Dynamic Model of Serotonin Presynapse and Its Application to Suicide Attempt in Patients with Bipolar Disorder
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics of Study Participants and Genetic Association Study
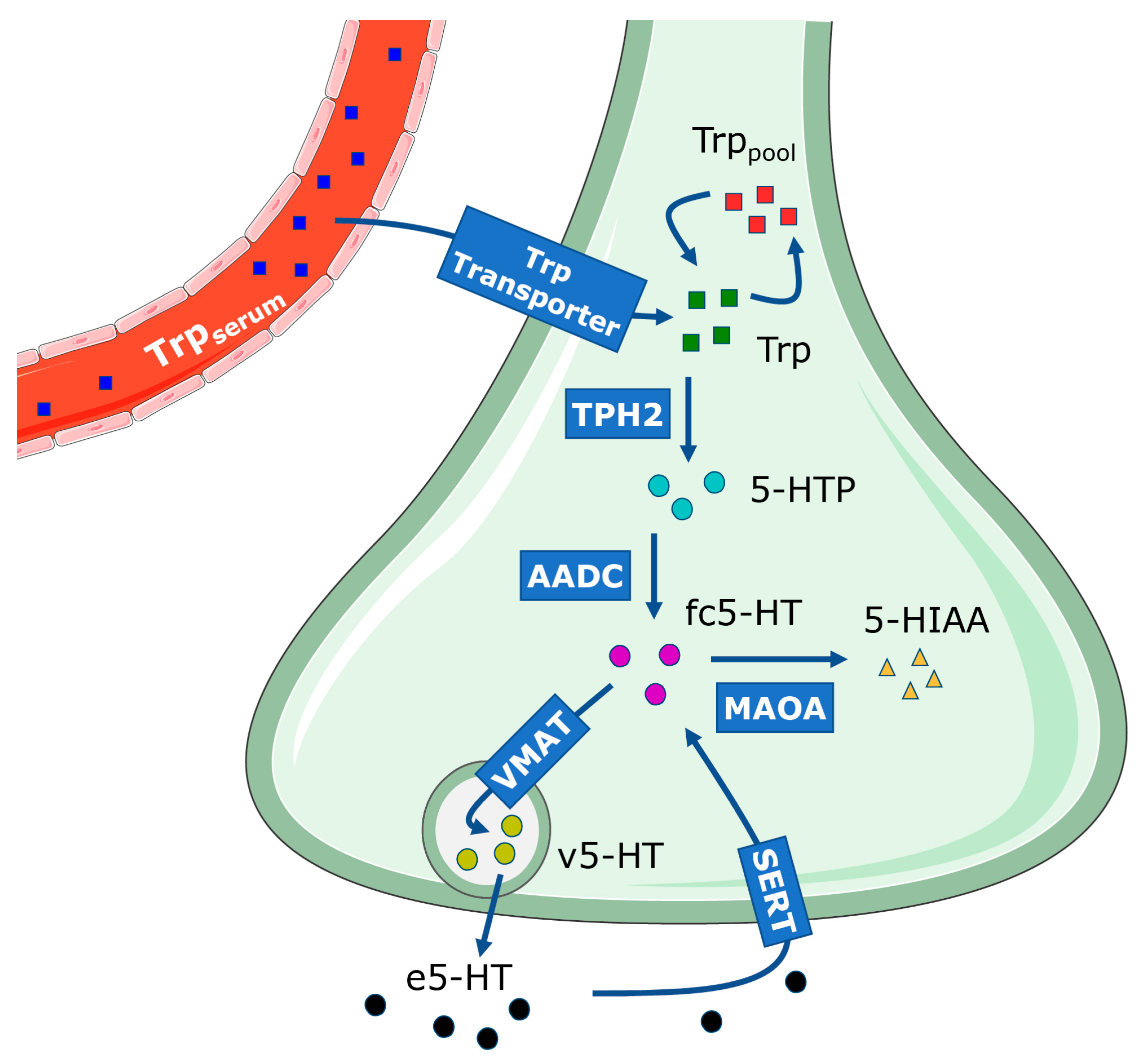
2.2. Description of the Dynamic Model of 5-HT Presynapse
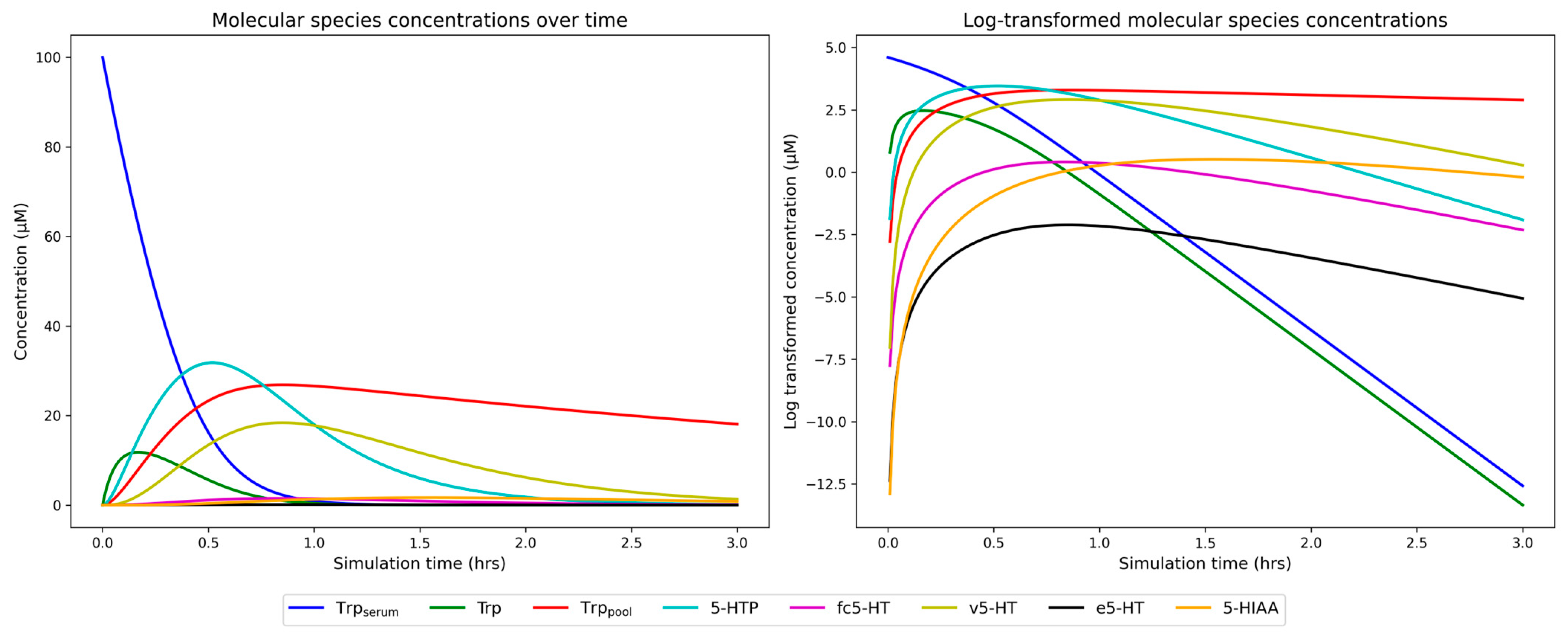
2.3. Simulation of Dynamic 5-HT Presynapse Model on Unaffected Individuals
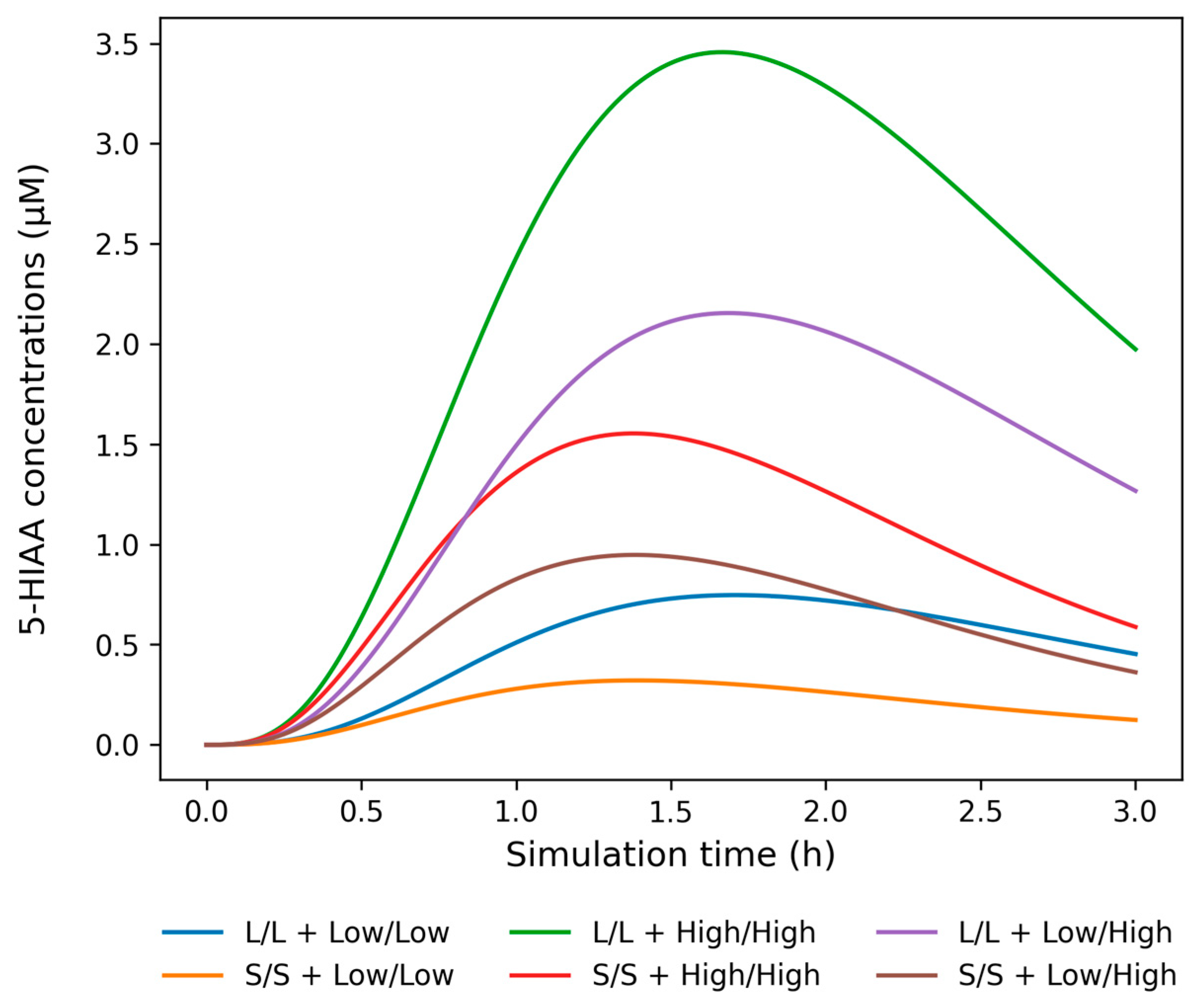
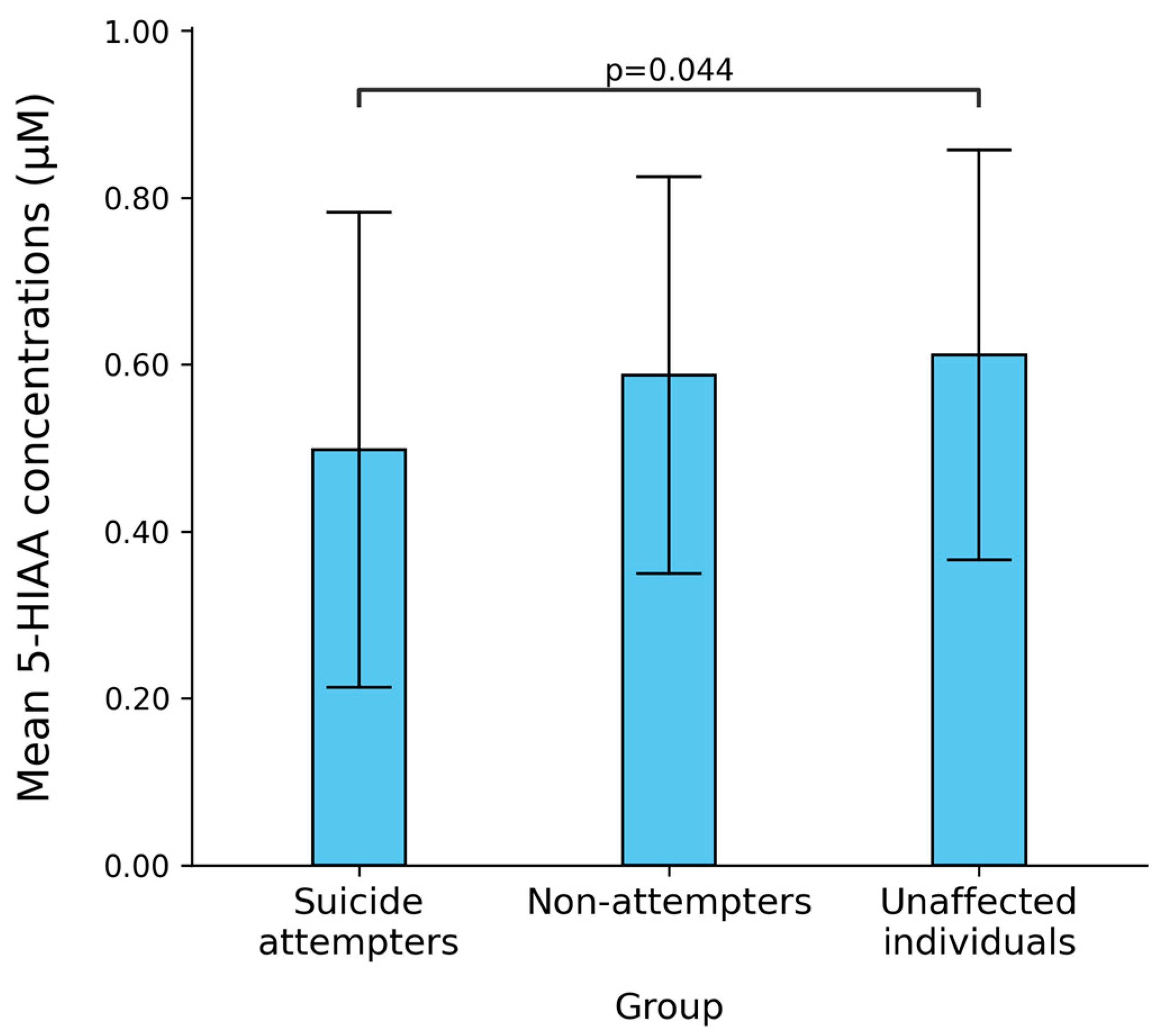
2.4. Simulation on Bipolar Disorder Patients with and Without Suicide Attempt History
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Genotyping and Genetic Association
4.3. Dynamic Model of the 5-HT Presynapse
4.3.1. Model Equations
- (1)
- The production of 5-HT from Trpserum is a complex process influenced by the binding of the Trp transporter to serum albumin, which can limit its availability in the presynapse, as well as competition with other amino acids for cellular uptake. For simplicity, we modeled the transport of Trp into the presynapse as a single step with transporter kinetics as described by Best et al. [45]. We introduced a single input of 100 µM Trpserum, based on findings from [45], who reported a concentration of 96 µM, allowing for consistency across comparisons. Given that Trp levels vary across brain regions as well as with dietary intake [36,80,81], we chose to use a higher concentration to ensure measurable effects of genetic variants. In the equationd[Trpserum]/dt = −Vtrpin,Vtrpin represents the transport rate of Trp from serum into the cell, with a negative sign indicating continuous depletion of the substrate. As our model represents an approximation of the 5-HT presynapse with a focus on 5-HT synthesis, reuptake, and degradation, we did not explicitly model the transport of Trp through the cytoplasm of the cell. Instead, Trp availability was captured through the change of Trpserum, Trppool, and Trp molecular species.
- (2)
- Inside the cell, Trp can be used either in 5-HT synthesis or in various other cellular processes (Trppool):d[Trppool]/dt = Vpool_forward − Vpool_reverse − [Trppool] × kpool_removal.Here, Vpool_forward and Vpool_reverse represent the rates of filling and leakage from the Trppool into Trp available for 5-HT synthesis. The model assumes that the rate of depletion of Trppool is proportional to the amount of Trp present, determined by the kpool_removal constant, where kpool_removal = 0.2 h−1 [45].Table 3. Reaction rates of model differential equations and their parameter values.
Reaction Rate Equations Parameter Parameter Value (Unit) Reference Vtrpin = (Vmaxtrpin × [Trpserum])/(Kmtrpin + [Trpserum]) Kmtrpin
Vmaxtrpin64 (µM)
400 (µM/h)[45] Vpool_forward = k1 × [Trp] k1 6 (µM/h) [45] Vpool_reverse = kminus1 × [Trp] kminus1 0.6 (µM/h) [45] VTPH2 = (VmaxTPH2 × [Trp])/(KmTPH2 + [Trp]) KmTPH2
VmaxTPH244 (µM)
/ 1[82] VAADC = (VmaxAADC × [5-HTP])/(KmAADC + [5-HTP]) KmAADC
VmaxAADC160 (µM)
400(µM/h)[45] VVMAT = (VmaxVMAT × [fc5-HT])/(KmVMAT + [fc5-HT]) KmVMAT
VmaxVMAT19 (µM)
3500 (µM/h)[23]
[45]Vrelease = krelease × [v5-HT] krelease 20 1/h [45] VSERT = (VmaxSERT × [e5-HT])/(KmSERT + [e5-HT]) KmSERT
VmaxSERT0.2605 (µM)
/ 1[51] VMAOA = (VmaxMAOA × [fc5-HT])/(KmMAOA + [fc5-HT]) KmMAOA
VmaxMAOA86 (µM)
/ 1[83] 1 Slash corresponding to Vmax for TPH2, SERT and MAOA indicates that this value was calculated based on variant genotypes. The calculated Vmax values for appropriate variant genotypes are given in Table S3. Reaction rates Vpool_forward, Vpool_reverse and Vrelease are proportional to the amount of substrate involved, and are described as the law of mass action, expressed as V = k × [S], where k is the mass constant and [S] is the substrate concentration. All other reactions mediated by Tryptophan transporter, TPH2, AADC, VMAT, SERT and MAOA, are described using Michaelis-Menten rate law, expressed as V = (Vmax × [S])/(Km + [S]), where Vmax and Km are the functional properties of the protein involved. - (3)
- The majority of Trp was stored in the Trppool, while approximately 2% is used for 5-HT synthesis by the enzyme TPH2 [21]. TPH2 possesses dual activity and converts both Trp and tetrahydrobiopterin into 5-HTP and dihydrobiopterin, respectively. For simplicity, we modeled TPH2 activity as a single-substrate reaction, with Trp as the substrate and 5-HTP as the product:d[Trp]/dt = Vtrpin − Vpool_forward + Vpool_reverse − [Trp] × ktrp_removal − VTPH2.Trp available for 5-HT synthesis originates from Trpserum, which enters the system through Vtrpin and bypasses Trppool. It also includes some leakage from the Trppool, while a portion is metabolized or removed from the system, indicated by the mass constant ktrp_removal = 0.2 h−1 [45]. However, its primary use is by TPH2 at a rate VTPH2 to produce 5-HTP.
- (4)
- 5-HTP is decarboxylated by AADC into fc5-HT:d[5-HTP]/dt = VTPH2 − VAADC.5-HTP levels in the cell depend on the rate of TPH2 activity (VTPH2) during which it is created and the AADC activity (VAADC) that catalyzes the conversion of 5-HTP into fc5-HT.
- (5)
- fc5-HT is rapidly transported into vesicles via VMAT:d[fc5-HT]/dt = VAADC − VVMAT + [v5-HT] × kout + VSERT − VMAOA.The majority of 5-HT synthesized by AADC is stored inside the vesicles at the rate VVMAT, maintaining a low level of fc5-HT, as described in [23]. We assumed a passive leakage of v5-HT into the cellular compartment at a rate proportional to the mass constant kout = 40 h−1 [45]. Additionally, the concentration of fc5-HT was influenced by SERT activity (VSERT) and MAOA activity (VMAOA).
- (6)
- v5-HT is released into the synaptic cleft through exocytosis:v5-HT available for exocytosis depends on VMAT activity (VVMAT), its leakage from vesicles, and the exocytosis rate (Vrelease). We approximated 5-HT release into the synaptic cleft as a continuous process at a rate of 20 µM/h. As the rate of exocytosis varies by brain region due to differences in vesicle types, sizes, and densities [84,85], we selected a relatively high exocytosis rate of 20 µM/h to ensure it was not a limiting factor in the simulation.d[v5-HT]/dt = VVMAT − [v5-HT] × kout − Vrelease.
- (7)
- In the synaptic cleft, e5-HT is involved in impulse propagation and later undergoes reuptake into the presynaptic neuron via SERT transporters:d[e5-HT]/dt = Vrelease − VSERT − [e5-HT] × k5-HT_removal.The concentration of e5-HT depends on the rate of 5-HT release into the synaptic cleft (Vrelease) and the action of SERT (VSERT). Furthermore, a portion of e5-HT undergoes catabolism and removal from the synaptic cleft, represented by the mass constant k5-HT_removal = 400 h−1 [45].
- (8)
- Recycled 5-HT re-enters the pool of available free 5-HT and can be stored again in vesicles. However, a portion of 5-HT undergoes enzymatic breakdown into 5-hydroxy-3-indole acetaldehyde by the enzyme MAOA, followed by rapid conversion into 5-HIAA that is removed from the system [24]. We grouped these two processes into a single reaction with MAOA kinetics:d[5-HIAA]/dt = VMAOA − 1 × [5-HIAA].The concentration of 5-HIAA primarily depends on the MAOA reaction rate (VMAOA). In the rat brain, 5-HIAA levels were shown to be stable for at least five hours, with a mean basal concentration of 1.45 ± 0.12 µM [86]. Its removal from the CSF approximates first-order kinetics, where the rate of 5-HIAA removal is proportional to its concentration in the system. This is consistent with the rate constant of 5-HIAA disappearance of 0.81 ± 0.06 h−1, measured in the rat dorsal raphe nucleus [87]. In our model, 5-HIAA removal was approximated as a first-order reaction, with 1 µM catabolized per hour.
4.3.2. Calculating Vmax for TPH2, SERT, and MAOA
4.3.3. Incorporating Genotype into Dynamic Model
([NX-derived E] × kcat × [S])/(Km + [S]) =
([NX-derived E] × Cgenotype × kcat × [S])/(Km + [S]).
4.3.4. Simulation and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BD | bipolar disorder |
| 5-HT | serotonin |
| 5-HIAA | serotonin degradation product 5-hydroxy-3-indolacetic acid |
| CSF | cerebrospinal fluid |
| GWAS | genome-wide association studies |
| Trp | tryptophan |
| Trpserum | serum tryptophan |
| Trppool | tryptophan pool used in processes other than serotonin synthesis |
| TPH2 | tryptophan hydroxylase 2 |
| 5-HTP | serotonin precursor 5-hydroxytryptophan |
| AADC | aromatic L-amino acid decarboxylase |
| fc-5HT | free cellular serotonin |
| v5-HT | serotonin stored in the synaptic vesicle |
| VMAT | vesicular monoamine transporter |
| e5-HT | extracellular serotonin |
| SERT | serotonin transporter |
| MAOA | monoamine oxidase A |
| 5-HTTLPR | 5-HT transporter linked polymorphic region |
| uVNTR | upstream variable number tandem repeat |
| Cgenotype | genotype-specific correction parameter for mRNA expression level |
| Vmax | maximum reaction rate of an enzyme |
| Km | Michaelis–Menten constant |
| [S] | substrate concentration |
| E | enzyme concentration |
| kcat | turnover number |
| NX | normalized expression units |
References
- Bach, H.; Arango, V. Neuroanatomy of Serotonergic Abnormalities in Suicide. In The Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-3881-5. [Google Scholar]
- Pare, C.M.; Yeung, D.P.; Price, K.; Stacey, R.S. 5-Hydroxytryptamine, Noradrenaline, and Dopamine in Brainstem, Hypothalamus, and Caudate Nucleus of Controls and of Patients Committing Suicide by Coal-Gas Poisoning. Lancet 1969, 2, 133–135. [Google Scholar] [CrossRef]
- Shaw, D.M.; Camps, F.E.; Eccleston, E.G. 5-Hydroxytryptamine in the Hind-Brain of Depressive Suicides. Br. J. Psychiatry 1967, 113, 1407–1411. [Google Scholar] [CrossRef]
- Stanley, M.; Stanley, B.; Traskman-Bendz, L.; Mann, J.J.; Meyendorff, E. Neurochemical Findings in Suicide Completers and Suicide Attempters. Suicide Life-Threat. Behav. 1986, 16, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.D.; Khaibulina, A.A.; Ellis, S.P.; Moran, A.; Rice, P.M.; Mann, J.J.; Arango, V. Morphometry of the Dorsal Raphe Nucleus Serotonergic Neurons in Suicide Victims. Biol. Psychiatry 1999, 46, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.; Wasserman, J.; Rujescu, D.; Sokolowski, M. Neurobiology and the Genetics of Suicide. In Oxford Textbook of Suicidology and Suicide Prevention; Wasserman, D., Wasserman, C., Wasserman, D., Eds.; Oxford University Press: Oxford, UK, 2021; p. 175. ISBN 978-0-19-883444-1. [Google Scholar]
- Boldrini, M.; Underwood, M.D.; Mann, J.J.; Arango, V. More Tryptophan Hydroxylase in the Brainstem Dorsal Raphe Nucleus in Depressed Suicides. Brain Res. 2005, 1041, 19–28. [Google Scholar] [CrossRef]
- Stanley, M.; Stanley, B. Biochemical Studies in Suicide Victims: Current Findings and Future Implications. Suicide Life-Threat. Behav. 1989, 19, 30–42. [Google Scholar] [CrossRef]
- Duarte, C.; Vaughan, L.; Beasley, T.; Tiwari, H. Multifactorial Inheritance and Complex Diseases. In Emery and Rimoin’s Principles and Practice of Medical Genetics; Academic Press: Cambridge, MA, USA, 2013; pp. 1–15. [Google Scholar] [CrossRef]
- Antypa, N.; Serretti, A.; Rujescu, D. Serotonergic Genes and Suicide: A Systematic Review. Eur. Neuropsychopharmacol. 2013, 23, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Karanović, J.; Šviković, S.; Pantović, M.; Durica, S.; Brajušković, G.; Damjanović, A.; Jovanović, V.; Ivković, M.; Romac, S.; Savić Pavićević, D. Joint Effect of ADARB1 Gene, HTR2C Gene and Stressful Life Events on Suicide Attempt Risk in Patients with Major Psychiatric Disorders. World J. Biol. Psychiatry 2015, 16, 261–271. [Google Scholar] [CrossRef]
- Mann, J.J.; Brent, D.A.; Arango, V. The Neurobiology and Genetics of Suicide and Attempted Suicide: A Focus on the Serotonergic System. Neuropsychopharmacology 2001, 24, 467–477. [Google Scholar] [CrossRef]
- Schild, A.H.E.; Pietschnig, J.; Tran, U.S.; Voracek, M. Genetic Association Studies between SNPs and Suicidal Behavior: A Meta-Analytical Field Synopsis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 36–42. [Google Scholar] [CrossRef]
- Ashley-Koch, A.E.; Kimbrel, N.A.; Qin, X.J.; Lindquist, J.H.; Garrett, M.E.; Dennis, M.F.; Hair, L.P.; Huffman, J.E.; Jacobson, D.A.; Madduri, R.K.; et al. Genome-Wide Association Study Identifies Four Pan-Ancestry Loci for Suicidal Ideation in the Million Veteran Program. PLoS Genet. 2023, 19, e1010623. [Google Scholar] [CrossRef]
- Docherty, A.R.; Mullins, N.; Ashley-Koch, A.E.; Qin, X.; Coleman, J.R.I.; Shabalin, A.; Kang, J.; Murnyak, B.; Wendt, F.; Adams, M.; et al. GWAS Meta-Analysis of Suicide Attempt: Identification of 12 Genome-Wide Significant Loci and Implication of Genetic Risks for Specific Health Factors. Am. J. Psychiatry 2023, 180, 723–738. [Google Scholar] [CrossRef]
- Kimbrel, N.A.; Ashley-Koch, A.E.; Qin, X.J.; Lindquist, J.H.; Garrett, M.E.; Dennis, M.F.; Hair, L.P.; Huffman, J.E.; Jacobson, D.A.; Madduri, R.K.; et al. Identification of Novel, Replicable Genetic Risk Loci for Suicidal Thoughts and Behaviors Among US Military Veterans. JAMA Psychiatry 2023, 80, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Shabalin, A.A.; DiBlasi, E.; Gopal, S.; Canuso, C.M.; Palotie, A.; Drevets, W.C.; Docherty, A.R.; Coon, H. Genome-Wide Association Study Meta-Analysis of Suicide Death and Suicidal Behavior. Mol. Psychiatry 2023, 28, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, B.P. Mathematical Modelling in Systems Biology: An Introduction; MIT Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Tyson, J.J.; Laomettachit, T.; Kraikivski, P. Modeling the Dynamic Behavior of Biochemical Regulatory Networks. J. Theor. Biol. 2019, 462, 514–527. [Google Scholar] [CrossRef] [PubMed]
- van Riel, N.A.W. Dynamic Modelling and Analysis of Biochemical Networks: Mechanism-Based Models and Model-Based Experiments. Brief. Bioinform. 2006, 7, 364–374. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Role of Blood-Brain Barrier Transport of Tryptophan and Other Neutral Amino Acids in the Regulation of Substrate-Limited Pathways of Brain Amino Acid Metabolism. J. Neural Transm. Suppl. 1979, 15, 43–54. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Oldendorf, W.H. Kinetic Analysis of Blood-Brain Barrier Transport of Amino Acids. Biochim. Biophys. Acta 1975, 401, 128–136. [Google Scholar] [CrossRef]
- Wimalasena, K. Vesicular Monoamine Transporters: Structure-Function, Pharmacology, and Medicinal Chemistry: Vesicular Monoamine Transporters. Med. Res. Rev. 2011, 31, 483–519. [Google Scholar] [CrossRef]
- Bortolato, M.; Chen, K.; Shih, J.C. The Degradation of Serotonin: Role of MAO. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 203–218. ISBN 978-0-12-374634-4. [Google Scholar]
- Chen, G.-L.; Vallender, E.J.; Miller, G.M. Functional Characterization of the Human TPH2 5′ Regulatory Region: Untranslated Region and Polymorphisms Modulate Gene Expression in Vitro. Hum. Genet. 2008, 122, 645–657. [Google Scholar] [CrossRef]
- Denney, R.M.; Koch, H.; Craig, I.W. Association between Monoamine Oxidase A Activity in Human Male Skin Fibroblasts and Genotype of the MAOA Promoter-Associated Variable Number Tandem Repeat. Hum. Genet. 1999, 105, 542–551. [Google Scholar] [CrossRef]
- Heils, A.; Teufel, A.; Petri, S.; Stöber, G.; Riederer, P.; Bengel, D.; Lesch, K.P. Allelic Variation of Human Serotonin Transporter Gene Expression. J. Neurochem. 2002, 66, 2621–2624. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-E.; Pinsonneault, J.; Sadee, W.; Saffen, D. Tryptophan Hydroxylase 2 (TPH2) Haplotypes Predict Levels of TPH2 MRNA Expression in Human Pons. Mol. Psychiatry 2007, 12, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Sabol, S.Z.; Hu, S.; Hamer, D. A Functional Polymorphism in the Monoamine Oxidase A Gene Promoter. Hum. Genet. 1998, 103, 273–279. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Vázquez, G.H.; Tondo, L. Bipolar Depression: A Major Unsolved Challenge. Int. J. Bipolar Disord. 2020, 8, 1. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Pinna, M.; Nuñez, N.; Vázquez, G.H. Suicidal Risk Factors in Major Affective Disorders. Br. J. Psychiatry 2019, 215, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Bielau, H.; Krell, D.; Agelink, M.W.; Diekmann, S.; Wurthmann, C.; Trübner, K.; Bernstein, H.G.; Danos, P.; Bogerts, B. Circumscribed Numerical Deficit of Dorsal Raphe Neurons in Mood Disorders. Psychol. Med. 2002, 32, 93–103. [Google Scholar] [CrossRef]
- Kato, T. Current Understanding of Bipolar Disorder: Toward Integration of Biological Basis and Treatment Strategies. Psychiatry Clin. Neurosci. 2019, 73, 526–540. [Google Scholar] [CrossRef]
- Matthews, P.R.; Harrison, P.J. A Morphometric, Immunohistochemical, and in Situ Hybridization Study of the Dorsal Raphe Nucleus in Major Depression, Bipolar Disorder, Schizophrenia, and Suicide. J. Affect. Disord. 2012, 137, 125–134. [Google Scholar] [CrossRef]
- Goldman, N.; Glei, D.A.; Lin, Y.-H.; Weinstein, M. The Serotonin Transporter Polymorphism (5-HTTLPR): Allelic Variation and Links with Depressive Symptoms. Depress. Anxiety 2010, 27, 260–269. [Google Scholar] [CrossRef]
- Fernstrom, M.H.; Fernstrom, J.D. Brain Tryptophan Concentrations and Serotonin Synthesis Remain Responsive to Food Consumption after the Ingestion of Sequential Meals. Am. J. Clin. Nutr. 1995, 61, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, S.; Shirvan, A.; Linial, M. Vesicular Neurotransmitter Transporters: From Bacteria to Humans. Physiol. Rev. 1995, 75, 369–392. [Google Scholar] [CrossRef]
- Gibson, E.L. Tryptophan Supplementation and Serotonin Function: Genetic Variations in Behavioural Effects. Proc. Nutr. Soc. 2018, 77, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; Hindley, G.F.L.; Frei, O.; Smeland, O.B. New Insights from the Last Decade of Research in Psychiatric Genetics: Discoveries, Challenges and Clinical Implications. World Psychiatry 2023, 22, 4–24. [Google Scholar] [CrossRef]
- Almeida, H.S.; Mitjans, M.; Arias, B.; Vieta, E.; Ríos, J.; Benabarre, A. Genetic Differences between Bipolar Disorder Subtypes: A Systematic Review Focused in Bipolar Disorder Type II. Neurosci. Biobehav. Rev. 2020, 118, 623–630. [Google Scholar] [CrossRef]
- Amare, A.T.; Schubert, K.O.; Baune, B.T. Pharmacogenomics in the Treatment of Mood Disorders: Strategies and Opportunities for Personalized Psychiatry. EPMA J. 2017, 8, 211–227. [Google Scholar] [CrossRef]
- Mann, J.J. The Serotonergic System in Mood Disorders and Suicidal Behaviour. Phil. Trans. R. Soc. B 2013, 368, 20120537. [Google Scholar] [CrossRef]
- Chang, A.; Jeske, L.; Ulbrich, S.; Hofmann, J.; Koblitz, J.; Schomburg, I.; Neumann-Schaal, M.; Jahn, D.; Schomburg, D. BRENDA, the ELIXIR Core Data Resource in 2021: New Developments and Updates. Nucleic Acids Res. 2021, 49, D498–D508. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Best, J.; Nijhout, H.F.; Reed, M. Serotonin Synthesis, Release and Reuptake in Terminals: A Mathematical Model. Theor. Biol. Med. Model. 2010, 7, 34. [Google Scholar] [CrossRef]
- Best, J.; Duncan, W.; Sadre-Marandi, F.; Hashemi, P.; Nijhout, H.F.; Reed, M. Autoreceptor Control of Serotonin Dynamics. BMC Neurosci. 2020, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Stoltenberg, S.F. Epistasis among Presynaptic Serotonergic System Components. Behav. Genet. 2005, 35, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Stoltenberg, S.F. Serotonergic Agents and Alcoholism Treatment: A Simulation. Alcohol. Clin. Exp. Res. 2003, 27, 1853–1859. [Google Scholar] [CrossRef]
- Stoltenberg, S.F.; Nag, P. Description and Validation of a Dynamical Systems Model of Presynaptic Serotonin Function: Genetic Variation, Brain Activation and Impulsivity. Behav. Genet. 2010, 40, 262–279. [Google Scholar] [CrossRef]
- Bazhenova, E.Y.; Fursenko, D.V.; Kulikova, E.A.; Khotskin, N.V.; Sinyakova, N.A.; Kulikov, A.A. Effect of Photoperiodic Alterations on Depression-like Behavior and the Brain Serotonin System in Mice Genetically Different in Tryptophan Hydroxylase 2 Activity. Neurosci. Lett. 2019, 699, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.L.; Johnson, M.E.; Li, J.; Hutcheson, J.; Carr, B.M.; Corley, K.M.; Gowans, A.B.; Smith, J. Serotonin Transport Kinetics Correlated between Human Platelets and Brain Synaptosomes. Psychopharmacology 2005, 180, 391–398. [Google Scholar] [CrossRef]
- Bunin, M.A.; Prioleau, C.; Mailman, R.B.; Wightman, R.M. Release and Uptake Rates of 5-Hydroxytryptamine in the Dorsal Raphe and Substantia Nigra Reticulata of the Rat Brain. J. Neurochem. 2002, 70, 1077–1087. [Google Scholar] [CrossRef]
- Åsberg, M. Neurotransmitters and Suicidal Behavior. The Evidence from Cerebrospinal Fluid Studies. Ann. N. Y. Acad. Sci. 1997, 836, 158–181. [Google Scholar] [CrossRef]
- Roy, A.; Pollack, S. Are Cerebrospinal Fluid or Urinary Monoamine Metabolite Measures Stronger Correlates of Suicidal Behavior in Depression? Neuropsychobiology 2008, 29, 164–167. [Google Scholar] [CrossRef]
- Mann, J.J. Neurobiology of Suicidal Behaviour. Nat. Rev. Neurosci. 2003, 4, 819–828. [Google Scholar] [CrossRef]
- Bach, H.; Huang, Y.-Y.; Underwood, M.D.; Dwork, A.J.; Mann, J.J.; Arango, V. Elevated Serotonin and 5-HIAA in the Brainstem and Lower Serotonin Turnover in the Prefrontal Cortex of Suicides. Synapse 2014, 68, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Sher, L.; Carballo, J.J.; Grunebaum, M.F.; Burke, A.K.; Zalsman, G.; Huang, Y.; John Mann, J.; Oquendo, M.A. A Prospective Study of the Association of Cerebrospinal Fluid Monoamine Metabolite Levels with Lethality of Suicide Attempts in Patients with Bipolar Disorder. Bipolar Disord. 2006, 8, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Pålsson, E.; Sellgren, C.; Rydén, E.; Kizza, R.; Pelanis, A.; Zetterberg, H.; Blennow, K.; Landén, M. Cerebrospinal Fluid Monoamine Metabolite Profiles in Bipolar Disorder, ADHD, and Controls. J. Neural Transm. 2017, 124, 1135–1143. [Google Scholar] [CrossRef]
- Young, L.T.; Warsh, J.J.; Kish, S.J.; Shannak, K.; Hornykeiwicz, O. Reduced Brain 5-HT and Elevated NE Turnover and Metabolites in Bipolar Affective Disorder. Biol. Psychiatry 1994, 35, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.M.; Ichise, M.; Fromm, S.J.; Nugent, A.C.; Rollis, D.; Gandhi, S.K.; Klaver, J.M.; Charney, D.S.; Manji, H.K.; Drevets, W.C. Serotonin Transporter Binding in Bipolar Disorder Assessed Using [11C]DASB and Positron Emission Tomography. Biol. Psychiatry 2006, 60, 207–217. [Google Scholar] [CrossRef]
- Miller, J.M.; Everett, B.A.; Oquendo, M.A.; Ogden, R.T.; Mann, J.J.; Parsey, R.V. Positron Emission Tomography Quantification of Serotonin Transporter Binding in Medication-Free Bipolar Disorder: 5-HTT Pet Imaging in Bipolar Disorder. Synapse 2016, 70, 24–32. [Google Scholar] [CrossRef]
- Blakely, R.D.; Ramamoorthy, S.; Schroeter, S.; Qian, Y.; Apparsundaram, S.; Galli, A.; DeFelice, L.J. Regulated Phosphorylation and Trafficking of Antidepressant-Sensitive Serotonin Transporter Proteins. Biol. Psychiatry 1998, 44, 169–178. [Google Scholar] [CrossRef]
- Schmitz, D.; Gloveli, T.; Empson, R.M.; Draguhn, A.; Heinemann, U. Serotonin Reduces Synaptic Excitation in the Superficial Medial Entorhinal Cortex of the Rat via a Presynaptic Mechanism. J. Physiol. 1998, 508 Pt 1, 119–129. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin Modulation of Cortical Neurons and Networks. Front. Integr. Neurosci. 2013, 7, 25. [Google Scholar] [CrossRef]
- Grohmann, M.; Hammer, P.; Walther, M.; Paulmann, N.; Büttner, A.; Eisenmenger, W.; Baghai, T.C.; Schüle, C.; Rupprecht, R.; Bader, M.; et al. Alternative Splicing and Extensive RNA Editing of Human TPH2 Transcripts. PLoS ONE 2010, 5, e8956. [Google Scholar] [CrossRef]
- Karanović, J.; Ivković, M.; Jovanović, V.M.; Šviković, S.; Pantović-Stefanović, M.; Brkušanin, M.; Damjanović, A.; Brajušković, G.; Savić-Pavićević, D. Effect of Childhood General Traumas on Suicide Attempt Depends on TPH2 and ADARB1 Variants in Psychiatric Patients. J. Neural Transm. 2017, 124, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Woulfe, D.; Kilic, F. Post-Translational Modifications of Serotonin Transporter. Pharmacol. Res. 2019, 140, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, A.; James, G.M.; Philippe, C.; Gryglewski, G.; Bauer, A.; Hienert, M.; Spies, M.; Kautzky, A.; Vanicek, T.; Hahn, A.; et al. Association of Protein Distribution and Gene Expression Revealed by PET and Post-Mortem Quantification in the Serotonergic System of the Human Brain. Cereb. Cortex 2017, 27, 117–130. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Zondervan, K.T.; Cardon, L.R. Designing Candidate Gene and Genome-Wide Case-Control Association Studies. Nat. Protoc. 2007, 2, 2492–2501. [Google Scholar] [CrossRef]
- Mann, J.J.; Arango, V.A.; Avenevoli, S.; Brent, D.A.; Champagne, F.A.; Clayton, P.; Currier, D.; Dougherty, D.M.; Haghighi, F.; Hodge, S.E.; et al. Candidate Endophenotypes for Genetic Studies of Suicidal Behavior. Biol. Psychiatry 2009, 65, 556–563. [Google Scholar] [CrossRef]
- Boldrini, M.; Xiao, Y.; Sing, T.; Zhu, C.; Jabbi, M.; Pantazopoulos, H.; Gürsoy, G.; Martinowich, K.; Punzi, G.; Vallender, E.J.; et al. Omics Approaches to Investigate the Pathogenesis of Suicide. Biol. Psychiatry 2024, S0006-3223(24)01352-0. [Google Scholar] [CrossRef] [PubMed]
- Riera-Serra, P.; Navarra-Ventura, G.; Castro, A.; Gili, M.; Salazar-Cedillo, A.; Ricci-Cabello, I.; Roldán-Espínola, L.; Coronado-Simsic, V.; García-Toro, M.; Gómez-Juanes, R.; et al. Clinical Predictors of Suicidal Ideation, Suicide Attempts and Suicide Death in Depressive Disorder: A Systematic Review and Meta-Analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1543–1563. [Google Scholar] [CrossRef]
- Sesso, G.; Bargnesi, F.; Olzi, F.; Mutti, G.; Berloffa, S.; Viglione, V.; Fantozzi, P.; Tolomei, G.; Guccione, F.; Milone, A.; et al. Efficacy and Safety of Lithium for Suicide and Suicide-Related Behaviors in Youth: A Review of the Literature. Brain Sci. 2024, 14, 1139. [Google Scholar] [CrossRef]
- Pompili, M.; Baldessarini, R.J.; Forte, A.; Erbuto, D.; Serafini, G.; Fiorillo, A.; Amore, M.; Girardi, P. Do Atypical Antipsychotics Have Antisuicidal Effects? A Hypothesis-Generating Overview. Int. J. Mol. Sci. 2016, 17, 1700. [Google Scholar] [CrossRef]
- Pincus, H.A.; First, M.; Frances, A.; McQueen, L. Reviewing DSM-IV. Am. J. Psychiatry 1996, 153, 850. [Google Scholar] [CrossRef]
- Sjöberg, R.L.; Ducci, F.; Barr, C.S.; Newman, T.K.; Dell’Osso, L.; Virkkunen, M.; Goldman, D. A Non-Additive Interaction of a Functional MAO-A VNTR and Testosterone Predicts Antisocial Behavior. Neuropsychopharmacology 2008, 33, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Diet-Induced Changes in Plasma Amino Acid Pattern: Effects on the Brain Uptake of Large Neutral Amino Acids, and on Brain Serotonin Synthesis. J. Neural Transm. Suppl. 1979, 15, 55–67. [Google Scholar] [CrossRef]
- Zahar, S.; Schneider, N.; Makwana, A.; Chapman, S.; Corthesy, J.; Amico, M.; Hudry, J. Dietary Tryptophan-Rich Protein Hydrolysate Can Acutely Impact Physiological and Psychological Measures of Mood and Stress in Healthy Adults. Nutr. Neurosci. 2023, 26, 303–312. [Google Scholar] [CrossRef]
- Winge, I.; McKinney, J.A.; Knappskog, P.M.; Haavik, J. Characterization of Wild-Type and Mutant Forms of Human Tryptophan Hydroxylase 2: Human Tryptophan Hydroxylase Mutations. J. Neurochem. 2007, 100, 1648–1657. [Google Scholar] [CrossRef]
- Geha, R.M.; Chen, K.; Wouters, J.; Ooms, F.; Shih, J.C. Analysis of Conserved Active Site Residues in Monoamine Oxidase A and B and Their Three-Dimensional Molecular Modeling. J. Biol. Chem. 2002, 277, 17209–17216. [Google Scholar] [CrossRef] [PubMed]
- Bruns, D.; Jahn, R. Real-Time Measurement of Transmitter Release from Single Synaptic Vesicles. Nature 1995, 377, 62–65. [Google Scholar] [CrossRef]
- Tamir, H.; Gershon, M.D. Storage of Serotonin and Serotonin Binding Protein in Synaptic Vesicles. J. Neurochem. 1979, 33, 35–44. [Google Scholar] [CrossRef]
- Mignot, E.; Serrano, A.; Laude, D.; Elghozi, J.L.; Dedek, J.; Scatton, B. Measurement of 5-HIAA Levels in Ventricular CSF (by LCEC) and in Striatum (by in Vivo Voltammetry) during Pharmacological Modifications of Serotonin Metabolism in the Rat. J. Neural Transm. 1985, 62, 117–124. [Google Scholar] [CrossRef]
- Echizen, H.; Freed, C.R. Measurement of Serotonin Turnover Rate in Rat Dorsal Raphe Nucleus by in Vivo Electrochemistry. J. Neurochem. 1984, 42, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Windahl, M.S.; Boesen, J.; Karlsen, P.E.; Christensen, H.E.M. Expression, Purification and Enzymatic Characterization of the Catalytic Domains of Human Tryptophan Hydroxylase Isoforms. Protein J. 2009, 28, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Vintém, A.P.B.; Price, N.T.; Silverman, R.B.; Ramsay, R.R. Mutation of Surface Cysteine 374 to Alanine in Monoamine Oxidase A Alters Substrate Turnover and Inactivation by Cyclopropylamines. Bioorg. Med. Chem. 2005, 13, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Bunin, M.A.; Wightman, R.M. Quantitative Evaluation of 5-Hydroxytryptamine (Serotonin) Neuronal Release and Uptake: An Investigation of Extrasynaptic Transmission. J. Neurosci. 1998, 18, 4854–4860. [Google Scholar] [CrossRef]
- Fanelli, G.; Serretti, A. The Influence of the Serotonin Transporter Gene 5-HTTLPR Polymorphism on Suicidal Behaviors: A Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 88, 375–387. [Google Scholar] [CrossRef]
| Total Sample n = 241 | Suicide Attempters n = 46 | Suicide Non-Attempters n = 55 | Unaffected Individuals n = 140 | p-Value | |
|---|---|---|---|---|---|
| Mean age ± SD | 40.69 ± 11.32 | 43.74 ± 11.80 | 39.05 ± 10.96 | 40.34 ± 11.19 | 0.134 1 |
| Sex (n, %) | |||||
| Male | 72 (29.88%) | 12 (26.09%) | 12 (21.82%) | 48 (34.29%) | 0.190 2 |
| Female | 169 (70.12%) | 34 (73.91%) | 43 (78.18%) | 92 (65.71%) |
| Molecular Species | Attempters (n = 46) (mean ± SD, nM) | Non-Attempters (n = 55) (mean ± SD, nM) | p-Value MWU 1 | Unaffected Individuals (n = 140) (mean ± SD, nM) | p-Value KW 2 |
|---|---|---|---|---|---|
| 5-HTP | 13,621.7 ± 207.0 | 13,635.1 ± 203.8 | 0.321 | 13,626.5 ± 225.9 | 0.488 |
| fc5-HT | 940.4 ± 272.7 | 850.4 ± 248.0 | 0.048 | 886.9 ± 260.0 | 0.105 |
| v5-HT | 2631.6 ± 707.7 | 2397.6 ± 646.6 | 0.047 | 2492.5 ± 675.2 | 0.103 |
| e5-HT | 70.8 ± 1.3 | 70.6 ± 1.2 | 0.604 | 70.5 ± 1.3 | 0.233 |
| 5-HIAA | 497.6 ± 284.5 | 587.1 ± 237.8 | 0.054 | 611.5 ± 245.3 | 0.045 |
| Gene | Expression Level (NX) 1 | kcat (1/s) | kcat (1/h) 2 | kcat Reference | Vmax 3 (µM/h) | Calculated E 4 (µM) | NX-Derived E 5 (µM) |
|---|---|---|---|---|---|---|---|
| TPH2 | 46.2 | 5.03 | 18,108 | [88] | 400 | 0.022 | 0.046 |
| MAOA | 18.6 | 18.6 | 66,960 | [89] | 1000 | 0.014 | 0.018 |
| SLC6A4 | 11.2 | 198.4 6 | 714,285 6 | / 7 | 8000 6 | / 7 | 0.0112 |
| Gene | Genetic Variant | Allele | Allele Frequency | Effect on mRNA Expression | Normalized Allelic Effect 1 |
|---|---|---|---|---|---|
| TPH2 | rs11178998 | A | 0.95 | Minor allele G increases TPH2 mRNA expression ~3 times [25] | 1 |
| G | 0.05 | 3 | |||
| rs4290270 | T | 0.63 | Minor allele A decreases TPH2 mRNA expression 1.43 times [28] | 1 | |
| A | 0.37 | 0.7 | |||
| rs7305115 | G | 0.60 | Minor allele A increases TPH2 mRNA expression 1.74 times [28] | 1 | |
| A | 0.40 | 1.7 | |||
| SLC6A4 | 5-HTTLPR | L | 0.58 | Major allele L increases SLC6A4 mRNA expression 3 times [27] | 1 |
| S | 0.42 | 0.3 | |||
| MAOA | uVNTR 2 | 3R (Low) | 0.302 | Alleles 4R and 3.5R increase MAOA mRNA expression 5–6 times [26,29] | 0.2 |
| 3.5R (High) | 0.004 | 1 | |||
| 4R (High) | 0.685 | 1 | |||
| 5R (Low) | 0.009 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radenković, L.; Karanović, J.; Pantović-Stefanović, M.; Lazić, D.; Brajušković, G.; Ivković, M.; Pešović, J.; Savić-Pavićević, D. Dynamic Model of Serotonin Presynapse and Its Application to Suicide Attempt in Patients with Bipolar Disorder. Int. J. Mol. Sci. 2025, 26, 4085. https://doi.org/10.3390/ijms26094085
Radenković L, Karanović J, Pantović-Stefanović M, Lazić D, Brajušković G, Ivković M, Pešović J, Savić-Pavićević D. Dynamic Model of Serotonin Presynapse and Its Application to Suicide Attempt in Patients with Bipolar Disorder. International Journal of Molecular Sciences. 2025; 26(9):4085. https://doi.org/10.3390/ijms26094085
Chicago/Turabian StyleRadenković, Lana, Jelena Karanović, Maja Pantović-Stefanović, Dušan Lazić, Goran Brajušković, Maja Ivković, Jovan Pešović, and Dušanka Savić-Pavićević. 2025. "Dynamic Model of Serotonin Presynapse and Its Application to Suicide Attempt in Patients with Bipolar Disorder" International Journal of Molecular Sciences 26, no. 9: 4085. https://doi.org/10.3390/ijms26094085
APA StyleRadenković, L., Karanović, J., Pantović-Stefanović, M., Lazić, D., Brajušković, G., Ivković, M., Pešović, J., & Savić-Pavićević, D. (2025). Dynamic Model of Serotonin Presynapse and Its Application to Suicide Attempt in Patients with Bipolar Disorder. International Journal of Molecular Sciences, 26(9), 4085. https://doi.org/10.3390/ijms26094085






