The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
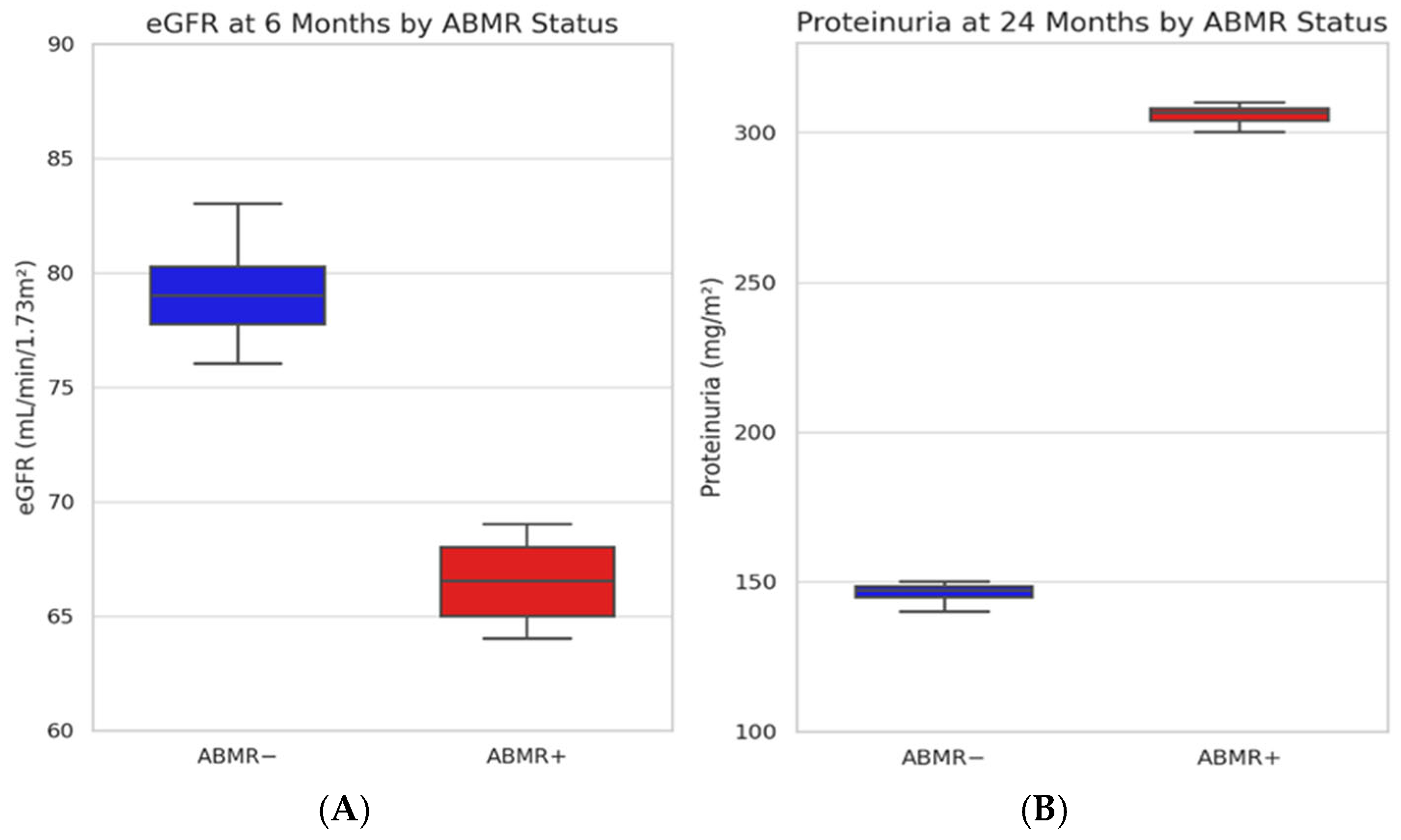
2.2. Kidney Function and Proteinuria over Time
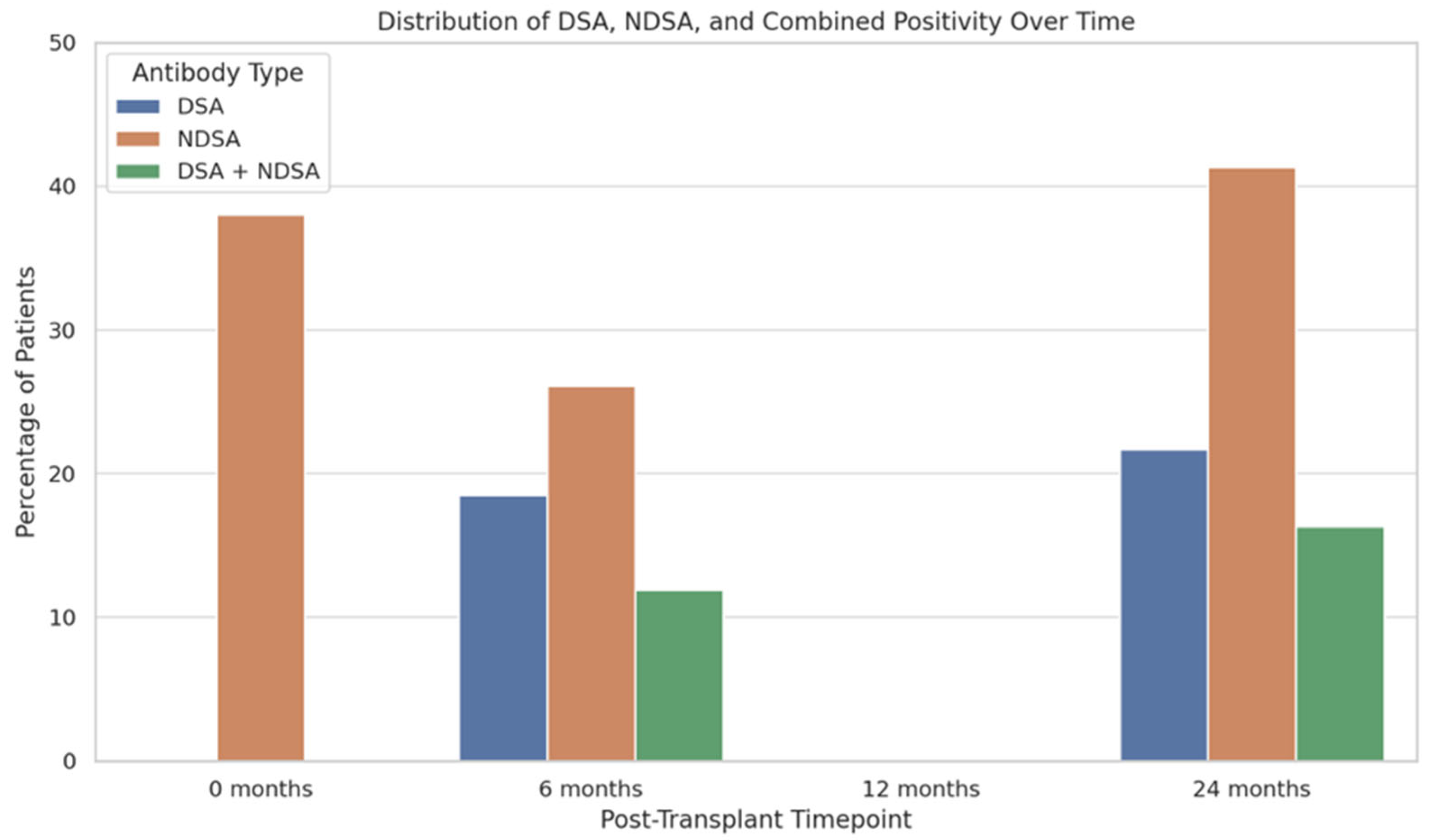
2.3. Incidence and Characterization of HLA Antibodies
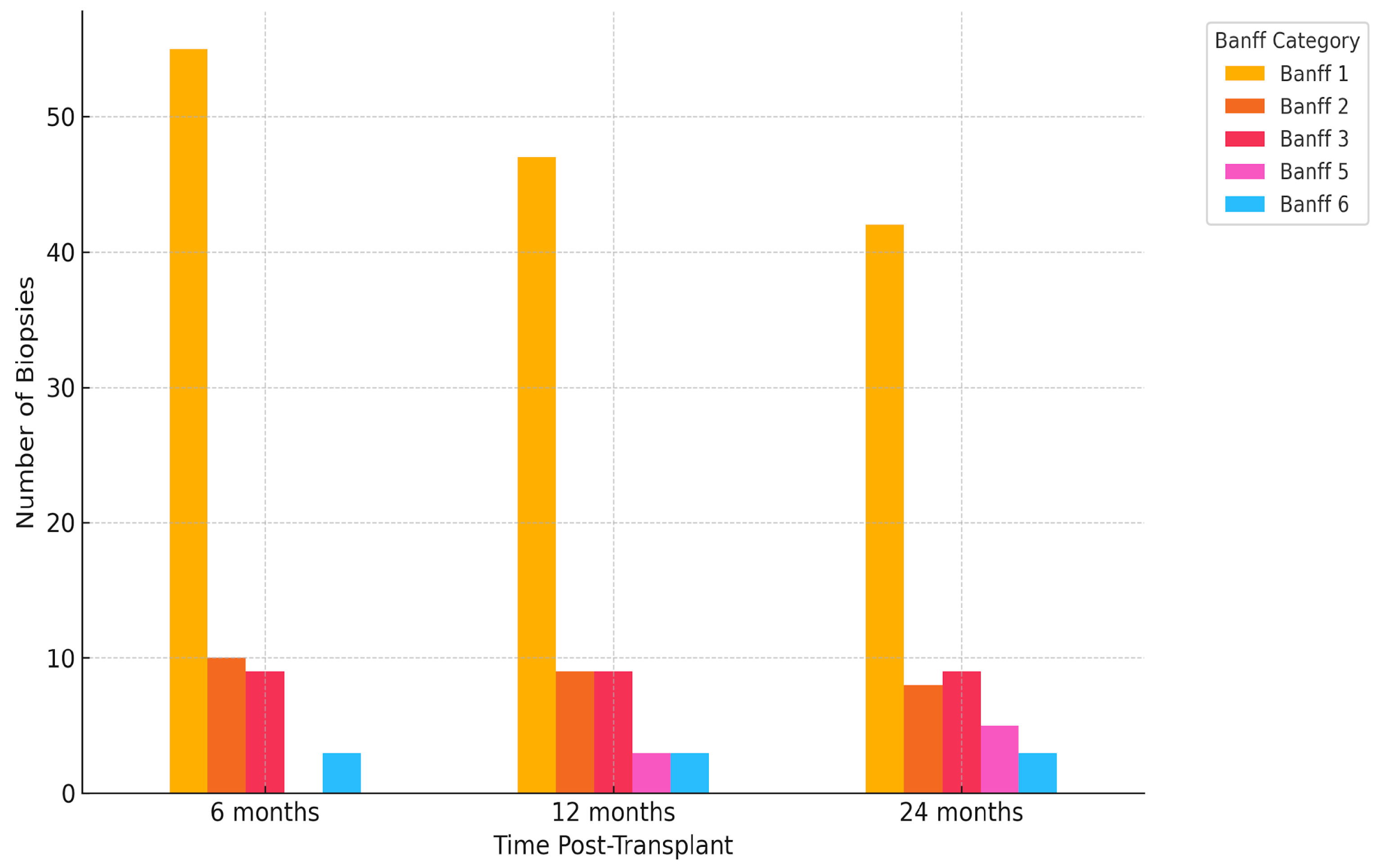
2.4. Histological Findings
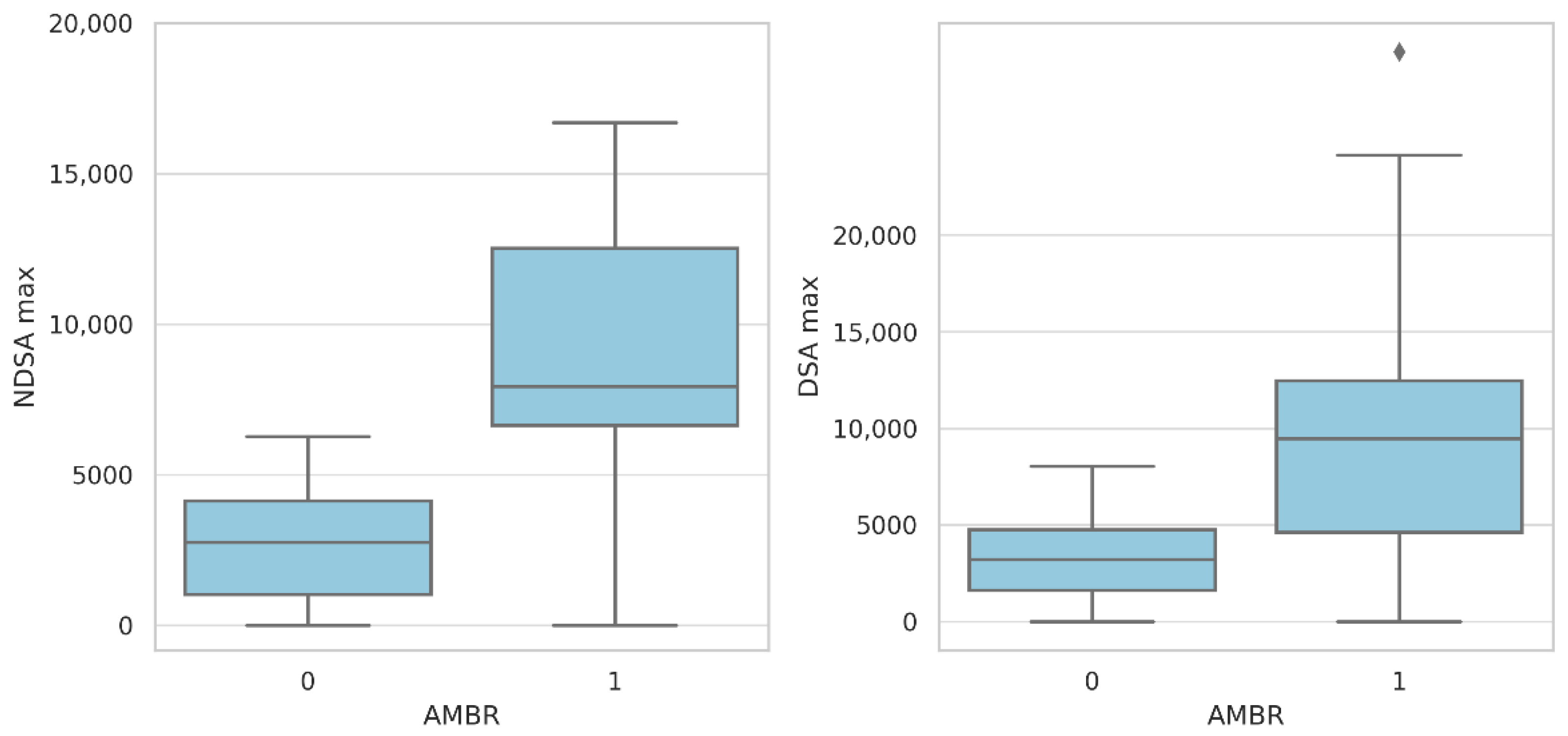
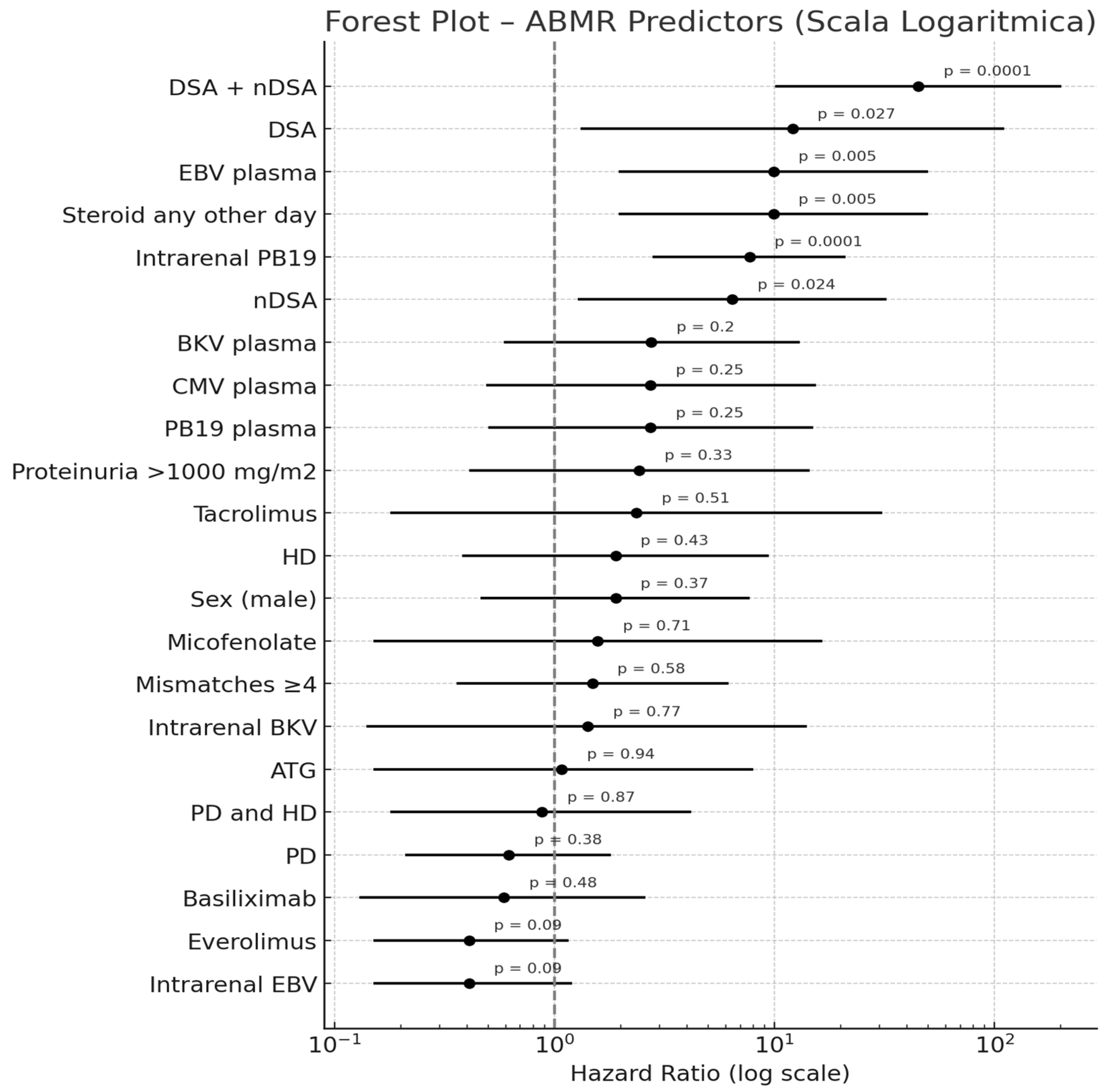
2.5. Predictors of Antibody-Mediated Rejection
3. Discussion
4. Materials and Methods
4.1. Patients and Methods
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| nDSAs | non-donor-specific antibodies |
| DSAs | donor-specific antibodies |
| ABMR | antibody-mediated rejection |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| CAKUT | congenital anomalies of the kidney and urinary tract |
| CKD | chronic kidney disease |
| MFI | mean fluorescence intensity |
| CNI | calcineurin inhibitor |
| PD | peritoneal dialysis |
| HD | hemodialysis |
| PVB19 | Parvovirus B19 |
| CMV | cytomegalovirus |
| EBV | Epstein–Barr virus |
| BKV | BK polyomavirus |
| ATG | anti-thymocyte globulin |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| CERTAIN | Cooperative European Paediatric Renal Transplant Initiative |
| RT-PCR | real-time polymerase chain reaction |
References
- Pape, L.; Becker, J.U.; Immenschuh, S.; Ahlenstiel, T. Acute and chronic antibody-mediated rejection in pediatric kidney transplantation. Pediatr. Nephrol. 2015, 30, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Zhu, L.; Terasaki, P.I.; Everly, M.J. HLA-specific anti bodies developed in the first year posttransplant are predictive of chronic rejection and renal graft loss. Transplantation 2009, 88, 568–574. [Google Scholar] [CrossRef]
- Bachelet, T.; Martinez, C.; Del Bello, A.; Couzi, L.; Kejji, S.; Guidicelli, G.; Lepreux, S.; Visentin, J.; Congy-Jolivet, N.; Rostaing, L.; et al. Deleterious impact of donor-specific anti-HLA antibodies toward HLA-Cw and HLA-DP in kidney transplantation. Transplantation 2016, 100, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, A.; Gauché, L.; Süsal, C.; Tran, T.H.; Waldherr, R.; Krupka, K.; Guzzo, I.; Carraro, A.; Oh, J.; Zirngibl, M.; et al. Incidence, risk factors, management strategies, and outcomes of antibody-mediated rejection in pediatric kidney transplant recipients—A multicenter analysis of the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN). Pediatr. Nephrol. 2025, 40, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Michelo, C.M.; van Cranenbroek, B.; Touw, P.; Claas, F.H.J.; van der Meer, A.; Joosten, I. Human cytomegalovirus infection increases both antibody- and non-antibody-dependent cellular reactivity by natural killer cells. Transpl. Direct 2017, 3, e335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oomen, L.; Bootsma-Robroeks, C.; Cornelissen, E.; de Wall, L.; Feitz, W. Pearls and pitfalls in pediatric kidney transplantation after 5 decades. Front. Pediatr. 2022, 10, 856630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rianthavorn, P.; Ettenger, R.B. Medication non-adherence in the adolescent renal transplant recipient: A clinician’s viewpoint. Pediatr. Transplant. 2005, 9, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Piazza, A.; Poggi, E.; Ozzella, G.; Borrelli, L.; Scornajenghi, A.; Iaria, G.; Tisone, G.; Adorno, D. Post-transplant donor-specific antibody production and graft outcome in kidney transplantation: Results of sixteen-year monitoring by flow cytometry. Clin. Transpl. 2006, 18, 323–336. [Google Scholar]
- Hourmant, M.; Cesbron-Gautier, A.; Terasaki, P.I.; Mizutani, K.; Moreau, A.; Dantal, J.; Giral, M.; Blancho, G.; Cantarovich, D.; Karam, G.; et al. Frequency and clinical implications of development of donor-specific and nondonor-specific HLA antibodies after kidney transplantation. J. Am. Soc. Nephrol. 2005, 16, 2804–2812. [Google Scholar] [CrossRef]
- Cioni, M.; Comoli, P.; Tagliamacco, A.; Innocente, A.; Basso, S.; Fontana, I.; Magnasco, A.; Trivelli, A.; Nocco, A.; Macchiagodena, M.; et al. Post-transplant de novo non donor-specific HLA antibodies are not associated with poor graft outcome in non-sensitized pediatric recipients of kidney transplantation. Pediatr. Transplant. 2021, 25, e14000. [Google Scholar] [CrossRef]
- Süsal, C.; Wettstein, D.; Döhler, B.; Morath, C.; Ruhenstroth, A.; Scherer, S.; Tran, T.H.; Gombos, P.; Schemmer, P.; Wagner, E.; et al. Association of kidney graft loss with de novo produced donor-specific and non-donor-specificHLA antibodies detected by single antigen testing. Transplantation 2015, 99, 1976–1980. [Google Scholar] [CrossRef]
- de Sousa, M.V.; Gonçalez, A.C.; Zollner, R.L.; Mazzali, M. Effect of preformed or denovo anti-HLA antibodies on function and graft survival in kidney transplant recipients. Ann. Transplant. 2018, 23, 457–466. [Google Scholar] [CrossRef]
- Cai, J.; Terasaki, P.I.; Mao, Q.; Pham, T.; El-Awar, N.; Lee, J.H.; Rebellato, L. Development of nondonor-specific HLA-DR antibodies in allograft recipients is associated with shared epitopes with mismatched donor DR antigens. Am. J. Transplant. 2006, 6, 2947–2954. [Google Scholar] [CrossRef]
- VanSickle, J.S.; Warady, B.A. Chronic Kidney Disease in Children. Pediatr. Clin. N. Am. 2022, 69, 1239–1254. [Google Scholar] [CrossRef]
- Yılmaz, S.; Özçakar, Z.B.; Taktak, A.; Kurt-Şükür, E.D.; Çakar, N.; Yalçınkaya, F. Proteinuria in pediatric renal transplant recipients. Pediatr. Transplant. 2018, 22, e13068. [Google Scholar] [CrossRef]
- Naesens, M.; Lerut, E.; Emonds, M.P.; Herelixka, A.; Evenepoel, P.; Claes, K.; Bammens, B.; Sprangers, B.; Meijers, B.; Jochmans, I.; et al. Proteinuria as a noninvasive marker for renal allograft histology and failure: An observational cohort study. J. Am. Soc. Nephrol. 2013, 24, 1576–1583. [Google Scholar] [CrossRef]
- Von Moos, S.; Cippà, P.E.; van Breemen, R. HLA antibodies are associated with deterioration of kidney allograft function irrespective of donor specificity. Hum. Immunol. 2021, 82, 19–24. [Google Scholar] [CrossRef]
- Claisse, G.; Absi, L.; Cognasse, F.; Alamartine, E.; Mariat, C.; Maillard, N. Relationship between mean fluorescence intensity and C1q/C3d-fixing capacities of anti-HLA antibodies. Hum. Immunol. 2017, 78, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Bertazza Partigiani, N.; Negrisolo, S.; Carraro, A.; Marzenta, D.; Manaresi, E.; Gallinella, G.; Barzon, L.; Benetti, E. Pre-existing intrarenal Parvovirus B19 infection may relate to antibody-mediated rejection in pediatric kidney transplant patients. Int. J. Mol. Sci. 2023, 24, 9147. [Google Scholar] [CrossRef]
- Bentata, Y. Parvovirus B19 in kidney transplantation: Key points and essential pitfalls to know. Expert. Rev. Anti Infect. Ther. 2021, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.B.; Calabrese, F.; Rigotti, P.; Furian, L.; Marino, S.; Cusinato, R.; Valente, M. Acute rejection and graft survival in renal transplanted patients with viral diseases. Mod. Pathol. 2004, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Banff Roufosse, C.; Simmonds, N.; Clahsen-van Groningen, M.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef]
- Jung, S.; Oh, E.J.; Yang, C.W.; Ahn, W.S.; Kim, Y.; Park, Y.J.; Han, K. Comparative evaluation of ELISA and Luminex panel reactive antibody assays for HLA alloantibody screening. Korean J. Lab. Med. 2009, 29, 473–480. [Google Scholar] [CrossRef][Green Version]
- The Jamovi Project. Jamovi, version 2.6; Computer Software; Jamovi: Sydney, Australia, 2025. Available online: https://www.jamovi.org (accessed on 14 April 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 14 April 2025).

| CHARACTERISTICS | ||
|---|---|---|
| Male sex (%) | 59 | 64 |
| Age at transplant, mean (IQR) | 11.5 | 2–21 |
| Weight at transplant kg, mean (IQR) | 26.5 | 7–83 |
| Mismatch, mean (IQR) | 4 | 1–6 |
| First transplant, % | 82 | 89 |
| PD, % | 38 | 41 |
| HD, % | 21 | 22 |
| PD + HD, % | 15 | 16 |
| No dialysis, % | 18 | 19 |
| 1st transplant, % | 82 | 89 |
| 2nd transplant, % | 9 | 9 |
| 3rd transplant, % | 1 | 1 |
| Deceased donor, % | 68 | 73 |
| Baseline nDSAs, % | 38 | |
| Cause of ESKD, % | ||
| CAKUT | 44 | 47 |
| glomerulopathy | 19 | 20 |
| ciliopathy | 15 | 16 |
| unknown | 4 | 4 |
| inborn error of metabolism | 3 | 3 |
| chronic TIN | 3 | 3 |
| perinatal asphyxia | 3 | 3 |
| HIV related | 1 | 1 |
| Immunosuppressive induction, % | ||
| basiliximab | 84 | 91 |
| ATG | 13 | 14 |
| tacrolimus | 49 | 53 |
| mycophenolate mofetil | 80 | 86 |
| steroid | 92 | 100 |
| ciclosporin | 38 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangermano, M.; Soncin, V.; Auciello, M.; Ciabattoni, F.; Negrisolo, S.; Marinelli, E.; Bertazza Partigiani, N.; Benetti, E. The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients. Int. J. Mol. Sci. 2025, 26, 5870. https://doi.org/10.3390/ijms26125870
Sangermano M, Soncin V, Auciello M, Ciabattoni F, Negrisolo S, Marinelli E, Bertazza Partigiani N, Benetti E. The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients. International Journal of Molecular Sciences. 2025; 26(12):5870. https://doi.org/10.3390/ijms26125870
Chicago/Turabian StyleSangermano, Maria, Vittoria Soncin, Maria Auciello, Francesco Ciabattoni, Susanna Negrisolo, Elena Marinelli, Nicola Bertazza Partigiani, and Elisa Benetti. 2025. "The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients" International Journal of Molecular Sciences 26, no. 12: 5870. https://doi.org/10.3390/ijms26125870
APA StyleSangermano, M., Soncin, V., Auciello, M., Ciabattoni, F., Negrisolo, S., Marinelli, E., Bertazza Partigiani, N., & Benetti, E. (2025). The Impact of Non-Donor-Specific HLA Antibodies on Antibody-Mediated Rejection in Pediatric Kidney Transplant Recipients. International Journal of Molecular Sciences, 26(12), 5870. https://doi.org/10.3390/ijms26125870






