Challenges and Opportunities for Post-COVID Pulmonary Disease: A Focused Review of Immunomodulation
Abstract
1. Introduction
2. Materials and Literature Search Results
3. Discussion
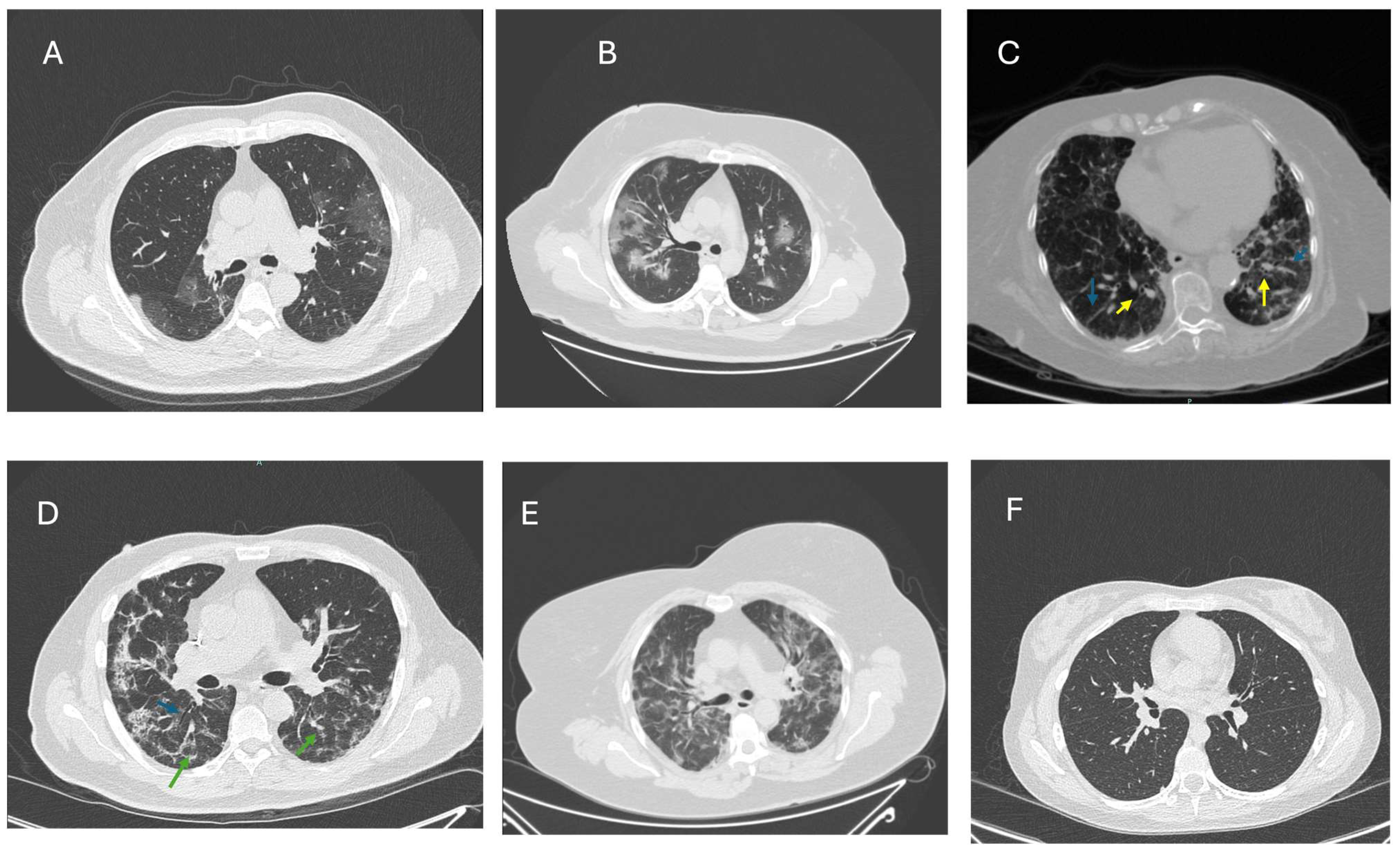
3.1. PCS-Related Pulmonary Sequelae
3.2. Current PCS Therapy
3.3. PCS and Immunomodulatory Treatment with Macrolides
3.4. Future Perspectives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-2 | Angiotensin Converting Enzyme-2 |
| ARDS | Acute Respiratory Distress Syndrome |
| AZM | Azithromycin |
| CAM | Clarithromycin |
| CRP | C-Reactive Protein |
| CRS | Cytokine Release Syndrome |
| CS | Corticosteroids |
| CT | Computed Tomography |
| DLCO | Diffusing Capacity for Carbon Monoxide |
| ECM | Extracellular Matrix |
| ERY | Erythromycin |
| FILD | Fibrotic interstitial lung disease |
| IPF | Idiopathic pulmonary fibrosis |
| PCS | Post-COVID Syndrome |
| PFT | Pulmonary Function test |
| PASC | Post-Acute Sequelae of Coronavirus |
| PC19-ILD | Post-COVID-19 Interstitial Lung Disease |
| PC19-PF | Post-COVID-19 Pulmonary Fibrosis |
References
- Yousefifard, M.; Zali, A.; Zarghi, A.; Madani Neishaboori, A.; Hosseini, M.; Safari, S. Non-steroidal anti-inflammatory drugs in management of COVID-19; A systematic review on current evidence. Int. J. Clin. Pract. 2020, 74, e13557. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Welson, N.N. Pathophysiology of Post-COVID syndromes: A new perspective. Virol. J. 2022, 19, 158. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Chee, Y.J.; Fan, B.E.; Young, B.E.; Dalan, R.; Lye, D.C. Clinical trials on the pharmacological treatment of long COVID: A systematic review. J. Med. Virol. 2023, 95, e28289. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Adorjan, K.; Behrends, U.; Ertl, G.; Suttorp, N.; Lehmann, C. Post-COVID Syndrome. Dtsch. Arztebl. Int. 2023, 120, 48–55. [Google Scholar] [CrossRef]
- Tran, V.T.; Porcher, R.; Pane, I.; Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef]
- Han, M.; Liu, Q.; Ji, Z.; Jin, L.; Jin, W.; Gao, Z. Use of pirfenidone in fibrotic interstitial lung diseases and beyond: A review. Front. Med. 2024, 11, 1411279. [Google Scholar] [CrossRef]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front. Neurol. 2023, 14, 1090747. [Google Scholar] [CrossRef]
- Munblit, D.; Nicholson, T.R.; Needham, D.M.; Seylanova, N.; Parr, C.; Chen, J.; Kokorina, A.; Sigfrid, L.; Buonsenso, D.; Bhatnagar, S.; et al. Studying the post-COVID-19 condition: Research challenges, strategies, and importance of Core Outcome Set development. BMC Med. 2022, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.C.; Xiao, J.; Liu, W.; Li, Y.; Chen, G. Long COVID and its Management. Int. J. Biol. Sci. 2022, 18, 4768–4780. [Google Scholar] [CrossRef]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmarinas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098. [Google Scholar] [CrossRef] [PubMed]
- Patton, M.J.; Benson, D.; Robison, S.W.; Raval, D.; Locy, M.L.; Patel, K.; Grumley, S.; Levitan, E.B.; Morris, P.; Might, M.; et al. Characteristics and determinants of pulmonary long COVID. JCI Insight 2024, 9, e177518. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ram-Mohan, N.; Kim, D.; Rogers, A.J.; Blish, C.A.; Nadeau, K.C.; Blomkalns, A.L.; Yang, S. Association Between SARS-CoV-2 RNAemia and Post-Acute Sequelae of COVID-19. medRxiv 2021, 9, ofab646. [Google Scholar] [CrossRef]
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020, 78, e83–e102. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Fesu, D.; Polivka, L.; Barczi, E.; Foldesi, M.; Horvath, G.; Hidvegi, E.; Bohacs, A.; Muller, V. Post-COVID interstitial lung disease in symptomatic patients after COVID-19 disease. Inflammopharmacology 2023, 31, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, S.; Peris, A.; De Gaudio, A.R.; Geppetti, P. SARS-CoV-2 and COVID-19: From the Bench to the Bedside. Physiol. Rev. 2020, 100, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.J.; Solomon, J.J.; Lee, J.E.; Choi, H.; Chae, K.J.; Lee, K.S.; Lynch, D.A. Chronic Lung Injury after COVID-19 Pneumonia: Clinical, Radiologic, and Histopathologic Perspectives. Radiology 2024, 310, e231643. [Google Scholar] [CrossRef]
- Ribeiro Carvalho, C.R.; Lamas, C.A.; Visani de Luna, L.A.; Chate, R.C.; Salge, J.M.; Yamada Sawamura, M.V.; Toufen, C.; Garcia, M.L.; Scudeller, P.G.; Nomura, C.H.; et al. Post-COVID-19 respiratory sequelae two years after hospitalization: An ambidirectional study. Lancet Reg. Health Am. 2024, 33, 100733. [Google Scholar] [CrossRef]
- John, A.E.; Joseph, C.; Jenkins, G.; Tatler, A.L. COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts. Immunol. Rev. 2021, 302, 228–240. [Google Scholar] [CrossRef]
- Antonogiannaki, E.M.; Grigoropoulos, I.; Manali, E.D.; Thomas, K.; Kallieri, M.; Alexopoulou, P.; Papaioannou, A.I.; Prountzos, S.; Karachaliou, A.; Kontopoulou, C.; et al. Long-Term Lung Sequelae in Survivors of Severe/Critical COVID-19 Pneumonia: The “Non-Steroid”, “Non-Interventional” Approach. J. Clin. Med. 2025, 14, 347. [Google Scholar] [CrossRef]
- Lazar, M.; Barbu, E.C.; Chitu, C.E.; Buzoianu, M.; Petre, A.C.; Tiliscan, C.; Arama, S.S.; Arama, V.; Ion, D.A.; Olariu, M.C. Surviving COVID-19 and Battling Fibrosis: A Retrospective Cohort Study Across Three Pandemic Waves. Diagnostics 2024, 14, 2811. [Google Scholar] [CrossRef]
- Banu, N.; Panikar, S.S.; Leal, L.R.; Leal, A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: Therapeutic implications. Life Sci. 2020, 256, 117905. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Y.; Li, Z.; Hu, L.; Chen, Y.; Zhu, S.; Hu, J.; Huai, T.; Li, M.; Zhang, G.; et al. Pulmonary redox imbalance drives early fibroproliferative response in moderate/severe coronavirus disease-19 acute respiratory distress syndrome and impacts long-term lung abnormalities. Ann. Intensive Care 2024, 14, 72. [Google Scholar] [CrossRef]
- Narasaraju, T.; Neeli, I.; Criswell, S.L.; Krishnappa, A.; Meng, W.; Silva, V.; Bila, G.; Vovk, V.; Serhiy, Z.; Bowlin, G.L.; et al. Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID. Biomolecules 2024, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Oatis, D.; Simon-Repolski, E.; Balta, C.; Mihu, A.; Pieretti, G.; Alfano, R.; Peluso, L.; Trotta, M.C.; D’Amico, M.; Hermenean, A. Cellular and Molecular Mechanism of Pulmonary Fibrosis Post-COVID-19: Focus on Galectin-1, -3, -8, -9. Int. J. Mol. Sci. 2022, 23, 8210. [Google Scholar] [CrossRef]
- Gerayeli, F.V.; Park, H.Y.; Milne, S.; Li, X.; Yang, C.X.; Tuong, J.; Eddy, R.L.; Vahedi, S.M.; Guinto, E.; Cheung, C.Y.; et al. Single-cell sequencing reveals cellular landscape alterations in the airway mucosa of patients with pulmonary long COVID. Eur. Respir. J. 2024, 64, 2301947. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, A.; Karadogan, D.; Hursoy, N.; Telatar, T.G.; Kose Kabil, N.; Marim, F.; Kaya, I.; Er, A.B.; Ercelik, M.; Polat Yulug, D.; et al. Post-COVID Interstitial Lung Disease: How do We Deal with This New Entity? Balk. Med. J. 2024, 41, 377–386. [Google Scholar] [CrossRef]
- Yu, D.; Yin, G.; Lei, J.; Gong, Y.; Zheng, L.; He, D.; Lei, L.; Sun, L. The correlation between serum levels of laminin, type IV collagen, type III procollagen N-terminal peptide and hyaluronic acid with the progression of post-COVID-19 pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1382244. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.; Wong, M.; Ouwerkerk, W.; Wu, M.Z.; Ren, Q.W.; Chandramouli, C.; Teramoto, K.; Teng, K.T.; Huang, J.; To, K.K.; et al. The association between baseline viral load and long-term risk in patients with COVID-19 in Hong Kong: A territory-wide study. Sci. Rep. 2024, 14, 30644. [Google Scholar] [CrossRef]
- Maguire, C.; Chen, J.; Rouphael, N.; Pickering, H.; Phan, H.V.; Glascock, A.; Chu, V.; Dandekar, R.; Corry, D.; Kheradmand, F.; et al. Chronic Viral Reactivation and Associated Host Immune Response and Clinical Outcomes in Acute COVID-19 and Post-Acute Sequelae of COVID-19. bioRxiv 2024. [Google Scholar] [CrossRef]
- Klos, K.; Jaskola-Polkowska, D.; Plewka-Barcik, K.; Rozynska, R.; Pietruszka-Waleka, E.; Zabicka, M.; Kania-Pudlo, M.; Maliborski, A.; Plicht, K.; Angielski, G.; et al. Pulmonary Function, Computed Tomography Lung Abnormalities, and Small Airway Disease after COVID-19: 3-, 6-, and 9-Month Follow-Up. J. Clin. Med. 2024, 13, 2733. [Google Scholar] [CrossRef]
- Babar, M.; Jamil, H.; Mehta, N.; Moutwakil, A.; Duong, T.Q. Short- and Long-Term Chest-CT Findings after Recovery from COVID-19: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 621. [Google Scholar] [CrossRef]
- Watanabe, A.; So, M.; Iwagami, M.; Fukunaga, K.; Takagi, H.; Kabata, H.; Kuno, T. One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis. Respirology 2022, 27, 605–616. [Google Scholar] [CrossRef]
- Caruso, D.; Guido, G.; Zerunian, M.; Polidori, T.; Lucertini, E.; Pucciarelli, F.; Polici, M.; Rucci, C.; Bracci, B.; Nicolai, M.; et al. Post-Acute Sequelae of COVID-19 Pneumonia: Six-month Chest CT Follow-up. Radiology 2021, 301, E396–E405. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00063. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Tang, X.X. Virus infection induced pulmonary fibrosis. J. Transl. Med. 2021, 19, 496. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Peluso, M.J.; Rodgers, K.; Aberg, J.A.; Patterson, T.F.; Tamburro, R.; Baizer, L.; Goldman, J.D.; Rouphael, N.; Deitchman, A.; et al. Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 2023, 14, 1129459. [Google Scholar] [CrossRef]
- Hafezi, B.; Chan, L.; Knapp, J.P.; Karimi, N.; Alizadeh, K.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells. Cells 2021, 10, 1761. [Google Scholar] [CrossRef]
- Al Turkey, H.Y.; Abdullah, A.S.; Ahmed, H.K.; Amin, B.J.H.; Mahmood, Y.M.; Kakamad, S.H.; Qadir, A.N.; Mohammed, H.S.; Bayz, H.H.; Mustafa, S.M. Post COVID-19 Pulmonary Fibrosis Management: A Systematic Review. Barw. Med. J. 2024, 2, 69–70. [Google Scholar]
- Boshra, M.S.; Abou Warda, A.E.; Sayed, M.A.; Elkomy, M.H.; Alotaibi, N.H.; Mohsen, M.; Sarhan, R.M. Effect of Pirfenidone on Risk of Pulmonary Fibrosis in COVID-19 Patients Experiencing Cytokine Storm. Healthcare 2022, 10, 2387. [Google Scholar] [CrossRef]
- Tanvir, M.; Wagay, I.; Nisar, S.; Ahmed, R.N.; Maqbool, M.; Kareem, O.; Muzaffer, U. Early intervention with anti-fibrotic pirfenidone is effective than corticosteroids in preventing pulmonary fibrosis in severe COVID pneumonia patients. Curr. Med. Res. Pract. 2022, 12, 53–60. [Google Scholar] [CrossRef]
- Shu, Y.; He, L.; Liu, C. Impact of anti-fibrotic medications on post-COVID-19 pulmonary fibrosis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2024, 147, 107193. [Google Scholar] [CrossRef]
- Cano, E.J.; Fonseca Fuentes, X.; Corsini Campioli, C.; O’Horo, J.C.; Abu Saleh, O.; Odeyemi, Y.; Yadav, H.; Temesgen, Z. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest 2021, 159, 1019–1040. [Google Scholar] [CrossRef] [PubMed]
- Griesel, M.; Wagner, C.; Mikolajewska, A.; Stegemann, M.; Fichtner, F.; Metzendorf, M.I.; Nair, A.A.; Daniel, J.; Fischer, A.L.; Skoetz, N. Inhaled corticosteroids for the treatment of COVID-19. Cochrane Database Syst. Rev. 2022, 3, CD015125. [Google Scholar] [CrossRef]
- Mizera, J.; Genzor, S.; Sova, M.; Stanke, L.; Burget, R.; Jakubec, P.; Vykopal, M.; Pobeha, P.; Zapletalova, J. The effectiveness of glucocorticoid treatment in post-COVID-19 pulmonary involvement. Pneumonia 2024, 16, 2. [Google Scholar] [CrossRef]
- Sun, G.; Lin, K.; Ai, J.; Zhang, W. The efficacy of antivirals, corticosteroids, and monoclonal antibodies as acute COVID-19 treatments in reducing the incidence of long COVID: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Zhang, J.; Wu, J.-J.; Guo, W.-W.; Tang, F.-S. Strengthening pharmacotherapy research for COVID-19-induced pulmonary fibrosis. World J. Clin. Cases 2024, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Micheletto, C.; Izquierdo, J.L.; Avdeev, S.N.; Rada Escobar, R.A.; Pacheco Gallego, M.C. N-acetylcysteine as a therapeutic approach to post-COVID-19 pulmonary fibrosis adjunctive treatment. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4872–4880. [Google Scholar] [CrossRef]
- Mylvaganam, R.J.; Bailey, J.I.; Sznajder, J.I.; Sala, M.A.; Northwestern Comprehensive, C.C.C. Recovering from a pandemic: Pulmonary fibrosis after SARS-CoV-2 infection. Eur. Respir. Rev. 2021, 30, 210194. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Machuca, T.N.; Querrey, M.; Kurihara, C.; Garza-Castillon, R., Jr.; Kim, S.; Manerikar, A.; Pelaez, A.; Pipkin, M.; Shahmohammadi, A.; et al. Early outcomes after lung transplantation for severe COVID-19: A series of the first consecutive cases from four countries. Lancet Respir. Med. 2021, 9, 487–497. [Google Scholar] [CrossRef]
- Gazzaniga, G.; Voltini, M.; Carletti, A.; Lenta, E.; Meloni, F.; Briganti, D.F.; Avanzini, M.A.; Comoli, P.; Belliato, M. Potential application of mesenchymal stromal cells as a new therapeutic approach in acute respiratory distress syndrome and pulmonary fibrosis. Respir. Res. 2024, 25, 170. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, M.; Wu, H.; Su, W.; Wang, Y.; Li, P. Potential Beneficial Effects of Naringin and Naringenin on Long COVID-A Review of the Literature. Microorganisms 2024, 12, 332. [Google Scholar] [CrossRef]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The Immunomodulatory Effects of Macrolides-A Systematic Review of the Underlying Mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Jang, Y.J. Macrolide therapy in respiratory viral infections. Mediat. Inflamm. 2012, 2012, 649570. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamamoto, S.; Ogasawara, N.; Takano, K.; Shiraishi, T.; Sato, T.; Miyata, R.; Kakuki, T.; Kamekura, R.; Kojima, T.; et al. Clarithromycin prevents human respiratory syncytial virus-induced airway epithelial responses by modulating activation of interferon regulatory factor-3. Pharmacol. Res. 2016, 111, 804–814. [Google Scholar] [CrossRef]
- Yamaya, M.; Kikuchi, A.; Sugawara, M.; Nishimura, H. Anti-inflammatory effects of medications used for viral infection-induced respiratory diseases. Respir. Investig. 2023, 61, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Shinahara, W.; Takahashi, E.; Sawabuchi, T.; Arai, M.; Hirotsu, N.; Takasaki, Y.; Shindo, S.; Shibao, K.; Yokoyama, T.; Nishikawa, K.; et al. Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: A retrospective analysis. PLoS ONE 2013, 8, e70060. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Wan, K.S.; Liu, Y.C.; Huang, C.S.; Su, Y.M. Effects of low-dose clarithromycin added to fluticasone on inflammatory markers and pulmonary function among children with asthma: A randomized clinical trial. Allergy Rhinol. 2016, 7, 131–134. [Google Scholar] [CrossRef]
- Fouka, E.; Lamprianidou, E.; Arvanitidis, K.; Filidou, E.; Kolios, G.; Miltiades, P.; Paraskakis, E.; Antoniadis, A.; Kotsianidis, I.; Bouros, D. Low-dose clarithromycin therapy modulates Th17 response in non-cystic fibrosis bronchiectasis patients. Lung 2014, 192, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Mancia Leon, W.R.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef]
- Madrid, P.B.; Panchal, R.G.; Warren, T.K.; Shurtleff, A.C.; Endsley, A.N.; Green, C.E.; Kolokoltsov, A.; Davey, R.; Manger, I.D.; Gilfillan, L.; et al. Evaluation of Ebola Virus Inhibitors for Drug Repurposing. ACS Infect. Dis. 2015, 1, 317–326. [Google Scholar] [CrossRef]
- Gielen, V.; Johnston, S.L.; Edwards, M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010, 36, 646–654. [Google Scholar] [CrossRef]
- Yan, Y.; Wu, L.; Li, X.; Zhao, L.; Xu, Y. Immunomodulatory role of azithromycin: Potential applications to radiation-induced lung injury. Front. Oncol. 2023, 13, 966060. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: Retrospective cohort study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef]
- Tsiakos, K.; Tsakiris, A.; Tsibris, G.; Voutsinas, P.M.; Panagopoulos, P.; Kosmidou, M.; Petrakis, V.; Gravvani, A.; Gkavogianni, T.; Klouras, E.; et al. Early Start of Oral Clarithromycin Is Associated with Better Outcome in COVID-19 of Moderate Severity: The ACHIEVE Open-Label Single-Arm Trial. Infect. Dis. Ther. 2021, 10, 2333–2351. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Al-Gareeb, A.I.; Saad, H.M.; Al-Kuraishy, H.M. COVID-19 and corticosteroids: A narrative review. Inflammopharmacology 2022, 30, 1189–1205. [Google Scholar] [CrossRef]
- Horwitz, L.I.; Thaweethai, T.; Brosnahan, S.B.; Cicek, M.S.; Fitzgerald, M.L.; Goldman, J.D.; Hess, R.; Hodder, S.L.; Jacoby, V.L.; Jordan, M.R.; et al. Researching COVID to Enhance Recovery (RECOVER) adult study protocol: Rationale, objectives, and design. PLoS ONE 2023, 18, e0286297. [Google Scholar] [CrossRef]
- Hama Amin, B.J.; Kakamad, F.H.; Ahmed, G.S.; Ahmed, S.F.; Abdulla, B.A.; Mohammed, S.H.; Mikael, T.M.; Salih, R.Q.; Ali, R.K.; Salh, A.M.; et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann. Med. Surg. 2022, 77, 103590. [Google Scholar] [CrossRef]
- Kazama, I. Stabilizing mast cells by commonly used drugs: A novel therapeutic target to relieve post-COVID syndrome? Drug Discov. Ther. 2020, 14, 259–261. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Jian, H.; Lin, J. Effects of macrolides on airway microbiome and cytokine of children with bronchiolitis: A systematic review and meta-analysis of randomized controlled trials. Microbiol. Immunol. 2019, 63, 343–349. [Google Scholar] [CrossRef]
- Tahan, F.; Ozcan, A.; Koc, N. Clarithromycin in the treatment of RSV bronchiolitis: A double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 2007, 29, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.N.; To, K.K.W.; Chan, J.F.W.; Cheng, V.C.C.; Liu, K.S.H.; Tam, A.; Chan, T.C.; Zhang, A.J.; Li, P.; Wong, T.L.; et al. Efficacy of Clarithromycin-Naproxen-Oseltamivir Combination in the Treatment of Patients Hospitalized for Influenza A(H3N2) Infection: An Open-label Randomized, Controlled, Phase IIb/III Trial. Chest 2017, 151, 1069–1080. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbeeck Mendez, S.; Do Orozco, I.L.; Gavilanez-Chavez, G.E.; Nava-Zavala, A.H.; Zavala-Cerna, M.G. Challenges and Opportunities for Post-COVID Pulmonary Disease: A Focused Review of Immunomodulation. Int. J. Mol. Sci. 2025, 26, 3850. https://doi.org/10.3390/ijms26083850
Verbeeck Mendez S, Do Orozco IL, Gavilanez-Chavez GE, Nava-Zavala AH, Zavala-Cerna MG. Challenges and Opportunities for Post-COVID Pulmonary Disease: A Focused Review of Immunomodulation. International Journal of Molecular Sciences. 2025; 26(8):3850. https://doi.org/10.3390/ijms26083850
Chicago/Turabian StyleVerbeeck Mendez, Steffi, Isabella L. Do Orozco, Guadalupe E. Gavilanez-Chavez, Arnulfo Hernán Nava-Zavala, and Maria G. Zavala-Cerna. 2025. "Challenges and Opportunities for Post-COVID Pulmonary Disease: A Focused Review of Immunomodulation" International Journal of Molecular Sciences 26, no. 8: 3850. https://doi.org/10.3390/ijms26083850
APA StyleVerbeeck Mendez, S., Do Orozco, I. L., Gavilanez-Chavez, G. E., Nava-Zavala, A. H., & Zavala-Cerna, M. G. (2025). Challenges and Opportunities for Post-COVID Pulmonary Disease: A Focused Review of Immunomodulation. International Journal of Molecular Sciences, 26(8), 3850. https://doi.org/10.3390/ijms26083850





