Tolerogenic Therapies in Cardiac Transplantation
Abstract
1. Introduction
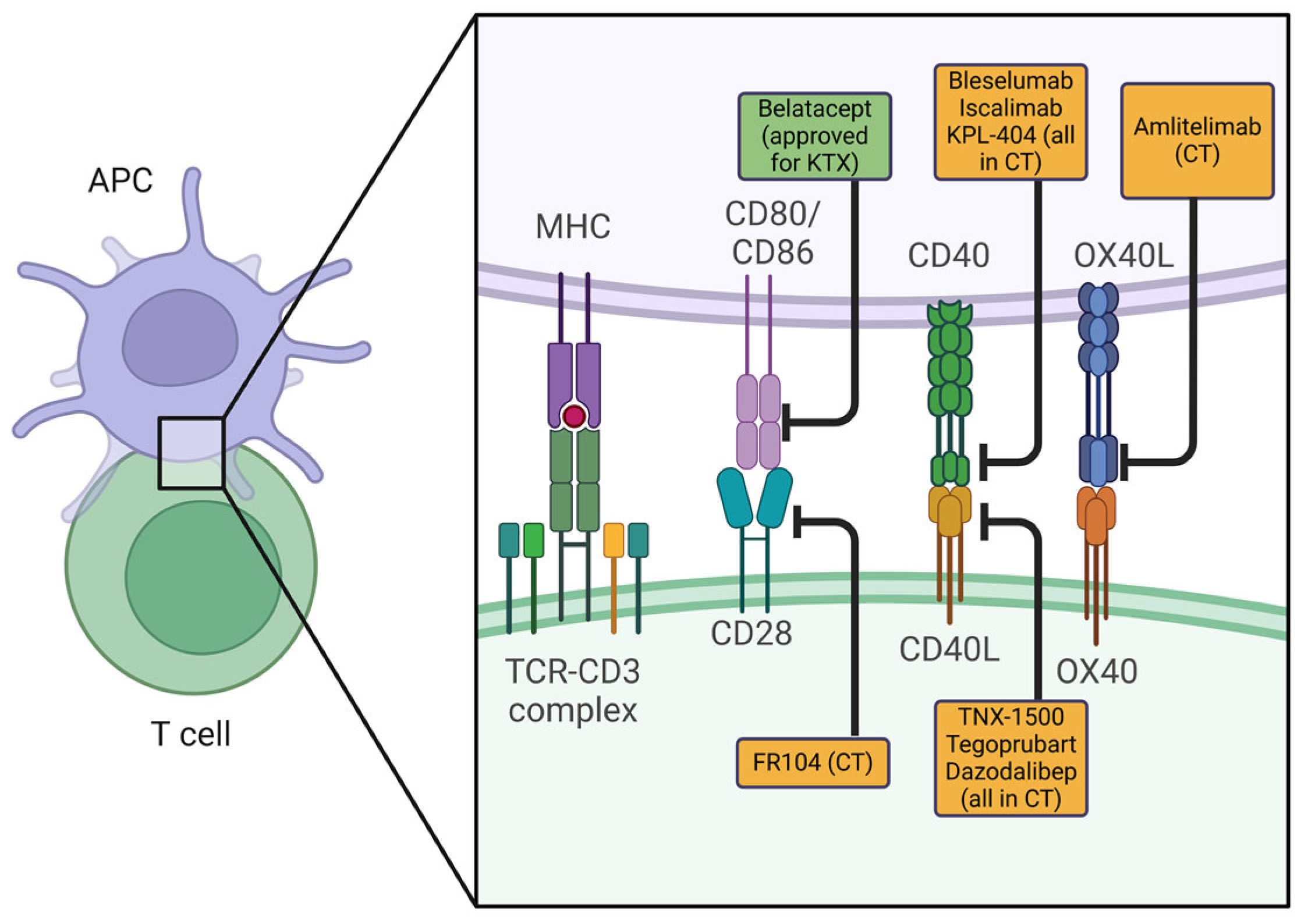
2. Co-Stimulation Blockade
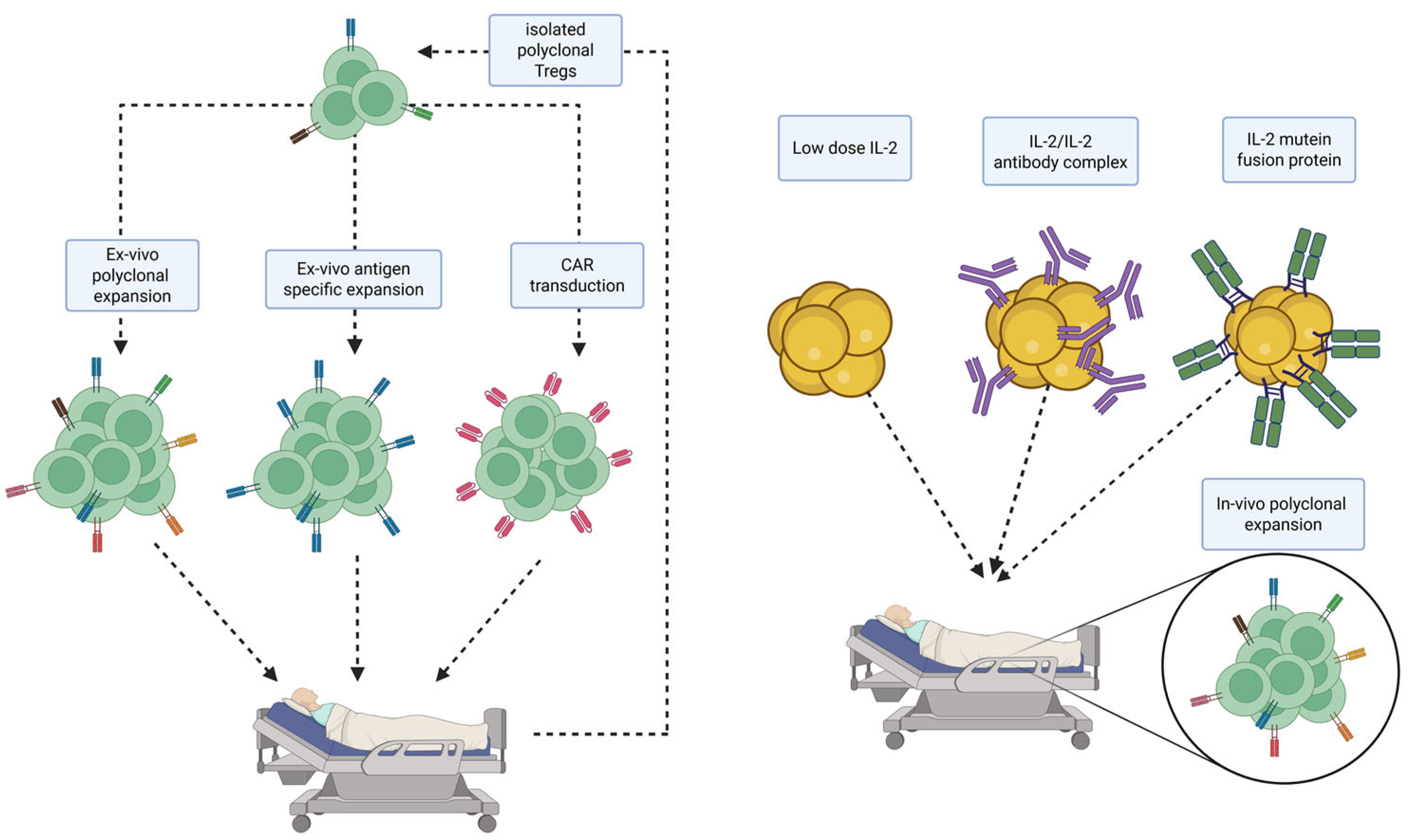
3. Regulatory (T) Cell-Based Therapies
4. Mixed Chimerism
5. Thymus Transplantation
6. Double Organ Transplantation
7. Innovative Advancements for the Extension of the Donor Pool
AB0-Incompatible Transplantation
8. Discussion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lodhi, S.A.; Lamb, K.E.; Meier-Kriesche, H.U. Solid organ allograft survival improvement in the United States: The long-term does not mirror the dramatic short-term success. Am. J. Transplant. 2011, 11, 1226–1235. [Google Scholar] [CrossRef]
- Pierson, R.N., 3rd. Tolerance in heart transplantation: The Holy Grail, or an attainable goal? Heart Fail. Clin. 2007, 3, 17–29. [Google Scholar] [CrossRef]
- Unger, L.W.; Muckenhuber, M.; Mahr, B.; Schwarz, C.; Pilat, N.; Granofszky, N.; Regele, H.; Wekerle, T. Chronic CD40L blockade is required for long-term cardiac allograft survival with a clinically relevant CTLA4-Ig dosing regimen. Front. Immunol. 2022, 13, 1060576. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Ezzelarab, M.B. A Tale of Two Pathways: Renewing the Promise of Anti-CD40L Blockade. Am. J. Transplant. 2017, 17, 1156–1157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pilat, N.; Wekerle, T. Transplantation tolerance through mixed chimerism. Nat. Rev. Nephrol. 2010, 6, 594–605. [Google Scholar] [CrossRef]
- Harden, P.N.; Game, D.S.; Sawitzki, B.; Van der Net, J.B.; Hester, J.; Bushell, A.; Issa, F.; Brook, M.O.; Alzhrani, A.; Schlickeiser, S.; et al. Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am. J. Transplant. 2021, 21, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.J.; Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003, 3, 199–210. [Google Scholar] [CrossRef]
- Madariaga, M.L.; Kreisel, D.; Madsen, J.C. Organ-specific differences in achieving tolerance. Curr. Opin. Organ. Transplant. 2015, 20, 392–399. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Quan, D.; Garcia, B.; Ozcay, N.; Duff, J.; Stiller, C.; Lazarovits, A.; Grant, D.; Zhong, R. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation 1996, 62, 1267–1272. [Google Scholar] [CrossRef]
- Massart, A.; Ghisdal, L.; Abramowicz, M.; Abramowicz, D. Operational tolerance in kidney transplantation and associated biomarkers. Clin. Exp. Immunol. 2017, 189, 138–157. [Google Scholar] [CrossRef]
- Pierson, R.N., 3rd; Chang, A.C.; Blum, M.G.; Blair, K.S.; Scott, M.A.; Atkinson, J.B.; Collins, B.J.; Zhang, J.P.; Thomas, D.W.; Burkly, L.C.; et al. Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation 1999, 68, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Elwood, E.T.; Alexander, D.Z.; Ritchie, S.C.; Hendrix, R.; Tucker-Burden, C.; Cho, H.R.; Aruffo, A.; Hollenbaugh, D.; Linsley, P.S.; et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996, 381, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Chen, J.; Wang, F.; Lan, T.; Wang, Y.; Xia, J.; Li, Z.; Xie, Q.; Huang, R.; Qi, Z. Monoclonal antibody treatment to prolong the secondary cardiac allograft survival in alloantigen-primed mice. Scand. J. Immunol. 2010, 71, 345–352. [Google Scholar] [CrossRef]
- Pearl, J.P.; Parris, J.; Hale, D.A.; Hoffmann, S.C.; Bernstein, W.B.; McCoy, K.L.; Swanson, S.J.; Mannon, R.B.; Roederer, M.; Kirk, A.D. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am. J. Transplant. 2005, 5, 465–474. [Google Scholar] [CrossRef]
- Henn, V.; Slupsky, J.R.; Grafe, M.; Anagnostopoulos, I.; Forster, R.; Muller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef]
- Miura, S.; Habibabady, Z.A.; Pollok, F.; Ma, M.; Rosales, I.A.; Kinoshita, K.; Pratts, S.; McGrath, G.; Chaban, R.; Fogarty, S.; et al. TNX-1500, a crystallizable fragment-modified anti-CD154 antibody, prolongs nonhuman primate cardiac allograft survival. Am. J. Transplant. 2023, 23, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- de Graav, G.N.; Udomkarnjananun, S.; Baan, C.C.; Reinders, M.E.J.; Roodnat, J.I.; de Winter, B.C.M.; Hesselink, D.A. New Developments and Therapeutic Drug Monitoring Options in Costimulatory Blockade in Solid Organ Transplantation: A Systematic Critical Review. Ther. Drug Monit. 2025, 47, 64–76. [Google Scholar] [CrossRef]
- Pilat, N.; Klaus, C.; Schwarz, C.; Hock, K.; Oberhuber, R.; Schwaiger, E.; Gattringer, M.; Ramsey, H.; Baranyi, U.; Zelger, B.; et al. Rapamycin and CTLA4Ig synergize to induce stable mixed chimerism without the need for CD40 blockade. Am. J. Transplant. 2015, 15, 1568–1579. [Google Scholar] [CrossRef]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef]
- Fu, N.; Xie, F.; Sun, Z.; Wang, Q. The OX40/OX40L Axis Regulates T Follicular Helper Cell Differentiation: Implications for Autoimmune Diseases. Front. Immunol. 2021, 12, 670637. [Google Scholar] [CrossRef]
- Kitchens, W.H.; Dong, Y.; Mathews, D.V.; Breeden, C.P.; Strobert, E.; Fuentes, M.E.; Larsen, C.P.; Ford, M.L.; Adams, A.B. Interruption of OX40L signaling prevents costimulation blockade-resistant allograft rejection. JCI Insight 2017, 2, e90317. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Yao, Z.; Ning, F.; Zhang, L.; Fang, J.; Li, G.; Xu, L.; Xiong, Y.; Liu, L.; Chen, R.; et al. Blockade of OX40/OX40L pathway combined with ethylene-carbodiimide-fixed donor splenocytes induces donor-specific allograft tolerance in presensitized recipients. Ann. Transl. Med. 2020, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Peng, F.; Lin, M.; Xia, J.; Yu, S.; Lan, G.; Wang, Y.; Xie, X.; Fang, C.; Corbascio, M.; et al. Anti-OX40L monoclonal antibody prolongs secondary heart allograft survival based on CD40/CD40L and LFA-1/ICAM-1 blockade. Transpl. Immunol. 2015, 32, 84–91. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Tian, W.; Liu, T.; Han, H.; Garcia, B.; Li, X.C.; Du, C. Memory T Cells Mediate Cardiac Allograft Vasculopathy and are Inactivated by Anti-OX40L Monoclonal Antibody. Cardiovasc. Drugs Ther. 2014, 28, 115–122. [Google Scholar] [CrossRef]
- Saghari, M.; Gal, P.; Gilbert, S.; Yateman, M.; Porter-Brown, B.; Brennan, N.; Quaratino, S.; Wilson, R.; Grievink, H.W.; Klaassen, E.S.; et al. OX40L Inhibition Suppresses KLH-driven Immune Responses in Healthy Volunteers: A Randomized Controlled Trial Demonstrating Proof-of-Pharmacology for KY1005. Clin. Pharmacol. Ther. 2022, 111, 1121–1132. [Google Scholar] [CrossRef]
- Greenfield, E.A.; Nguyen, K.A.; Kuchroo, V.K. CD28/B7 costimulation: A review. Crit. Rev. Immunol. 1998, 18, 389–418. [Google Scholar] [CrossRef]
- Haspot, F.; Seveno, C.; Dugast, A.S.; Coulon, F.; Renaudin, K.; Usal, C.; Hill, M.; Anegon, I.; Heslan, M.; Josien, R.; et al. Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. Am. J. Transplant. 2005, 5, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Vincenti, F. Costimulation blockade in autoimmunity and transplantation. J. Allergy Clin. Immunol. 2008, 121, 299–306. [Google Scholar] [CrossRef]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef]
- Masson, P.; Henderson, L.; Chapman, J.R.; Craig, J.C.; Webster, A.C. Belatacept for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 2014, CD010699. [Google Scholar] [CrossRef] [PubMed]
- Oren, D.; Uriel, M.; Moeller, C.M.; Valledor, A.F.; DeFilippis, E.M.; Lotan, D.; Colombo, P.C.; Yuzefpolskaya, M.; Topkara, V.K.; Clerkin, K.J.; et al. Utility of a fusion protein T-cell co-stimulation blocker Belatacept in heart transplant recipients: Real world experience from a high volume center. Clin. Transplant. 2024, 38, e15251. [Google Scholar] [CrossRef]
- Fida, N.; Eagar, T.N.; Yun, A.N.; Rogers, A.W.; Nguyen, D.T.; Graviss, E.A.; Ishaq, F.; DiPaola, N.R.; Kim, J.; Janardhana, G.; et al. Effectiveness of combined plasma cell therapy and costimulation blockade based desensitization regimen in heart transplant candidates. Clin. Transplant. 2024, 38, e15249. [Google Scholar] [CrossRef] [PubMed]
- Magee, C.N.; Murakami, N.; Borges, T.J.; Shimizu, T.; Safa, K.; Ohori, S.; Cai, S.; Uffing, A.; Azzi, J.; Elyaman, W.; et al. Notch-1 Inhibition Promotes Immune Regulation in Transplantation Via Regulatory T Cell-Dependent Mechanisms. Circulation 2019, 140, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, C.R.; Tong, K.P.; Liu, D.; Laurie, S.J.; Ford, M.L. TIGIT agonism alleviates costimulation blockade-resistant rejection in a regulatory T cell-dependent manner. Am. J. Transplant. 2023, 23, 180–189. [Google Scholar] [CrossRef]
- Duneton, C.; Winterberg, P.D.; Ford, M.L. Activation and regulation of alloreactive T cell immunity in solid organ transplantation. Nat. Rev. Nephrol. 2022, 18, 663–676. [Google Scholar] [CrossRef]
- Pilat, N.; Lefsihane, K.; Brouard, S.; Kotsch, K.; Falk, C.; Steiner, R.; Thaunat, O.; Fusil, F.; Montserrat, N.; Amarelli, C.; et al. T- and B-cell therapy in solid organ transplantation: Current evidence and future expectations. Transpl. Int. 2021, 34, 1594–1606. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Gorochov, G.; Ehrenstein, M.; Musset, L.; Sakaguchi, S.; Amoura, Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 2011, 10, 744–755. [Google Scholar] [CrossRef]
- Mikami, N.; Sakaguchi, S. Regulatory T cells in autoimmune kidney diseases and transplantation. Nat. Rev. Nephrol. 2023, 19, 544–557. [Google Scholar] [CrossRef]
- Tsang, J.Y.; Tanriver, Y.; Jiang, S.; Xue, S.A.; Ratnasothy, K.; Chen, D.; Stauss, H.J.; Bucy, R.P.; Lombardi, G.; Lechler, R. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Investig. 2008, 118, 3619–3628. [Google Scholar] [CrossRef]
- Pilat, N.; Steiner, R.; Sprent, J. Treg Therapy for the Induction of Immune Tolerance in Transplantation-Not Lost in Translation? Int. J. Mol. Sci. 2023, 24, 1752. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Kawakami, R.; Mikami, N. Treg-based immunotherapy for antigen-specific immune suppression and stable tolerance induction: A perspective. Immunother. Adv. 2023, 3, ltad007. [Google Scholar] [CrossRef] [PubMed]
- Ezzelarab, M.B.; Zhang, H.; Sasaki, K.; Lu, L.; Zahorchak, A.F.; van der Windt, D.J.; Dai, H.; Perez-Gutierrez, A.; Bhama, J.K.; Thomson, A.W. Ex Vivo Expanded Donor Alloreactive Regulatory T Cells Lose Immunoregulatory, Proliferation, and Antiapoptotic Markers After Infusion Into ATG-lymphodepleted, Nonhuman Primate Heart Allograft Recipients. Transplantation 2021, 105, 1965–1979. [Google Scholar] [CrossRef]
- Lee, L.M.; Zhang, H.; Lee, K.; Liang, H.; Merleev, A.; Vincenti, F.; Maverakis, E.; Thomson, A.W.; Tang, Q. A Comparison of Ex Vivo Expanded Human Regulatory T Cells Using Allogeneic Stimulated B Cells or Monocyte-Derived Dendritic Cells. Front. Immunol. 2021, 12, 679675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019, 10, 2250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, W.; Liang, C.L.; Chen, Y.; Liu, H.; Qiu, F.; Dai, Z. Chimeric Antigen Receptor (CAR) Treg: A Promising Approach to Inducing Immunological Tolerance. Front. Immunol. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Wagner, J.C.; Tang, Q. CAR-Tregs as a Strategy for Inducing Graft Tolerance. Curr. Transplant. Rep. 2020, 7, 205–214. [Google Scholar] [CrossRef]
- Stucchi, A.; Maspes, F.; Montee-Rodrigues, E.; Fousteri, G. Engineered Treg cells: The heir to the throne of immunotherapy. J. Autoimmun. 2024, 144, 102986. [Google Scholar] [CrossRef]
- MacDonald, K.G.; Hoeppli, R.E.; Huang, Q.; Gillies, J.; Luciani, D.S.; Orban, P.C.; Broady, R.; Levings, M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Investig. 2016, 126, 1413–1424. [Google Scholar] [CrossRef]
- Sicard, A.; Lamarche, C.; Speck, M.; Wong, M.; Rosado-Sanchez, I.; Blois, M.; Glaichenhaus, N.; Mojibian, M.; Levings, M.K. Donor-specific chimeric antigen receptor Tregs limit rejection in naive but not sensitized allograft recipients. Am. J. Transplant. 2020, 20, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.V.; Kelley, T.W. Induction of granzyme B expression in T-cell receptor/CD28-stimulated human regulatory T cells is suppressed by inhibitors of the PI3K-mTOR pathway. BMC Immunol. 2009, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M. The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 2019, 211, 13–22. [Google Scholar] [CrossRef]
- Wermke, M.; Kraus, S.; Ehninger, A.; Bargou, R.C.; Goebeler, M.E.; Middeke, J.M.; Kreissig, C.; von Bonin, M.; Koedam, J.; Pehl, M.; et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood 2021, 137, 3145–3148. [Google Scholar] [CrossRef]
- Ellis, G.I.; Deng, M.Z.; Winn, D.W.; Coker, K.E.; Shukla, D.; Bhoj, V.; Milone, M.C.; Duran-Struuck, R.; Riley, J.L. Generation of non-human primate CAR Tregs using artificial antigen-presenting cells, simian tropic lentiviral vectors, and antigen-specific restimulation. STAR Protoc. 2022, 3, 101784. [Google Scholar] [CrossRef] [PubMed]
- Raffin, C.; Vo, L.T.; Bluestone, J.A. T(reg) cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020, 20, 158–172. [Google Scholar] [CrossRef]
- Todo, S.; Yamashita, K.; Goto, R.; Zaitsu, M.; Nagatsu, A.; Oura, T.; Watanabe, M.; Aoyagi, T.; Suzuki, T.; Shimamura, T.; et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 2016, 64, 632–643. [Google Scholar] [CrossRef]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef]
- Brook, M.O.; Hester, J.; Petchey, W.; Rombach, I.; Dutton, S.; Bottomley, M.J.; Black, J.; Abdul-Wahab, S.; Bushell, A.; Lombardi, G.; et al. Transplantation Without Overimmunosuppression (TWO) study protocol: A phase 2b randomised controlled single-centre trial of regulatory T cell therapy to facilitate immunosuppression reduction in living donor kidney transplant recipients. BMJ Open 2022, 12, e061864. [Google Scholar] [CrossRef]
- San Segundo, D.; Fabrega, E.; Lopez-Hoyos, M.; Pons, F. Reduced numbers of blood natural regulatory T cells in stable liver transplant recipients with high levels of calcineurin inhibitors. Transplant. Proc. 2007, 39, 2290–2292. [Google Scholar] [CrossRef]
- Segundo, D.S.; Ruiz, J.C.; Izquierdo, M.; Fernandez-Fresnedo, G.; Gomez-Alamillo, C.; Merino, R.; Benito, M.J.; Cacho, E.; Rodrigo, E.; Palomar, R.; et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 2006, 82, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, G.; Gray, E.; Mastoridis, S.; Merritt, E.; Kodela, E.; Yang, J.H.M.; Danger, R.; Mairal, M.; Christakoudi, S.; Lozano, J.J.; et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc. Natl. Acad. Sci. USA 2017, 114, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Unger, L.; Mahr, B.; Aumayr, K.; Regele, H.; Farkas, A.M.; Hock, K.; Pilat, N.; Wekerle, T. The Immunosuppressive Effect of CTLA4 Immunoglobulin Is Dependent on Regulatory T Cells at Low But Not High Doses. Am. J. Transplant. 2016, 16, 3404–3415. [Google Scholar] [CrossRef]
- Furukawa, A.; Wisel, S.A.; Tang, Q. Impact of Immune-Modulatory Drugs on Regulatory T Cell. Transplantation 2016, 100, 2288–2300. [Google Scholar] [CrossRef]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Lim, T.Y.; Perpinan, E.; Londono, M.C.; Miquel, R.; Ruiz, P.; Kurt, A.S.; Kodela, E.; Cross, A.R.; Berlin, C.; Hester, J.; et al. Low dose interleukin-2 selectively expands circulating regulatory T cells but fails to promote liver allograft tolerance in humans. J. Hepatol. 2023, 78, 153–164. [Google Scholar] [CrossRef]
- Webster, K.E.; Walters, S.; Kohler, R.E.; Mrkvan, T.; Boyman, O.; Surh, C.D.; Grey, S.T.; Sprent, J. In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 2009, 206, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Pilat, N.; Wiletel, M.; Weijler, A.M.; Steiner, R.; Mahr, B.; Warren, J.; Corpuz, T.M.; Wekerle, T.; Webster, K.E.; Sprent, J. Treg-mediated prolonged survival of skin allografts without immunosuppression. Proc. Natl. Acad. Sci. USA 2019, 116, 13508–13516. [Google Scholar] [CrossRef]
- Trotta, E.; Bessette, P.H.; Silveria, S.L.; Ely, L.K.; Jude, K.M.; Le, D.T.; Holst, C.R.; Coyle, A.; Potempa, M.; Lanier, L.L.; et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 2018, 24, 1005–1014. [Google Scholar] [CrossRef]
- Efe, O.; Gassen, R.B.; Morena, L.; Ganchiku, Y.; Al Jurdi, A.; Lape, I.T.; Ventura-Aguiar, P.; LeGuern, C.; Madsen, J.C.; Shriver, Z.; et al. A humanized IL-2 mutein expands Tregs and prolongs transplant survival in preclinical models. J. Clin. Investig. 2024, 134, e173107. [Google Scholar] [CrossRef]
- Wekerle, T.; Kurtz, J.; Sayegh, M.; Ito, H.; Wells, A.; Bensinger, S.; Shaffer, J.; Turka, L.; Sykes, M. Peripheral deletion after bone marrow transplantation with costimulatory blockade has features of both activation-induced cell death and passive cell death. J. Immunol. 2001, 166, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Fouzia, N.A.; Edison, E.S.; Lakshmi, K.M.; Korula, A.; Velayudhan, S.R.; Balasubramanian, P.; Abraham, A.; Viswabandya, A.; George, B.; Mathews, V.; et al. Long-term outcome of mixed chimerism after stem cell transplantation for thalassemia major conditioned with busulfan and cyclophosphamide. Bone Marrow Transplant. 2018, 53, 169–174. [Google Scholar] [CrossRef]
- Wekerle, T.; Kurtz, J.; Ito, H.; Ronquillo, J.V.; Dong, V.; Zhao, G.; Shaffer, J.; Sayegh, M.H.; Sykes, M. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat. Med. 2000, 6, 464–469. [Google Scholar] [CrossRef]
- Pilat, N.; Farkas, A.M.; Mahr, B.; Schwarz, C.; Unger, L.; Hock, K.; Oberhuber, R.; Aumayr, K.; Wrba, F.; Wekerle, T. T-regulatory cell treatment prevents chronic rejection of heart allografts in a murine mixed chimerism model. J. Heart Lung Transplant. 2014, 33, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sachs, D.H.; Kawai, T.; Sykes, M. Induction of tolerance through mixed chimerism. Cold Spring Harb. Perspect. Med. 2014, 4, a015529. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, T.R.; Delmonico, F.; Tolkoff-Rubin, N.; McAfee, S.; Sackstein, R.; Saidman, S.; Colby, C.; Sykes, M.; Sachs, D.H.; Cosimi, A.B. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: The induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation 1999, 68, 480–484. [Google Scholar] [CrossRef]
- Sykes, M. Mixed chimerism and transplant tolerance. Immunity 2001, 14, 417–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwarze, M.L.; Menard, M.T.; Fuchimoto, Y.; Huang, C.A.; Houser, S.; Mawulawde, K.; Allison, K.S.; Sachs, D.H.; Madsen, J.C. Mixed hematopoietic chimerism induces long-term tolerance to cardiac allografts in miniature swine. Ann. Thorac. Surg. 2000, 70, 131–138, discussion 138–139. [Google Scholar] [CrossRef]
- Kawai, T.; Cosimi, A.B.; Wee, S.L.; Houser, S.; Andrews, D.; Sogawa, H.; Phelan, J.; Boskovic, S.; Nadazdin, O.; Abrahamian, G.; et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation 2002, 73, 1757–1764. [Google Scholar] [CrossRef]
- Shinoda, K.; Akiyoshi, T.; Chase, C.M.; Farkash, E.A.; Ndishabandi, D.K.; Raczek, C.M.; Sebastian, D.P.; Pelle, P.D.; Russell, P.S.; Madsen, J.C.; et al. Depletion of foxp3(+) T cells abrogates tolerance of skin and heart allografts in murine mixed chimeras without the loss of mixed chimerism. Am. J. Transplant. 2014, 14, 2263–2274. [Google Scholar] [CrossRef]
- Sommer, W.; O, J.M.; Pruner, K.B.; Dehnadi, A.; Ha Huh, K.; Robinson, K.A.; Hanekamp, I.; Rosales, I.; Bean, A.S.; Paster, J.; et al. A Mixed-chimerism Protocol Utilizing Thymoglobulin and Belatacept Did Not Induce Lung Allograft Tolerance, Despite Previous Success in Renal Allotransplantation. Transplant. Direct 2021, 7, e705. [Google Scholar] [CrossRef]
- Tonsho, M.; O, J.M.; Ahrens, K.; Robinson, K.; Sommer, W.; Boskovic, S.; Patel, P.M.; Becerra, D.C.; Huh, K.H.; Miller, C.L.; et al. Cardiac allograft tolerance can be achieved in nonhuman primates by donor bone marrow and kidney cotransplantation. Sci. Transl. Med. 2025, 17, eads0255. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Nakao, A.; Zhang, W.; Gorantla, V.; Zheng, X.X. Heart allograft tolerance induced and maintained by vascularized hind-limb transplant in rats. Clin. Dev. Immunol. 2013, 2013, 483856. [Google Scholar] [CrossRef]
- Wachsmuth, L.P.; Patterson, M.T.; Eckhaus, M.A.; Venzon, D.J.; Gress, R.E.; Kanakry, C.G. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J. Clin. Investig. 2019, 129, 2357–2373. [Google Scholar] [CrossRef]
- Crocchiolo, R.; Bramanti, S.; Vai, A.; Sarina, B.; Mineri, R.; Casari, E.; Tordato, F.; Mauro, E.; Timofeeva, I.; Lugli, E.; et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl. Infect. Dis. 2015, 17, 242–249. [Google Scholar] [CrossRef]
- Lin, C.J.; Vader, J.M.; Slade, M.; DiPersio, J.F.; Westervelt, P.; Romee, R. Cardiomyopathy in patients after posttransplant cyclophosphamide-based hematopoietic cell transplantation. Cancer 2017, 123, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Kabore, M.D.; McElrath, C.C.; Ali, M.A.E.; Almengo, K.; Gangaplara, A.; Fisher, C.; Barreto, M.A.; Shaikh, A.; Olkhanud, P.B.; Xu, X.; et al. Low dose post-transplant cyclophosphamide and sirolimus induce mixed chimerism with CTLA4-Ig or lymphocyte depletion in an MHC-mismatched murine allotransplantation model. Bone Marrow Transplant. 2024, 59, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.M.; Rao, A.S.; Zeevi, A.; Kormos, R.L.; McCurry, K.R.; Hattler, B.G.; Fung, J.J.; Starzl, T.E.; Griffith, B.P. A clinical trial combining donor bone marrow infusion and heart transplantation: Intermediate-term results. J. Thorac. Cardiovasc. Surg. 2000, 119, 673–681. [Google Scholar] [CrossRef]
- Fitch, Z.W.; Kang, L.; Li, J.; Knechtle, S.J.; Turek, J.W.; Kirk, A.D.; Markert, M.L.; Kwun, J. Introducing thymus for promoting transplantation tolerance. J. Allergy Clin. Immunol. 2022, 150, 549–556. [Google Scholar] [CrossRef]
- Markert, M.L.; Gupton, S.E.; McCarthy, E.A. Experience with cultured thymus tissue in 105 children. J. Allergy Clin. Immunol. 2022, 149, 747–757. [Google Scholar] [CrossRef]
- Gupton, S.E.; McCarthy, E.A.; Markert, M.L. Care of Children with DiGeorge Before and After Cultured Thymus Tissue Implantation. J. Clin. Immunol. 2021, 41, 896–905. [Google Scholar] [CrossRef]
- Kwun, J.; Li, J.; Rouse, C.; Park, J.B.; Farris, A.B.; Kuchibhatla, M.; Turek, J.W.; Knechtle, S.J.; Kirk, A.D.; Markert, M.L. Cultured thymus tissue implantation promotes donor-specific tolerance to allogeneic heart transplants. JCI Insight 2020, 5, e129983. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Markert, M.L.; Turek, J.W. Induction of donor-specific tolerance to heart transplantation: From concept to clinical translation. J. Thorac. Cardiovasc. Surg. 2023, 165, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Mohiuddin, M.; Goldstein, C.; Yokoyama, H.; DiSesa, V.J. Durability of donor-specific and organ-specific heart transplant tolerance induced by intrathymic pretreatment with allogeneic spleen cells. J. Thorac. Cardiovasc. Surg. 1996, 111, 429–431. [Google Scholar] [CrossRef]
- Shen, Z.; Kline, G.; Mohiuddin, M.; DiSesa, V.J. Histocompatibility differences and cardiac transplant tolerance produced by intrathymic pretreatment. J. Thorac. Cardiovasc. Surg. 1994, 107, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Djamali, A.; Waller, K.R.; McAnulty, J.; Hullett, D.; Becker, B.N.; Odorico, J.S. Intrathymic injection of anti-Fas monoclonal antibody prolongs murine non-vascularized cardiac allograft survival. Transpl. Int. 2004, 17, 301–309. [Google Scholar] [CrossRef]
- Menard, M.T.; Schwarze, M.L.; Allan, J.S.; Johnston, D.R.; Mawulawde, K.; Shimizu, A.; Yamada, K.; Houser, S.L.; Allison, K.S.; Sachs, D.H.; et al. Composite “thymoheart” transplantation improves cardiac allograft survival. Am. J. Transplant. 2004, 4, 79–86. [Google Scholar] [CrossRef]
- Nobori, S.; Samelson-Jones, E.; Shimizu, A.; Hisashi, Y.; Yamamoto, S.; Kamano, C.; Teranishi, K.; Vagefi, P.A.; Nuhn, M.; Okumi, M.; et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation 2006, 81, 26–35. [Google Scholar] [CrossRef]
- Johnston, D.R.; Muniappan, A.; Hoerbelt, R.; Guenther, D.A.; Shoji, T.; Houser, S.L.; Sachs, D.H.; Madsen, J.C. Heart and en-bloc thymus transplantation in miniature swine. J. Thorac. Cardiovasc. Surg. 2005, 130, 554–559. [Google Scholar] [CrossRef]
- Oh, B.; Furtmuller, G.J.; Malek, V.; Fryer, M.L.; Brayton, C.; Walczak, P.; Janowsky, M.; Brandacher, G.; Dorafshar, A.H. Split Tolerance in a Murine Model of Heterotopic En Bloc Chest Wall Transplantation. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1595. [Google Scholar] [CrossRef]
- Narula, J.; Bennett, L.E.; DiSalvo, T.; Hosenpud, J.D.; Semigran, M.J.; Dec, G.W. Outcomes in recipients of combined heart-kidney transplantation: Multiorgan, same-donor transplant study of the International Society of Heart and Lung Transplantation/United Network for Organ Sharing Scientific Registry. Transplantation 1997, 63, 861–867. [Google Scholar] [CrossRef]
- Sampaio, N.Z.; Faleiro, M.D.; Vieira, L.; Lech, G.E.; Viana, S.W.; Tavares, C.P.O.; Mattiazzi, A.D.; Burke, G.W., 3rd. Simultaneous Heart and Kidney Transplantation: A Systematic Review and Proportional Meta-Analysis of Its Characteristics and Long-Term Variables. Transpl. Int. 2024, 37, 12750. [Google Scholar] [CrossRef]
- Williams, K.A.; Hart, D.N.; Fabre, J.W.; Morris, P.J. Distribution and quantitation of HLA-ABC and DR (Ia) antigens on human kidney and other tissues. Transplantation 1980, 29, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Mawulawde, K.; Menard, M.T.; Shimizu, A.; Aretz, H.T.; Choo, J.K.; Allison, K.S.; Slisz, J.K.; Sachs, D.H.; Madsen, J.C. Mechanisms of tolerance induction and prevention of cardiac allograft vasculopathy in miniature swine: The effect of augmentation of donor antigen load. J. Thorac. Cardiovasc. Surg. 2000, 119, 709–719. [Google Scholar] [CrossRef]
- Yang, C.; Ge, J.; Rosales, I.; Yuan, Q.; Szuter, E.; Acheampong, E.; Russell, P.S.; Madsen, J.C.; Colvin, R.B.; Alessandrini, A. Kidney-induced systemic tolerance of heart allografts in mice. JCI Insight 2020, 5, e139331. [Google Scholar] [CrossRef] [PubMed]
- Raichlin, E.; Daly, R.C.; Rosen, C.B.; McGregor, C.G.; Charlton, M.R.; Frantz, R.P.; Clavell, A.L.; Rodeheffer, R.J.; Pereira, N.L.; Kremers, W.K.; et al. Combined heart and liver transplantation: A single-center experience. Transplantation 2009, 88, 219–225. [Google Scholar] [CrossRef]
- Alexopoulos, S.P.; Wu, W.K.; Ziogas, I.A.; Matsuoka, L.K.; Rauf, M.A.; Izzy, M.; Perri, R.; Schlendorf, K.H.; Menachem, J.N.; Shah, A.S. Adult Combined Heart-Liver Transplantation: The United States Experience. Transpl. Int. 2021, 35, 10036. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Raichlin, E.; Hasin, T.; Boilson, B.A.; Schirger, J.A.; Pereira, N.L.; Edwards, B.S.; Clavell, A.L.; Rodeheffer, R.J.; Frantz, R.P.; et al. Combined heart and liver transplant attenuates cardiac allograft vasculopathy compared with isolated heart transplantation. Transplantation 2013, 95, 859–865. [Google Scholar] [CrossRef]
- Atluri, P.; Gaffey, A.; Howard, J.; Phillips, E.; Goldstone, A.B.; Hornsby, N.; MacArthur, J.W.; Cohen, J.E.; Gutsche, J.; Woo, Y.J. Combined heart and liver transplantation can be safely performed with excellent short- and long-term results. Ann. Thorac. Surg. 2014, 98, 858–862. [Google Scholar] [CrossRef]
- Dec, G.W.; Narula, J. Toward Immunomodulation in Heart Transplantation: 2 Organs Are Better Than 1. J. Am. Coll. Cardiol. 2021, 77, 1341–1343. [Google Scholar] [CrossRef]
- Urschel, S.; West, L.J. ABO-incompatible heart transplantation. Curr. Opin. Pediatr. 2016, 28, 613–619. [Google Scholar] [CrossRef]
- Bansal, N.; West, L.J.; Simmonds, J.; Urschel, S. ABO-incompatible heart transplantation-evolution of a revolution. J. Heart Lung Transplant. 2024, 43, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Bacha, E.; Farr, M. The Future of Pediatric and Adult Heart Transplantation: Perspective from the United States. Circulation 2024, 149, 339–341. [Google Scholar] [CrossRef]
- Irving, C.; Gennery, A.; Kirk, R. Pushing the boundaries: The current status of ABO-incompatible cardiac transplantation. J. Heart Lung Transplant. 2012, 31, 791–796. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, S.; Hosgood, S.A.; Walker-Panse, L.; Rahfeld, P.; Macdonald, S.S.; Kizhakkedathu, J.N.; Withers, S.G.; Nicholson, M.L. Enzymatic conversion of human blood group A kidneys to universal blood group O. Nat. Commun. 2024, 15, 2795. [Google Scholar] [CrossRef]
- Wang, A.; Ribeiro, R.V.P.; Ali, A.; Brambate, E.; Abdelnour-Berchtold, E.; Michaelsen, V.; Zhang, Y.; Rahfeld, P.; Moon, H.; Gokhale, H.; et al. Ex vivo enzymatic treatment converts blood type A donor lungs into universal blood type lungs. Sci. Transl. Med. 2022, 14, eabm7190. [Google Scholar] [CrossRef] [PubMed]
- Rummler, S.; Bauschke, A.; Barthel, E.; Jutte, H.; Maier, K.; Ziehm, P.; Malessa, C.; Settmacher, U. Current techniques for AB0-incompatible living donor liver transplantation. World J. Transplant. 2016, 6, 548–555. [Google Scholar] [CrossRef]
- Song, G.W.; Lee, S.G.; Hwang, S.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Kwon, S.W.; Ko, G.Y.; Kim, K.W. Dual living donor liver transplantation with ABO-incompatible and ABO-compatible grafts to overcome small-for-size graft and ABO blood group barrier. Liver Transpl. 2010, 16, 491–498. [Google Scholar] [CrossRef]
- Issitt, R.; Crook, R.; Shaw, M.; Robertson, A. The Great Ormond Street Hospital immunoadsorption method for ABO-incompatible heart transplantation: A practical technique. Perfusion 2021, 36, 34–37. [Google Scholar] [CrossRef]
- Singh, A.K.; Goerlich, C.E.; Shah, A.M.; Zhang, T.; Tatarov, I.; Ayares, D.; Horvath, K.A.; Mohiuddin, M.M. Cardiac Xenotransplantation: Progress in Preclinical Models and Prospects for Clinical Translation. Transpl. Int. 2022, 35, 10171. [Google Scholar] [CrossRef]
- Griffith, B.P.; Grazioli, A.; Singh, A.K.; Tully, A.; Galindo, J.; Saharia, K.K.; Shah, A.; Strauss, E.R.; Odonkor, P.N.; Williams, B.; et al. Transplantation of a genetically modified porcine heart into a live human. Nat. Med. 2025, 31, 589–598. [Google Scholar] [CrossRef] [PubMed]
| Trial | Trial Name | ClinicalTrials.gov ID | Phase | Condition | Status | Key Findings |
|---|---|---|---|---|---|---|
| Belatacept | Belatacept Evaluation of Nephroprotection and Efficacy as First-Line Immunosuppression (BENEFIT) | NCT00256750 | Phase III | Kidney transplantation | Completed | Better renal function than Cyclosporine but with higher acute rejection rates. |
| KY1005 (Amlitelimab) | A Study of Subcutaneous KY1005 in Healthy Volunteers | NCT04449939 | Phase I | Healthy volunteers | Completed | Safe, well-tolerated, and showed promising pharmacokinetics. |
| TNX-1500 (Anti-CD40L mAb) | TNX-1500 (Fc-modified Humanized Anti-CD40L mAb) Single Ascending Dose Study in Healthy Participants | Not available (pending NCT) | Phase I | Healthy volunteers (kidney transplantation dosing) | Completed (Feb 2025) | Blocked antibody responses, no thromboembolic events, and supports monthly dosing. Phase 2 planned. |
| Tegoprubart | Long-Term Safety and Efficacy of Tegoprubart in Kidney Transplant Recipients | NCT06126380 | Phase II | Kidney transplantation | Ongoing | Non-thrombogenic, Phase II ongoing for efficacy assessment. |
| Dazodalibep + Belatacept | A Study to Evaluate the Safety and Efficacy of Dual Co-Stimulation Blockade With VIB4920 and Belatacept for Prophylaxis of Allograft Rejection in Adults Receiving a Kidney Transplant | NCT04046549 | Phase II | Kidney transplantation | Ongoing | Dual blockade approach under Phase II evaluation. |
| Belatacept | Belatacept in Heart Transplant Recipients at Risk for Kidney Failure | NCT04180085 | Recruiting | Heart transplantation + kidney failure risk | Recruiting | Assessing Belatacept’s efficacy in heart transplant patients with kidney risk. |
| Belatacept | Belatacept in Heart Transplant Recipients at Risk for Kidney Failure (Second Cohort) | NCT04477629 | Recruiting | Heart transplantation + kidney failure risk | Recruiting | Ongoing trial similar to NCT04180085, focusing on safety and efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolner, L.; William-Olsson, J.; Podesser, B.K.; Zuckermann, A.; Pilat, N. Tolerogenic Therapies in Cardiac Transplantation. Int. J. Mol. Sci. 2025, 26, 3968. https://doi.org/10.3390/ijms26093968
Wolner L, William-Olsson J, Podesser BK, Zuckermann A, Pilat N. Tolerogenic Therapies in Cardiac Transplantation. International Journal of Molecular Sciences. 2025; 26(9):3968. https://doi.org/10.3390/ijms26093968
Chicago/Turabian StyleWolner, Laurenz, Johan William-Olsson, Bruno K. Podesser, Andreas Zuckermann, and Nina Pilat. 2025. "Tolerogenic Therapies in Cardiac Transplantation" International Journal of Molecular Sciences 26, no. 9: 3968. https://doi.org/10.3390/ijms26093968
APA StyleWolner, L., William-Olsson, J., Podesser, B. K., Zuckermann, A., & Pilat, N. (2025). Tolerogenic Therapies in Cardiac Transplantation. International Journal of Molecular Sciences, 26(9), 3968. https://doi.org/10.3390/ijms26093968







