1. Introduction
Adult neurogenesis refers to the generation and integration of new functional neurons into the neural circuitry of the adult brain, a process that has garnered significant interest in recent decades [
1]. While previously believed to occur exclusively during embryonic development, research has demonstrated that neurogenesis persists at low levels throughout postnatal and adult life in mammals [
2,
3,
4]. In adult mammals, this process is primarily restricted to three regions: the subgranular zone (SGZ) of the hippocampal dentate gyrus [
5,
6], the subventricular zone (SVZ) of the subpallium [
7], and, more recently, the hypothalamic region [
8,
9,
10].
Neurons generated in the SGZ remain within the dentate gyrus and contribute to hippocampal function [
11], whereas those produced in the SVZ migrate via the rostral migratory stream to the olfactory bulb, supporting olfactory neuron homeostasis. These neurogenic niches are maintained by glial-like stem cells of embryonic origin, known as radial glial cells (RGs), characterized by their distinctive morphology—a rounded cell body with minimal dendrites and a long radial axon extending through the telencephalic mass [
12]. Recent findings also indicate that adult neurogenesis occurs in the hypothalamus, where tanycytes give rise to new neurons [
13].
Both embryonic and adult neurogenesis share a similar molecular framework, making them comparable processes. In both cases, RGs generate immature precursors that differentiate into functional neurons [
14,
15,
16,
17]. This evolutionary conservation underscores the fundamental significance of neurogenesis in maintaining neural plasticity and function throughout life.
Despite its spatial limitations in mammals, adult neurogenesis appears to be necessary for optimal adult brain function, facilitating the integration of newly generated neurons into existing circuits [
18]. Adult neurogenesis is closely related to physiological brain plasticity [
19], and reduced levels of neurogenesis have been associated with pathological conditions such as Alzheimer’s disease [
20,
21]. The activation of radial glial cells and the subsequent production of new neurons are essential for maintaining olfactory bulb homeostasis; studies involving the genetic ablation of these cells in mice have demonstrated a progressive reduction of granule cells in the olfactory bulb, accompanied by impaired contextual and spatial memory, learning, and pattern recognition [
22,
23].
In the murine hippocampus, the rate of adult neurogenesis varies across the lifespan, with a notable decline observed during aging [
21,
24]. However, the extent to which hippocampal adult neurogenesis declines with age in humans remains a topic of debate, with some studies suggesting a decline in neurogenesis with age [
25], while others indicate that adult neurogenesis or neural progenitor cell (NPC) numbers may not decrease despite a reduction in the progenitor pool [
26,
27]. Despite the ongoing discussion regarding the decline of neurogenesis in humans, correlations between cognitive status and the number of newborn neurons in both healthy and pathological states suggest that appropriate levels of adult neurogenesis are crucial for maintaining brain functionality [
20,
28]. Compiled data suggest that new neurons could be derived not only from stem cells but also from a population of neuroblasts displaying a protracted maturation.
Examining adult neurogenesis outside the group of mammals could provide valuable insights into the mechanisms that regulate this process, as the situation varies significantly among other vertebrate groups. Studies on songbirds have demonstrated that adult neurogenesis occurs seasonally in the ventricular zone when they learn new songs, particularly in the hyperstriatum accessorium and hyperstriatum ventralis [
29,
30]. Various studies have been conducted on different species of bony fish, such as
Danio rerio [
31], the killifish
Nothobranchius furzeri [
32], the knifefish
Apteronotus leptorhynchus, and the rainbow trout
Oncorhynchus mykiss [
33,
34], all demonstrating the presence of several active neurogenic niches localized throughout their brains.
Adult neurogenesis appears to be absent in cyclostomes [
35,
36], while recent studies in chondrichthyans have revealed extensive neurogenic activity in adult brains [
37,
38], suggesting that adult neurogenesis could be considered a basal trait of gnathostomes. This observation is particularly interesting because, from a neuroanatomical perspective, chondrichthyans are more similar to mammals than to teleosts. This reasoning stems from the two distinct ways in which telencephalon morphogenesis occurs in these groups: evagination, observed in both chondrichthyans and sarcopterygian tetrapods, versus eversion, commonly seen in actinopterygians, including teleosts [
39].
Analyses of the cellular processes of adult neurogenesis and in-depth comparisons of this process between mammals and fish have been extensively reported in the literature (Cacialli and Lucini 2019; Fernández-Hernández and Rhiner 2015; Diotel et al. 2020) [
37,
40,
41,
42] and we direct the readers to the cited articles for deeper discussions about this topic.
During evagination, the dorsal pallium folds inward to form a median septum and symmetric lateral ventricles. In contrast, during eversion, the pallium folds outward, causing the area corresponding to the ventricle to extend into the dorso-lateral region [
39]. This leads to distinctly different anatomical localizations within the adult telencephalon that can be attributed to the same embryonic origin.
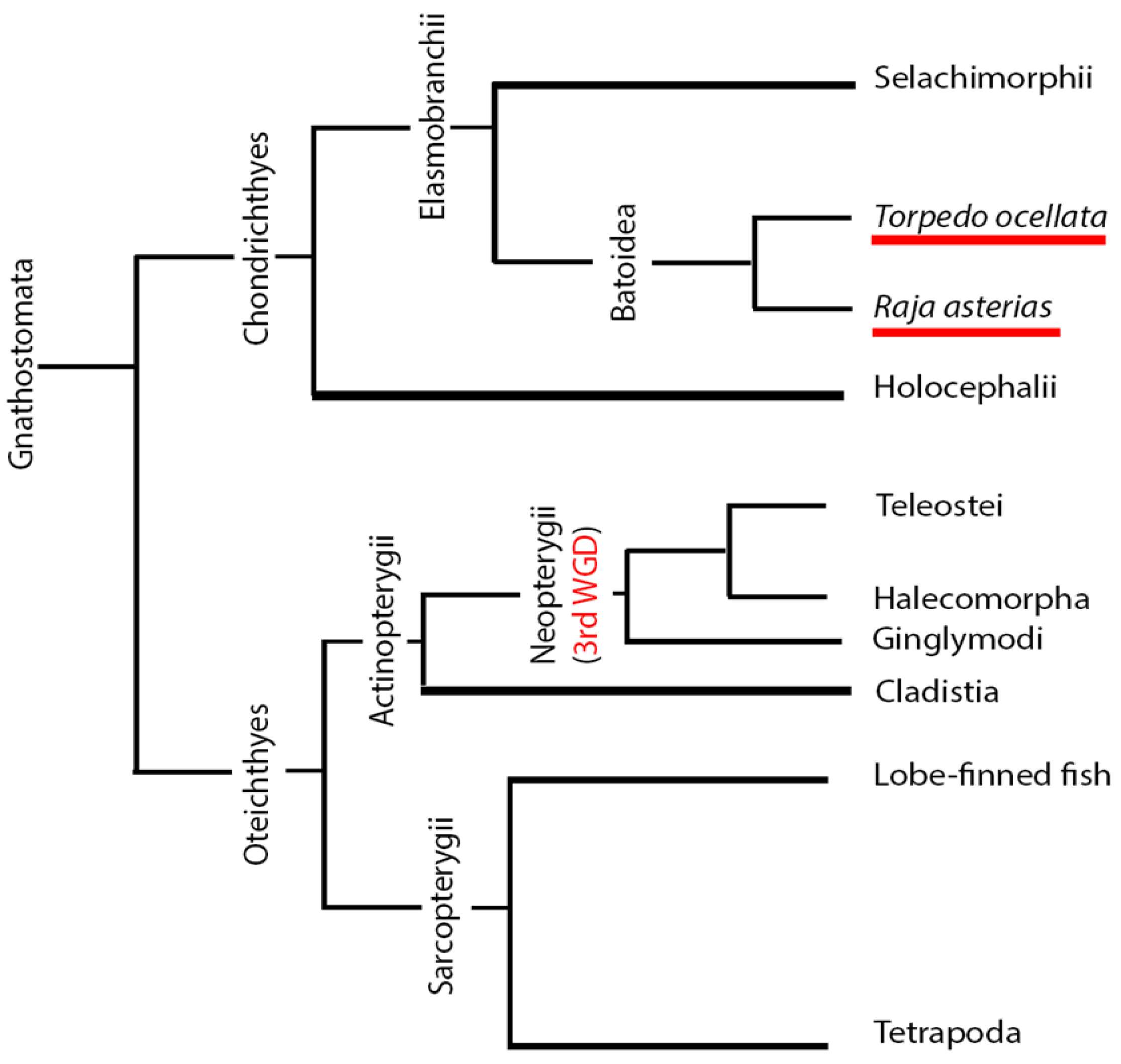
The sharing of anatomical features between cartilaginous fishes and mammals can be explained from a phylogenetic perspective (
Figure 1). Chondrichthyans are basal to both actinopterygian and tetrapod clades, which can be considered sister groups. Osteichthyes and Chondrichthyes form their own clades that evolved independently from a common Gnathostomata ancestor. Within the Osteichthyes group, the clade of
Sarcopterygii evolved, from which tetrapods originated. The sister clade to the
Sarcopterygii is the
Actinopterygii, from which the
Teleostei group emerged. Within the
Sarcopterygii group, tetrapods and teleosts evolved independently, while chondrichthyans are basal to both and have retained many ancestral features. In contrast, actinopterygians, specifically teleosts, have developed numerous unique traits that distinguish them from sarcopterygians. For this reason, some neuroanatomical features of chondrichthyans may be more similar to those of mammals than to those of Osteichthyes. This is one of two main arguments supporting the choice of chondrichthyans as a better alternative model to teleosts for comparative neuroanatomical studies with mammals.
The other important observation supporting this choice is that after the divergence of their clade from that of tetrapods and chondrichthyans,
Actinopterygii underwent an additional event of whole genome duplication (WGD), further increasing the differences between them and mammals [
43] (
Figure 1). These arguments validate the use of chondrichthyans as a reference group for studying the evolution of conserved traits such as adult neurogenesis.
The widespread occurrence of adult neurogenesis in the brains of Osteichthyes, contrasted with its absence in tetrapods, raises the question of whether this trait is ancestral and has been lost in tetrapods or if it evolved independently within the Osteichthyes lineage. Recent studies on chondrichthyans may provide insights into this question, and further investigation into the distribution of adult neurogenesis in other chondrichthyan groups is essential to determine whether it can be considered an ancestral feature. To date, insufficient research has been conducted on cartilaginous fishes to definitively ascertain whether adult neurogenesis is a basal trait of gnathostomes.
Currently, the only species in which adult neurogenesis has been characterized is the small-spotted catshark,
Scyliorhinus canicula, which belongs to the
Galeomorphii group, a part of the
Selachimorpha subclass within the class
Elasmobranchii [
37,
38]. This class also includes batoideans, which remain poorly characterized in terms of adult neurogenesis.
Considering the lack of knowledge in the context of batoidean adult neurogenesis, in this study, we decided to expand our understanding of this process in Raja asterias (R. asterias) and Torpedo ocellata (T. ocellata). R. asterias, commonly known as the Mediterranean starry ray, is a small saltwater fish that reaches a maximum length of 75 cm and is found in the Mediterranean Sea. In contrast, T. ocellata is distributed throughout the Mediterranean Sea and along the western coast of Africa, reaching a maximum length of 60 cm.
In general, batoids as rays, skates, and torpedoes are a diverse group of elasmobranchs that exhibit a dorsoventrally compressed body plan. Their nervous system organization presents a unique opportunity to study the evolution of conserved traits, such as adult neurogenesis [
44]. Studies on batoid brain morphology and function have revealed adaptations related to their ecological niches, such as larger olfactory bulbs in species inhabiting deep sea or murky waters [
45]. Further research on the nervous system of batoids, including
R. asterias and
T. ocellata, can provide valuable insights into the evolution and diversity of neural circuits in vertebrates.
The aim of this study on
R. asterias and
T. ocellata was to provide a qualitative description and characterization of adult neurogenic niches along the rostro-caudal axis of the brain and compare these with the well-described organization of adult neurogenic niches in
S. canicula. To this end, we employed immunohistochemical techniques and in situ hybridization that enabled the localization of putative neurogenic niches. As already carried out by our group for
S. canicula [
37,
38,
46,
47,
48], we utilized antibodies against proliferating cell nuclear antigen (PCNA) as markers for proliferative cells, S100B as typical glial markers, Musashi-1 (Msi1) as markers for neuronal progenitors, and phospho-H3 (pH3) as mitotic markers. Additionally, we performed in situ hybridization for
Notch1,
Notch3, and
Sox2, which are expressed by neural stem cells, as evidenced by studies performed in zebrafish. Active radial glial cells in zebrafish express both
Notch1 and
Notch3, while quiescent radial glial cells express only
Notch3; the deletion of
Notch1 affects cell division, while the removal of
Notch3 promotes proliferation [
46,
47,
48]. We used the combination of
Notch1 and
Notch3 as markers for active stem cells, while the expression of
Notch3 alone served as a marker for quiescent stem cells.
Sox2 is known to universally label neural progenitors and stem cells throughout the central nervous system [
49,
50,
51].
3. Discussion
The aim of this study was to increase our knowledge about the phenomenon of adult neurogenesis in the chondrichthyans group, with particular attention to the Batoidea group, to understand whether widespread neurogenesis could represent an ancestral trait and how neurogenic niches are shaped in response to morphogenic variations across brain macrostructure. In particular, rays were characterized by a comparatively larger telencephalon and much smaller ventricle surface as well as a larger and more folded cerebellum. T. ocellata on the other hand was characterized by the Lobus Electricus, a structure that innervates its electric organ.
3.1. Telencephalon
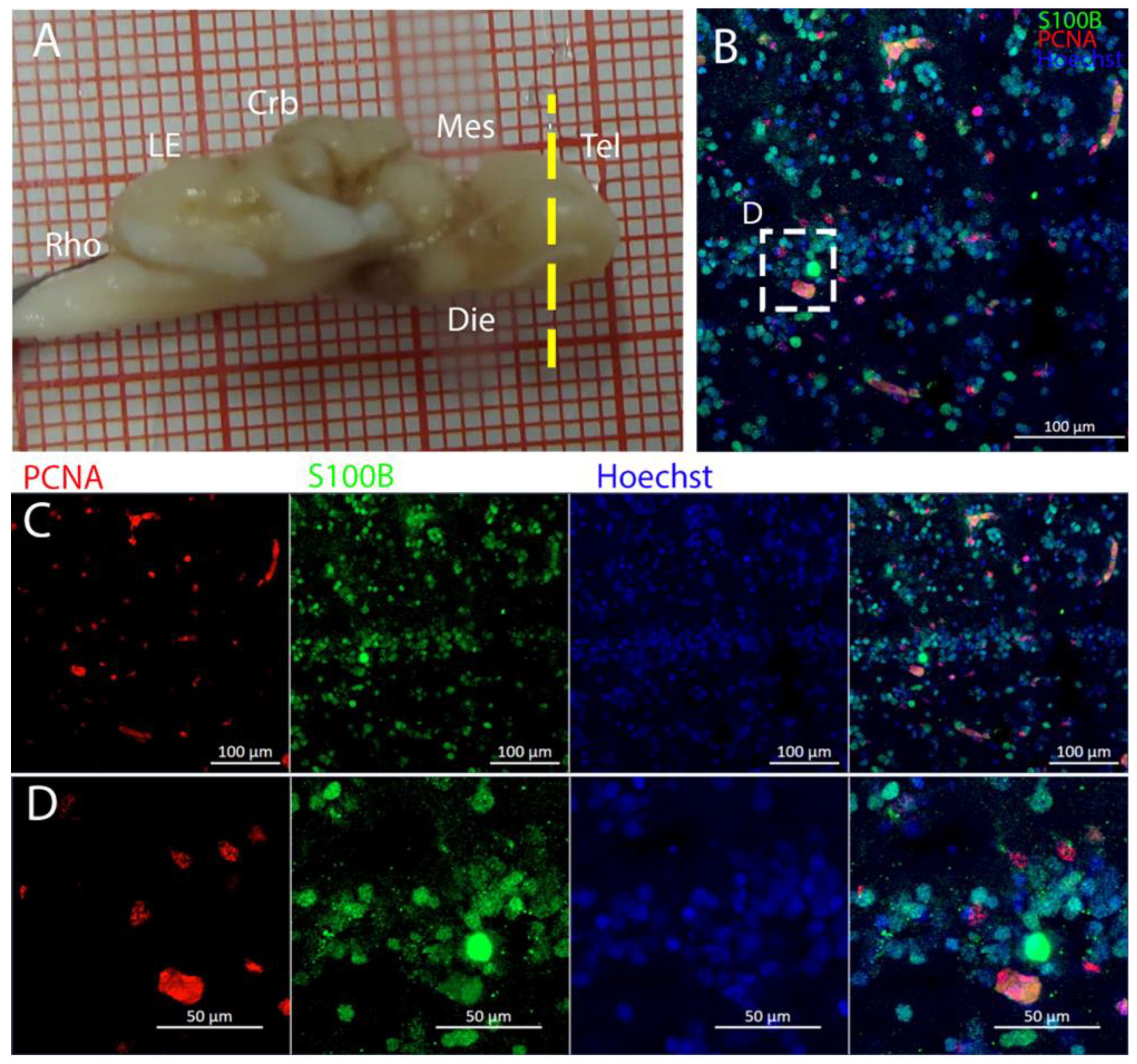
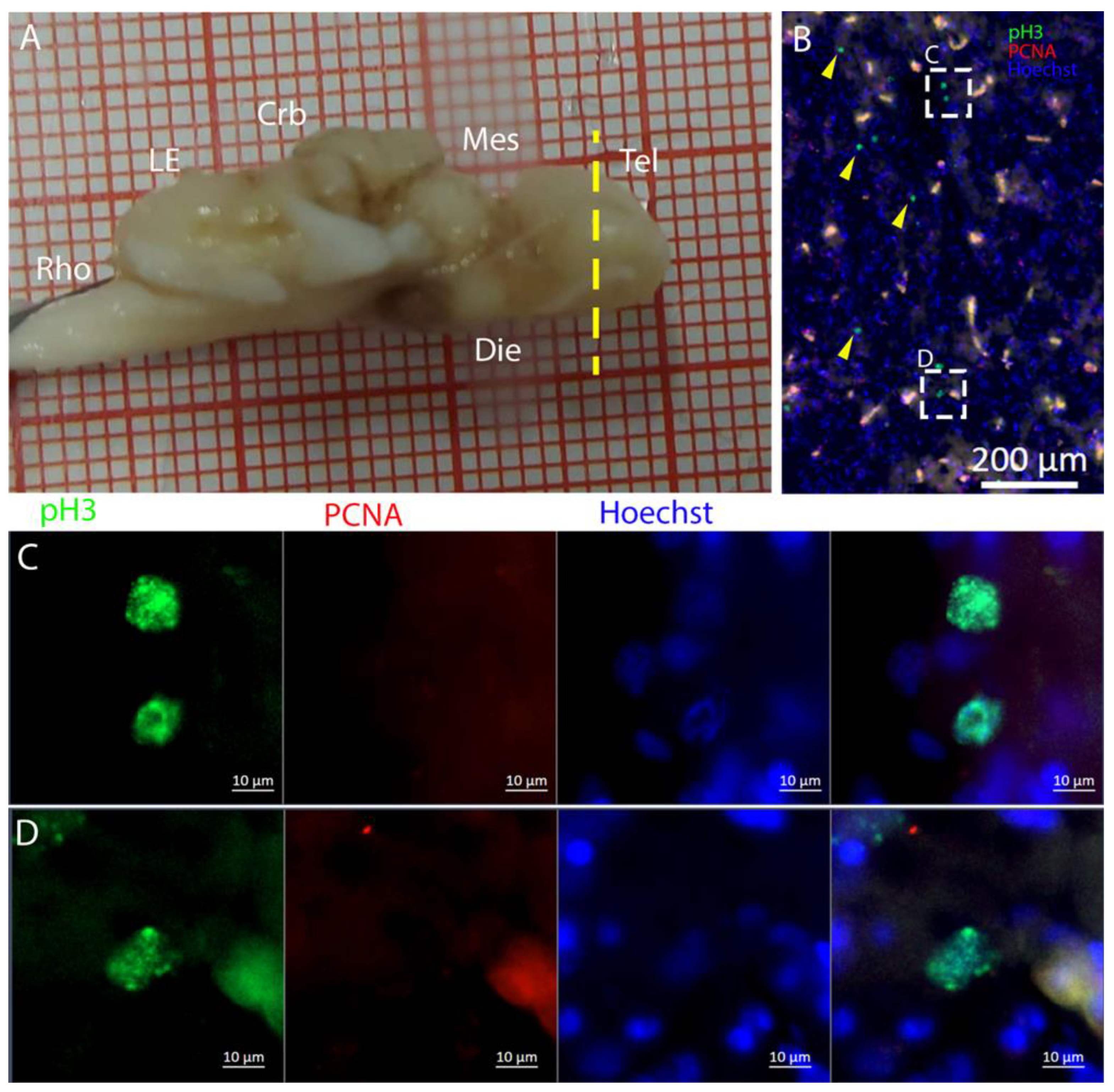
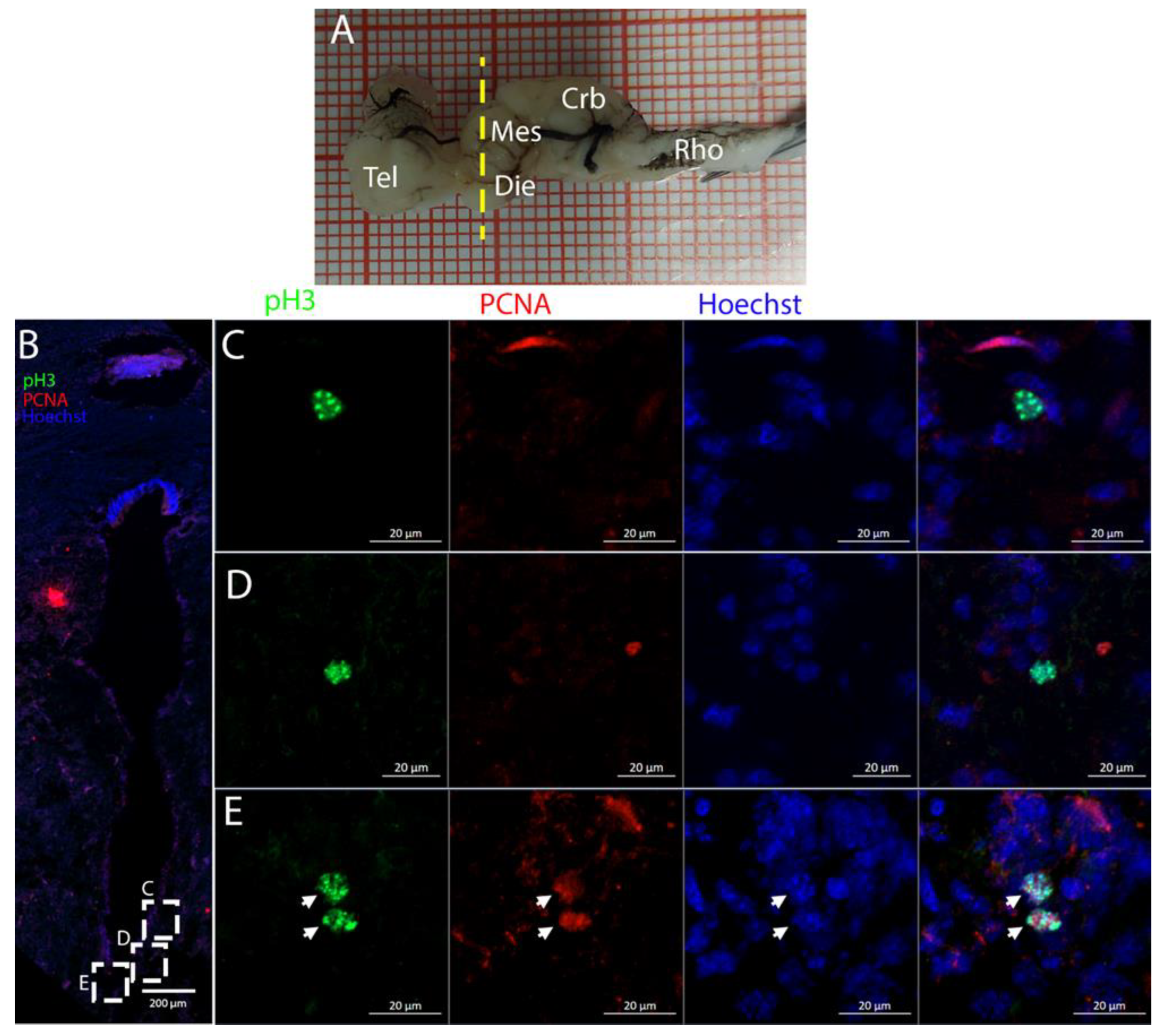
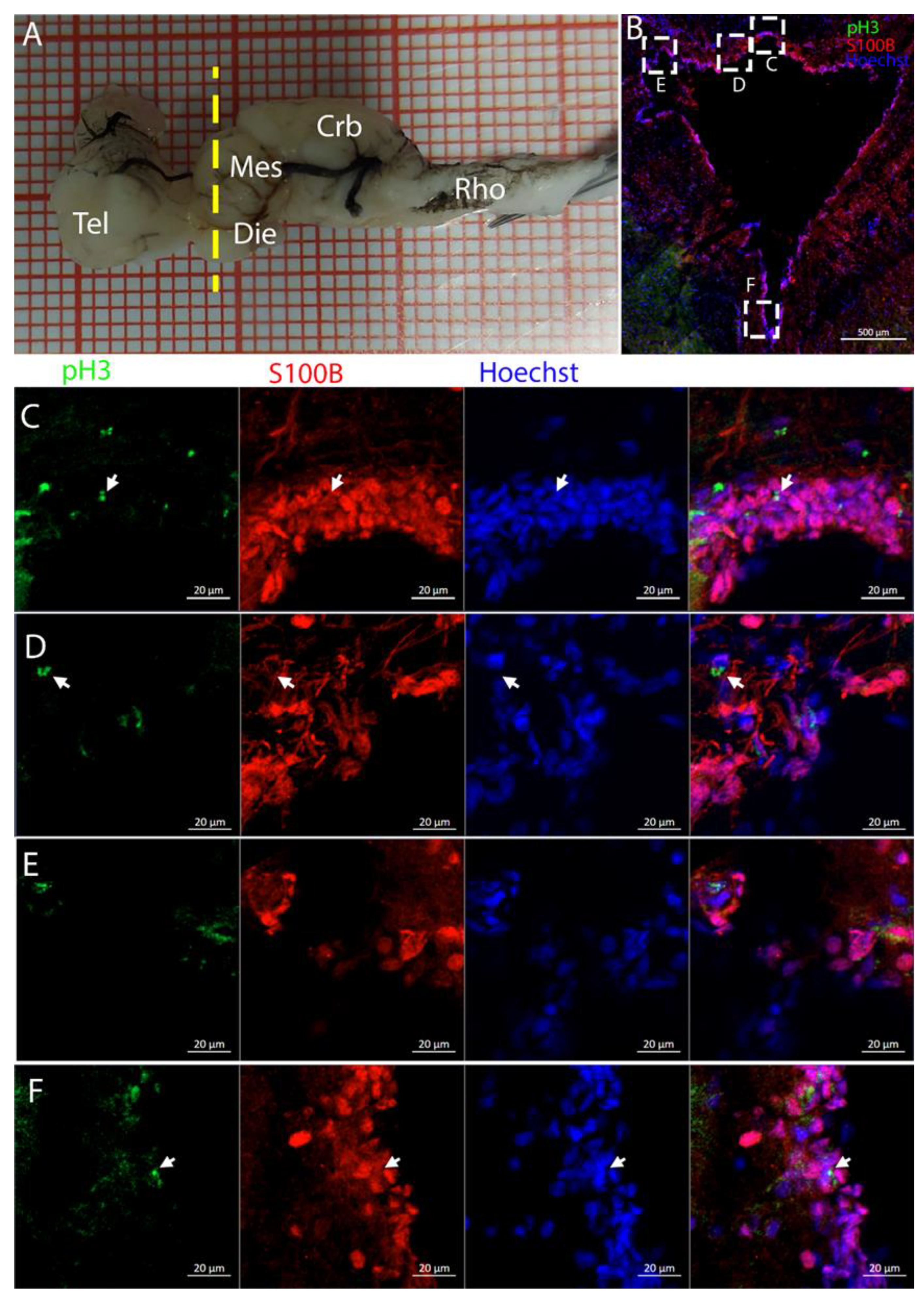
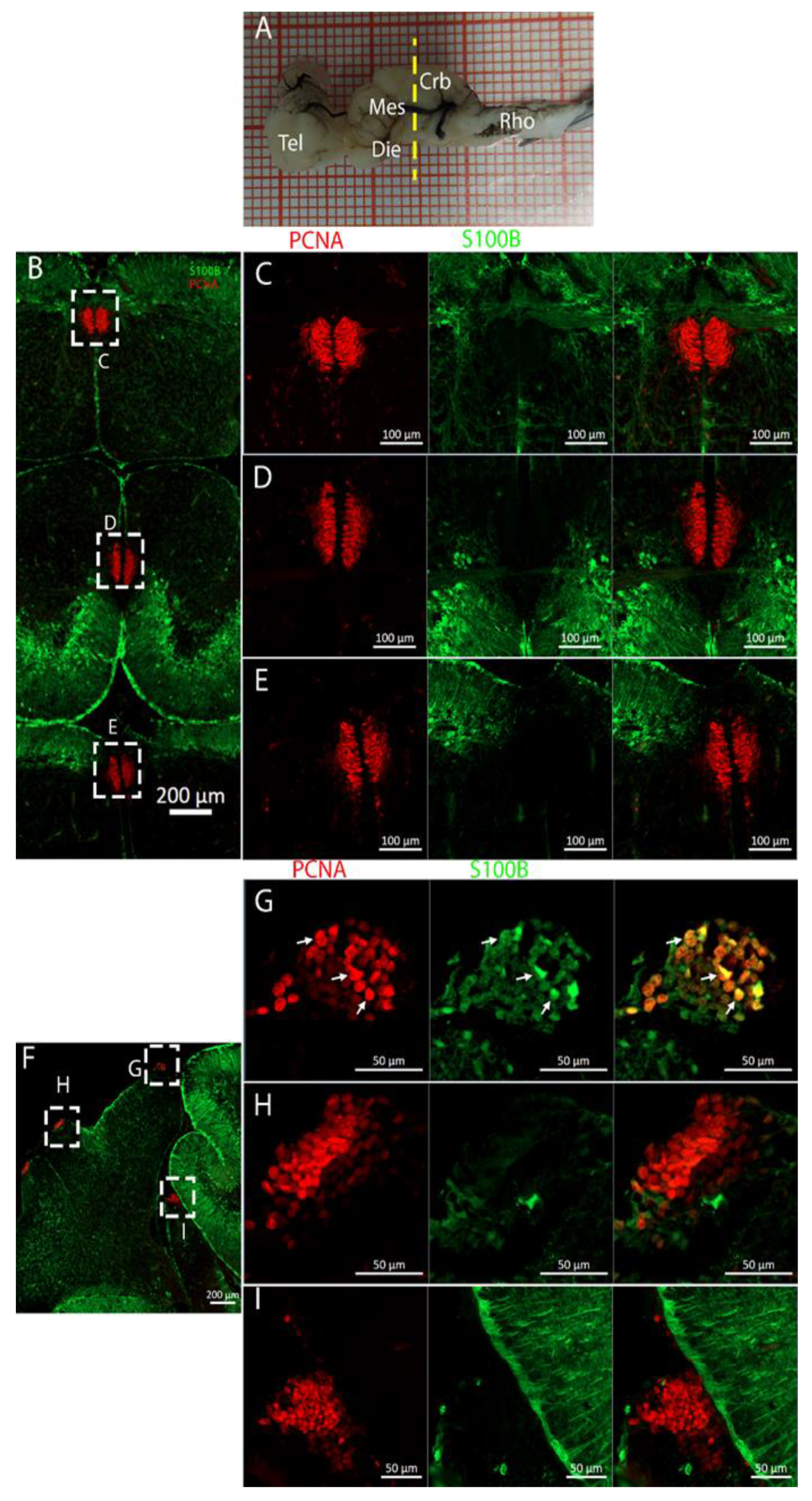
The telencephalon of both
R. asterias and
T. ocellata presented two symmetrical ventricles that appeared partially collapsed, with a reduced luminal cavity. In both species, we found the presence of neurogenic markers along the ventricular surface throughout the rostro-caudal axis, presenting positive signals for both S100B and Msi1. These cells showed very clear processes that extended from the ventricle to the parenchyma, an indication of their RG nature. To confirm the presence of actively dividing cells, we also performed staining for pH3. The presence of pH3+ cells confirmed that mitotic events occurred around the ventricles. Actively dividing cells were also present in the parenchyma, but their nature was not investigated. They could represent transit-amplifying neural progenitors, glial progenitors, or oligodendrocyte progenitors. It should be noted that the density of S100B cells in the parenchyma was much higher than in
S. canicula and S100B+ cells in rays, which have been suggested to have an astrocyte-like morphology [
50,
51]. It is possible that these astrocytes retain the possibility of dividing in the adult brain of rays, explaining the high density of dividing cells in the parenchyma. To further characterize the neurogenic niche in
R. asterias, we performed in situ hybridizations for
Notch1,
Notch3, and
Sox2. Probes were designed based on homology since, at the time, no genomic data were available for
R. asterias. We thus used reference sequences from genes of the phylogenetically close species,
Amblyraja radiata.
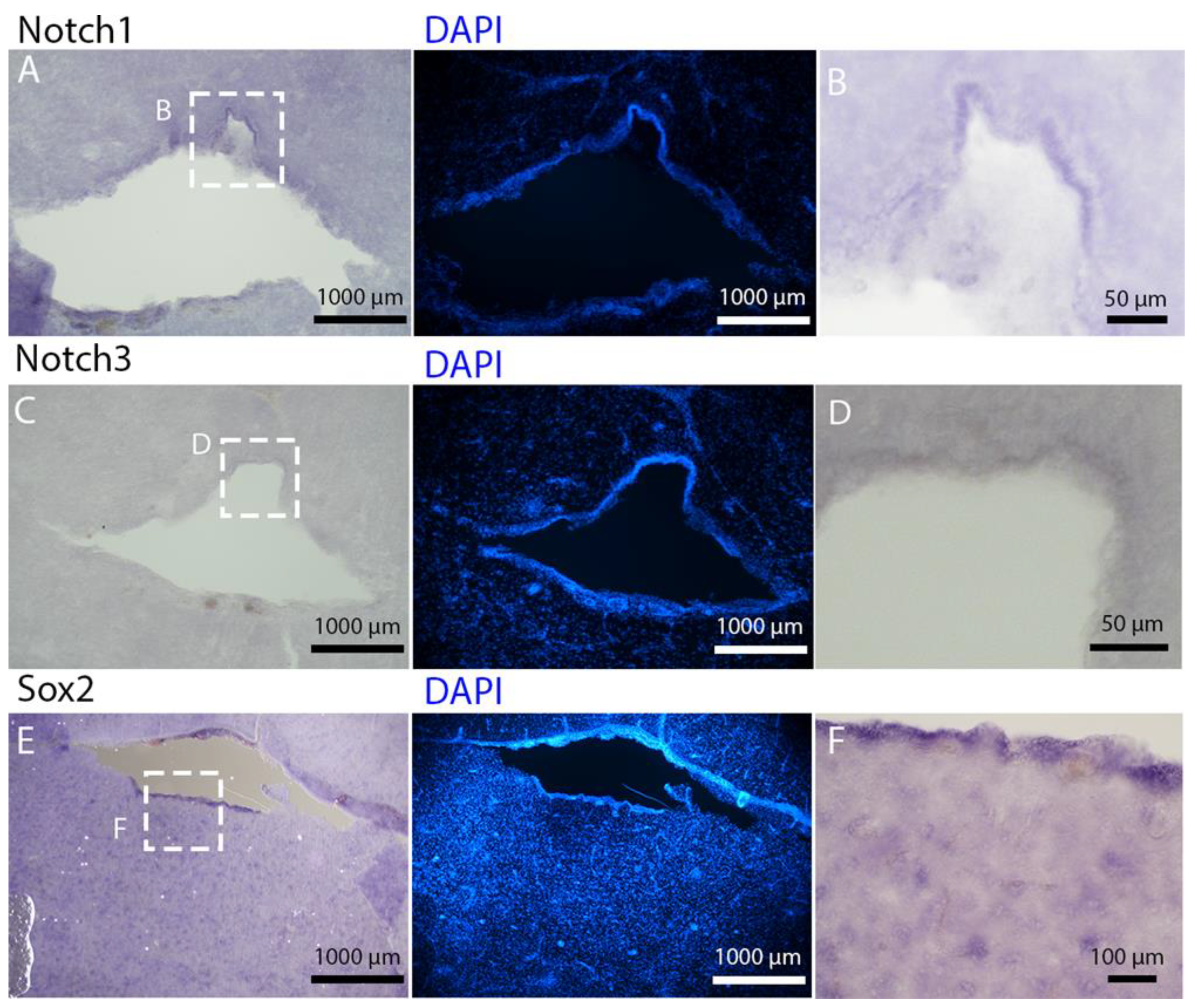
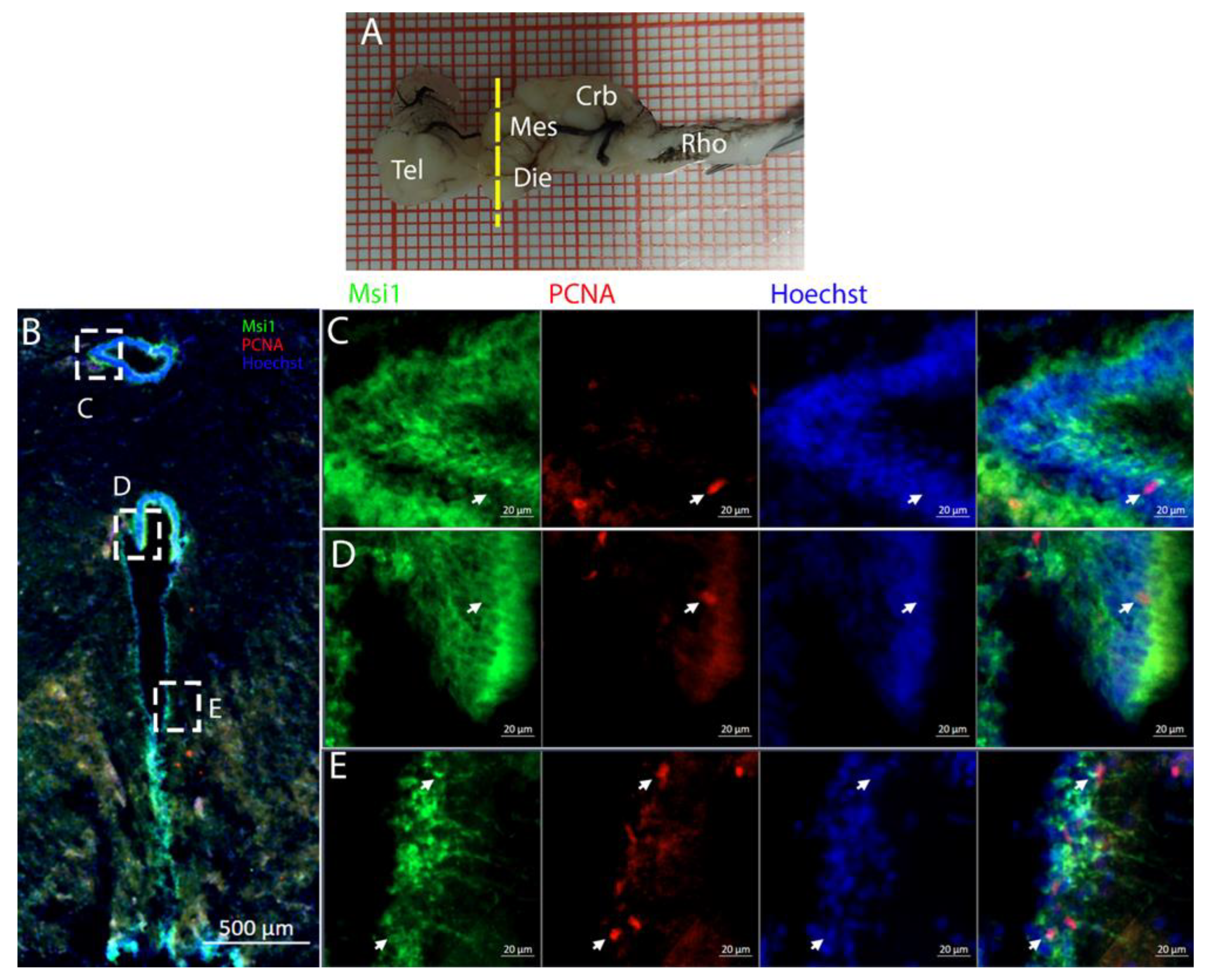
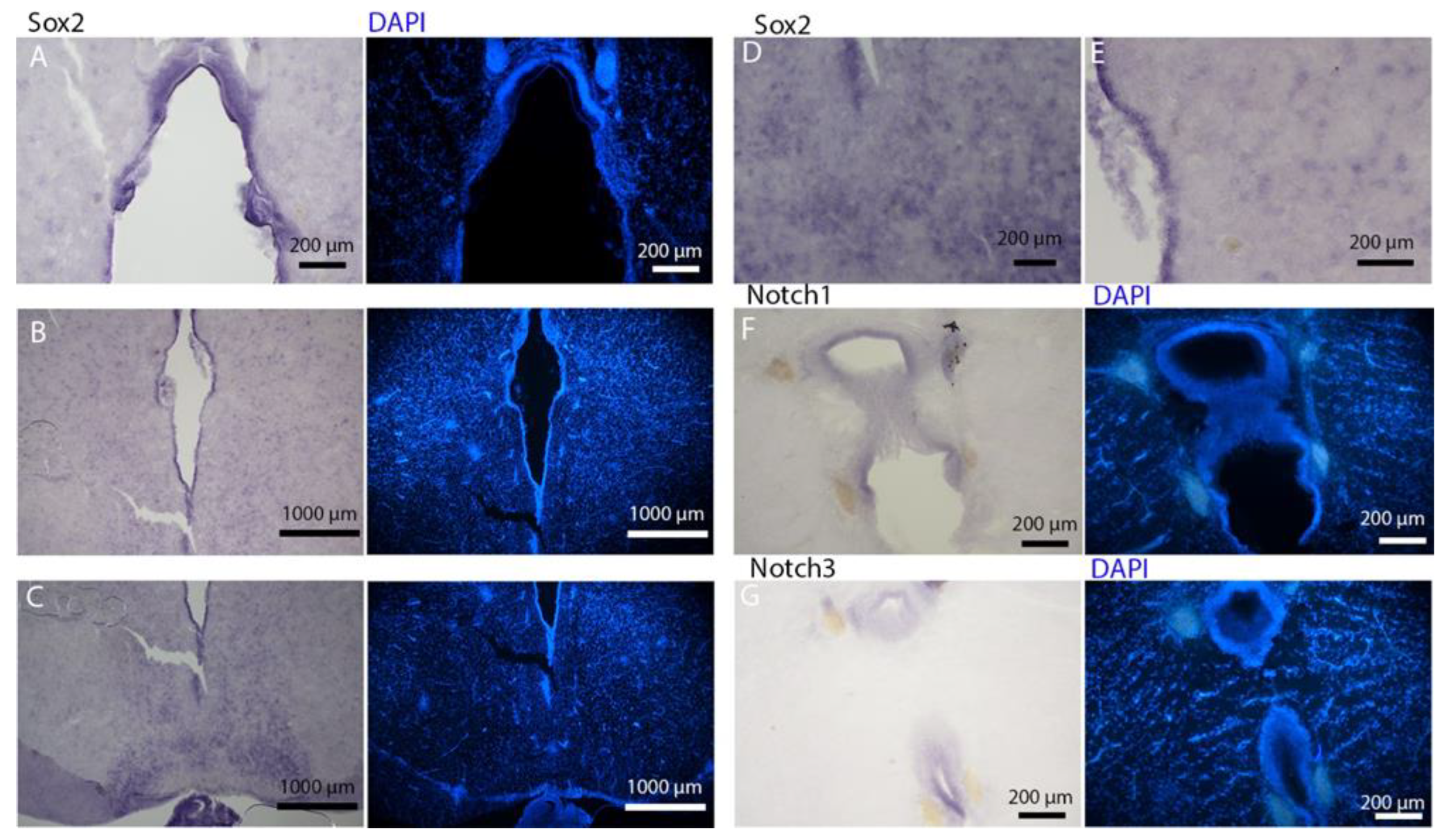
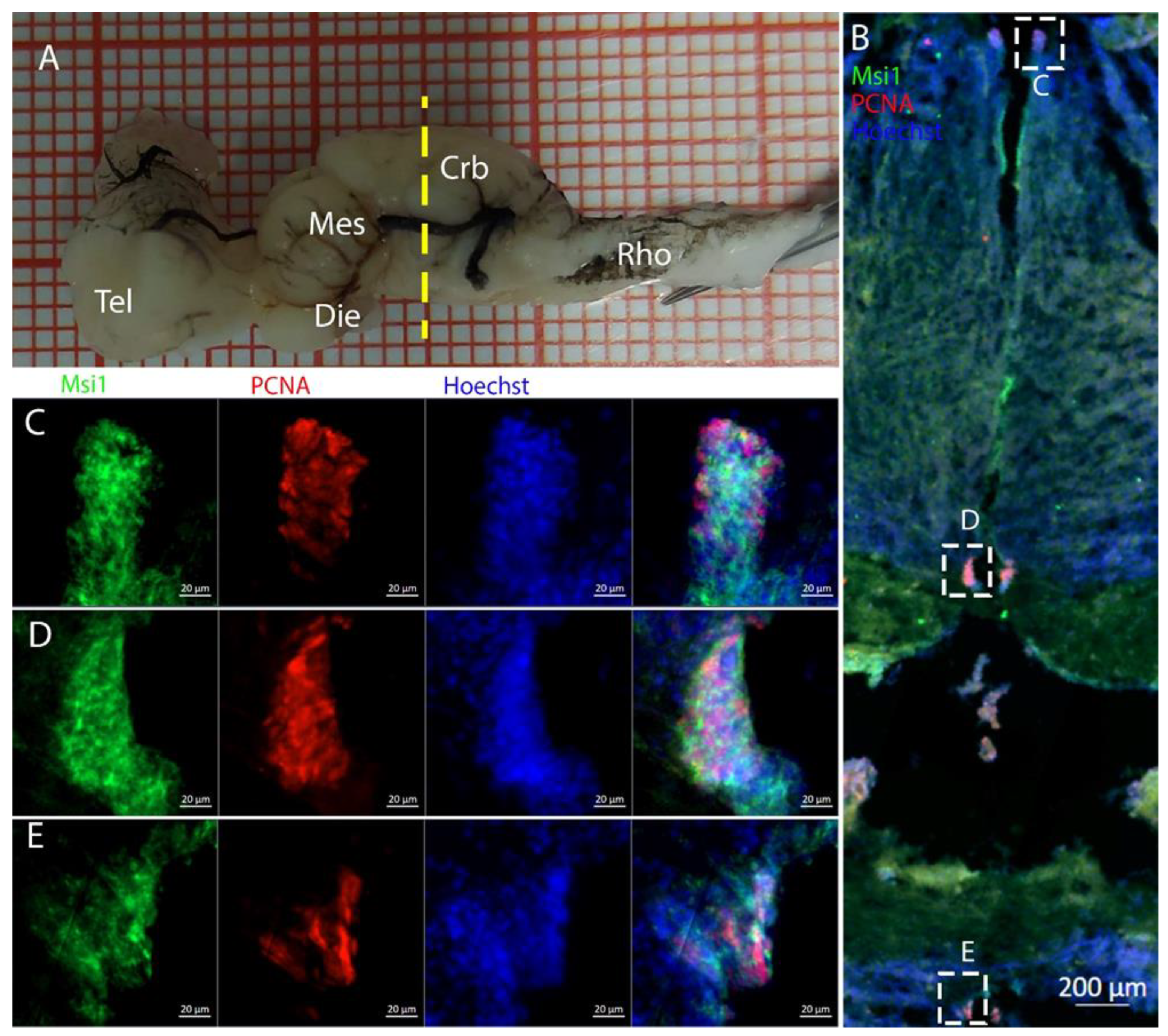
Notch1 and
Notch3 revealed a patterning of the telencephalic ventricles as they were expressed only in its dorsal part, in a spatially limited region (
Figure 6A–D), and no signal from the remaining part of the ventricle was detectable. The coexpression of
Notch1 and
Notch3 is commonly linked to active neurogenic stem cells and is present in very early precursors [
44,
45,
46]. The
Sox2 signal is also linked to neurogenic stem cells but is only expressed in RG and immature neuronal progenitors [
47,
48,
49]. The expression of
Sox2 was more distributed alongside the surface of the ventricle, confirming the stem cell nature of the cells in the subventricular zone. Together, these data indicate a difference between a more dorsal area of primordial cells expressing
Notch1 and
Notch3 and a distribution of different types of stem cells around the rest of the ventricle.
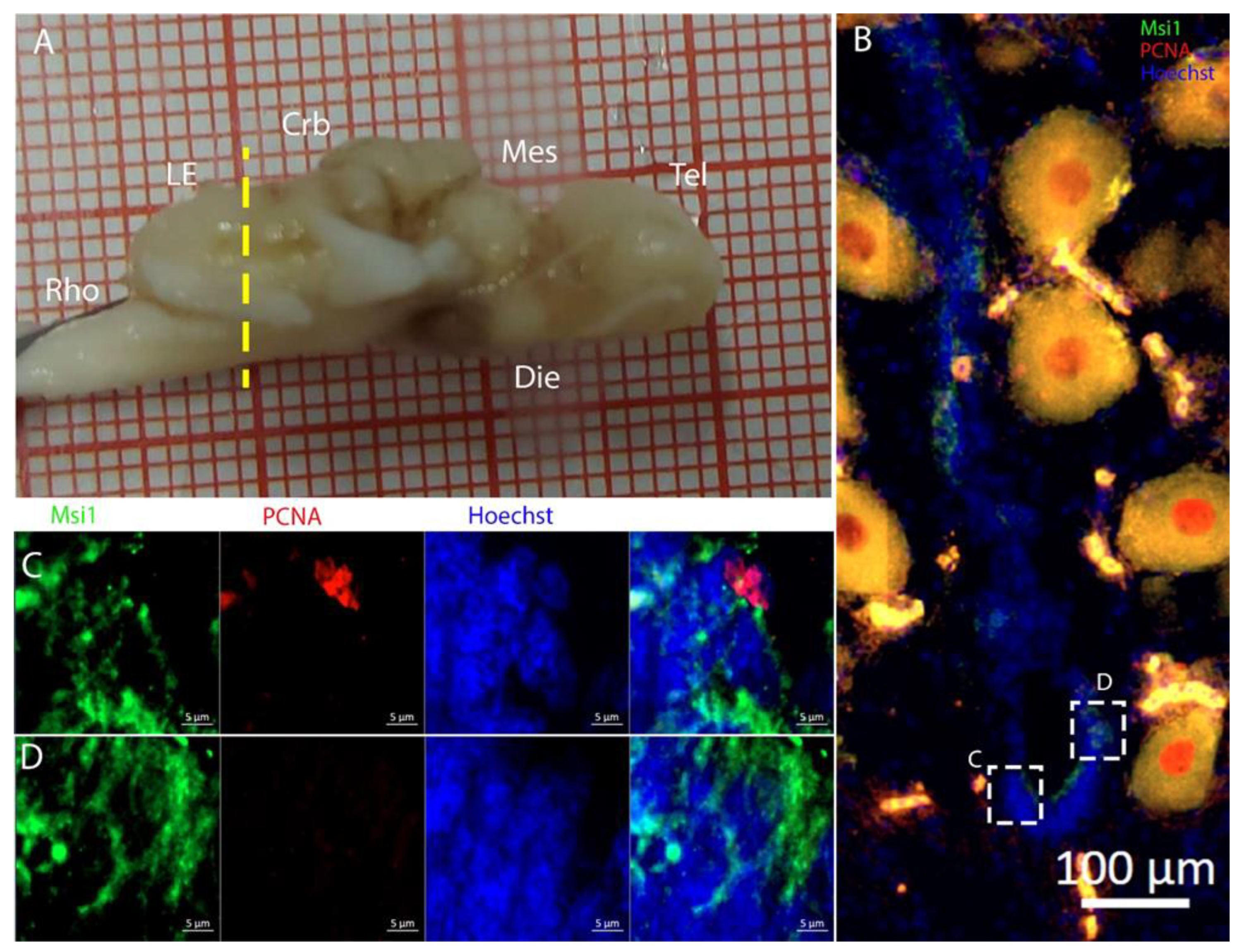
Sox2 was also detectable and abundant in the parenchyma, in line with the observations of Docampo-Seara et al. [
37], who detected
Sox2+ cells in the telencephalic parenchyma of
S. canicula. These
Sox2+ cells appear to be more abundant in rays and may represent transit progenitors or a specialized population of astrocytes. Further investigations are needed to characterize the nature of these cells. The nature of the neurogenic niche appears to be different in
T. ocellata. S100B+ cells did not present the typical shape of RG but appeared to be spherical, with no prolonged processes apart from in rare cases (
Figure 7C). There was an abundant presence of S100B+ cells along the ventricle and into the parenchyma, but only a few of those appeared to be also PCNA+. This may indicate a limited presence of actively dividing progenitors, confirmed by the limited number of pH3+ cells (
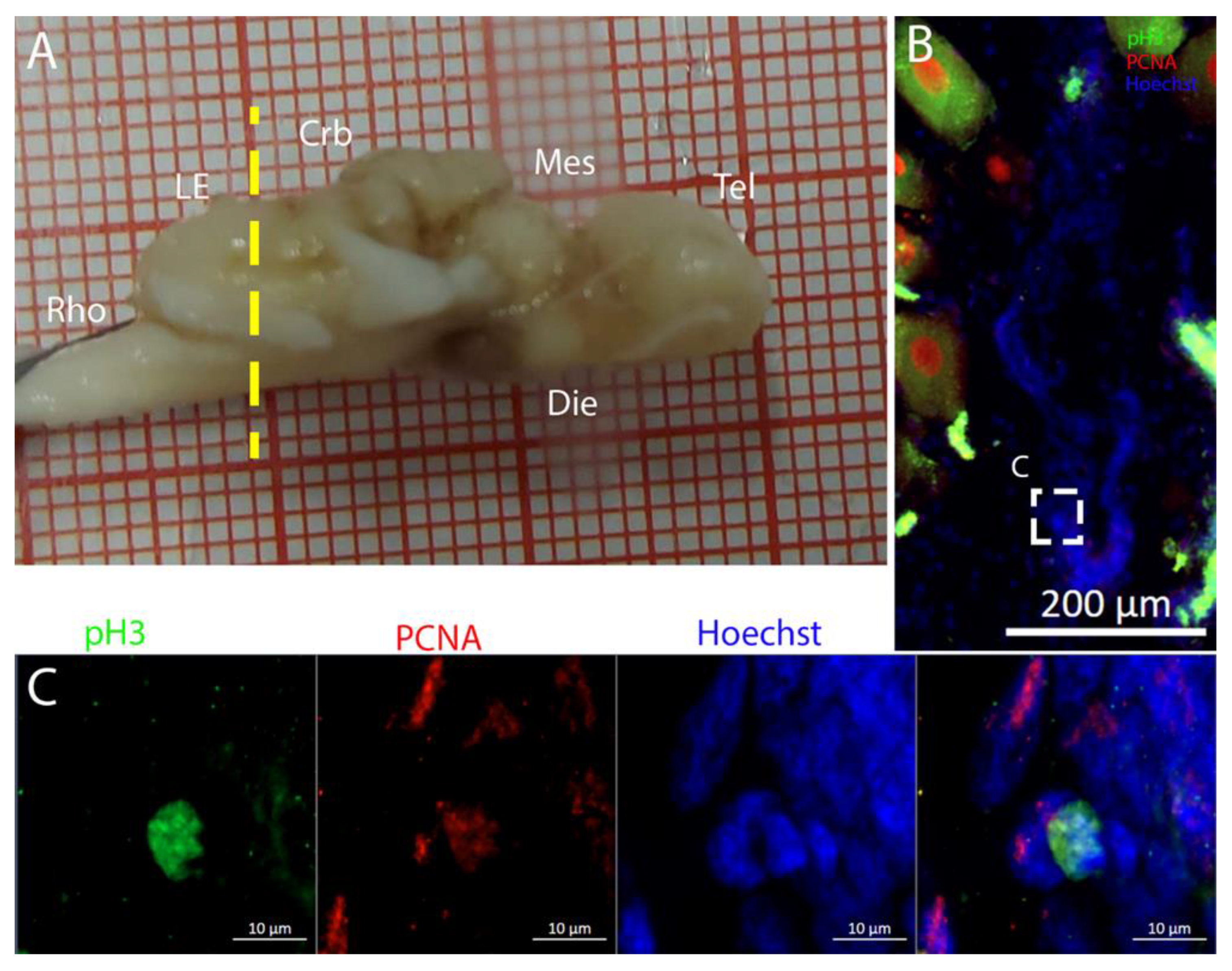
Figure 8B). As discussed for
R. asterias, the presence of pH3+ cells (both positive and negative for PCNA staining) in the parenchyma could indicate their identity as transient amplifying progenitors (TAP) or glial or oligodendrocyte progenitors. To better understand the nature of the parenchymal PCNA+ cells and their distribution, we plan to repeat and improve this characterization.
3.2. Mesencephalon
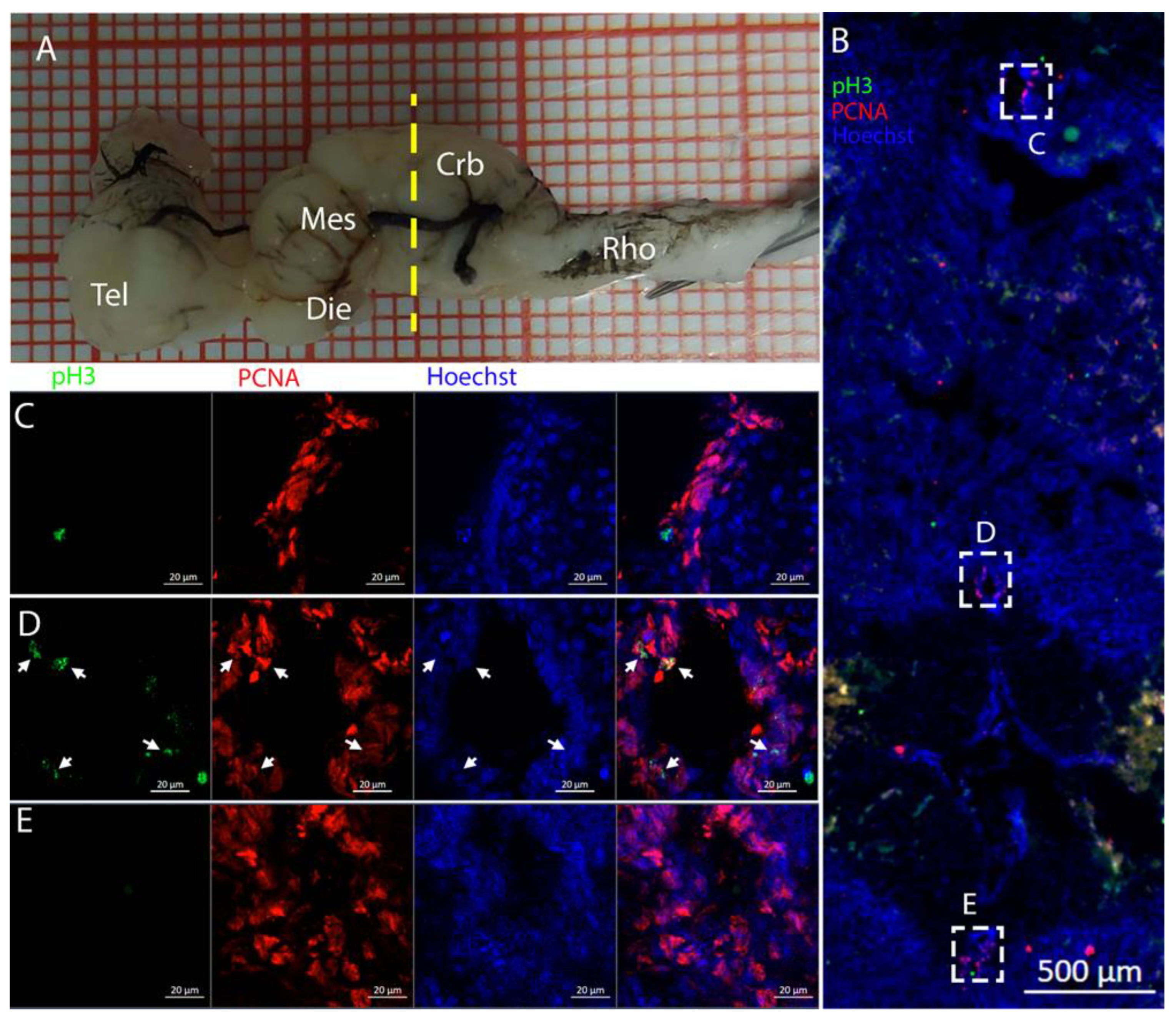
The optic tectum is a large and prominent region in this species. Unlike mammals, fish have evolved this area more extensively than the telencephalon, probably reflecting their adaptation to their ecological niche [
52]. In the mesencephalon, the situation of both species appears to be more similar: at this level, there is a big central ventricle surrounded by S100B+ cells with numerous PCNA+ cells (
Figure 9,
Figure 12 and
Figure 14). In the dorsal area in the
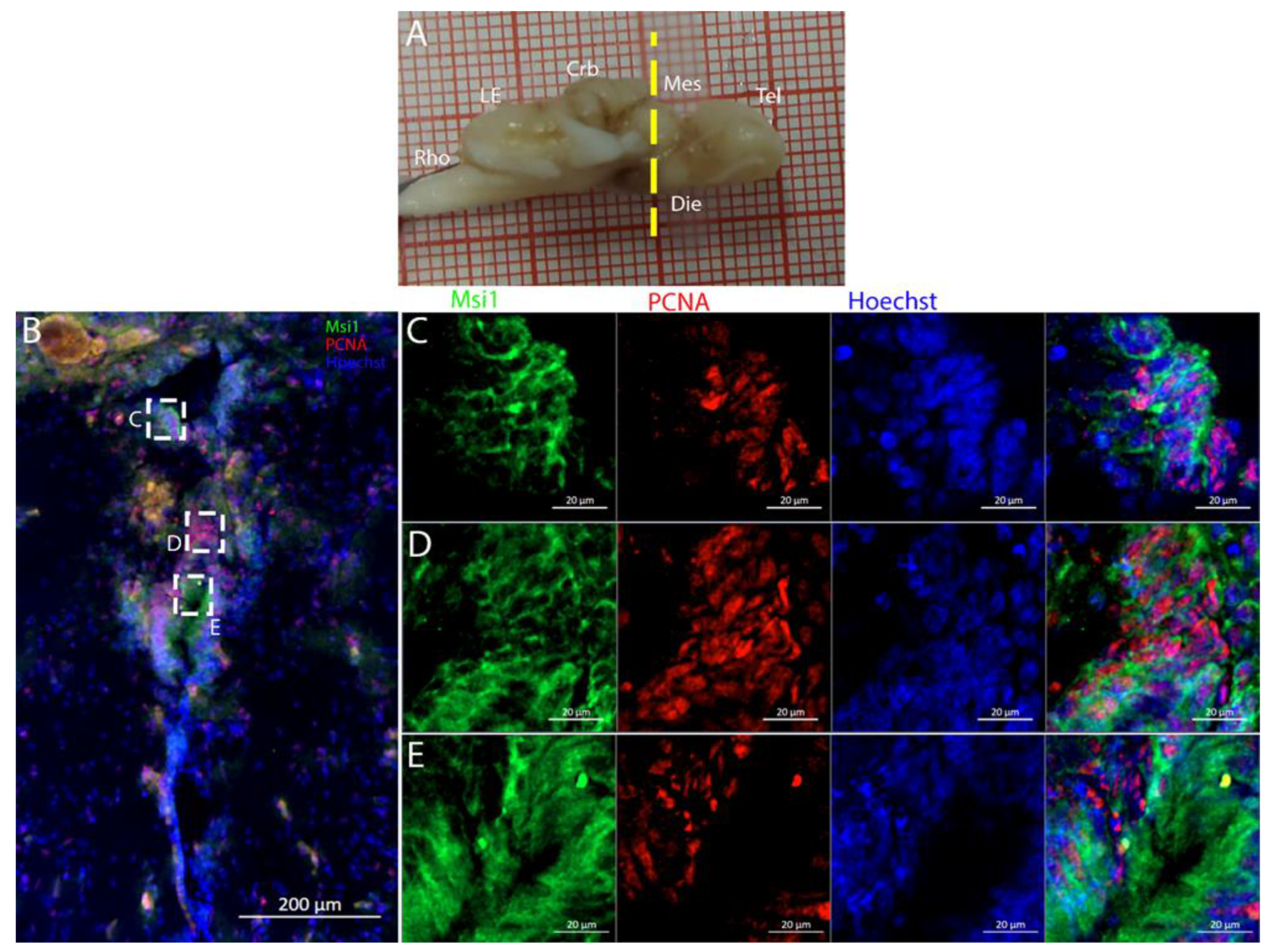
R. asterias dorsal ventricle, there was a region of high-density cells that were S100B-. We could clearly see how S100B+ cells localized all around the ventricle except for this area. Despite the absence of S100B+ cells, there were numerous PCNA+ cells, more than in the rest of the ventricular surface. These dorsally located cells were strongly Msi1+ (
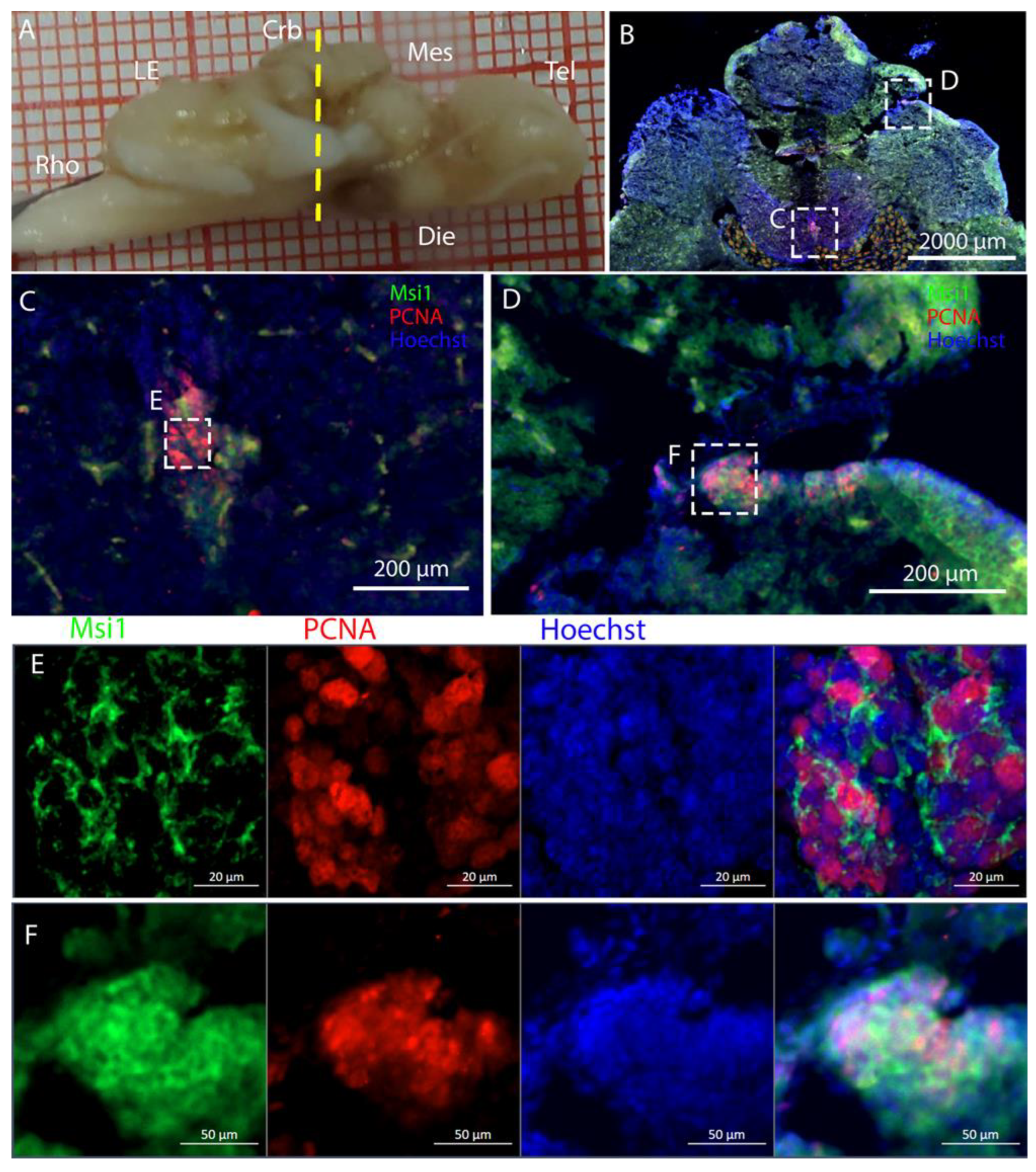
Figure 10), clearly indicating their neurogenic nature. These cells were the only cells of the niche expressing both
Notch1 and
Notch3. These cells were also positive for
Sox2+, together with the other cells localized on the rest of the ventricular surface. Considering the differential labeling of this area, the rest of the ventricular surface, and the parenchyma, we hypothesize that there is a main area of neurogenesis, sustained by a cell type different than RGs, probably made by neuroepithelial cells, as observed in
S. canicula in [
38]. This observation is sustained by the presence of S100B-/PCNA+ cells. The remaining part of the ventricle is probably composed of RGs, with them being S100B+ and positive for other markers like
Sox2 and Msi1 but negative for
Notch1 and
Notch3. Some of these cells are active and probably divide with asymmetric divisions, generating immature neuronal precursors that are visible as
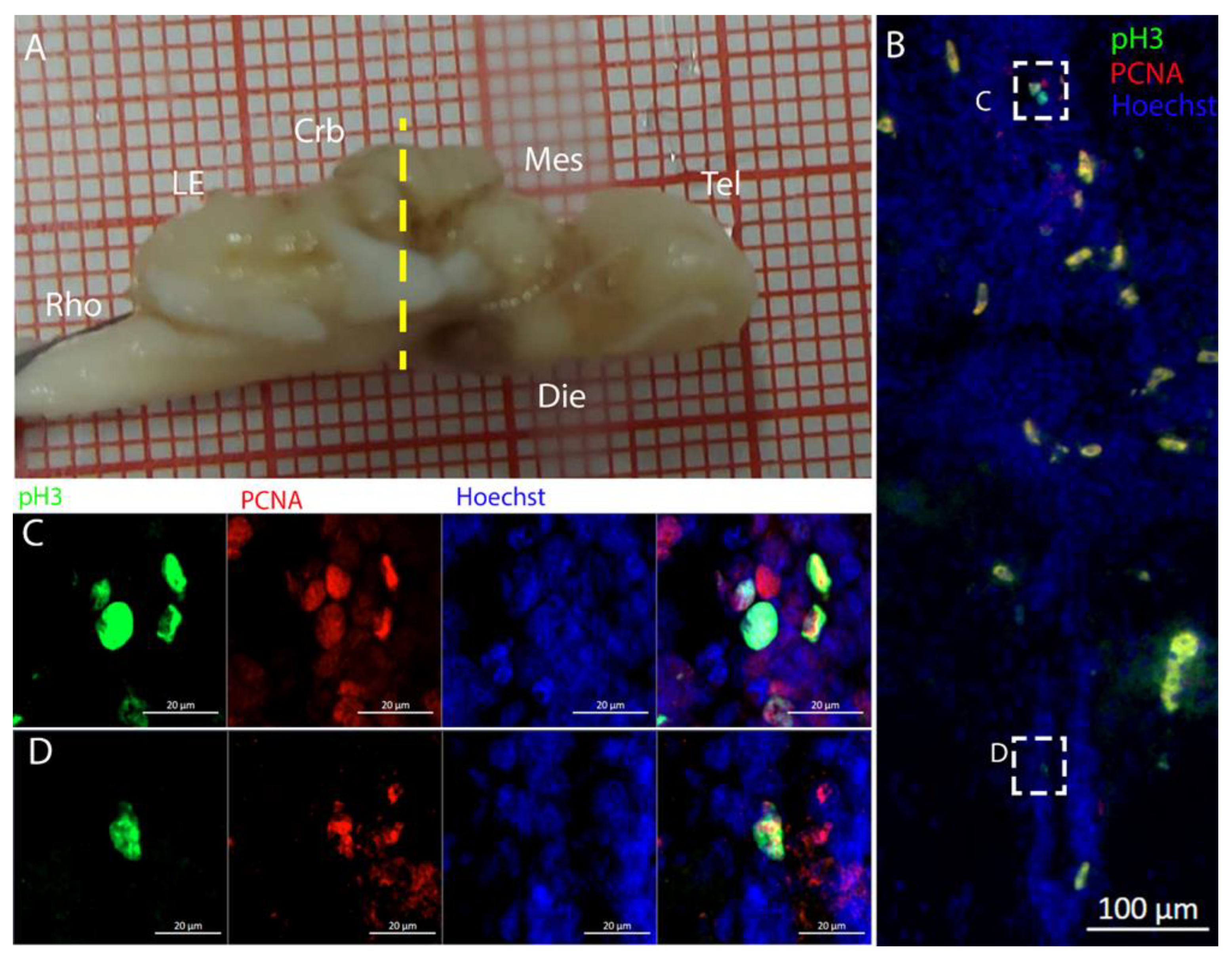
Sox2+ or PCNA+ cells in the parenchyma. The mesencephalic niche seemed to have mixed features of a neuroepithelial niche and a radial glia. Several cells were also stained for the marker of division pH3, as observed in the ventral mesencephalon (
Figure 11). In particular, the presence of two recently divided cells (
Figure 11E, white arrows) suggested a fast turnover of this proliferative cell population. The situation in
T. ocellata was different since we were not able to identify a region with densely packed cells. All the ventricular surfaces appeared to be homogeneous, with double-positive S100B+/PCNA+ cells and Msi1+ cells distributed all around the ventricle. The main difference from the
R. asterias mesencephalic ventricle was the absence of strong S100B positivity in all the ventricular cells: while all cells appeared to be Msi1+, not all of them showed the expression of S100B. This indicated that the niche was composed of a mixed population of RGs and other types of progenitor cells.
3.3. Cerebellum
The cerebellum of
Raja asterias anteriorly exhibited seven distinct neurogenic niches, with three located in the medial region and four in the lateral area. Notably, two of these niches were situated in the
Corpus Cerebelli, one was in the medial and dorsal
Octavolateralis Nuclei (MON and DON), and four were in the cerebellar auricles (
Figure 16). The identification of multiple neurogenic niches in both medial and lateral regions of the cerebellum, including those in the
Corpus Cerebelli and cerebellar auricles, underscores the complexity of the neural development of this species in these areas. The only niche where cells co-expressed PCNA and S100B was located dorsally in the dorsal auricles (
Figure 16G), while all the other cerebellar niches showed mutually exclusive staining for these markers, similar to what has been described for
Scyliorhinus canicula [
38]. These S100+/PCNA- cells resembled the morphology of the Bergmann glia, a type of radial astrocyte found in the human cerebellar cortex: this similarity is consistent with previous studies highlighting the role of Bergmann glia in guiding neuronal migration and supporting neural development in the cerebellum [
53,
54]. All PCNA+ cells in the medial line of the cerebellum were also Msi1+, indicating their role as neural progenitors and their putative neuroepithelial origin (
Figure 17). Moreover, the expression of Msi1 in niches along the medial line of the cerebellum, particularly in PCNA+ cells, indicated that Msi1 may play a role in identifying and maintaining neural progenitor cells within these regions, as observed in other chondrichthyans species [
38]. The absence of S100B staining and a wall-like, shaped, densely packed distribution of these cells reinforce the idea of their possible neuroepithelial origin. Msi1 is widely recognized as a marker for neural progenitor cells, including neuroepithelial stem cells [
55,
56,
57]. However, further investigation with additional neuroepithelial and epithelial markers—not available in our laboratory—would be necessary to confirm this observation. More posteriorly, the number of cerebellar niches increased to nine, including the bilateral auricular niches, not detectable more rostrally. The auricular niches appeared to be similar in nature to the cerebellar ones, showing the classic, mutually exclusive labeling of PCNA and S100B (
Figure 16G). We were not able to apply the in situ hybridization experiment to the cerebellum slices, so we plan to do this in the future. The presence of double-positive cells for PCNA and pH3 in the ventral cerebellar and DON niches suggests active cell division and neurogenesis in these areas. However, the absence of S100B+/pH3+ cells in the cerebellum indicated that differentiated cells may not have been actively dividing, consistent with their role in supporting neural function rather than proliferation (
Figure 18). For technical reasons, we could not observe S100B+ cells in the cerebellum of
T. ocellata. and plan to collect these data in the future. We did collect evidence of labeling for Msi1+, PCNA+, and pH3+. At this level, the ventricular structure became smaller but kept the same characteristics as before. Cells around the ventricle were Msi1+, with a high concentration of PCNA+ cells (
Figure 19) and pH3+ cells (
Figure 20), which demonstrated how, even if the niche was smaller, it was still perfectly active. Comparing the cerebellar structure of
R. asterias and
T. ocellata, we observed differences in the distribution and number of neurogenic niches. While
R. asterias exhibited multiple niches in both the
corpus cerebelli and cerebellar auricles,
T. ocellata showed a more restricted pattern, with niches primarily in the DON and auricular regions. This variation highlights species-specific adaptations in neural development and organization. At the very posterior end of the
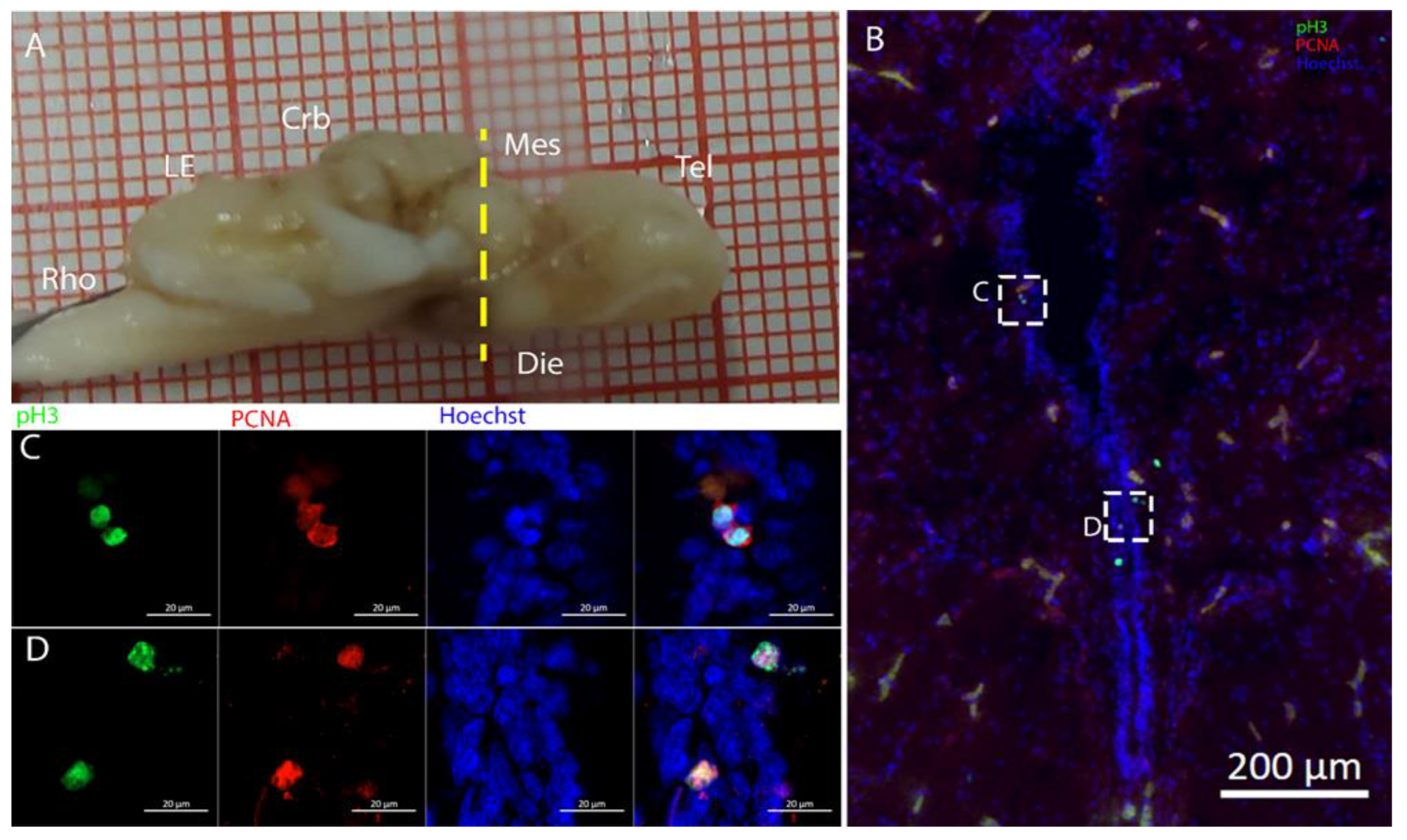
T. ocellata brain, in the
Lobus electricus (LE), a little ventricle was still present, and it maintained positivity to the Msi1 signal. We were even able to find a pH3+/PCNA+ cell, a sign that, even if in smaller proportions, adult neurogenesis is still present in the most caudal region of the
LE (
Figure 21 and
Figure 22).
3.4. Conclusions and Perspectives
This study provides valuable insights into the phenomenon of adult neurogenesis in chondrichthyans, with a particular focus on the Batoidea group, including Raja asterias and Torpedo ocellata. Our findings suggest that adult neurogenesis in these species is widespread and potentially represents an ancestral trait within the Chondrichthyan lineage. We observed distinct neurogenic niches in various regions of the brain, including the telencephalon, mesencephalon, and cerebellum, with significant variations between species.
In R. asterias, neurogenic activity was prominent across the telencephalon, mesencephalon, and cerebellum, with a marked presence of neurogenic markers such as S100B, Musashi1 (Msi1), and Sox2. The high density of S100B+ cells in the parenchyma, coupled with active cell division, suggests that these cells may contribute to maintaining neural plasticity in the adult brain. Additionally, the multiple neurogenic niches in the cerebellum, particularly in the corpus cerebelli and auricles, further emphasize the complexity and plasticity of neural development in R. asterias.
In contrast, T. ocellata exhibited a more restricted neurogenic pattern in the cerebellum, with fewer niches primarily in the dorsal octavolateralis nucleus and auricular regions. Despite a smaller number of niches, T. ocellata still showed active neurogenesis, as evidenced by the presence of Msi1+, PCNA+, and pH3+ cells. The existence of neurogenic activity in the caudal region of the Lobus electricus, as evidenced by the presence of pH3+/PCNA+ cells, suggests that neurogenesis may play a role even in specialized structures such as the electric organ.
These findings highlight both conserved and species-specific features of adult neurogenesis in chondrichthyans and provide a foundation for future studies aimed at better understanding the cellular and molecular mechanisms underlying neurogenic processes in these ancient vertebrates. Further investigations, particularly in the characterization of different progenitor cell populations and the functional significance of these neurogenic niches, will be essential to fully elucidate the role of neurogenesis in brain plasticity and adaptation across species.
Future studies—which are beyond the scope of the present manuscript—could focus on identifying the molecular pathways that regulate neurogenesis in chondrichthyans, including the role of key signaling pathways such as Notch, Wnt, and fibroblast growth factors in controlling neural progenitor cell behavior. Understanding the interactions between these pathways will be crucial for deciphering the mechanisms that guide the maintenance and differentiation of progenitor cells in the adult brain.
Moreover, examining the functional significance of neurogenesis in different brain regions will provide insights into the ecological and behavioral adaptations of Chondrichthyan species. For instance, how neurogenesis contributes to sensory processing in species like T. ocellata, with its electric organ, could lead to a better understanding of how neurogenesis is adapted to specialized brain functions.
Additionally, comparative studies across other Chondrichthyan species and different vertebrate groups will help clarify the evolutionary conservation, or rather the diversity of neurogenic processes. By identifying conserved markers and cell types across species, future research could explore how these mechanisms evolved and how they contribute to brain plasticity in response to environmental demands.
In parallel, further research into the spatial organization and lineage of neural precursors in these species could provide new insights into the dynamics of brain regeneration and plasticity. This is particularly relevant in the context of understanding how neurogenic niches respond to injury or other environmental challenges, which could open up avenues for regenerative medicine applications. Ultimately, these studies could provide new perspectives on the potential for harnessing the regenerative capabilities of the adult brain, not only in chondrichthyans but also in other vertebrate groups, including humans.
4. Materials and Methods
4.1. Tissue Collection and Preparation
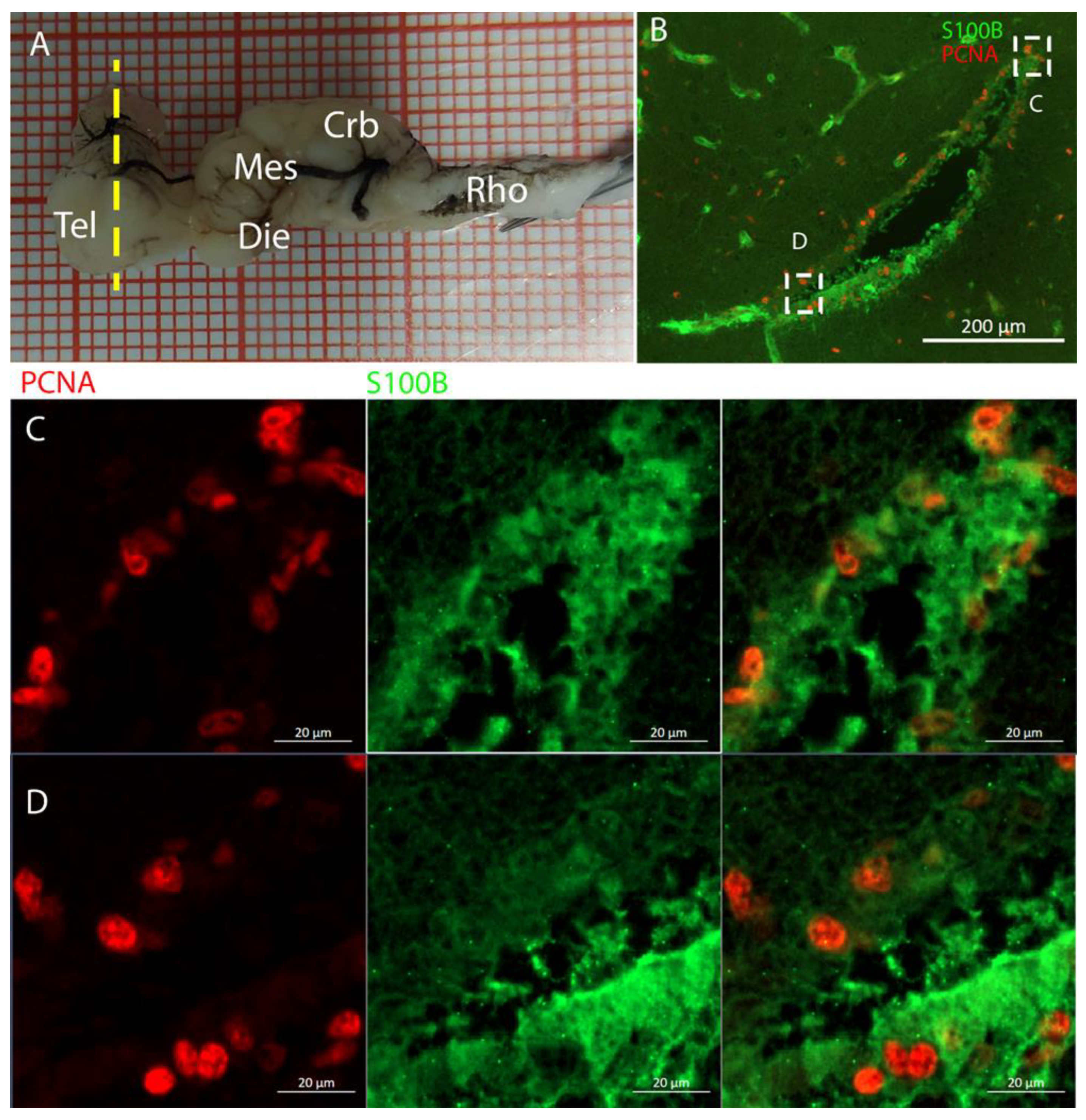
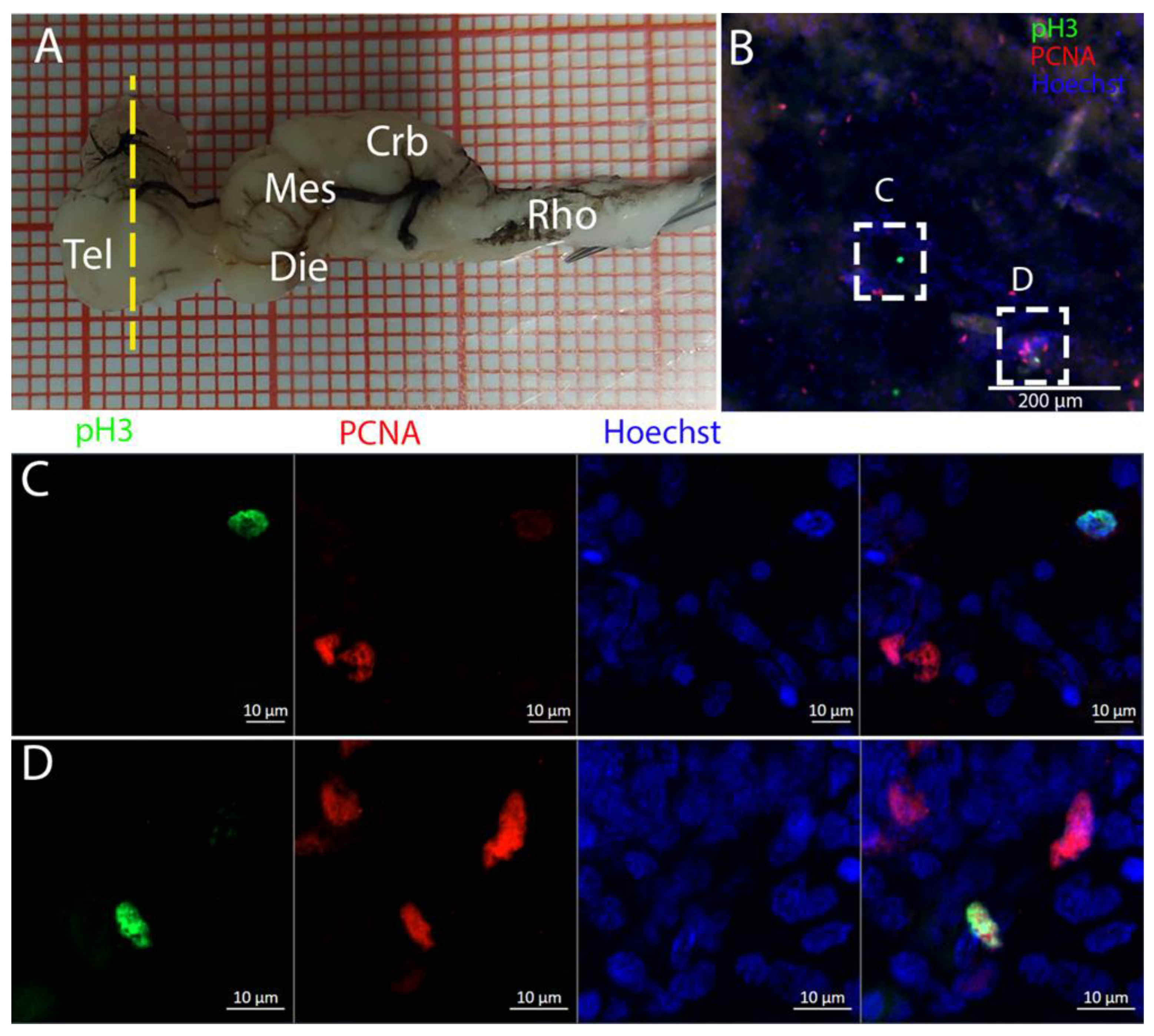
Adult specimens of R. asterias and T. ocellata were provided fresh by local fishermen. Brains were dissected immediately and fixed in 4% PFA (paraformaldehyde) overnight, in accordance with the approval of the Italian Ministry of Health (cod. B290E.N.TU2). After 24 h in 4% PFA, tissues were transferred to 30% sucrose for cryoprotection and allowed to equilibrate for 24–48 h at 4 °C. Following this period, brains were embedded in OCT (Tissue-Tek O.C.T. Compound; Sakura Finetek, Venice, Italy) and snap-frozen in isopentane. Once embedded, we sectioned the brains into 45 µm slices using a Leica cryostat and mounted them onto Superfrost Plus glass slides (Thermo Fisher Scientific, Monza, Italy)
4.2. Total RNA Extraction and cDNA Preparation
We used the RNeasy Mini Kit (Qiagen, Milan, Italy) for total RNA extraction, according to the manufacturer’s protocol. We quantified RNA using an FC-3100 (Nanoready, Hangzhou city, Zhejiang, China) spectrophotometer and checked the quality using agarose gel in RNAse-free conditions. From the total RNA, we performed retrotranscription, synthesizing cDNA using the Reverse Transcriptase Core Kit (Eurogentec, Segrate, Italy).
4.3. DIG-Labeled Riboprobe Synthesis
Templates for R. asterias Notch1, Notch3, and Sox2 were obtained from cDNA using forward, Fw: 5′-ACGCTGTGAAATGGACATCA-3′ and reverse carrying a T7 promoter sequence on its 5′ end, Re: 5′-TAATACGACTCACTATAGGGCGTTCTGACAGGGTTGACTC-3′ for Notch1; forward Fw: 5′-GGATCTGGTGAACAAGTACA-3′ and reverse with T7 promoter, Re: 5′-TAATACGACTCACTATAGGGAATCAGGACGTTCTCAC-3′ for Notch3; and forward Fw 5′-CAAGATGCACAACTCGGAGA-3′ and reverse with T7 promoter, Re: 5′-TAATACGACTCACTATAGGGTCCAAGTTCTGTGCTTTGCT-3′ for Sox2. PCR products were then purified with the Wizard® SV Gel and PCR Clean-Up System (Promega, Milano, Italy) and verified by Sanger sequencing (Eurofins Genomics, Milano, Italy). We used 50 ng of PCR as a template to transcribe DIG-labeled probes using the T7 polymerase (Thermofisher Scientific, Monza, Italy) and digoxygenated RNTP mix (Roche, Darmstadt, Germany) for 2 h at 37 °C. The resulting DIG-labeled riboprobe was precipitated with 1/10 of the volume of 5 M LiCl and 2.5 volumes of cold ethanol at −20 °C overnight, washed with 70% cold ethanol, resuspended in nuclease-free water, and stored at −80 °C.
4.4. Free-Floating In Situ Hybridization (ff-ISH)
ff-ISHs were performed according to [
58], with some modifications, as explained in the following paragraph. Briefly, sections (100 µm) were rehydrated in PBS (phosphate buffered saline), detached from the glass slice, and recovered in a 2 mL safelock tube (one section each). Sections were directly prehybridized for 30 min at 66 °C and then incubated with a digoxigenin DIG-labeled probe at 66 °C overnight. Immediately before incubation, the probe was denatured at 80 °C for 3 min. Sections were washed twice for 15 min at 66 °C, first with 2× SSC and then with 0.2× SSC. Sections were then treated with TMN solution (Tris-MgCl2-NaCl buffer) 3 times for 5 min and then stained with BM-Purple (Roche, Darmstadt, Germany). The staining was constantly monitored under a stereomicroscope (M80 Leica, Wetzlar, Germany) equipped with an LED-light O-ring and blocked with 1% PBST (1× PBS + 1% Triton X-100). Once the color was fully developed, sections were postfixed in 4% PFA overnight at 4 °C and coverslipped.
4.5. Double Immunofluorescence
To perform double immunofluorescence staining, we began by rinsing the slides with 1× PBS and then proceeded with acid antigen unmasking (citrate buffer, pH 6) for three minutes. The tissue was then blocked with a solution containing 5% BSA (bovine serum albumin) and 0.3% Triton-X 100 in 1× PBS for two hours at room temperature. We incubated the slides overnight at 4 °C with a combination of primary antibodies, each at its proper dilution (
Table 2). The following day, we rinsed the slides with PBS 1× three times and incubated them with the required combination of secondary antibodies, diluted 1:500, for two hours at room temperature. We then rinsed the sections again three times in 1× PBS 1×, and nuclei were counterstained with Hoechst 33342 (Invitrogen, Waltham, MA, USA), diluted 1:5000 in 1× PBS for one minute. Finally, the slides were mounted with Fluoroshield mounting medium (Sigma, St. Louis, MO, USA). All incubations were performed in a humid chamber.
4.6. Immunofluorescence Using Monovalent Fab Fragment Secondary Antibodies
To use two primary antibodies generated from the same host species simultaneously, we utilized monovalent Fab fragments (AffiniPure Fab Fragment, Jackson ImmunoResearch, West Grove, PA, USA) (
Table 2). These monovalent fragments block immunoglobulins, allowing the use of a second primary antibody from the same host. This process involved two successive steps: we first applied the first primary antibody and blocked it with Fab fragments; then, we added the second primary antibody and labeled it with a conventional secondary antibody. The process is similar to standard double immunofluorescence but with a few differences. After incubating the tissue overnight at 4 °C with the first primary antibody and removing the unbound leftover the following day, we applied the appropriate Fab fragment (instead of the secondary antibody) at a 1:400 dilution. After a 2 h incubation at room temperature, we rinsed the Fab fragments and incubated the antibody overnight at 4 °C with the second primary antibody. The following day, we rinsed off the antibody and incubated the tissues with the appropriate secondary antibody, following the standard double immunofluorescence protocol.
4.7. Whole Brain Immunofluorescence
The raja asterias whole brain was dissected, fixed in 4% PFA, dehydrated with increasing concentrations of ethanol (i.e., 50% and 70%), and stored at 4 °C until processing. Consequently, the brain was dehydrated in 100% ethanol for 1 h in agitation, chilled in ice for 20 min, and left in agitation at 4 °C in 66% DCM (dichloromethane)/33% ethanol overnight for lipid removal. The next day, it was rehydrated gradually at 80%, 60%, 40%, and 20% for 1 h at room temperature. Once hydrated, the brain was washed with 1× PBS and incubated in 2% PBSGT (1× PBS, 0.2% Gelatin, 2% Triton X-100) at 37 °C for two days. At the end of the blocking process, the brain was incubated with the primary antibodies (anti-PCNA (M0879)) at the proper dilution (
Table 2) in 2% PBSGT in agitation at 37 °C for five days. The brain was rinsed six times with 1% PBST for one hour at room temperature and then incubated in 1% PBSGT (1× PBS + 0.2% gelatin + 1% Triton X-100) with the conjugated secondary antibodies for three days at 37 °C. The remaining unbounded antibodies were washed away six times with 1% PBST and left in 1% PBST overnight. In order to clarify the brain, the sample was dehydrated with increasing concentrations of ethanol (20%, 40%, 60%, 80%, and 100%) and incubated with 66% DCM (dichloromethane)/33% ethanol overnight. The next day, the brain was washed twice with 100% DCM for 20 min, transferred to a dark glass tube with 100% DBE (dibenzylether), and incubated at room temperature overnight. On the final day, the DBE was exchanged twice with MACS
® Imaging Solution (catalog n° 130-128-511, Miltenyi, North Rhine-Westphalia, Germany) and incubated for three hours until imaging at room temperature.
4.8. Imaging
Images of immunofluorescence-stained samples were acquired using a Zeiss LSM900 Airyscan confocal microscope, a Zeiss AxioScan microscope equipped with an Apotome slide, and a Nikon Eclipse Ts2R equipped with a DS-Qi2 camera. The anatomical maps were obtained by imaging several tiles along the x-y axis using the Zeiss Axioscan with a 10× objective. The magnification of single areas was realized using 20× or 40× objectives, acquiring multiple z-plans of each area distanced by the recommended distance and collapsing them together into a Maximum Projection single image using the Zen suite. Images acquired by Nikon were acquired using only a single Z plane. ff-ISH whole-panoramic-view images were acquired with a Nikon Eclipse600 microscope equipped with a DS-Fi3 color camera (Nikon, Tokyo, Japan) supplied with a double-LED lightO-ring. All images were adjusted for contrast and brightness using either the Zen Blue 3. 11 suite or ImageJ 1.54p. Panels were realized in Adobe Photoshop.
Whole-brain immunofluorescence of R. asterias was acquired using the UltraMicroscope Blaze™ light sheet microscope (Miltenyi, North Rhine-Westphalia, Germany) with a 1× objective.