feng, J.; Liu, T.; Zhang, J.; et al. Alleviation of hepatic ischemia reperfusion injury by oleanolic acid pretreating via reducing HMGB1 release and inhibiting apoptosis and autophagy. Mediat. Inflamm. 2019, 2019, 3240713–3240722. [Google Scholar] [CrossRef]
- Chen, F.F.; Wang, J.T.; Zhang, L.X.; Xing, S.F.; Wang, Y.X.; Wang, K.; Deng, S.L.; Zhang, J.Q.; Tang, L.; Wu, H.S. Oleanolic acid derivative DKS26 exerts anti-diabetic and hepatoprotective effects in diabetic mice and promotes glucagon-like peptide-1 secretion and expression in intestinal cells. Br. J. Pharmacol. 2017, 174, 2912–2928. [Google Scholar] [CrossRef]
- Zhao, D.; Shu, B.; Wang, C.; Zhao, Y.; Cheng, W.; Sha, N.; Li, C.; Wang, Q.; Lu, S.; Wang, Y. Oleanolic acid exerts inhibitory effects on the late stage of osteoclastogenesis and prevents bone loss in osteoprotegerin knockout mice. J. Cell Biochem. 2020, 121, 152–164. [Google Scholar] [CrossRef]
- Melo, T.S.; Gattass, C.R.; Soares, D.C.; Cunha, M.R.; Ferreira, C.; Tavares, M.T.; Saraiva, E.; Parise-Filho, R.; Braden, H.; Delorenzi, J.C. Oleanolic acid (OA) as an antileishmanial agent: Biological evaluation and in silico mechanistic insights. Parasitol. Int. 2016, 65, 227–237. [Google Scholar] [CrossRef]
- Córdova, C.; Gutiérrez, B.; Martínez-García, C.; Martín, R.; Gallego-Muñoz, P.; Hernández, M.; Nieto, M.L. Oleanolic acid controls allergic and inflammatory responses in experimental allergic conjunctivitis. PLoS ONE 2014, 9, e91282. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Lee, Y.C. Oleanolic acid suppresses ovalbumin-induced airway inflammation and Th2-mediated allergic asthma by modulating the transcription factors T-bet, GATA-3, RORγt and Foxp3 in asthmatic mice. Int. Immunopharmacol. 2014, 18, 311–324. [Google Scholar] [CrossRef]
- Madlala, H.P.; Metzinger, T.; van Heerden, F.R.; Musabayane, C.T.; Mubagwa, K.; Dessy, C. Vascular endothelium-dependent and independent actions of oleanolic acid and its synthetic oleanane derivatives as possible mechanisms for hypotensive effects. PLoS ONE 2016, 11, e0147395. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Q.; Shen, J.; Chen, C.P.; Ma, X.; Lin, C.; Ouyang, Q.; Xuan, C.X.; Liu, J.; Sun, H.B.; Liu, J. Lipid-lowering effects of oleanolic acid in hyperlipidemic patients. Chin. J. Nat. Med. 2018, 16, 339–346. [Google Scholar] [CrossRef]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK–p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, C.C.; Hong, Y.R.; Zhu, X.C. Anticancer activity of novel oleanolic acid methyl ester derivative in HeLa cervical cancer cells is mediated through apoptosis induction and reactive oxygen species production. Bangladesh J. Pharmacol. 2015, 10, 896–902. [Google Scholar] [CrossRef]
- Lùcio, K.A.; da Graça Rocha, G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Potočnjak, I.; Šimić, L.; Vukelić, I.; Batičić, L.; Domitrović, R. Oleanolic acid induces HCT116 colon cancer cell death through the p38/FOXO3a/Sirt6 pathway. Chem. Biol. Interact. 2022, 363, 110010. [Google Scholar] [CrossRef]
- Ovesná, Z.; Kozics, K.; Slameňová, D. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mutat. Res. 2006, 600, 131–137. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Chang, J.Y.; Kuo, C.C.; Chang, C.Y.; Kuo, Y.H. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry 2005, 66, 495–501. [Google Scholar] [CrossRef]
- Gülçin, I.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant activity of lignans from fringe 579 tree (Chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S. Oxidized LDL and atherogenesis. Ann. N. Y. Acad. Sci. 1999, 874, 134–137. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Liu, R.; Chen, H.; Bai, H.; Liang, X.; Zhang, X.; Wang, Z.; Li, W.; Hai, C. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Boyd, L.; Riva, F. Triterpenoids as reactive oxygen species modulators of cell fate. Chem. Res. Toxicol. 2022, 35, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Makuch, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Pełech, R.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of betulin and its derivatives. Molecules 2021, 26, e3435. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Zaprutko, L.; Ruszkowski, P.; Hładoń, B. Anti-cancer effect of A-ring or/and C-ring modified oleanolic acid derivatives on KB, MCF-7 and HeLa cell lines. Org. Biomol. Chem. 2012, 10, 2201–2205. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Atamanyuk, D.; Lesyk, R.; Zaprutko, L. Hybrids of oleanolic acid with norbornene-2,3-dicarboximide-N-carboxylic acids as potential anticancer agents. Acta Pol. Pharm. Drug Res. 2017, 74, 827–835. [Google Scholar]
- Günther, A.; Zalewski, P.; Sip, S.; Ruszkowski, P.; Bednarczyk-Cwynar, B. Oleanolic acid dimers with potential application in medicine—Design, synthesis, physico-chemical characteristics, cytotoxic and antioxidant activity. Int. J. Mol. Sci. 2024, 25, 6989. [Google Scholar] [CrossRef]
- Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic acid co-amorphous systems with amino acids for improved solubility and antioxidant activity. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef] [PubMed]
- García-Granados, G.; López, P.E.; Melguizo, E.; Parra, A.; Simeó, Y. Partial synthesis of C-ring derivatives from oleanolic and maslinic acids. Formation of several triene systems by chemical and photochemical isomerisation processes. Tetrahedron 2004, 60, 1491–1503. [Google Scholar] [CrossRef]
- Hichri, F.; Jannet, H.B.; Cheriaa, J.; Jegham, S.; Mighri, Z. Antibacterial activities of a few prepared derivatives of oleanolic acid and of other natural triterpenic compounds. C. R. Chim. 2003, 6, 473–483. [Google Scholar] [CrossRef]
- Ali, M.S.; Jahangir, M.; ul Hussan, S.S.; Choudhary, M.I. Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 2002, 60, 295–299. [Google Scholar] [CrossRef]
- Froelich, A.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Gzella, A. Beckmann rearrangement within the ring C of oleanolic acid lactone: Synthesis, structural study and reaction mechanism analysis. J. Mol. Struct. 2017, 1136, 173–181. [Google Scholar] [CrossRef]
- Jakubowska, K.; Guzińska-Ustymowicz, K.; Famulski, W.; Cepowicz, D.; Jagodzińska, D.; Pryczynicz, A. Reduced expression of caspase-8 and cleaved caspase-3 in pancreatic ductal adenocarcinoma cells. Oncol. Lett. 2016, 11, 1879–1884. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Napolitano, G. The role of the transcription factor nuclear factor-kappa B in thyroid autoimmunity and cancer. Front. Endocrinol. 2018, 9, 471–478. [Google Scholar] [CrossRef]
- Potapskyi, E.; Kustrzyńska, K.; Łażewski, D.; Skupin-Mrugalska, P.; Lesyk, R.; Wierzchowski, M. Introducing bromine in the molecular structure as a good strategy to the drug design. J. Med. Sci. 2024, 93, e1128. [Google Scholar] [CrossRef]
- Tihanyi, B.; Nyitray, L. Recent advances in CHO cell line development for recombinant protein production. Drug Discov. Today Technol. 2020, 38, 25–34. [Google Scholar] [CrossRef]
- Sukandar, E.R.; Kaennakam, S.; Raab, P.; Nost, X.; Rassamee, K.; Bauer, R.; Siripong, P.; Ersam, T.; Tip-pyang, S.; Chavasiri, W. Cytotoxic and anti-inflammatory activities of dihydroisocoumarin and xanthone derivatives from Garcinia picrorhiza. Molecules 2021, 26, 6626. [Google Scholar] [CrossRef]
- Jebli, N.; Gupta, G.K. Recent Advances on Oximes Derivatives: Green Catalysis and Various Synthetic Routes. Curr. Green Chem. 2024, 12, 170–184. [Google Scholar] [CrossRef]
- Golubev, N.; Denisov, G.; Gindin, V.; Ligay, S.; Limbach, H.; Smirnov, S. The role of short hydrogen bonds in mechanisms of enzymatic action. J. Mol. Struct. 1994, 322, 83–91. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Zaprutko, L. Oleanolic acid A-lactams inhibit the growth of HeLa, KB, MCF-7 and Hep-G2 cancer cell lines at micromolar concentrations. Anticancer Agents Med. Chem. 2016, 16, 579–592. [Google Scholar] [CrossRef]
- Peńa-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Delgado, V.; Sepúlveda, S.; Benites, J.; Theoduloz, C.; Calderon, P.B.; Muccioli, G.G. Synthesis and cytotoxic activity on human cancer cells of novel isoquinolinequinone-amino acid derivatives. Molecules 2016, 21, 1199. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Yalçın, G. Antioxidant capacity of a turkish traditional alcoholic drink, Raki. Pol. J. Food Nutr. Sci. 2016, 66, 167–171. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Mencherini, T.; Celano, R.; Mouhoubi, Z.; Tamendjari, A.; Aquino, R.P.; Rastrelli, L. Chemical composition and antioxidant activity of algerian propolis. J. Agric. Food Chem. 2013, 61, 5080–5088. [Google Scholar] [CrossRef]
- Available online: https://admetmesh.scbdd.com (accessed on 10 December 2024).
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A review. Curr. Drug Targets. 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, B.C.; Kuszak, A.J.; Bloss, G.; Fukagawa, N.K.; Hoffman, F.A.; Jafari, M.; Barrett, B.; Brown, P.N.; Bushman, F.D.; Casper, S.J.; et al. Improving natural product research translation: From source to clinical trial. FASEB J. 2020, 34, 41–65. [Google Scholar] [CrossRef]
- Dzobo, K. The role of natural products as sources of therapeutic agents for innovative drug discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Wrzeciono, U.; Małecki, I.; Budzianowski, J.; Kieryłowicz, H.; Zaprutko, L.; Beimcik, E.; Kostępska, H. Nitrogenous triterpene derivatives. 10. Hemisuccinates of some oleanolic acid derivatives and their antiulcer effect. Die Pharm. 1985, 40, 542–544. [Google Scholar]
- Ahmad, V.U.; Atta-ur-Rahman, A.U.R. Handbook of Natural Products Data. Volume 2. Pentacyclic Triterpenoids; Elsevier: Amsterdam, The Netherlands, 1994; pp. 1–509. [Google Scholar]
- Lewis, K.G.; Tucker, D.J. The separation of substituted olean-12-en-28-oic acids from the corresponding urs-12-en-28-oic acid isomers. Aust. J. Chem. 1983, 36, 2297–2305. [Google Scholar] [CrossRef]
- Martinez, A.; Perojil, A.; Rivas, F.; Medina-O’Donnell, M.; Parra, A. Semi-synthesis of taraxerane triterpenoids from oleanolic acid. Tetrahedron 2014, 71, 792–800. [Google Scholar] [CrossRef]
- Available online: https://way2drug.com (accessed on 20 November 2024).
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.Y. Prediction of biological activity spectra for substances evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef]
- Poroikov, V.; Filimonov, D.A.; Gloriozova, T.; Lagunin, A. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Bull. Acad. Sci. USSR Div. Chem. Sci. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jarosz, T.; Krukiewicz, K. Enhancing anticancer activity through the combination of bioreducing agents and triterpenes. Future Med. Chem. 2018, 10, 511–525. [Google Scholar] [CrossRef]
- Available online: https://www.cheminfo.org/Chemistry/Cheminformatics/FormatConverter/index.html (accessed on 10 November 2024).
- Bednarczyk-Cwynar, B.; Ruszkowski, P. Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies. Pharmaceutics 2024, 16, 86. [Google Scholar] [CrossRef]
| 1.000–0.950 | 0.949–0.900 | 0.899–0.850 | 0.849–0.800 | 0.799–0.750 | 0.749–0.700 | <0.700 |
| Activity | Pa Factor (and Pi Factor) of Compounds 1–8 (Pa ≥ 0.700) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 (OA) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Acylcarnitine hydrolase inhibitor | ≤0.700 | 0.731 (0.022) | 0.779 (0.016) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Antileukemic | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | 0.755 (0.005) | ≤0.700 |
| Antineoplastic | 0.876 (0.005) | 0.934 (0.004) | 0.928 (0.005) | 0.951 (0.004) | 0.940 (0.004) | 0.947 (0.004) | 0.951 (0.004) | 0.939 (0.004) |
| Antineoplastic (colon cancer) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | 0.841 (0.004) | ≤0.700 | 0.801 (0.004) |
| Antineoplastic (colourectal cancer) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | 0.843 (0.004) | ≤0.700 | 0.803 (0.004) |
| Antineoplastic (lung cancer) | 0.766 (0.005) | 0.863 (0.003) | 0.855 (0.004) | 0.848 (0.004) | 0.825 (0.004) | 0.808 (0.004) | 0.815 (0.004) | 0.775 (0.004) |
| Antinociceptive | ≤0.700 | ≤0.700 | 0.710 (0.003) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Apoptosis agonist | 0.901 (0.004) | 0.879 (0.005) | 0.889 (0.004) | 0.898 (0.004) | 0.898 (0.004) | 0.881 (0.005) | 0.921 (0.004) | 0.884 (0.005) |
| Caspase 3 stimulant | 0.984 (0.002) | 0.882 (0.004) | 0.923 (0.003) | 0.905 (0.003) | 0.962 (0.003) | 0.920 (0.003) | 0.764 (0.008) | 0.969 (0.002) |
| Caspase 8 stimulant | 0.914 (0.001) | 0.835 (0.001) | 0.830 (0.001) | 0.846 (0.001) | 0.854 (0.001) | 0.724 (0.003) | 0.747 (0.003) | 0.735 (0.003) |
| Chemopreventive | 0.937 (0.002) | 0.742 (0.005) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| CYP2J substrate | 0.808 (0.019) | ≤0.700 | 0.734 (0.039) | ≤0.700 | 0.774 (0.027) | ≤0.700 | ≤0.700 | ≤0.700 |
| Hepatic disorders treatment | 0.834 (0.004) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Hepatoprotectant | 0.930 (0.002) | 0.755 (0.005) | 0.705 (0.007) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Hypolipemic | ≤0.700 | 0.805 (0.007) | 0.757 (0.010) | 0.783 (0.008) | 0.774 (0.009) | 0.753 (0.010) | ≤0.700 | 0.732 (0.011) |
| Insulin promotor | 0.869 (0.004) | ≤0.700 | 0.760 (0.004) | ≤0.700 | 0.753 (0.004) | ≤0.700 | ≤0.700 | ≤0.700 |
| Membrane integrity antagonist | 0.928 (0.002) | 0.776 (0.009) | ≤0.700 | 0.704 (0.014) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Mucomembranous protector | 0.894 (0.005) | 0.727 (0.045) | ≤0.700 | 0.742 (0.038) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Nitric oxide antagonist | ≤0.700 | 0.752 (0.003) | 0.775 (0.003) | 0.781 (0.003) | 0.777 (0.003) | 0.701 (0.003) | 0.823 (0.002) | 0.702 (0.003) |
| Oxidoreductase inhibitor | 0.904 (0.002) | 0.774 (0.008) | 0.714 (0.014) | 0.801 (0.006) | 0.773 (0.008) | ≤0.700 | ≤0.700 | ≤0.700 |
| Phosphatase inhibitor | 0.894 (0.001) | 0.711 (0.011) | 0.756 (0.005) | 0.712 (0.011) | 0.761 (0.005) | ≤0.700 | ≤0.700 | 0.729 (0.008) |
| T-17β-dehydratase (NADP+) inhibitor | 0.892 (0.007) | ≤0.700 | 0.746 (0.040) | ≤0.700 | 0.789 (0.028) | ≤0.700 | ≤0.700 | ≤0.700 |
| Transcription factor NF kappa B stimulant | 0.954 (0.001) | 0.817 (0.002) | 0.878 (0.002) | 0.827 (0.002) | 0.876 (0.002) | 0.784 (0.003) | 0.881 (0.002) | 0.844 (0.002) |
| Transcription factor stimulant | 0.954 (0.001) | 0.817 (0.002) | 0.878 (0.002) | 0.827 (0.002) | 0.876 (0.002) | 0.784 (0.003) | 0.881 (0.002) | 0.844 (0.002) |
| 1.000–0.950 | 0.949–0.900 | 0.899–0.850 | 0.849–0.800 | 0.799–0.750 | 0.749–0.700 | <0.700 |
| Activity | Pa Factor (and Pi Factor) of Compounds 9–14 (Pa ≥ 0.700) | |||||
|---|---|---|---|---|---|---|
| 9 | 10 | 11 | 12 | 13 | 14 | |
| Acylcarnitine hydrolase inhibitor | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Antileukemic | 0.707 (0.005) | 0.784 (0.004) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Antineoplastic | 0.904 (0.005) | 0.916 (0.005) | 0.871 (0.005) | 0.860 (0.006) | 0.785 (0.014) | 0.797 (0.012) |
| Antineoplastic (colon cancer) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Antineoplastic (colourectal cancer) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Antineoplastic (lung cancer) | 0.769 (0.005) | 0.738 (0.005) | ≤0.700 | 0.746 (0.005) | ≤0.700 | ≤0.700 |
| Antinociceptive | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Apoptosis agonist | 0.872 (0.005) | 0.895 (0.004) | 0.825 (0.006) | 0.724 (0.013) | ≤0.700 | ≤0.700 |
| Caspase 3 stimulant | 0.811 (0.005) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Caspase 8 stimulant | 0.777 (0.002) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Chemopreventive | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| CYP2J substrate | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Hepatic disorders treatment | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Hepatoprotectant | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Insulin promotor | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Interleukin 2 agonist | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | 0.732 (0.002) |
| Membrane integrity antagonist | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Mucomembranous protector | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Nitric oxide antagonist | 0.706 (0.003) | 0.762 (0.003) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Oxidoreductase inhibitor | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Phosphatase inhibitor | 0.740 (0.007) | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| T-17β-dehydratase (NADP+) inhibitor | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 | ≤0.700 |
| Transcription factor NF kappa B stimulant | 0.857 (0.002) | 0.849 (0.002) | 0.747 (0.003) | 0.786 (0.003) | ≤0.700 | ≤0.700 |
| Transcription factor stimulant | 0.857 (0.002) | 0.849 (0.002) | 0.747(0.003) | 0.786 (0.003) | ≤0.700 | ≤0.700 |
| ≤5.00 μM | 5.01–10.00 μM | 10.01–20.00 μM | 20.01–50.00 μM | ≥50.01 μM |
| Comp. No. | IC50 [μM] (±s) | ||||
|---|---|---|---|---|---|
| CHO | A-549 | PC-3 | SKOV-3 | HDFs | |
| 1 | 9.16 (0.16) | 8.79 (±0.20) * | 18.63 (±0.05) ** | 18.81 (±0.09) ** | 24.87 (±0.04) ** |
| 2 | 4.19 (0.03) | 4.68 (0.24) | 3.26 (0.06) | 2.96 (0.01) | 7.51 (0.09) |
| 3 | 46.96 (0.17) | 23.27 (0.15) | 21.88 (0.08) | 21.09 (0.17) | 38.27 (0.19) |
| 4 | 52.66 (0.18) | 24.67 (0.16) | 25.91 (0.16) | 22.81 (0.15) | 41.95 (0.12) |
| 5 | 38.24 (0.17) | 38.03 (0.17) | 31.02 (0.07) | 36.99 (0.02) | 51.84 (0.11) |
| 6 | 5.49 (0.15) | 1.27 (0.01) | 1.88 (0.01) | 1.73 (0.04) | 3.91 (0.04) |
| 7 | >100 | 66.14 (0.17) | >100 | >100 | >100 |
| 8 | >100 | >100 | >100 | >100 | >100 |
| 9 | 42.01 (0.15) | 29.87 (0.15) | 26.09 (0.03) | 26.41 (0.13) | 51.03 (0.19) |
| 10 | 21.36 (0.11) | 15.55 (0.21) | 12.94 (0.06) | 12.05 (0.11) | 39.11 (0.11) |
| 11 | 55.41 (0.13) | 34.63 (0.12) | 25.13 (0.11) | 21.75 (0.09) | 28.94 (0.15) |
| 12 | 9.23 (0.04) | 7.88 (0.11) | 6.27 (0.03) | 6.22 (0.01) | 9.13 (0.06) |
| 13 | >100 | 89.32 (0.24) | 73.09 (0.18) | 75.83 (0.19) | 89.37 (0.16) |
| 14 | 86.95 (0.22) | 66.23 (0.24) | 65.88 (0.12) | 68.31 (0.18) | 84.09 (0.11) |
| 7.00–7.99 | 6.00–6.99 | 5.00–5.00 | 4.00–4.99 |
| Comp. No. | Cell Line, AI | |
|---|---|---|
| PC-3 | SKOV-3 | |
| 1 | 5.27 (0.04) | 5.16 (0.01) |
| 2 | 4.76 (0.02) | 4.71 (0.11) |
| 6 | 7.28 (0.02) | 7.22 (0.06) |
| 10 | 5.82 (0.04) | 5.05 (0.03) |
| 12 | 5.43 (0.05) | 5.11 (0.01) |
| CurPocket ID | Cavity Volume (Å3) | Centre (x, y, z) | Cavity Size (x, y, z) | Comments |
|---|---|---|---|---|
| C1 | 991 | 36, 8, 51 | 14, 17, 13 | The largest pocket, a typical EGFR active site |
| C2 | 665 | 24, 0, 54 | 15, 12, 9 | Strong bonds, particularly for bromolactones |
| C3 | 416 | 31, −4, 40 | 10, 14, 7 | Smaller pocket, less favourable results |
| C4 | 184 | 40, 18, 67 | 8, 9, 5 | A small cavity with limited bonding possibilities |
| C5 | 173 | 3, 15, 63 | 6, 10, 7 | Small pocket, hydrophobic interactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk-Cwynar, B.; Günther, A.; Ruszkowski, P.; Sip, S.; Zalewski, P. Oleanolic Acid Lactones as Effective Agents in the Combat with Cancers—Cytotoxic and Antioxidant Activity, SAR Analysis, Molecular Docking and ADMETox Profile. Int. J. Mol. Sci. 2025, 26, 4099. https://doi.org/10.3390/ijms26094099
Bednarczyk-Cwynar B, Günther A, Ruszkowski P, Sip S, Zalewski P. Oleanolic Acid Lactones as Effective Agents in the Combat with Cancers—Cytotoxic and Antioxidant Activity, SAR Analysis, Molecular Docking and ADMETox Profile. International Journal of Molecular Sciences. 2025; 26(9):4099. https://doi.org/10.3390/ijms26094099
Chicago/Turabian StyleBednarczyk-Cwynar, Barbara, Andrzej Günther, Piotr Ruszkowski, Szymon Sip, and Przemysław Zalewski. 2025. "Oleanolic Acid Lactones as Effective Agents in the Combat with Cancers—Cytotoxic and Antioxidant Activity, SAR Analysis, Molecular Docking and ADMETox Profile" International Journal of Molecular Sciences 26, no. 9: 4099. https://doi.org/10.3390/ijms26094099
APA StyleBednarczyk-Cwynar, B., Günther, A., Ruszkowski, P., Sip, S., & Zalewski, P. (2025). Oleanolic Acid Lactones as Effective Agents in the Combat with Cancers—Cytotoxic and Antioxidant Activity, SAR Analysis, Molecular Docking and ADMETox Profile. International Journal of Molecular Sciences, 26(9), 4099. https://doi.org/10.3390/ijms26094099











