Acute Stress and Autoimmune Markers: Evaluating the Psychoneuroimmunology Axis in Firefighter Recruits
Abstract
:1. Introduction
2. Results
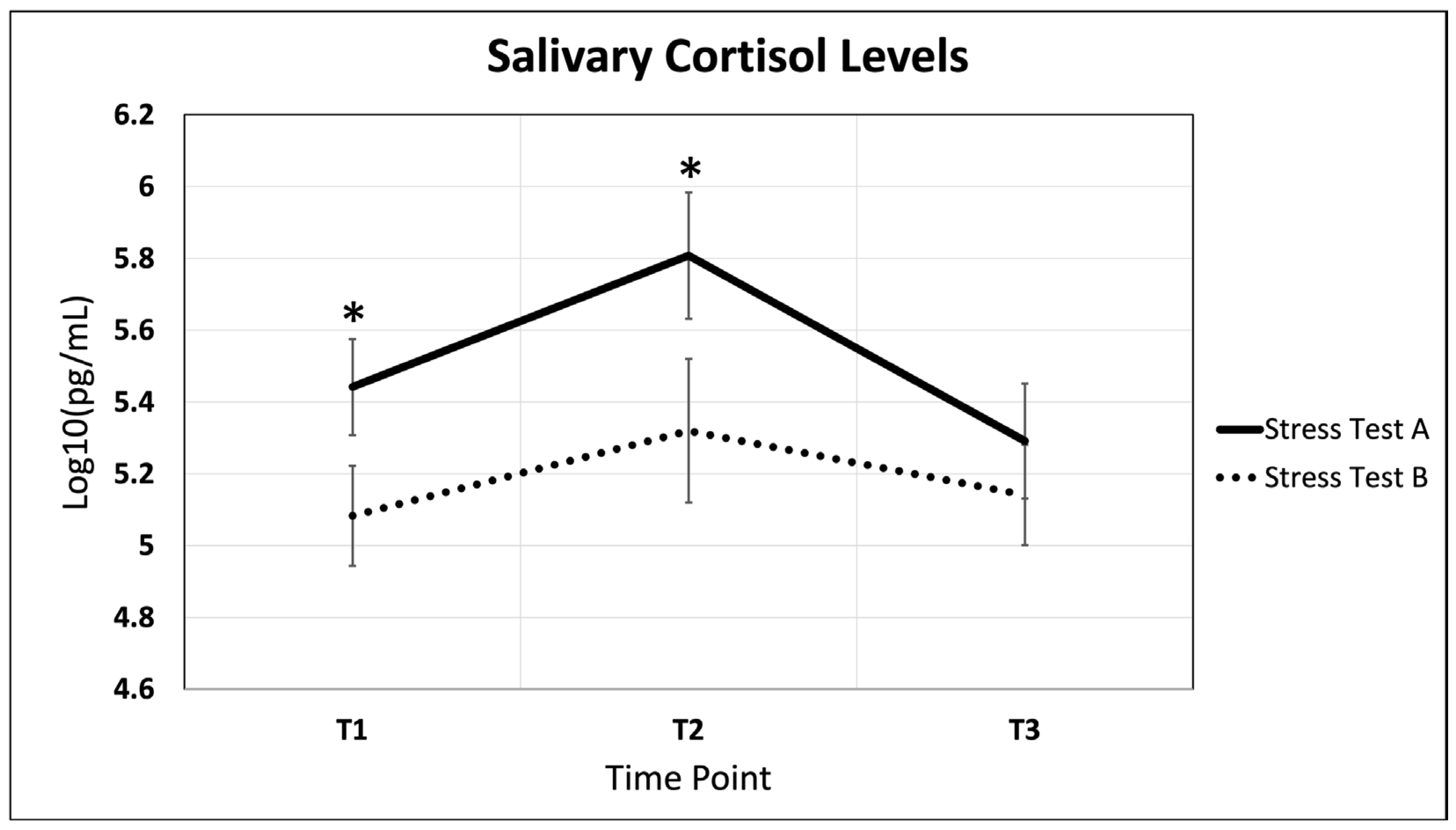
2.1. Salivary Cortisol Fluctuations Throughout Acute Stress and Recovery
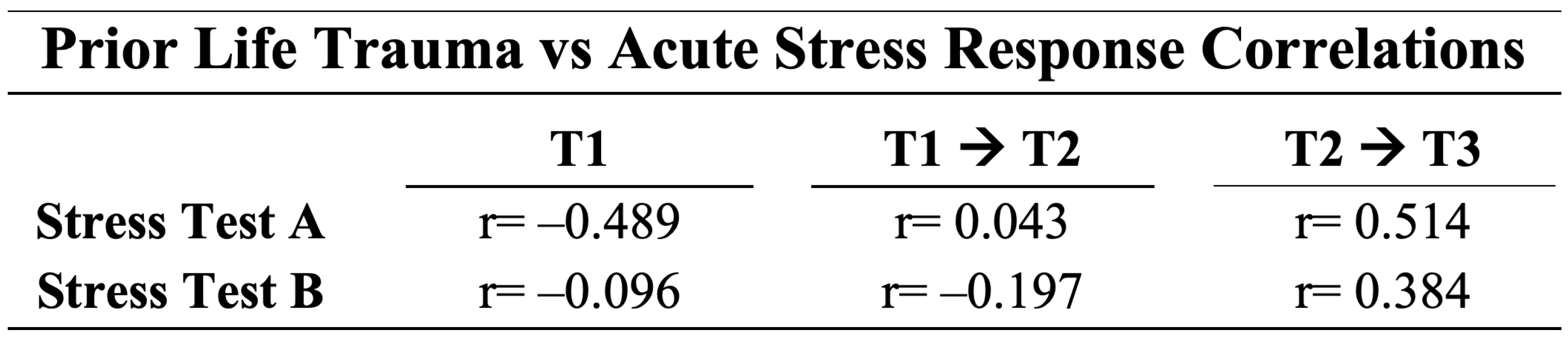
2.2. The Effects of Prior Life Trauma on the Acute Stress Response
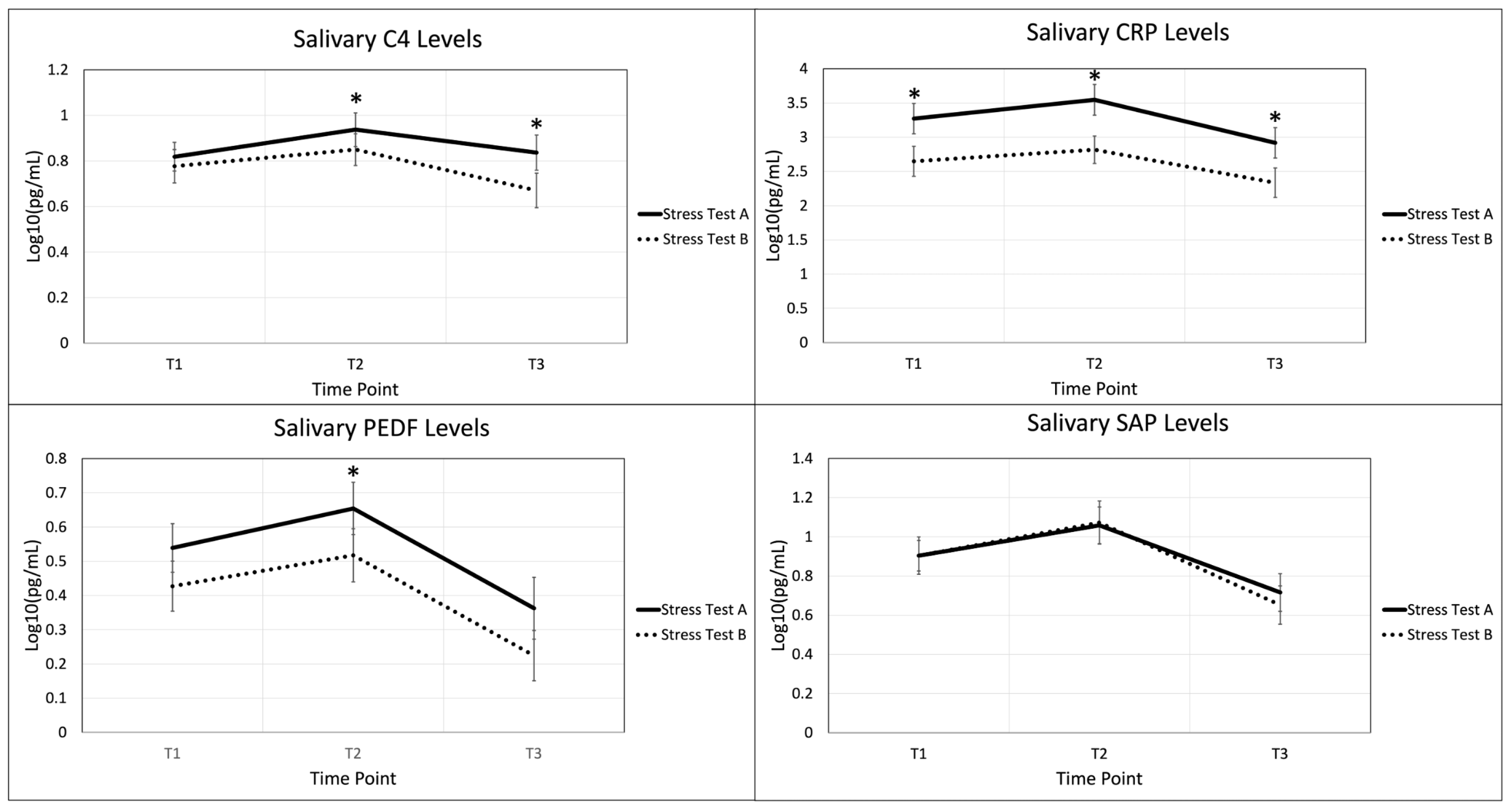
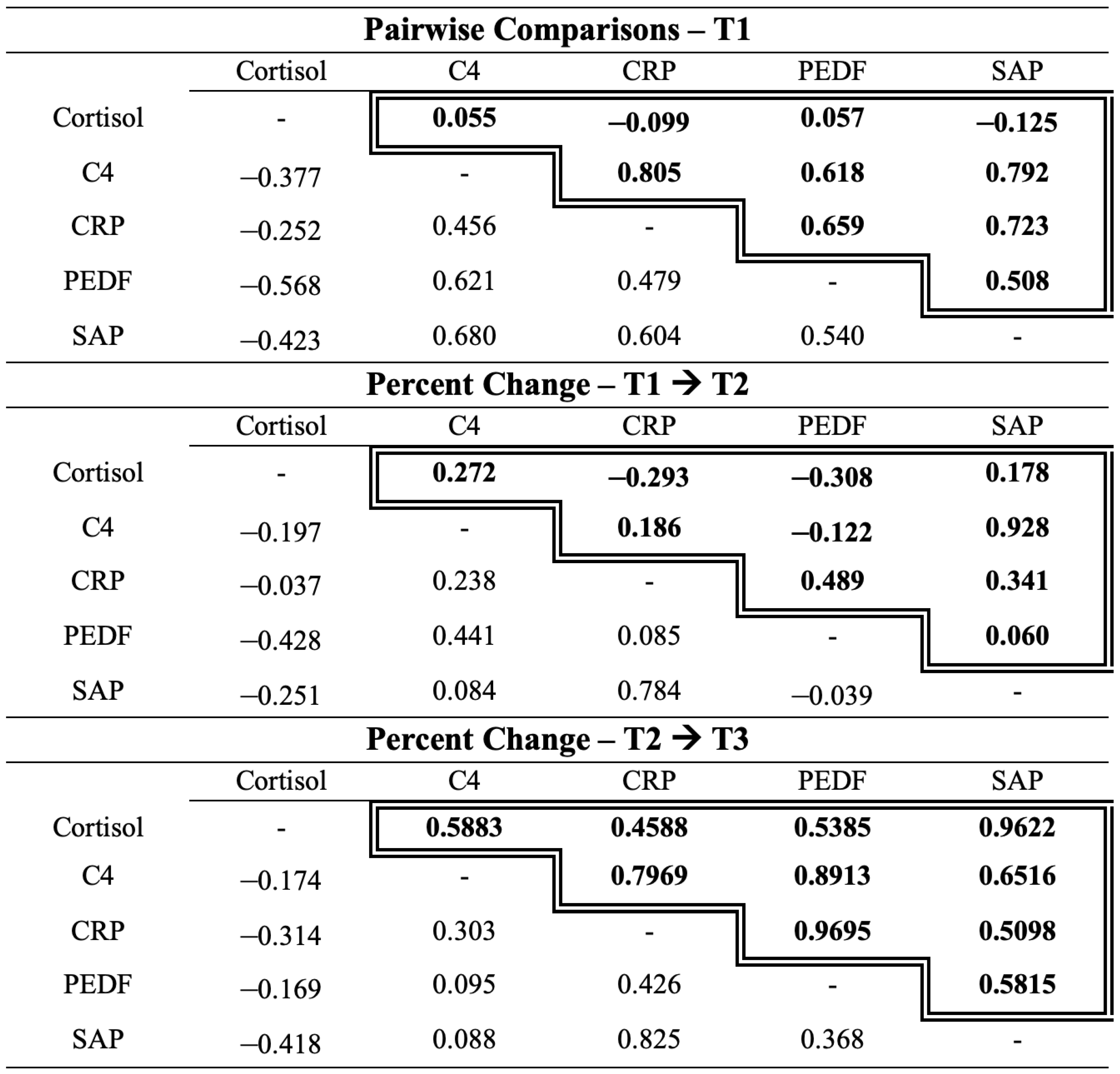
2.3. Autoimmune Biomarkers in the Acute Stress Response
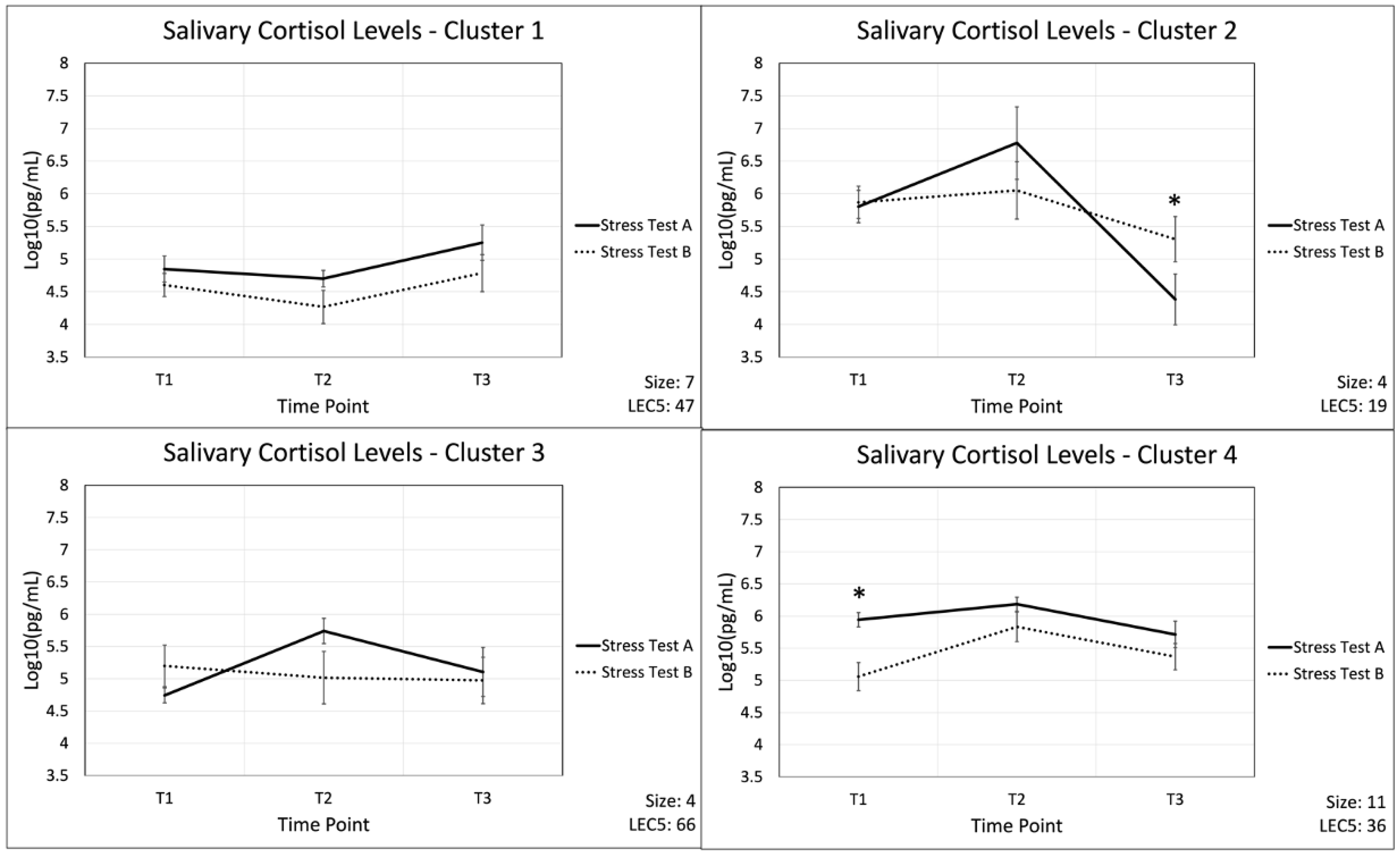
2.4. Cluster Analysis as a Means to Group Participants’ Cortisol Response
3. Discussion
3.1. Salivary Cortisol Fluctuations Throughout Acute Stress and Recovery
3.2. The Effects of Prior Life Trauma on the Acute Stress Response
3.3. Autoimmune Biomarkers in the Acute Stress Response
3.4. Limitations
3.5. Future Directions
4. Materials and Methods
4.1. Study Design
4.2. Participants
4.3. Demographic Data Collection
4.4. Life Events Checklist
4.5. Stress Test
4.6. Salivary Sample Collection
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASR | acute stress response |
| CRP | C-reactive protein |
| C4 | complement C4 |
| PEDF | pigment epithelium derived factor |
| SAP | serum amyloid P |
| PTSD | post-traumatic stress disorder |
| SNS | sympathetic nervous system |
| HPA | hypothalamus–pituitary–adrenal |
| AIM2 | absent in melanoma 2 |
| RA | rheumatoid arthritis |
| SLE | systemic lupus erythematosus |
| LEC-5 | life events checklist |
| ANOVA | analysis of variances |
References
- Sternberg, E.M.; Chrousos, G.P.; Wilder, R.L.; Gold, P.W. The Stress Response and the Regulation of Inflammatory Disease. Ann. Intern. Med. 1992, 117, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.S.; Slavich, G.M. Lifetime Stress Exposure and Health: A Review of Contemporary Assessment Methods and Biological Mechanisms. Soc. Personal. Psychol. Compass 2017, 11, e12335. [Google Scholar] [CrossRef] [PubMed]
- Solomon, Z.; Levin, Y.; Crompton, L.; Ginzburg, K. Is Acute Stress Reaction a Risk Factor for Early Mortality? Health Psychol. 2019, 38, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Hoerster, K.D.; Campbell, S.; Dolan, M.; Stappenbeck, C.A.; Yard, S.; Simpson, T.; Nelson, K.M. PTSD Is Associated with Poor Health Behavior and Greater Body Mass Index through Depression, Increasing Cardiovascular Disease and Diabetes Risk among U.S. Veterans. Prev. Med. Rep. 2019, 15, 100930. [Google Scholar] [CrossRef]
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the Relationships between Physiological and Psychosocial Stress, Cortisol and Cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid Regulation of Inflammation and Its Functional Correlates: From HPA Axis to Glucocorticoid Receptor Dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Yehuda, R. Status of Glucocorticoid Alterations in Post-traumatic Stress Disorder. Ann. N. Y. Acad. Sci. 2009, 1179, 56–69. [Google Scholar] [CrossRef]
- Alotiby, A. Immunology of Stress: A Review Article. J. Clin. Med. 2024, 13, 6394. [Google Scholar] [CrossRef]
- Gill, J.M.; Saligan, L.; Woods, S.; Page, G. PTSD Is Associated With an Excess of Inflammatory Immune Activities. Perspect. Psychiatr. Care 2009, 45, 262–277. [Google Scholar] [CrossRef]
- Spitzer, C.; Barnow, S.; Völzke, H.; Wallaschofski, H.; John, U.; Freyberger, H.J.; Löwe, B.; Grabe, H.J. Association of Posttraumatic Stress Disorder with Low-Grade Elevation of C-Reactive Protein: Evidence from the General Population. J. Psychiatr. Res. 2010, 44, 15–21. [Google Scholar] [CrossRef]
- Montero-López, E.; Santos-Ruiz, A.; González, R.; Navarrete-Navarrete, N.; Ortego-Centeno, N.; Martínez-Augustín, O.; Rodríguez-Blázquez, M.; Peralta-Ramírez, M.I. Analyses of Hair and Salivary Cortisol for Evaluating Hypothalamic–Pituitary–Adrenal Axis Activation in Patients with Autoimmune Disease. Stress 2017, 20, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Straub, R.H. Stress as a Risk Factor in the Pathogenesis of Rheumatoid Arthritis. Neuroimmunomodulation 2006, 13, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Villaggio, B.; Foppiani, L.; Briata, M.; Sulli, A.; Pizzorni, C.; Faelli, F.; Prete, C.; Felli, L.; Seriolo, B.; et al. The Hypothalamic-Pituitary-Adrenal and Gonadal Axes in Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 2000, 917, 835–843. [Google Scholar] [CrossRef]
- Tzioufas, A.G.; Tsonis, J.; Moutsopoulos, H.M. Neuroendocrine Dysfunction in Sjögren’s Syndrome. Neuroimmunomodulation 2008, 15, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Kostandi, M.; Moutsopoulos, H.M. Hypothalamic-Pituitary-Adrenal Axis Function in Sjögren’s Syndrome: Mechanisms of Neuroendocrine and Immune System Homeostasis. Ann. N. Y. Acad. Sci. 2006, 1088, 41–51. [Google Scholar] [CrossRef]
- Slominski, A.T.; Brożyna, A.A.; Tuckey, R.C. Cutaneous Glucocorticoidogenesis and Cortisol Signaling Are Defective in Psoriasis. J. Invest. Dermatol. 2017, 137, 1609–1611. [Google Scholar] [CrossRef]
- Weigl, B.A. The Significance of Stress Hormones (Glucocorticoids, Catecholamines) for Eruptions and Spontaneous Remission Phases in Psoriasis. Int. J. Dermatol. 2000, 39, 678–688. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Shirzad, N.; Moosavi, M.-S. The Association of Elevated Plasma Cortisol and Hashimoto’s Thyroiditis, a Neglected Part of Immune Response. Acta Clin. Belg. 2016, 71, 81–85. [Google Scholar] [CrossRef]
- Scheffer, M.; Becker, J.; De Azeredo, L.A.; Grassi-Oliveira, R.; De Almeida, R.M.M. Subjective and Physiological Stress Measurement in a Multiple Sclerosis Sample and the Relation with Executive Functions Performance. J. Neural Transm. 2019, 126, 613–622. [Google Scholar] [CrossRef]
- Pereira, G.M.; Soares, N.M.; Souza, A.R.D.; Becker, J.; Finkelsztejn, A.; Almeida, R.M.M.D. Basal Cortisol Levels and the Relationship with Clinical Symptoms in Multiple Sclerosis: A Systematic Review. Arq. Neuropsiquiatr. 2018, 76, 622–634. [Google Scholar] [CrossRef]
- Mathews, H.L.; Janusek, L.W. Epigenetics and Psychoneuroimmunology: Mechanisms and Models. Brain. Behav. Immun. 2011, 25, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.A.; Lapp, H.E.; Hunter, R.G. Epigenetic Mechanisms of the Glucocorticoid Receptor. Trends Endocrinol. Metab. 2019, 30, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Aiello, A.E.; Wildman, D.E.; Koenen, K.C.; Pawelec, G.; De Los Santos, R.; Goldmann, E.; Galea, S. Epigenetic and Immune Function Profiles Associated with Posttraumatic Stress Disorder. Proc. Natl. Acad. Sci. USA 2010, 107, 9470–9475. [Google Scholar] [CrossRef]
- Zieker, J.; Zieker, D.; Jatzko, A.; Dietzsch, J.; Nieselt, K.; Schmitt, A.; Bertsch, T.; Fassbender, K.; Spanagel, R.; Northoff, H.; et al. Differential Gene Expression in Peripheral Blood of Patients Suffering from Post-Traumatic Stress Disorder. Mol. Psychiatry 2007, 12, 116–118. [Google Scholar] [CrossRef]
- Segman, R.H.; Shefi, N.; Goltser-Dubner, T.; Friedman, N.; Kaminski, N.; Shalev, A.Y. Peripheral Blood Mononuclear Cell Gene Expression Profiles Identify Emergent Post-Traumatic Stress Disorder among Trauma Survivors. Mol. Psychiatry 2005, 10, 500–513. [Google Scholar] [CrossRef]
- Wolf, E.J.; Logue, M.W.; Zhao, X.; Daskalakis, N.P.; Morrison, F.G.; Escarfulleri, S.; Stone, A.; Schichman, S.A.; McGlinchey, R.E.; Milberg, W.P.; et al. PTSD and the Klotho Longevity Gene: Evaluation of Longitudinal Effects on Inflammation via DNA Methylation. Psychoneuroendocrinology 2020, 117, 104656. [Google Scholar] [CrossRef]
- Miller, M.W.; Maniates, H.; Wolf, E.J.; Logue, M.W.; Schichman, S.A.; Stone, A.; Milberg, W.; McGlinchey, R. CRP Polymorphisms and DNA Methylation of the AIM2 Gene Influence Associations between Trauma Exposure, PTSD, and C-Reactive Protein. Brain. Behav. Immun. 2018, 67, 194–202. [Google Scholar] [CrossRef]
- Hawn, S.E.; Neale, Z.; Wolf, E.J.; Zhao, X.; Pierce, M.; Fein-Schaffer, D.; Milberg, W.; McGlinchey, R.; Logue, M.; Miller, M.W. Methylation of the AIM2 Gene: An Epigenetic Mediator of PTSD-Related Inflammation and Neuropathology Plasma Biomarkers. Depress. Anxiety 2022, 39, 323–333. [Google Scholar] [CrossRef]
- Kumari, P.; Russo, A.J.; Shivcharan, S.; Rathinam, V.A. AIM2 in Health and Disease: Inflammasome and Beyond. Immunol. Rev. 2020, 297, 83–95. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Heim, C.M. A Short Review on the Psychoneuroimmunology of Posttraumatic Stress Disorder: From Risk Factors to Medical Comorbidities. Brain. Behav. Immun. 2011, 25, 6–13. [Google Scholar] [CrossRef]
- Speer, K.; Upton, D.; Semple, S.; McKune, A. Systemic Low-Grade Inflammation in Post-Traumatic Stress Disorder: A Systematic Review. J. Inflamm. Res. 2018, 11, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ren, Y.-H.; Zhao, F.-J.; Chen, Y.; Huang, Y.-F.; Yang, L.; You, X.-M. Cross-Sectional Study of the Effects of Job Burnout on Immune Function in 105 Female Oncology Nurses at a Tertiary Oncology Hospital. Med. Sci. Monit. 2021, 27, e929711-1. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Yang, Y.; Xu, B.; Qin, Y.; Zang, G.; Zhou, C.; Zheng, P.; Chen, J.; Cheng, K.; Chen, J.; et al. Pigment Epithelium-Derived Factor Alleviates Depressive-like Behaviors in Mice by Modulating Adult Hippocampal Synaptic Growth and Wnt Pathway. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109792. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Yu, H.; Chen, C.; Xu, X.; He, Y.; Wang, Y.; Tian, Y.; Wu, Z.; Lan, T.; Li, Y.; et al. Pigment Epithelium-Derived Factor May Induce Antidepressant Phenotypes in Mice by the Prefrontal Cortex. Neurosci. Lett. 2022, 771, 136423. [Google Scholar] [CrossRef]
- Gouin, J.-P.; Glaser, R.; Malarkey, W.B.; Beversdorf, D.; Kiecolt-Glaser, J. Chronic Stress, Daily Stressors, and Circulating Inflammatory Markers. Health Psychol. 2012, 31, 264–268. [Google Scholar] [CrossRef]
- Kennedy, E.; Niedzwiedz, C.L. The Association of Anxiety and Stress-Related Disorders with C-Reactive Protein (CRP) within UK Biobank. Brain Behav. Immun. Health 2022, 19, 100410. [Google Scholar] [CrossRef]
- Steptoe, A.; Hamer, M.; Chida, Y. The Effects of Acute Psychological Stress on Circulating Inflammatory Factors in Humans: A Review and Meta-Analysis. Brain. Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef]
- Miller, E.S.; Apple, C.G.; Kannan, K.B.; Funk, Z.M.; Plazas, J.M.; Efron, P.A.; Mohr, A.M. Chronic Stress Induces Persistent Low-Grade Inflammation. Am. J. Surg. 2019, 218, 677–683. [Google Scholar] [CrossRef]
- Johnson, A.J.; Urizar, G.G.; Nwabuzor, J.; Dinh, P. Racism, Shame, and Stress Reactivity among Young Black Women. Stress Health 2022, 38, 1001–1013. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef]
- Jenkins, A.; Zhang, S.X.; Gosmanova, A.; Aston, C.; Dashti, A.; Baker, M.Z.; Lyons, T.; Ma, J.-X. Increased Serum Pigment Epithelium Derived Factor Levels in Type 2 Diabetes Patients. Diabetes Res. Clin. Pract. 2008, 82, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Coss, S.L.; Zhou, D.; Chua, G.T.; Aziz, R.A.; Hoffman, R.P.; Wu, Y.L.; Ardoin, S.P.; Atkinson, J.P.; Yu, C.-Y. The Complement System and Human Autoimmune Diseases. J. Autoimmun. 2023, 137, 102979. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Agyemang, A.F.; Alimzhanov, M.B.; Degn, S.; Tsiftsoglou, S.A.; Alicot, E.; Jones, S.A.; Ma, M.; Carroll, M.C. Complement C 4 Maintains Peripheral B-cell Tolerance in a Myeloid Cell Dependent Manner. Eur. J. Immunol. 2013, 43, 2441–2450. [Google Scholar] [CrossRef]
- Vogt, L.M.; Talens, S.; Kwasniewicz, E.; Scavenius, C.; Struglics, A.; Enghild, J.J.; Saxne, T.; Blom, A.M. Activation of Complement by Pigment Epithelium–Derived Factor in Rheumatoid Arthritis. J. Immunol. 2017, 199, 1113–1121. [Google Scholar] [CrossRef]
- Gitlin, J.D.; Gitlin, J.I.; Gitlin, D. Localization of C-reactive Protein in Synovium of Patients with Rheumatoid Arthritis. Arthritis Rheum. 1977, 20, 1491–1499. [Google Scholar] [CrossRef]
- Kjellman, M.; Laurell, A.-B.; Löw, B.; Sjöholm, A.G. Homozygous Deficiency of C4 in a Child with a Lupus Erythematosus Syndrome. Clin. Genet. 1982, 22, 331–339. [Google Scholar] [CrossRef]
- Fielder, A.H.; Walport, M.J.; Batchelor, J.R.; Rynes, R.I.; Black, C.M.; Dodi, I.A.; Hughes, G.R. Family Study of the Major Histocompatibility Complex in Patients with Systemic Lupus Erythematosus: Importance of Null Alleles of C4A and C4B in Determining Disease Susceptibility. Br. Med. J. 1983, 286, 425–428. [Google Scholar] [CrossRef]
- Einav, S.; Pozdnyakova, O.O.; Ma, M.; Carroll, M.C. Complement C4 Is Protective for Lupus Disease Independent of C3. J. Immunol. 2002, 168, 1036–1041. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Blank, M.; Langevitz, P.; Slutsky, L.; Pras, M.; Levy, Y.; Shovman, O.; Witte, T.; Doria, A.; Rovensky, J.; et al. Anti-Serum Amyloid Component P Antibodies in Patients with Systemic Lupus Erythematosus Correlate with Disease Activity. Ann. Rheum. Dis. 2005, 64, 1698–1702. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Qiao, B.; Xu, W.; Xiong, S. Amelioration of Lupus Nephritis by Serum Amyloid P Component Gene Therapy with Distinct Mechanisms Varied from Different Stage of the Disease. PLoS ONE 2011, 6, e22659. [Google Scholar] [CrossRef] [PubMed]
- Bickerstaff, M.C.M.; Botto, M.; Hutchinson, W.L.; Herbert, J.; Tennent, G.A.; Bybee, A.; Mitchell, D.A.; Cook, H.T.; Butler, P.J.G.; Walport, M.J.; et al. Serum Amyloid P Component Controls Chromatin Degradation and Prevents Antinuclear Autoimmunity. Nat. Med. 1999, 5, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Liakouli, V.; Elies, J.; El-Sherbiny, Y.M.; Scarcia, M.; Grant, G.; Abignano, G.; Derrett-Smith, E.C.; Esteves, F.; Cipriani, P.; Emery, P.; et al. Scleroderma Fibroblasts Suppress Angiogenesis via TGF-β/Caveolin-1 Dependent Secretion of Pigment Epithelium-Derived Factor. Ann. Rheum. Dis. 2018, 77, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Głodkowska-Mrówka, E.; Górska, E.; Ciurzyński, M.; Stelmaszczyk-Emmel, A.; Bienias, P.; Irzyk, K.; Siwicka, M.; Lipińska, A.; Ciepiela, O.; Pruszczyk, P.; et al. Pro- and Antiangiogenic Markers in Patients with Pulmonary Complications of Systemic Scleroderma. Respir. Physiol. Neurobiol. 2015, 209, 69–75. [Google Scholar] [CrossRef]
- Rowe, P.; Wasserfall, C.; Croker, B.; Campbell-Thompson, M.; Pugliese, A.; Atkinson, M.; Schatz, D. Increased Complement Activation in Human Type 1 Diabetes Pancreata. Diabetes Care 2013, 36, 3815–3817. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Zhang, S.X.; Rowley, K.G.; Karschimkus, C.S.; Nelson, C.L.; Chung, J.S.; O’Neal, D.N.; Januszewski, A.S.; Croft, K.D.; Mori, T.A.; et al. Increased Serum Pigment Epithelium-derived Factor Is Associated with Microvascular Complications, Vascular Stiffness and Inflammation in Type 1 Diabetes. Diabet. Med. 2007, 24, 1345–1351. [Google Scholar] [CrossRef]
- Hudson, L.K.; Dancho, M.E.; Li, J.; Bruchfeld, J.B.; Ragab, A.A.; He, M.M.; Bragg, M.; Lenaghan, D.; Quinn, M.D.; Fritz, J.R.; et al. Emetine Di-HCl Attenuates Type 1 Diabetes Mellitus in Mice. Mol. Med. 2016, 22, 585–596. [Google Scholar] [CrossRef]
- Katakami, N.; Kaneto, H.; Yamasaki, Y.; Matsuhisa, M. Increased Serum Pigment Epithelium-Derived Factor Levels in Type 1 Diabetic Patients with Diabetic Retinopathy. Diabetes Res. Clin. Pract. 2008, 81, e4–e7. [Google Scholar] [CrossRef]
- Gilliam, B.E.; Wolff, A.E.; Moore, T.L. Partial C4 Deficiency in Juvenile Idiopathic Arthritis Patients. JCR J. Clin. Rheumatol. 2007, 13, 256–260. [Google Scholar] [CrossRef]
- McLean, R.H.; Wyatt, R.J.; Julian, B.A. Complement Phenotypes in Glomerulonephritis: Increased Frequency of Homozygous Null C4 Phenotypes in IgA Nephropathy and Henoch-Schönlein Purpura. Kidney Int. 1984, 26, 855–860. [Google Scholar] [CrossRef]
- Zipplies, J.K.; Hauck, S.M.; Schoeffmann, S.; Amann, B.; Stangassinger, M.; Ueffing, M.; Deeg, C.A. Serum PEDF Levels Are Decreased in a Spontaneous Animal Model for Human Autoimmune Uveitis. J. Proteome Res. 2009, 8, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Sugita, S.; Kamoi, K. Immunological Homeostasis of the Eye. Prog. Retin. Eye Res. 2013, 33, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Bravo, Y.; Cole, J.; Gobeil, K. Acute Psychosocial Stress Differentially Influences Salivary Endocrine and Immune Measures in Undergraduate Students. Physiol. Behav. 2012, 107, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Auer, B.J.; Calvi, J.L.; Jordan, N.M.; Schrader, D.; Byrd-Craven, J. Communication and Social Interaction Anxiety Enhance Interleukin-1 Beta and Cortisol Reactivity during High-Stakes Public Speaking. Psychoneuroendocrinology 2018, 94, 83–90. [Google Scholar] [CrossRef]
- Grissom, N.; Bhatnagar, S. Habituation to Repeated Stress: Get Used to It. Neurobiol. Learn. Mem. 2009, 92, 215–224. [Google Scholar] [CrossRef]
- Kühnel, A.; Kroemer, N.B.; Elbau, I.G.; Czisch, M.; Sämann, P.G.; Walter, M.; BeCome Working Group; Binder, E.B. Psychosocial Stress Reactivity Habituates Following Acute Physiological Stress. Hum. Brain Mapp. 2020, 41, 4010–4023. [Google Scholar] [CrossRef]
- Gerra, G.; Zaimovic, A.; Mascetti, G.G.; Gardini, S.; Zambelli, U.; Timpano, M.; Raggi, M.A.; Brambilla, F. Neuroendocrine Responses to Experimentally-Induced Psychological Stress in Healthy Humans. Psychoneuroendocrinology 2001, 26, 91–107. [Google Scholar] [CrossRef]
- Schommer, N.C.; Hellhammer, D.H.; Kirschbaum, C. Dissociation Between Reactivity of the Hypothalamus-Pituitary-Adrenal Axis and the Sympathetic-Adrenal-Medullary System to Repeated Psychosocial Stress. Psychosom. Med. 2003, 65, 450–460. [Google Scholar] [CrossRef]
- Von Majewski, K.; Kraus, O.; Rhein, C.; Lieb, M.; Erim, Y.; Rohleder, N. Acute Stress Responses of Autonomous Nervous System, HPA Axis, and Inflammatory System in Posttraumatic Stress Disorder. Transl. Psychiatry 2023, 13, 36. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Roelofs, K.; Tollenaar, M.S.; Bakvis, P.; Van Pelt, J.; Spinhoven, P. Diminished Cortisol Responses to Psychosocial Stress Associated with Lifetime Adverse Events. Psychoneuroendocrinology 2008, 33, 227–237. [Google Scholar] [CrossRef]
- Shapero, B.G.; Hamilton, J.L.; Stange, J.P.; Liu, R.T.; Abramson, L.Y.; Alloy, L.B. Moderate Childhood Stress Buffers Against Depressive Response to Proximal Stressors: A Multi-Wave Prospective Study of Early Adolescents. J. Abnorm. Child Psychol. 2015, 43, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.C.W.; Shields, G.S.; Trainor, B.C.; Slavich, G.M.; Yonelinas, A.P. Greater Lifetime Stress Exposure Predicts Blunted Cortisol but Heightened DHEA Responses to Acute Stress. Stress Health 2019, 35, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Barthel, M.-C.; Fricke, K.; Muehlhan, M.; Vogel, S.; Alexander, N. Habituation of the Biological Response to Repeated Psychosocial Stress: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2025, 169, 105996. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, M.; Bhat, A.; Peters, A. How Stress Can Change Our Deepest Preferences: Stress Habituation Explained Using the Free Energy Principle. Front. Psychol. 2022, 13, 865203. [Google Scholar] [CrossRef]
- McLoughlin, E.; Arnold, R.; Freeman, P.; Turner, J.E.; Roberts, G.A.; Fletcher, D.; Slavich, G.M.; Moore, L.J. Lifetime Stressor Exposure and Psychophysiological Reactivity and Habituation to Repeated Acute Social Stressors. J. Sport Exerc. Psychol. 2022, 44, 427–438. [Google Scholar] [CrossRef]
- Schreier, H.M.C.; Kuras, Y.I.; McInnis, C.M.; Thoma, M.V.; St Pierre, D.G.; Hanlin, L.; Chen, X.; Wang, D.; Goldblatt, D.; Rohleder, N. Childhood Physical Neglect Is Associated With Exaggerated Systemic and Intracellular Inflammatory Responses to Repeated Psychosocial Stress in Adulthood. Front. Psychiatry 2020, 11, 504. [Google Scholar] [CrossRef]
- Roy, M.P.; Kirschbaum, C.; Steptoe, A. Psychological, Cardiovascular, and Metabolic Correlates of Individual Differences in Cortisol Stress Recovery in Young Men. Psychoneuroendocrinology 2001, 26, 375–391. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Schmahl, C.G.; Vermetten, E.; Van Dyck, R.; Bremner, J.D. Higher Cortisol Levels Following Exposure to Traumatic Reminders in Abuse-Related PTSD. Neuropsychopharmacology 2003, 28, 1656–1665. [Google Scholar] [CrossRef]
- Aizpurua-Perez, I.; Arregi, A.; Labaka, A.; Martinez-Villar, A.; Perez-Tejada, J. Psychological Resilience and Cortisol Levels in Adults: A Systematic Review. Am. J. Hum. Biol. 2023, 35, e23954. [Google Scholar] [CrossRef]
- DiMenichi, B.C.; Lempert, K.M.; Bejjani, C.; Tricomi, E. Writing About Past Failures Attenuates Cortisol Responses and Sustained Attention Deficits Following Psychosocial Stress. Front. Behav. Neurosci. 2018, 12, 45. [Google Scholar] [CrossRef]
- Gianferante, D.; Thoma, M.V.; Hanlin, L.; Chen, X.; Breines, J.G.; Zoccola, P.M.; Rohleder, N. Post-Stress Rumination Predicts HPA Axis Responses to Repeated Acute Stress. Psychoneuroendocrinology 2014, 49, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Ehlert, U.; Hellhammer, D.H. The Potential Role of Hypocortisolism in the Pathophysiology of Stress-Related Bodily Disorders. Psychoneuroendocrinology 2000, 25, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Zaba, M.; Kirmeier, T.; Ionescu, I.A.; Wollweber, B.; Buell, D.R.; Gall-Kleebach, D.J.; Schubert, C.F.; Novak, B.; Huber, C.; Köhler, K.; et al. Identification and Characterization of HPA-Axis Reactivity Endophenotypes in a Cohort of Female PTSD Patients. Psychoneuroendocrinology 2015, 55, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R. Early Life Adversity Reduces Stress Reactivity and Enhances Impulsive Behavior: Implications for Health Behaviors. Int. J. Psychophysiol. 2013, 90, 8–16. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, K.Y.; Zhou, W.H.; Gu, G.Z.; Wu, Y.Y.; Yu, S.F. Correlation of occupational stress with serum levels of immunoglobulins and complement in police. Chin. J. Ind. Hyg. Occup. Dis. 2016, 34, 99–102. [Google Scholar] [CrossRef]
- Szalai, A.J.; van Ginkel, F.W.; Dalrymple, S.A.; Murray, R.; McGhee, J.R.; Volanakis, J.E. Testosterone and IL-6 Requirements for Human C-Reactive Protein Gene Expression in Transgenic Mice. J. Immunol. 1998, 160, 5294–5299. [Google Scholar] [CrossRef]
- Raum, D.; Awdeh, Z.; Alper, C.A. BF Types and the Mode of Inheritance of Insulin-Dependent Diabetes Mellitus (IDDM). Immunogenetics 1981, 12, 59–74. [Google Scholar] [CrossRef]
- Speakman, S.; White, K.; LaPorta, A.J.; Payton, M.E.; Gubler, K.D.; Ryznar, R.J. Cytokine Fluctuation during Acute Stress Is Correlated to Life Trauma. J. Trauma Acute Care Surg. 2023, 95, 535–541. [Google Scholar] [CrossRef]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The Effects of Acute Psychological Stress on Circulating and Stimulated Inflammatory Markers: A Systematic Review and Meta-Analysis. Brain. Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of Stress on Immune Function: The Good, the Bad, and the Beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Umei, H.; Yamagishi, S.-I.; Imaizumi, T. Positive Association of Serum Levels of Pigment Epithelium-Derived Factor with High-Sensitivity C-Reactive Protein in Apparently Healthy Unmedicated Subjects. J. Int. Med. Res. 2010, 38, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Xia, W.; Yang, S.; Ye, P.; Mei, M.; Song, Y.; Luo, M.; Li, Q. Association of Serum Pigment Epithelium-Derived Factor with High-Sensitivity C-Reactive Protein in Women with Polycystic Ovary Syndrome. J. Endocrinol. Invest. 2013, 36, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamagishi, S.; Nakamura, K.; Matsui, T.; Imaizumi, T.; Inoue, H.; Ueno, T.; Sata, M. Pigment Epithelium-Derived Factor (PEDF) Blocks the Interleukin-6 Signaling to C-Reactive Protein Expression in Hep3B Cells by Suppressing Rac-1 Activation. Life Sci. 2006, 79, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Sic, A.; Bogicevic, M.; Brezic, N.; Nemr, C.; Knezevic, N.N. Chronic Stress and Headaches: The Role of the HPA Axis and Autonomic Nervous System. Biomedicines 2025, 13, 463. [Google Scholar] [CrossRef]
- Ryznar, R.; Wong, C.; Onat, E.; Towne, F.; LaPorta, A.; Payton, M. Principal Component Analysis of Salivary Cytokines and Hormones in the Acute Stress Response. Front. Psychiatry 2022, 13, 957545. [Google Scholar] [CrossRef]
- Szabo, Y.Z.; Slavish, D.C.; Graham-Engeland, J.E. The Effect of Acute Stress on Salivary Markers of Inflammation: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2020, 88, 887–900. [Google Scholar] [CrossRef]
- Engeland, C.G.; Bosch, J.A.; Rohleder, N. Salivary Biomarkers in Psychoneuroimmunology. Curr. Opin. Behav. Sci. 2019, 28, 58–65. [Google Scholar] [CrossRef]
- Rzeszutek, M.; Lis-Turlejska, M.; Palich, H.; Szumiał, S. The Polish Adaptation of the Life Events Checklist (LEC-5) for PTSD Criteria from DSM-5. Psychiatr. Pol. 2018, 52, 499–510. [Google Scholar] [CrossRef]
- Weathers, F.W.; Blake, D.D.; Schnurr, P.P.; Kaloupek, D.G.; Marx, B.P.; Keane, T.M. The Life Events Checklist for DSM-5 (LEC-5). Available online: https://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp (accessed on 4 February 2025).
- Kaufman, L.; Rousseeuw, P.J. Finding Groups in Data: An Introduction to Cluster Analysis, 1st ed.; Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 1990; ISBN 978-0-471-87876-6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, A.; Andrews, N.; Yasuda, K.; Hodge, M.; Ryznar, R. Acute Stress and Autoimmune Markers: Evaluating the Psychoneuroimmunology Axis in Firefighter Recruits. Int. J. Mol. Sci. 2025, 26, 3945. https://doi.org/10.3390/ijms26093945
Schmitt A, Andrews N, Yasuda K, Hodge M, Ryznar R. Acute Stress and Autoimmune Markers: Evaluating the Psychoneuroimmunology Axis in Firefighter Recruits. International Journal of Molecular Sciences. 2025; 26(9):3945. https://doi.org/10.3390/ijms26093945
Chicago/Turabian StyleSchmitt, Andrea, Nathan Andrews, Krista Yasuda, Mitchell Hodge, and Rebecca Ryznar. 2025. "Acute Stress and Autoimmune Markers: Evaluating the Psychoneuroimmunology Axis in Firefighter Recruits" International Journal of Molecular Sciences 26, no. 9: 3945. https://doi.org/10.3390/ijms26093945
APA StyleSchmitt, A., Andrews, N., Yasuda, K., Hodge, M., & Ryznar, R. (2025). Acute Stress and Autoimmune Markers: Evaluating the Psychoneuroimmunology Axis in Firefighter Recruits. International Journal of Molecular Sciences, 26(9), 3945. https://doi.org/10.3390/ijms26093945





