Investigation of the Neurotoxicity Mechanisms of Ni2+ in Rat Neocortical Neurons Through Transcriptome Analysis
Abstract
1. Introduction
2. Results
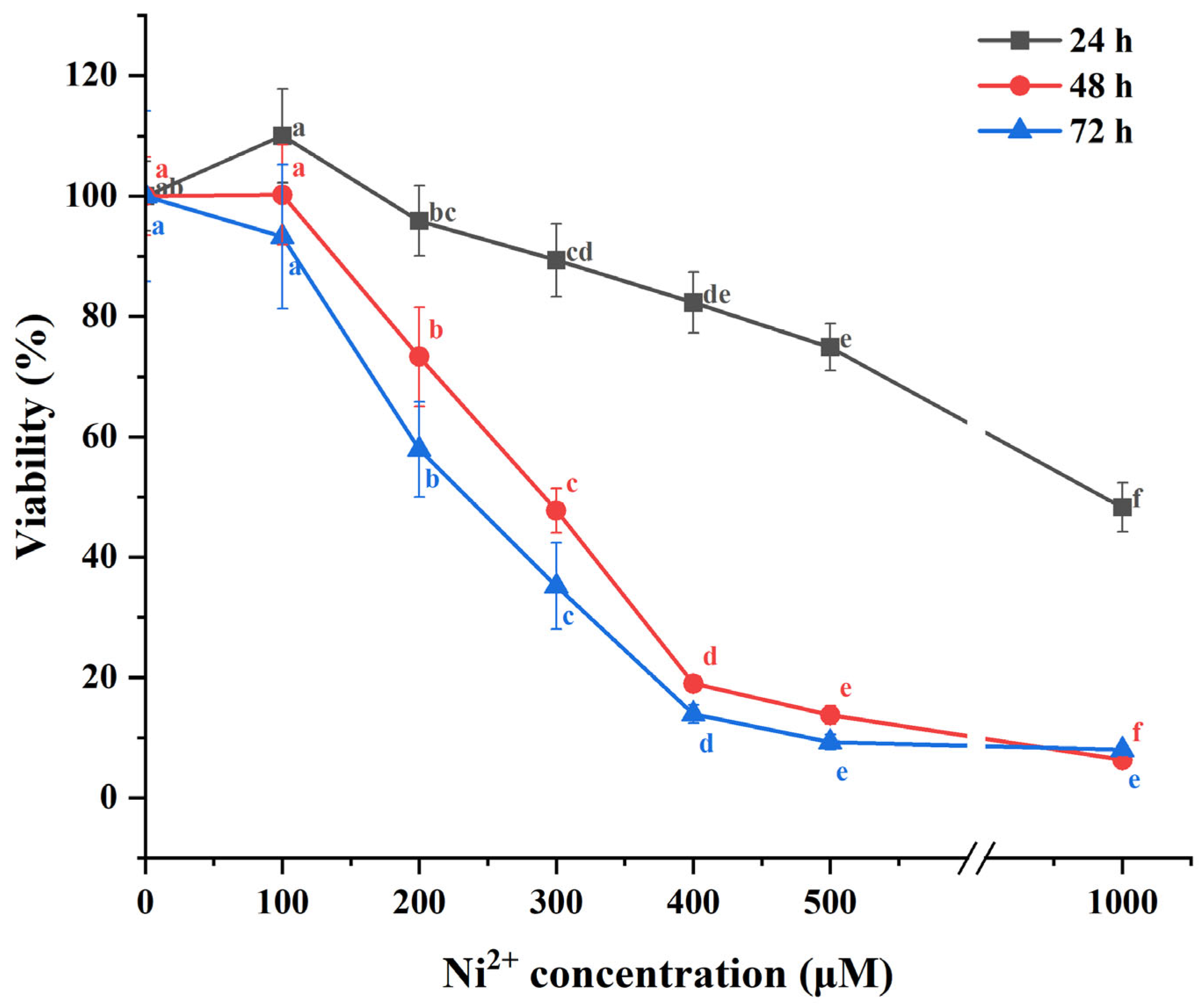
2.1. The Impact of Ni2+ on the Cell Viability of Neurons
2.2. The Effect of Ni2+ on the AIS Length of Neocortical Neurons
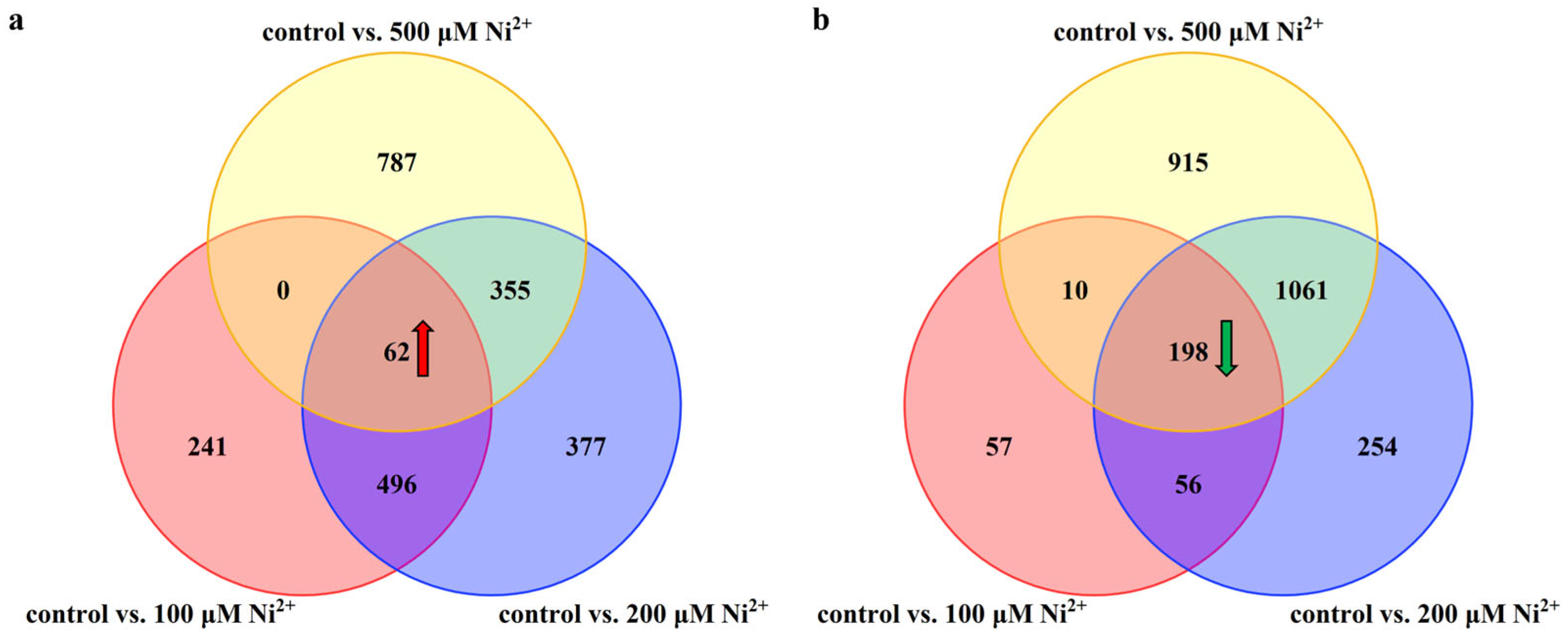
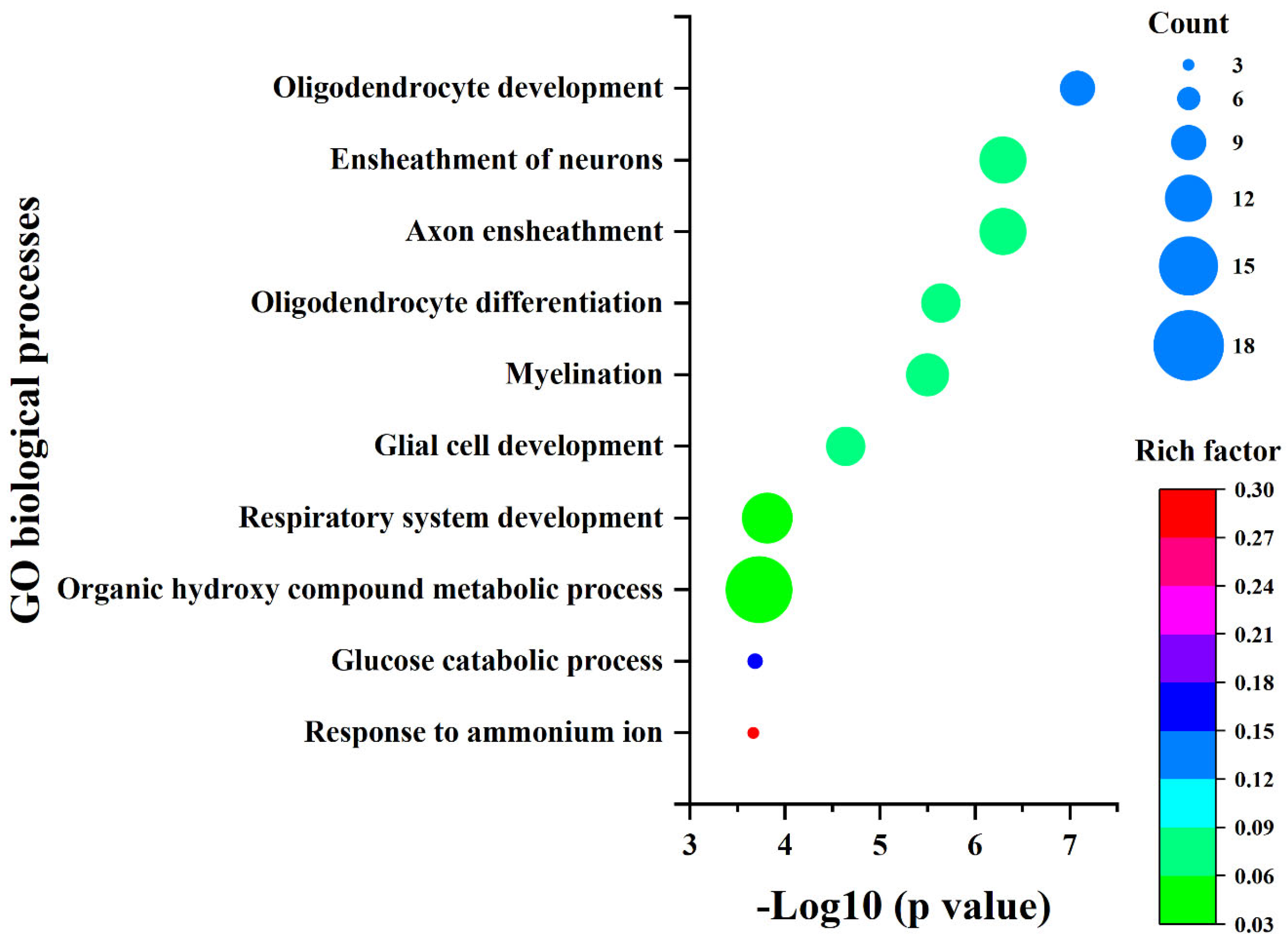
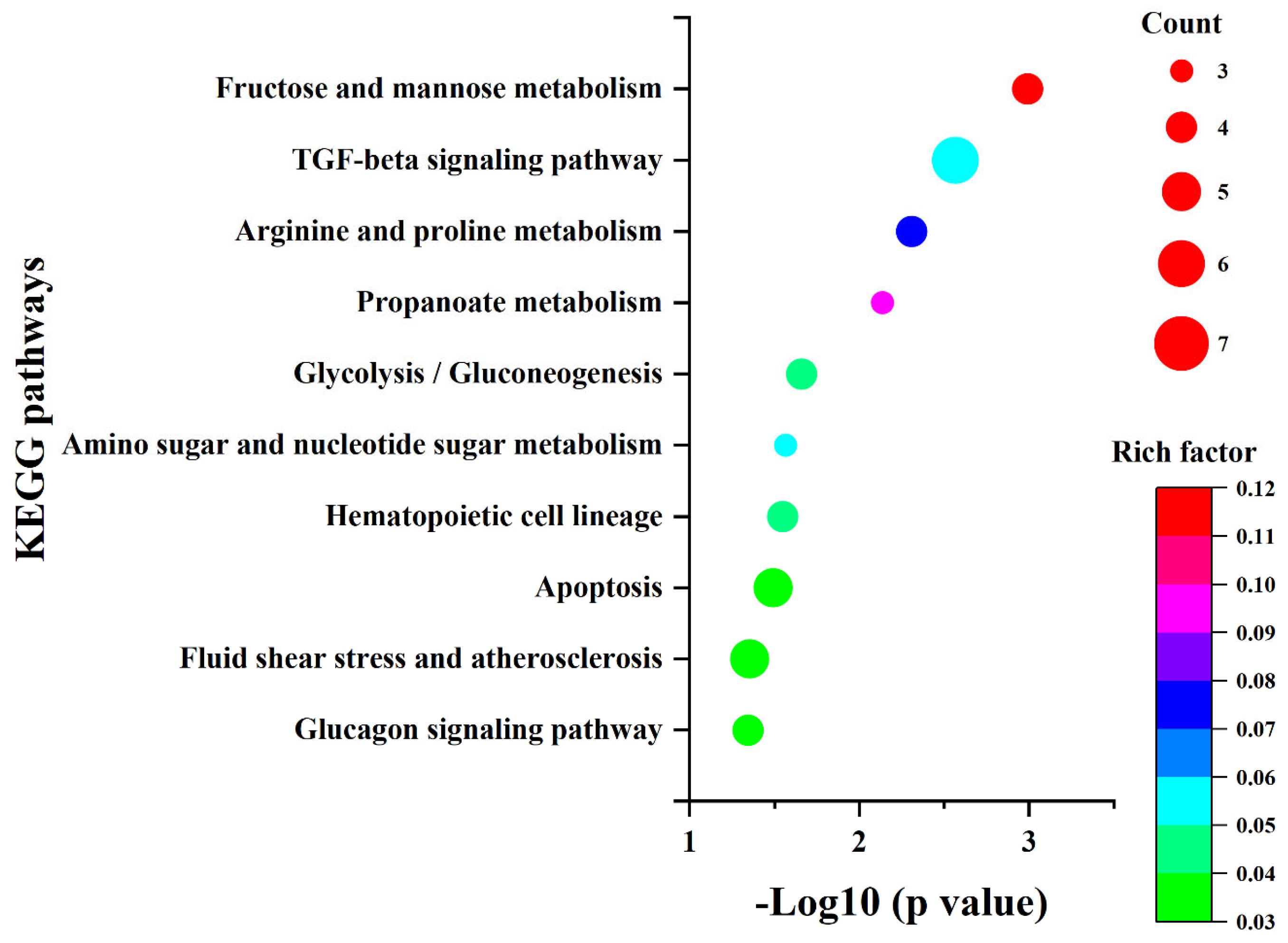
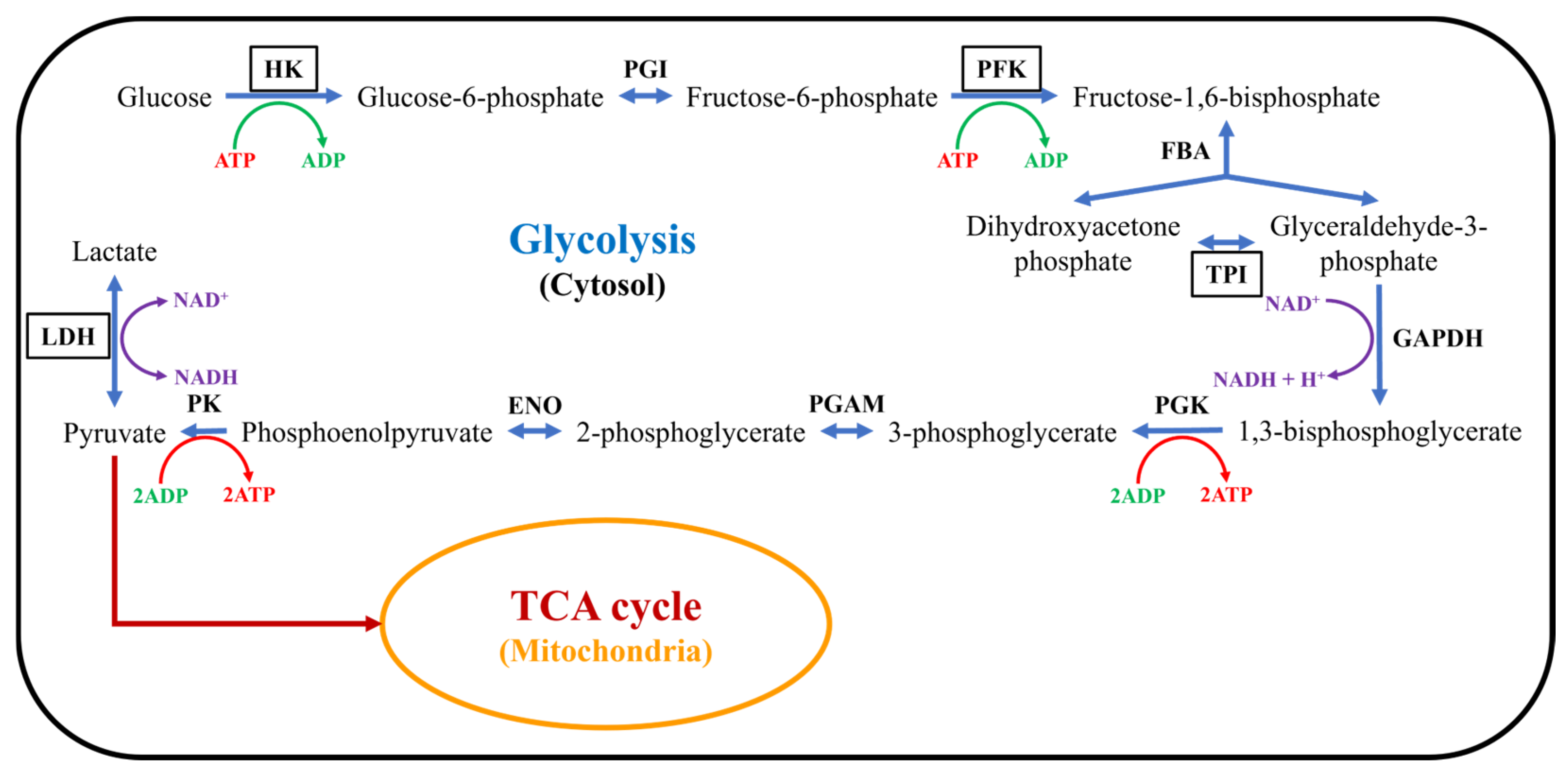
2.3. The Influence of Ni2+ on Gene Expression in Neocortical Neurons
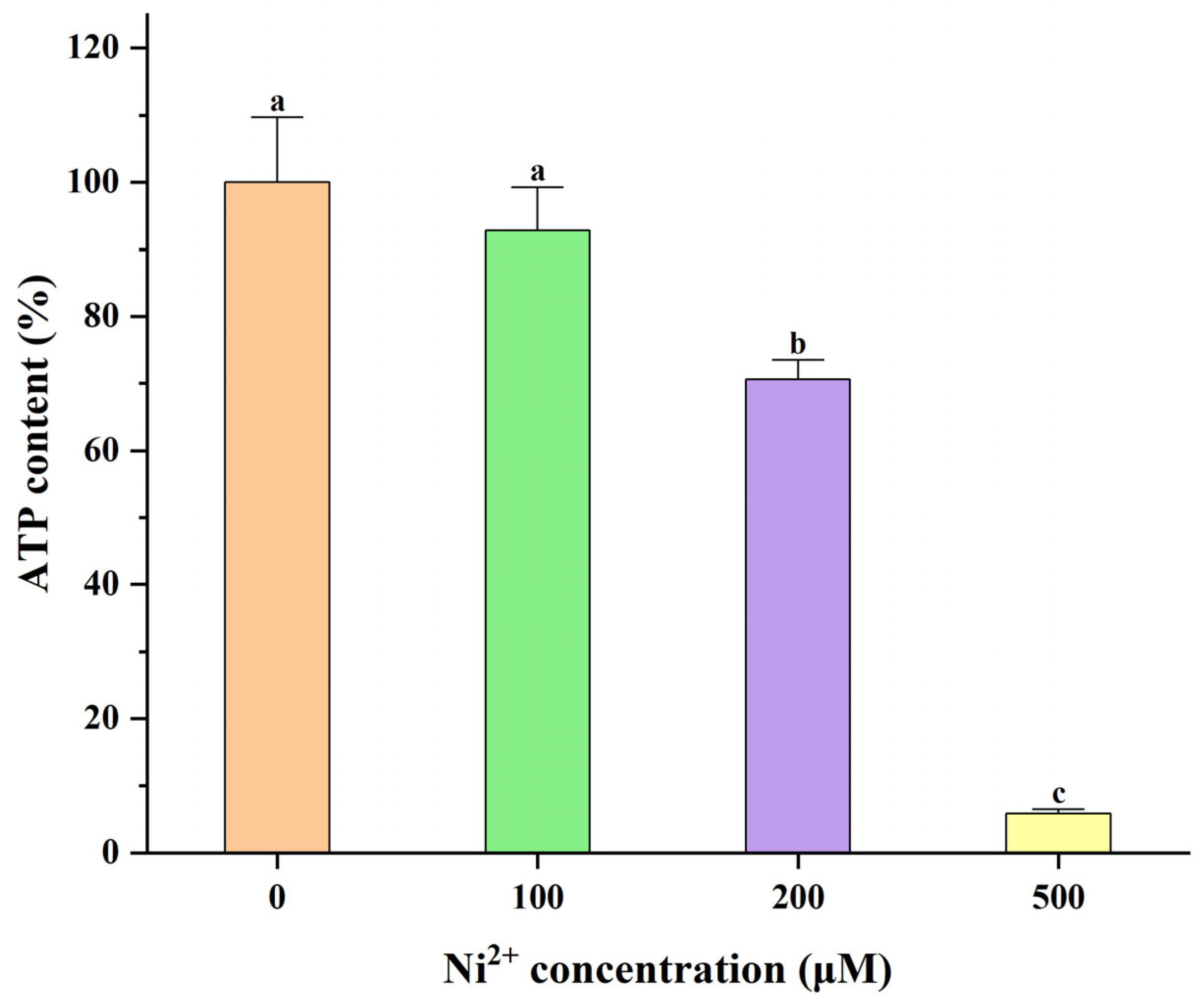
2.4. The Effect of Ni2+ on ATP Levels in Neocortical Neurons
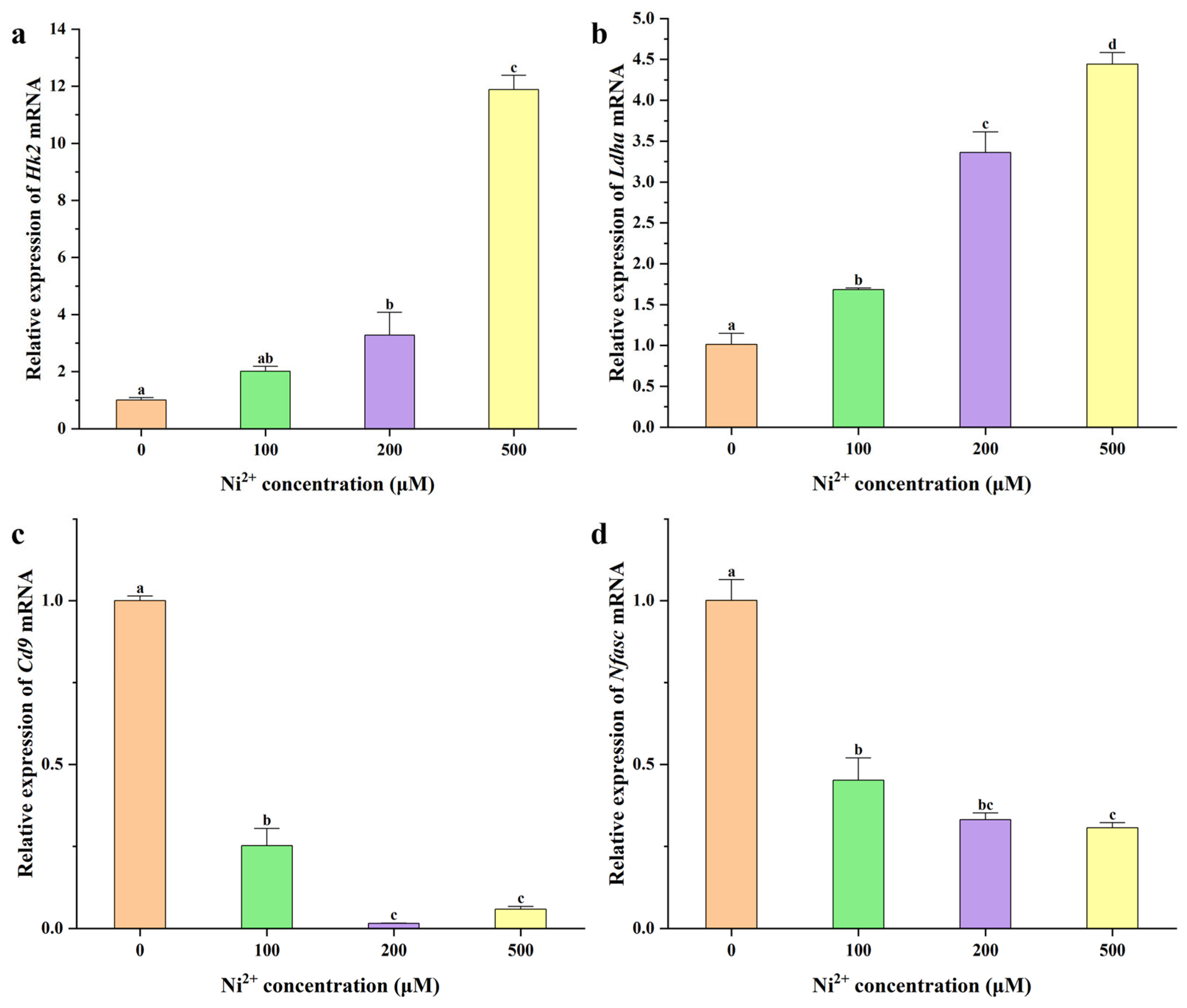
2.5. qRT-PCR Analysis
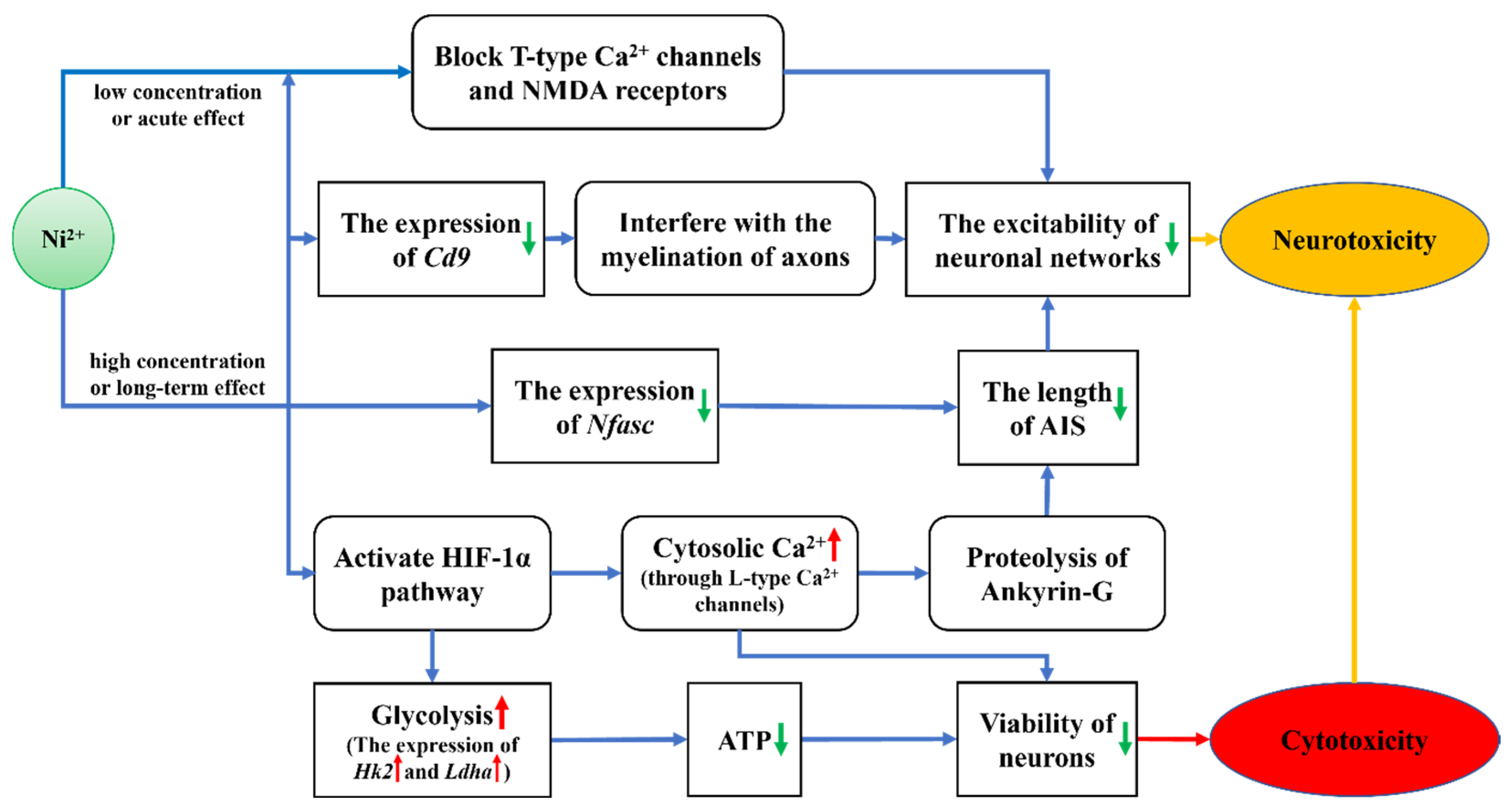
3. Discussion
3.1. The Acute and Long-Term Effects of Ni2+ on Neurons
3.2. Comparative Analysis of the Effects of Ni2+ on Gene Expression in Neurons and L929 Cells
4. Materials and Methods
4.1. The Isolation and Culture of Rat Neocortical Neurons
4.2. Cell Viability Assay
4.3. Measurement of the AIS’s Length
4.4. Transcriptome Sequencing and Bioinformatics Analyses
4.5. ATP Content Measurement
4.6. qRT-PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Available online: https://www.fda.gov/media/131150/download (accessed on 25 February 2025).
- Kimber, I.; Basketter, D.A. Allergic sensitization to nickel and implanted metal devices: A perspective. Dermatitis 2022, 33, 396–404. [Google Scholar] [CrossRef]
- Jiménez-Lamana, J.; Godin, S.; Aragonès, G.; Bladé, C.; Szpunar, J.; Lobinski, R. Nickel nanoparticles induce the synthesis of a tumor-related polypeptide in human epidermal keratinocytes. Nanomaterials 2020, 10, 992. [Google Scholar] [CrossRef]
- Cao, X.Y.; Tian, S.Q.; Fu, M.Y.; Li, Y.M.; Sun, Y.L.; Liu, J.L.; Liu, Y. Resveratrol protects human bronchial epithelial cells against nickel-induced toxicity via suppressing p38 MAPK, NF-κB signaling, and NLRP3 inflammasome activation. Environ. Toxicol. 2020, 35, 609–618. [Google Scholar] [CrossRef]
- EI Brouzi, M.Y.; Lamtai, M.; Fath, N.; Rezqaoui, A.; Zghari, O.; EI Hamzaoui, A.; Ibouzine-dine, L.; EI Hessni, A.; Mesfioui, A. Exploring the neuroprotective role of melatonin against nickel-induced neurotoxicity in the left hippocampus. Biometals 2024, 37, 1457–1469. [Google Scholar] [CrossRef]
- Lü, X.Y.; Bao, X.; Huang, Y.; Qu, Y.H.; Lu, H.Q.; Lu, Z.H. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials 2009, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.Y.; Lu, H.Q.; Zhao, L.F.; Yang, Y.M.; Lu, Z.H. Genome-wide pathways analysis of nickel ion-induced differential genes expression in fibroblasts. Biomaterials 2010, 31, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, F.D.; Zhang, Y.J.; Chen, R.; Lü, X.Y. Combination of gene/protein and metabolite multiomics to reveal biomarkers of nickel ion cytotoxicity and the underlying mechanism. Regen. Biomater. 2024, 11, rbae079. [Google Scholar] [CrossRef]
- Halwani, D.O.; Anderson, P.G.; Lemons, J.E.; Jordan, W.D.; Anayiotos, A.S.; Brott, B.C. In-vivo corrosion and local release of metallic ions from vascular stents into surrounding tissue. J. Invasive Cardiol. 2010, 22, 528–535. [Google Scholar]
- Saylor, D.M.; Craven, B.A.; Chandrasekar, V.; Simon, D.D.; Brown, R.P.; Sussman, E.M. Predicting patient exposure to nickel released from cardiovascular devices using multi-scale modeling. Acta Biomater. 2018, 70, 304–314. [Google Scholar] [CrossRef]
- Shotar, E.; Law-Ye, B.; Baronnet-Chauvet, F.; Zeidan, S.; Psimaras, D.; Bielle, F.; Pecquet, C.; Navarro, S.; Rosso, C.; Cohen, F.; et al. Non-ischemic cerebral enhancing lesions secondary to endovascular aneurysm therapy: Nickel allergy or foreign body reaction? Case series and review of the literature. Neuroradiology 2016, 58, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Nakagawa, I.; Yokoyama, S.; Wajima, D.; Wada, T.; Motoyama, Y.; Kichikawa, K.; Nakase, H. Nickel-associated delayed multiple white matter: Lesions after stent-assisted coil embolization of intracranial unruptured aneurysm. J. NeuroInterventional Surg. 2018, 10, e1. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Sullivan, S.J.L.; Stafford, P.R.; Lucas, A.D.; Malkin, E. Impact of nitinol stent surface processing on in-vivo nickel release and biological response. Acta Biomater. 2018, 72, 424–433. [Google Scholar] [CrossRef]
- Tsang, A.C.O.; Nicholson, P.; Pereira, V.M. Nickel-related adverse reactions in the treatment of cerebral aneurysms: A literature review. World Neurosurg. 2018, 115, 147–153. [Google Scholar] [CrossRef]
- Gupta, R.; Polaka, S.; Rajpoot, K.; Tekade, M.; Sharma, M.C.; Tekade, R.K. Importance of toxicity testing in drug discovery and research. In Pharmacokinetics and Toxicokinetic Considerations; Tekade, R.K., Ed.; Academic Press: San Diego, CA, USA, 2022; pp. 117–144. [Google Scholar]
- Jain, M.R.; Bandyopadhyay, D.; Sundar, R. Scientific and regulatory considerations in the development of in vitro techniques for toxicology. In In Vitro Toxicology; Dhawan, A., Kwon, S., Eds.; Academic Press: London, UK, 2018; pp. 165–185. [Google Scholar]
- Shanmugam, P.S.T.; Sampath, T.; Jagadeeswaran, I.; Thamizharasan, S.; Fathima, S.; Krithaksha, V. Cytotoxicity. In Biocompatibility Protocols for Medical Devices and Materials; Shanmugam, P.S.T., Sampath, T., Jagadeeswaran, I., Eds.; Academic Press: London, UK, 2023; pp. 1–18. [Google Scholar]
- U.S. Congress. Office of Technology Assessment. Summary, policy issues, and options for congressional action. In Neurotoxicity: Identifying and Controlling Poisons of the Nervous System, OTA-BA-436; U.S. Government Printing Office: Washington, DC, USA, 1990; pp. 3–42. [Google Scholar]
- Hou, K.; Meng, C.; Huang, Y.; Zhang, Z.Q.; Wang, Z.G.; Lü, X.Y. A Research on the role and mechanism of N-methyl-D-aspartate receptors in the effects of silver nanoparticles on the electrical excitability of hippocampal neuronal networks. J. Biomed. Nanotechnol. 2022, 18, 1423–1433. [Google Scholar] [CrossRef]
- Magee, J.C.; Johnston, D. Characterization of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J. Physiol. 1995, 487, 67–90. [Google Scholar] [CrossRef]
- Martínez, M.L.; Heredia, M.P.; Delgado, C. Expression of T-type Ca2+ channels in ventricular cells from hypertrophied rat hearts. J. Mol. Cell. Cardiol. 1999, 31, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Hobai, I.A.; Hancox, J.C.; Levi, A.J. Inhibition by nickel of the L-type Ca channel in guinea pig ventricular myocytes and effect of internal cAMP. Am. J. Physiol. 2000, 279, H692–H701. [Google Scholar] [CrossRef]
- Gavazzo, P.; Tedesco, M.; Chiappalone, M.; Zanardi, I.; Marchetti, C. Nickel modulates the electrical activity of cultured cortical neurons through a specific effect on N-methyl-d-aspartate receptor channels. Neuroscience 2011, 177, 43–55. [Google Scholar]
- Meng, C.; Lu, Y.; Huang, Y.; Lü, X.Y. Electrical excitability of neuronal networks based on the voltage threshold of electrical stimulation. Sci. Rep. 2024, 14, 31573. [Google Scholar] [CrossRef]
- Kuba, H.; Adachi, R.; Ohmori, H. Activity-dependent and activity-independent development of the axon initial segment. J. Neurosci. 2014, 34, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Kuba, H. Structural and functional plasticity at the axon initial segment. Front. Cell. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, C. The axon initial segment: An updated viewpoint. J. Neurosci. 2018, 38, 2135–2145. [Google Scholar] [CrossRef]
- Hamada, M.S.; Kole, M.H.P. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J. Neurosci. 2015, 35, 7272–7286. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 2003, 4, 2. [Google Scholar] [CrossRef]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef]
- Wolf, A.; Agnihotri, S.; Munoz, D.; Guha, A. Developmental profile and regulation of the glycolytic enzyme hexokinase 2 in normal brain and glioblastoma multiforme. Neurobiol. Dis. 2011, 44, 84–91. [Google Scholar] [CrossRef]
- Zheng, X.D.; Boyer, L.; Jin, M.J.; Mertens, J.; Kim, Y.S.; Ma, L.; Ma, L.; Hamm, M.; Gage, F.H.; Hunter, T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 2016, 5, e13374. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain glucose metabolism: Integration of energetics with function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Mekada, E.; Shishido, Y.; Ikenaka, K. Immune system-related CD9 is expressed in mouse central nervous system myelin at a very late stage of myelination. J. Neurosci. Res. 1997, 50, 312–320. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Stahl, R.C.; Carey, D.J. Tetraspanins are involved in Schwann cell-axon interaction. J. Neurosci. Res. 2013, 91, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, J.R.; Liu, X.Y.; Smith, A.; McNeil, K.; Weston, P.; Zhitkovich, A.; Hurt, R.; Kane, A.B. Bioavailability, intracellular mobilization of nickel, and HIF-1α activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles. Toxicol. Sci. 2011, 124, 138–148. [Google Scholar] [CrossRef]
- Sato, T.; Kishimoto, Y.; Asakawa, S.; Mizuno, N.; Hiratsuka, M.; Hirasawa, N. Involvement of COX-2 in nickel elution from a wire implanted subcutaneously in mice. Toxicology 2016, 363, 37–45. [Google Scholar] [CrossRef]
- Kasai, K.; Segawa, R.; Onodera, R.; Asakawa, S.; Hiratsuka, M.; Hirasawa, N. Lactate released from human fibroblasts enhances Ni elution from Ni plate. Toxicology 2021, 453, 152723. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Chen, Y.Y.; Gao, Y.; Zhang, D.; Jin, S.; Yao, W.X.; Li, L.N.; Yang, S.K.; Wu, Y.H. Metformin alleviates nickel-refining fumes-induced aerobic glycolysis via AMPK/GOLPH3 pathway in vitro and in vivo. Ecotox. Environ. Saf. 2022, 236, 113461. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, M.F.; Yang, G.; Tan, Q.R.; Chen, Y.B.; Li, G.; Zhang, Q.W.; Li, Y.K.; Wan, P.; Wu, J.G. Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 218. [Google Scholar] [CrossRef]
- Jiang, X.P.; Guo, X.W.; Xu, X.; Teng, M.; Huang, C.; Zhang, D.X.; Zhang, Q.; Zhang, J.P.; Huang, Y.S. Hypoxia regulates CD9-mediated keratinocyte migration via the P38/MAPK pathway. Sci. Rep. 2014, 4, 6304. [Google Scholar] [CrossRef]
- Hawke, L.G.; Whitford, M.K.M.; Ormiston, M.L. The production of pro-angiogenic VEGF-A isoforms by hypoxic human NK cells is independent of their TGF-β-mediated conversion to an ILC1-like phenotype. Front. Immunol. 2020, 11, 1903. [Google Scholar] [CrossRef]
- Warnier, G.; De Groote, E.; Britto, F.A.; Delcorte, O.; Nederveen, J.P.; Nilsson, M.I.; Pierreux, C.E.; Tarnopolsky, M.A.; Deldicque, L. Effects of an acute exercise bout in hypoxia on extracellular vesicle release in healthy and prediabetic subjects. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2022, 322, R112–R122. [Google Scholar] [CrossRef]
- Li, L.; Sun, B.; Sun, Y.P. Identification of functional TF-miRNA-hub gene regulatory network associated with ovarian endometriosis. Front. Genet. 2022, 13, 998417. [Google Scholar] [CrossRef] [PubMed]
- Buttermore, E.D.; Piochon, C.; Wallace, M.L.; Philpot, B.D.; Hansel, C.; Bhat, M.A. Pinceau organization in the cerebellum requires distinct functions of Neurofascin in purkinje and basket neurons during postnatal development. J. Neurosci. 2012, 32, 4724–4742. [Google Scholar] [CrossRef] [PubMed]
- Fréal, A.; Rai, D.; Tas, R.P.; Pan, X.X.; Katrukha, E.A.; van de Willige, D.; Stucchi, R.; Aher, A.; Yang, C.; Altelaar, A.F.M.; et al. Feedback-driven assembly of the axon initial segment. Neuron 2019, 104, 305–321. [Google Scholar] [CrossRef]
- He, L.P.; Jiang, W.L.; Li, J.C.; Wang, C. Crystal structure of Ankyrin-G in complex with a fragment of Neurofascin reveals binding mechanisms required for integrity of the axon initial segment. J. Biol. Chem. 2022, 298, 102272. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Liu, A.H.; Lu, N.; Li, Y.H.; Song, Q.L.; Yu, H.M.; Li, X.J. Inhibitive Effects of low oxygen and glucose deprivation on brain-pancreas relative protein expression via hypoxia- inducible factor-1 pathways. Cell. Physiol. Biochem. 2008, 22, 353–362. [Google Scholar] [CrossRef]
- Revuelta-López, E.; Cal, R.; Herraiz-Martínez, A.; de Gonzalo-Calvo, D.; Nasarre, L.; Roura, S.; Gálvez-Montón, C.; Bayes-Genis, A.; Badimon, L.; Hove-Madsen, L.; et al. Hypoxia-driven sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) downregulation depends on low-density lipoprotein receptor-related protein 1 (LRP1)-signalling in cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 85, 25–36. [Google Scholar] [CrossRef]
- Osuru, H.P.; Lavallee, M.; Thiele, R.H. Molecular and cellular response of the myocardium (H9C2 cells) towards hypoxia and HIF-1α inhibition. Front. Cardiovasc. Med. 2022, 9, 711421. [Google Scholar] [CrossRef]
- Harada, K.; Sorimachi, Y.; Yoshida, K. Proteolysis of ankyrin and Na+/K+-ATPase in postmortem rat brain: Is calpain involved? Forensic Sci. Int. 1997, 86, 77–85. [Google Scholar] [CrossRef]
- Schafer, D.P.; Jha, S.; Liu, F.D.; Akella, T.; McCullough, L.D.; Rasband, M.N. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 2009, 29, 13242–13254. [Google Scholar] [CrossRef] [PubMed]
- Barac-Nieto, M.; Constantinescu, A.; Pina-Benabou, M.H.; Rozental, R. Hypoxic rise in cytosolic calcium and renal proximal tubule injury mediated by a nickel-sensitive pathway. Exp. Biol. Med. 2004, 229, 1162–1168. [Google Scholar] [CrossRef]
- Nikonenko, I.; Bancila, M.; Bloc, A.; Muller, D.; Bijlenga, P. Inhibition of T-type calcium channels protects neurons from delayed ischemia-induced damage. Mol. Pharmacol. 2005, 68, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Godukhin, O.; Savin, A.; Kalemenev, S.; Levin, S. Neuronal hyperexcitability induced by repeated brief episodes of hypoxia in rat hippocampal slices: Involvement of ionotropic glutamate receptors and L-type Ca2+ channels. Neuropharmacology 2022, 42, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, G.; Liu, W.J.; Ding, W.W.; Dong, M.; Zheng, N.; Ye, H.Y.; Liu, J. Hypoxia-induced mitogenic factor promotes cardiac hypertrophy via calcium-dependent and hypoxia-inducible factor-1α mechanisms. Hypertension 2018, 72, 331–342. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 2011, 50, 211–221. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Available online: https://www.thermofisher.cn/cn/zh/home/references/molecular-probes-the-handbook/indicators-for-ca2-mg2-zn2-and-other-metal-ions.html (accessed on 25 February 2025).
- Huang, Y.; Lü, X.Y.; Ma, J.W. Toxicity of silver nanoparticles to human dermal fibroblasts on microRNA level. J. Biomed. Nanotechnol. 2014, 10, 3304–3317. [Google Scholar] [CrossRef]


| Gene Symbol | Primers | Sequence (5′ to 3′) | TM (°C) |
|---|---|---|---|
| Hk2 | Hk2-F | CCGTAGTGGACAAGATAAGAG | 62 |
| Hk2-R | TGGCAAAGTGAGGATGAAG | ||
| Ldha | Ldha-F | GCCGAGAGCATAATGAAGAA | 62 |
| Ldha-R | CGTCAGGAGTCAGTGTCA | ||
| Cd9 | Cd9-F | ATATGGCGTTGAACTGCTGTG | 62 |
| Cd9-R | GATGTGGAACTTGCTGTGGAA | ||
| Nfasc | Nfasc-F | TGCTTATCGTCTGCTTCAT | 62 |
| Nfasc-R | CTTGTTGTCCTCGTCACT | ||
| Gapdh (internal reference) | Gapdh-F | GCCATCACTGCCACTCAGAAGAC | 62 |
| Gapdh-R | ATGACCTTGCCCACAGCCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Lu, Y.; Huang, Y.; Lü, X. Investigation of the Neurotoxicity Mechanisms of Ni2+ in Rat Neocortical Neurons Through Transcriptome Analysis. Int. J. Mol. Sci. 2025, 26, 4014. https://doi.org/10.3390/ijms26094014
Meng C, Lu Y, Huang Y, Lü X. Investigation of the Neurotoxicity Mechanisms of Ni2+ in Rat Neocortical Neurons Through Transcriptome Analysis. International Journal of Molecular Sciences. 2025; 26(9):4014. https://doi.org/10.3390/ijms26094014
Chicago/Turabian StyleMeng, Chen, Yang Lu, Yan Huang, and Xiaoying Lü. 2025. "Investigation of the Neurotoxicity Mechanisms of Ni2+ in Rat Neocortical Neurons Through Transcriptome Analysis" International Journal of Molecular Sciences 26, no. 9: 4014. https://doi.org/10.3390/ijms26094014
APA StyleMeng, C., Lu, Y., Huang, Y., & Lü, X. (2025). Investigation of the Neurotoxicity Mechanisms of Ni2+ in Rat Neocortical Neurons Through Transcriptome Analysis. International Journal of Molecular Sciences, 26(9), 4014. https://doi.org/10.3390/ijms26094014






