Establishment of a Novel Platform for Developing Oral Vaccines Based on the Surface Display System of Yeast Spores
Abstract
:1. Introduction
2. Results
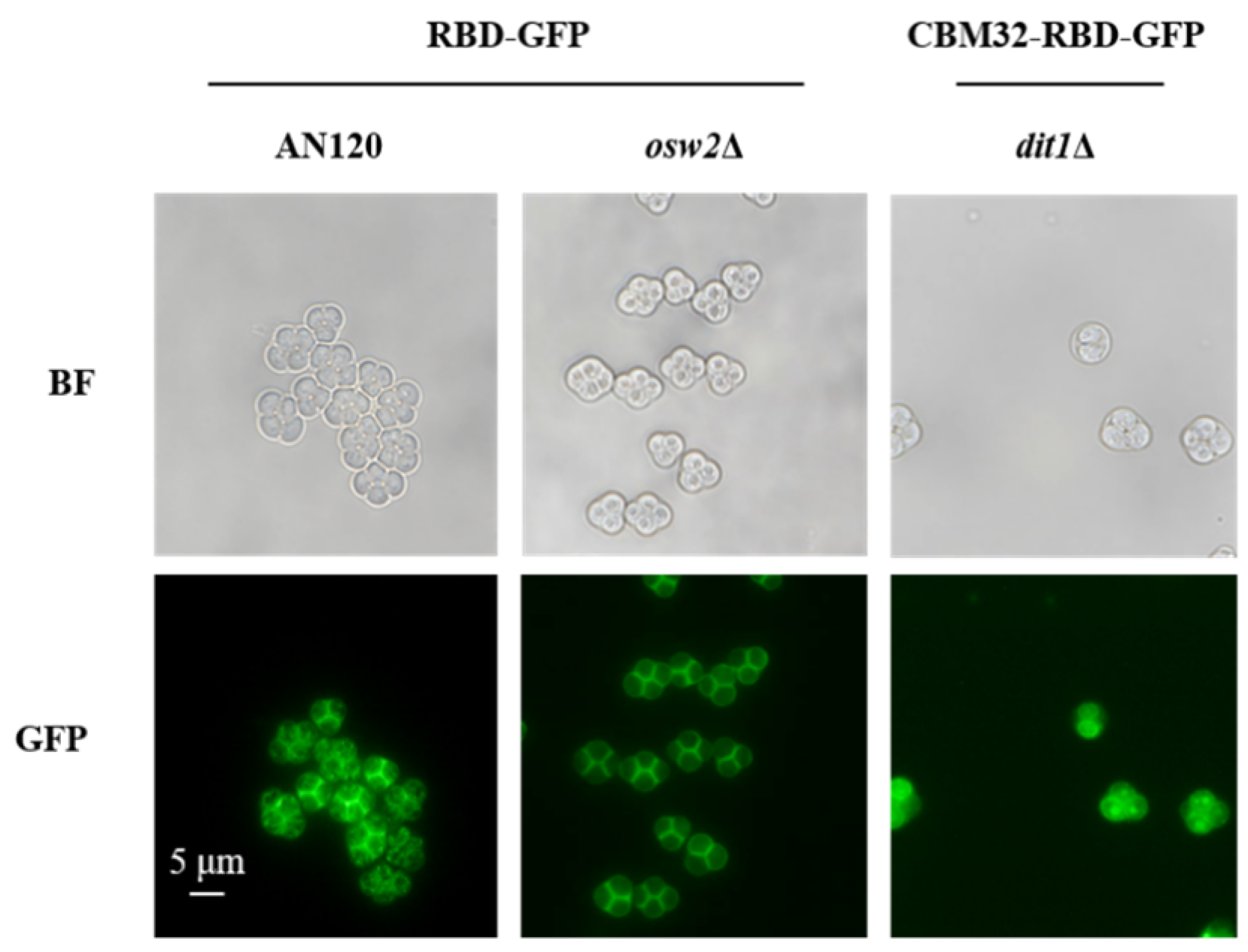
2.1. Expression and Localization of RBD on the Surface of S. cerevisiae Spores
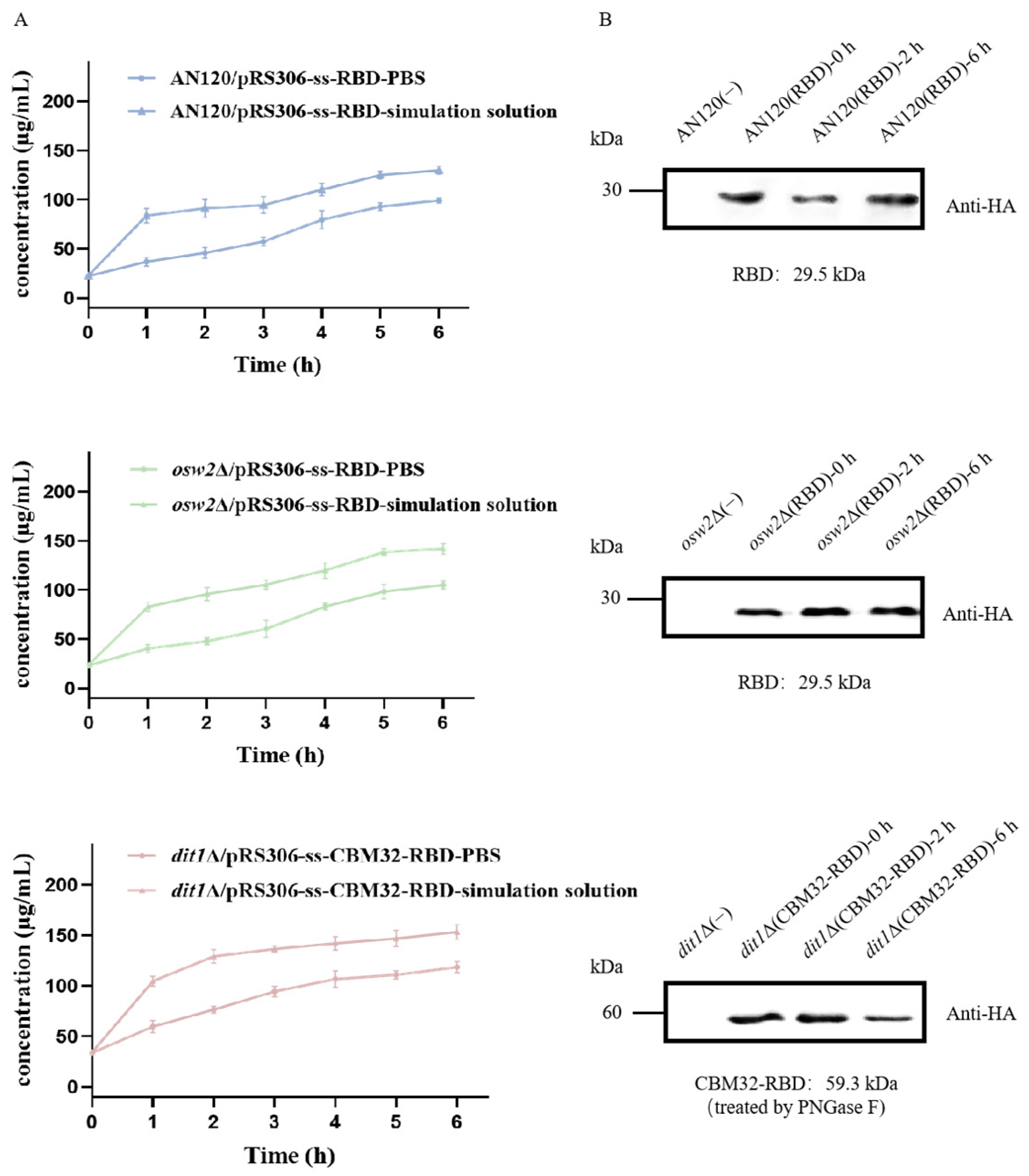
2.2. Stability of Spores by Stimulated Gastrointestinal Digestion
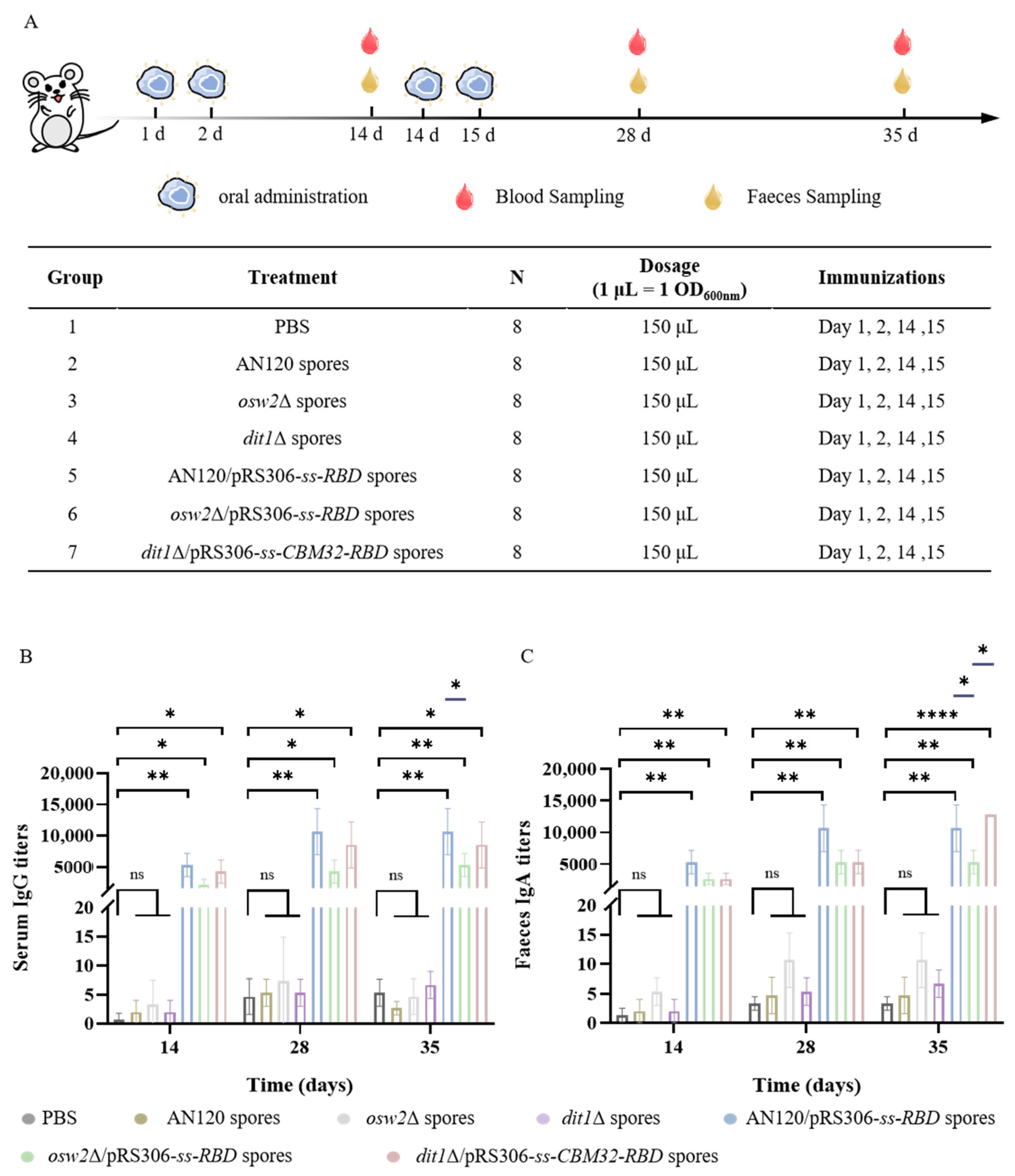
2.3. Measurement of the SARS-CoV-2 Antibodies
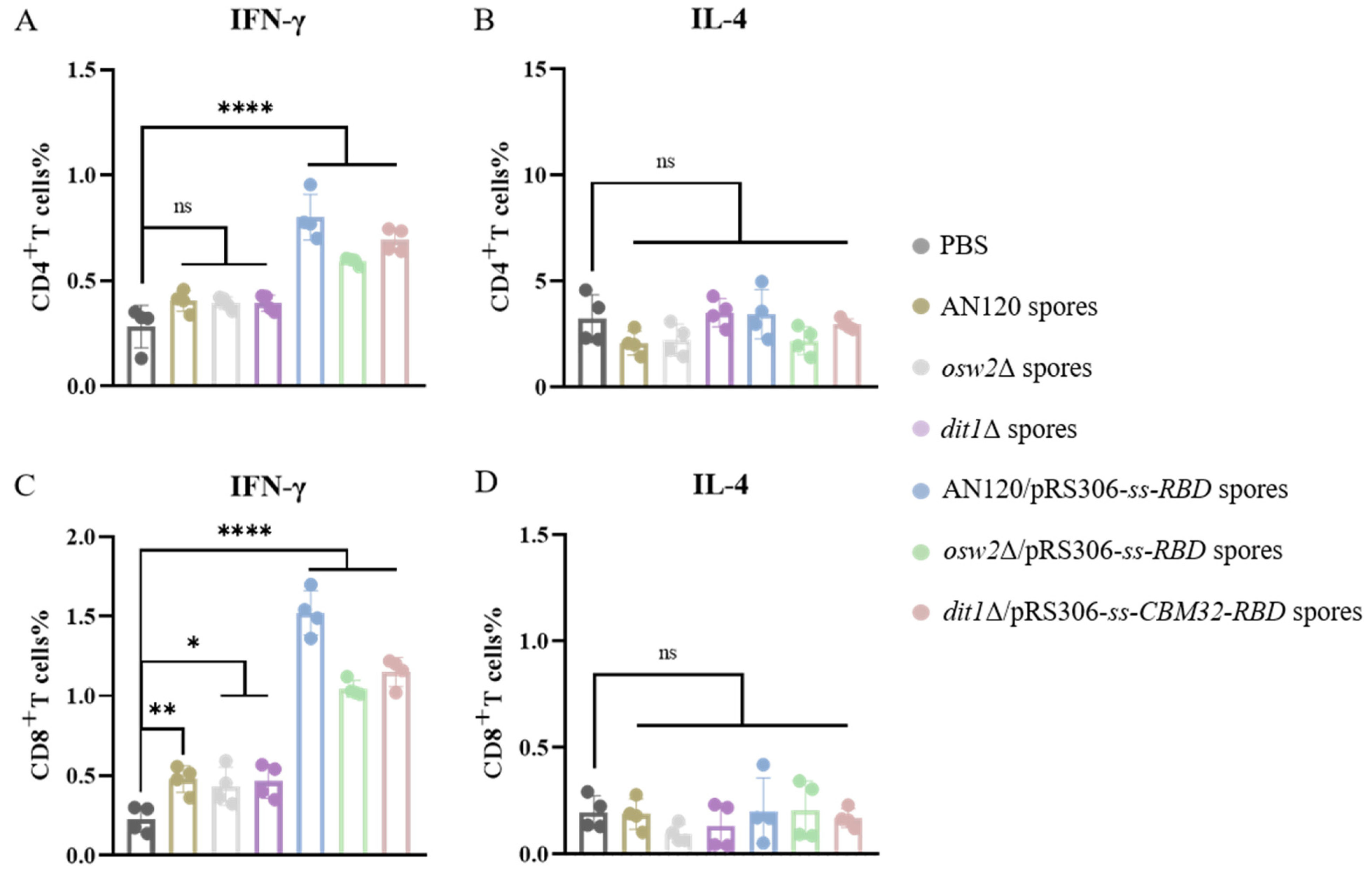
2.4. Th1-Skewing of the T Cell Response
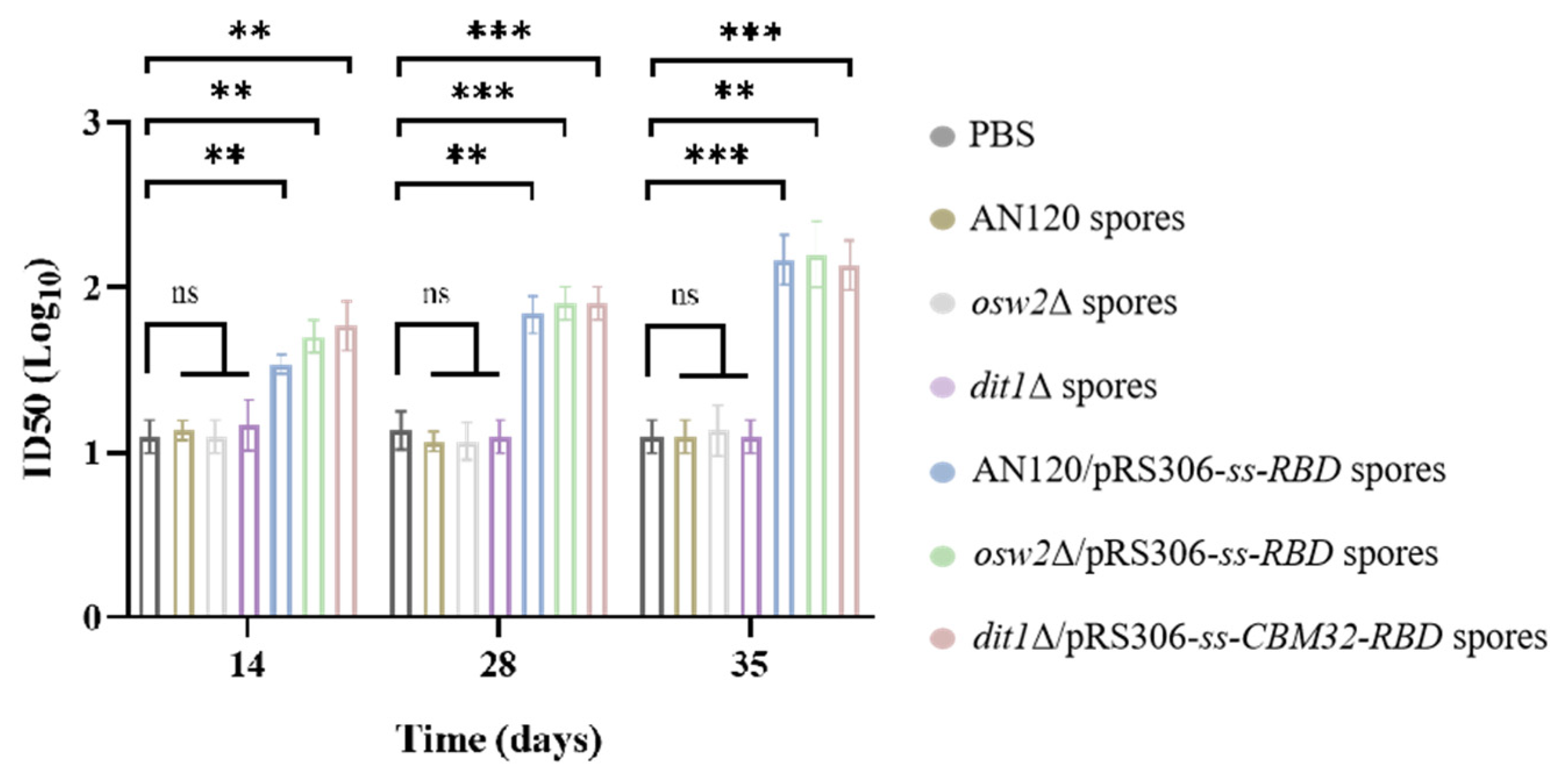
2.5. Antibody Neutralizing Activity
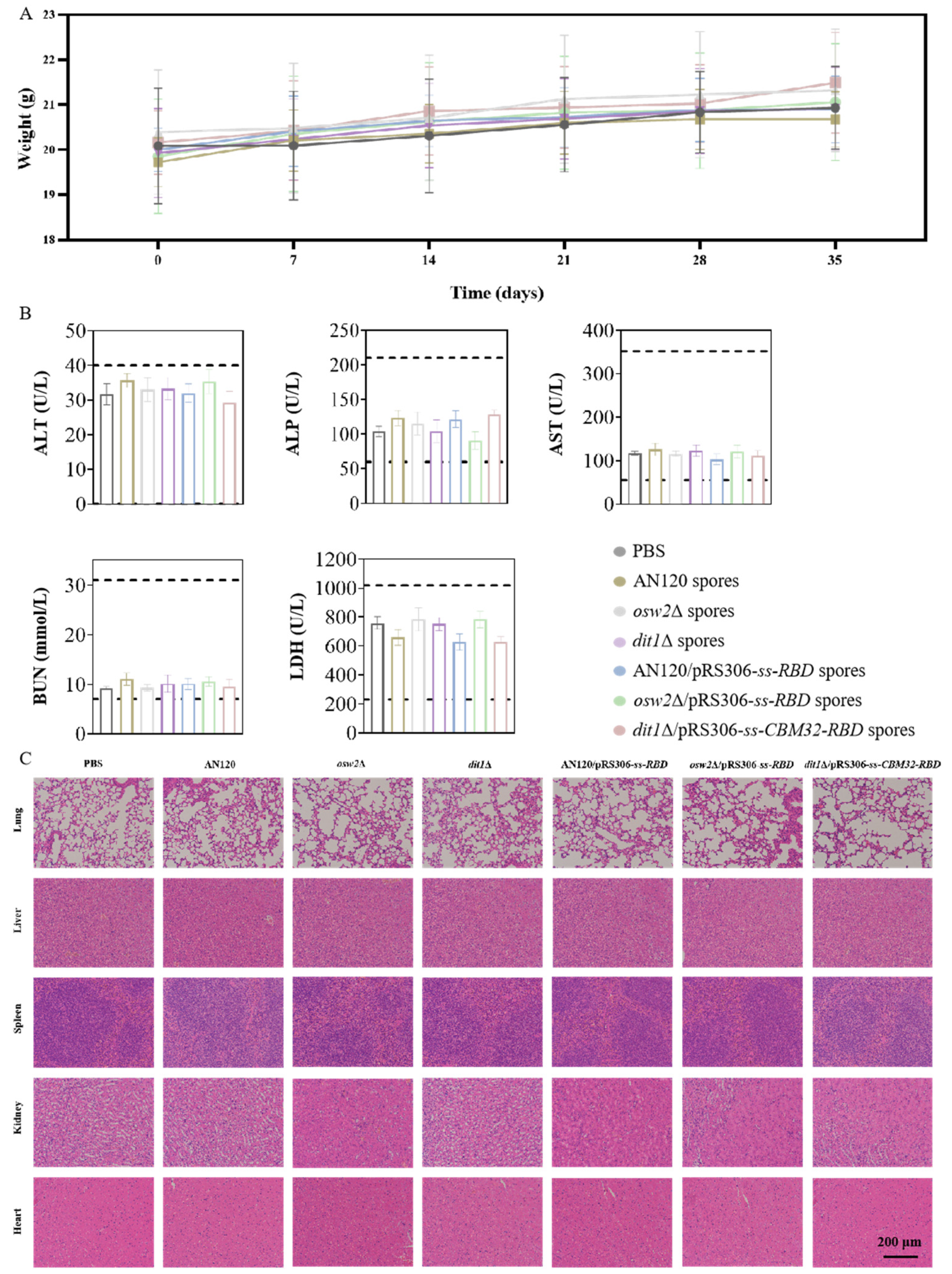
2.6. Reactogenicity Studies In Vivo
3. Discussion
4. Materials and Methods
4.1. Construction of Recombinant Strains and Plasmids
4.2. Production and Purification of Spores
4.3. Microscopy and Western Blot Analysis
4.4. Stimulated Gastrointestinal Digestion of Spores Displaying RBD In Vitro
4.5. Vaccine Formulation and Oral Immunization
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Preparation of Splenocytes
4.8. Flow Cytometry Analysis
4.9. Pseudovirus Neutralization Antibody Assay
4.10. Reactogenicity Assessment
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehl, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.D.; et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022, 14, eabn6868. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Qi, Y.D.; Chen, Y.G.; Zhang, Y.; Li, B.; Feng, J.; Zhang, X.Z. Tuning bacterial morphology to enhance anticancer vaccination. ACS Nano 2023, 17, 8815–8828. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.M.; Zou, K.Y.; Wiens, K.E.; Malembaka, E.B.; Bwire, G.; Okitayemba, P.W.; Hampton, L.M.; Azman, A.S.; Lee, E.C. Enhanced cholera surveillance to improve vaccination campaign efficiency. Nat. Med. 2024, 30, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Shakya, M.; Pollard, A.J. Typhoid conjugate vaccines: A step towards typhoid control. Lancet Glob. Health 2024, 12, e535–e536. [Google Scholar] [CrossRef]
- Barbosa, J.R.; de Carvalho, R.N., Jr. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef]
- Huang, X.N.; Ma, Y.Y.; Wang, Y.X.; Niu, C.; Liu, Z.M.; Yao, X.; Jiang, X.X.; Pan, R.H.; Jia, S.; Li, D.D.; et al. Oral probiotic vaccine expressing Koi Herpesvirus (KHV) ORF81 protein delivered by chitosan-alginate capsules is a promising strategy for mass oral vaccination of carps against KHV infection. J. Virol. 2021, 95, e00415-21. [Google Scholar] [CrossRef]
- Rivas-Aravena, A.; Sandino, A.M.; Spencer, E. Nanoparticles and microparticles of polymers and polysaccharides to administer fish vaccines. Biol. Res. 2013, 46, 407–419. [Google Scholar] [CrossRef]
- Wu, B.; Fan, H.P.; Zhang, X.Y.; Zheng, L.; Zhong, Q.F.; Zhang, G.Q.; Weng, Z.T. Preparation of the oral microencapsulated vaccine of Streptococcus agalaciate from tilapia and its immunological effect. J. Fish. China 2016, 40, 1258–1264. [Google Scholar]
- Fang, S.Q.; Liang, B.; Xiao, X.X.; Bai, L.; Tang, X.J.; Lojou, E.; Cosnier, S.; Liu, A.H. Controllable display of sequential enzymes on yeast surface with enhanced biocatalytic activity toward efficient enzymatic biofuel cells. J. Am. Chem. Soc. 2020, 142, 3222–3230. [Google Scholar]
- Bryan, J.T.; Buckland, B.; Hammond, J.; Jansen, K.U. Prevention of cervical cancer: Journey to develop the first human papillomavirus virus-like particle vaccine and the next generation vaccine. Curr. Opin. Chem. Biol. 2016, 32, 34–47. [Google Scholar] [CrossRef]
- Li, Z.J.; Liu, X.X.; Nakanishi, H.; Gao, X.D. Encapsulation of mannose-6-phosphate isomerase in yeast spores and its application in L-ribose production. J. Agric. Food Chem. 2020, 68, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Li, W.J.; Wang, Y.S.; Chen, Z.; Nakanishi, H.; Xu, X.Y.; Gao, X.D. Establishment of a novel cell surface display platform based on natural “chitosan beads” of yeast spores. J. Agric. Food Chem. 2022, 70, 7479–7489. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.J.; Tachikawa, H.; Gao, X.D.; Nakanishi, H. Use of yeast spores for microencapsulation of enzymes. Appl. Environ. Microb. 2014, 80, 4502–4510. [Google Scholar] [CrossRef] [PubMed]
- Koen, A.L.; Izu, A.; Baillie, V.; Kwatra, G.; Cutland, C.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of primary series AZD1222 (ChAdOx1 nCoV-19) vaccination against SARS-CoV-2 variants of concern: Final analysis of a randomized, placebo-controlled, phase 1b/2 study in South African adults (COV005). Vaccine 2023, 41, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Z.; Li, M.Y.; Wang, X.S. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.M.; Wan, L.G.; Xiang, T.X.; Le, A.P.; Liu, J.M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020, 20, 656. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Yeast-based vaccines: New perspective in vaccine development and application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef]
- Wasilenko, J.L.; Sarmento, L.; Spatz, S. Pantin-Jackwood, Cell surface display of highly pathogenic Avian Influenza virus hemagglutinin on the surface of Pichia pastoris cells uusing α-agglutinin for production of ooral vaccines. Biotechnol. Prog. 2010, 26, 542–547. [Google Scholar] [CrossRef]
- Upadhyaya, B.; Manjunath, R. Baker’s yeast expressing the Japanese encephalitis virus envelope protein on its cell surface: Induction of an antigen-specific but non-neutralizing antibody response. Yeast 2009, 26, 383–397. [Google Scholar] [CrossRef]
- Xing, H.G.; Zhu, L.Y.; Wang, P.P.; Zhao, G.P.; Zhou, Z.H.; Yang, Y.; Zou, H.; Yan, X. Display of receptor-binding domain of SARS-CoV-2 spike protein variants on the Saccharomyces cerevisiae cell surface. Front. Immunol. 2022, 13, 935573. [Google Scholar] [CrossRef] [PubMed]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Galvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.T.; Tong, J.C.; Wu, X.; Liu, S.; Wu, J.J.; Yu, Y.L.; Zhang, L.; Zhao, C.Y.; Lu, Q.; Nie, J.H.; et al. Development of a SARS-CoV-2 neutralization assay based on a pseudotyped virus using a HIV system. Medcomm 2024, 5, e517. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Pochard, P.; Lee, C.G.; Elias, J.A. Chitin particles are multifaceted immune adjuvants. Am. J. Respir. Crit. Care Med. 2010, 182, 1482–1491. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Doremalen, V.; Lambe, N.; Spencer, A. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- He, J.Y.; Yang, X.M.; He, W.Q.; Hong, H.; Lei, Z.M.; Chen, G.B.; Shen, L.; Yang, J.; Li, Z.L.; Wang, X.R.; et al. A bivalent recombinant vaccine targeting the S1 protein induces neutralizing antibodies against both SARS-CoV-2 variants and wild-type of the virus. Medcomm 2021, 2, 430–441. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, Q.; Liu, J.; Fang, Z.H.; Luo, L.P.; Li, S.; Lei, Y.X.; Li, Z.; Jin, J.; Xie, R.L.; et al. Development of bivalent mRNA vaccines against SARS-CoV-2 variants. Vaccines 2022, 10, 1807. [Google Scholar] [CrossRef]
- Gray, G.E.; Bekker, L.-G.; Laher, F.; Malahleha, M.; Allen, M.; Moodie, Z.; Grunenberg, N.; Huang, Y.; Grove, D.; Prigmore, B.; et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N. Engl. J. Med. 2021, 384, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Scott, D.A.; Gurtman, A.; Zareba, A.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Jiang, Q.; et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus Prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J. Infect. Dis. 2022, 225, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Dalvie, N.C.; Rodriguez-Aponte, S.A.; Hartwell, B.L.; Love, J.C. Engineered SARS-CoV-2 receptor-binding domain improves manufacturability in yeast and immunogenicity in mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2106845118. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, Y.S.; Chen, Z.; Nakanishi, H.; Xu, X.Y.; Gao, X.D.; Li, Z.J. Construction of a new Saccharomyces cerevisiae spore surface display system based on chitosan binding module. Acta Microbiol. Sin. 2022, 62, 4431–4446. [Google Scholar]
- Cao, L.; Diedrich, J.K.; Ma, Y.; Wang, N.; Pauthner, M.; Park, S.K.R.; Delahunty, C.M.; McLellan, J.S.; Burton, D.R.; Yates, J.R.; et al. Global site-specific analysis of glycoprotein N-glycan processing. Nat. Protoc. 2018, 13, 1196–1212. [Google Scholar] [CrossRef]
- Lin, A.; Apostolovic, D.; Jahnmatz, M.; Liang, F.; Ols, S.; Tecleab, T.; Wu, C.; van Hage, M.; Solovay, K.; Rubin, K.; et al. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J. Clin. Investig. 2020, 130, 2332–2346. [Google Scholar] [CrossRef]
- Paramithiotis, E.; Varaklis, C.; Pillet, S.; Shafiani, S.; Lancelotta, M.P.; Steinhubl, S.; Sugden, S.; Clutter, M.; Montamat-Sicotte, D.; Chermak, T.; et al. Integrated antibody and cellular immunity monitoring are required for assessment of the long term protection that will be essential for effective next generation vaccine development. Front. Immunol. 2023, 14, 1166059. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.F.; Zhu, Y.F.; Liu, C.X.; Gu, C.J.; Xu, S.Q.; Wang, Y.L.; Zhou, Y.; Wang, Y.X.; Han, W.Y.; et al. Development and structural basis of a two-MAb cocktail for treating SARS-CoV-2 infections. Nat. Commun. 2021, 12, 264. [Google Scholar] [CrossRef]
- Nie, J.H.; Li, Q.Q.; Wu, J.J.; Zhao, C.Y.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.L.; Qin, H.Y.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef]
- Nie, J.H.; Li, Q.Q.; Wu, J.J.; Zhao, C.Y.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.L.; Qin, H.Y.; Wang, M.; et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, C.; Bai, J.; Li, Y.; Li, Y.; Liu, Y.; Zhou, X.; Shi, J.; Nakanishi, H.; Li, Z. Establishment of a Novel Platform for Developing Oral Vaccines Based on the Surface Display System of Yeast Spores. Int. J. Mol. Sci. 2025, 26, 3615. https://doi.org/10.3390/ijms26083615
Si C, Bai J, Li Y, Li Y, Liu Y, Zhou X, Shi J, Nakanishi H, Li Z. Establishment of a Novel Platform for Developing Oral Vaccines Based on the Surface Display System of Yeast Spores. International Journal of Molecular Sciences. 2025; 26(8):3615. https://doi.org/10.3390/ijms26083615
Chicago/Turabian StyleSi, Chenyu, Jiawen Bai, Yuqing Li, Yang Li, Yishi Liu, Xiaoman Zhou, Jie Shi, Hideki Nakanishi, and Zijie Li. 2025. "Establishment of a Novel Platform for Developing Oral Vaccines Based on the Surface Display System of Yeast Spores" International Journal of Molecular Sciences 26, no. 8: 3615. https://doi.org/10.3390/ijms26083615
APA StyleSi, C., Bai, J., Li, Y., Li, Y., Liu, Y., Zhou, X., Shi, J., Nakanishi, H., & Li, Z. (2025). Establishment of a Novel Platform for Developing Oral Vaccines Based on the Surface Display System of Yeast Spores. International Journal of Molecular Sciences, 26(8), 3615. https://doi.org/10.3390/ijms26083615






