Allergen Immunotherapy: Pitfalls, Perks and Unexpected Allies
Abstract
:1. Introduction
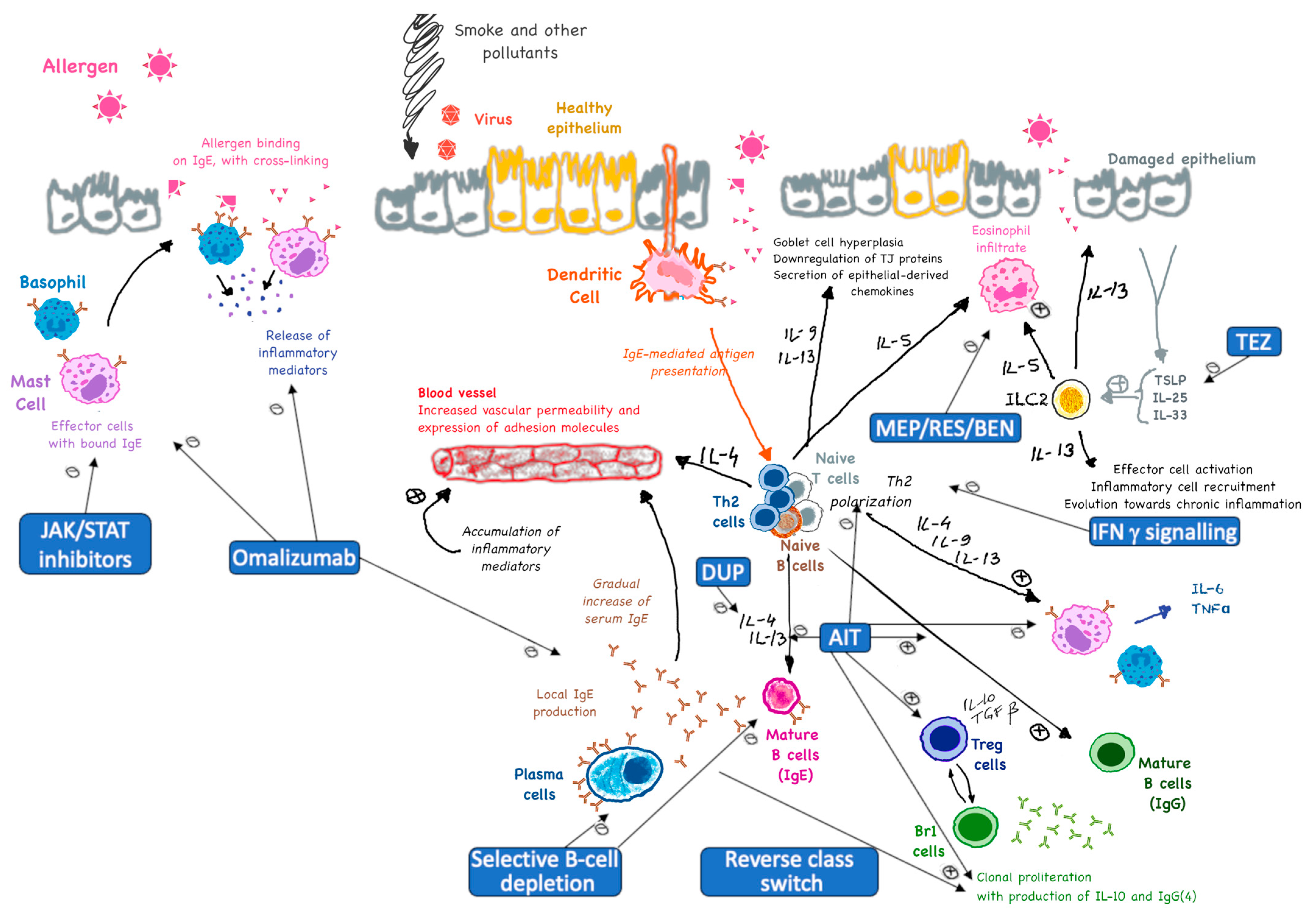
- Immune tolerance: AIT promotes immune tolerance by shifting the overall immune response from a T helper 2 (Th2)-dominated response (which is associated with allergies) to a Th1-dominated response. This shift reduces the production of allergen-specific IgE antibodies and increases the production of tolerogenic antibodies (mainly IgG), which act as blocking antibodies [10,11,12].
- Cytokine modulation: AIT modulates the production of cytokines, which are signaling molecules that regulate immune responses. It increases the production of anti-inflammatory cytokines like Interleukin (IL)-10 and Transforming Growth Factor (TGF)-β, while reducing the production of pro-inflammatory cytokines like IL-4, IL-5, and IL-13 [10,13,16,17,18].
2. Altered Antibody Responses
- Blocking role: IgG4 antibodies act as blocking antibodies that can prevent allergens from binding to IgE on the surface of mast cells and basophils. This helps reduce the release of histamine and other inflammatory mediators, thereby decreasing allergic symptoms [23].
- Tolerance induction: The production of IgG4 is associated with the induction of immune tolerance. IgG4 can compete with IgE for allergen binding, reducing the overall allergic response. This is particularly important in the context of long-term allergen exposure and repeated dosing during AIT [23,24].
3. Cellular Tolerance Network
- Immune tolerance induction: Tregs (specifically Tr1) help induce immune tolerance by suppressing the activity of effector T cells, such as Th2 cells, which are responsible for allergic reactions. This suppression reduces inflammation and allergic symptoms [17].
- Suppression of effector cells: Tregs inhibit the activation and function of mast cells, basophils, and eosinophils, which are key players in allergic reactions. This leads to a decrease in the release of histamine and other inflammatory mediators [37].
- Modulation of dendritic cells: Tregs can influence dendritic cells, which are antigen-presenting cells that play a critical role in initiating immune responses. By modulating dendritic cells in a cross-talking manner, Tregs help promote a more tolerogenic environment [38].
- Long-term persistence of immune tolerance: The presence and optimal activity of Tregs are essential for the long-term success of AIT. They contribute to the establishment of sustained immune tolerance, which can persist even after the discontinuation of treatment [39].
4. Effector Cell Suppression
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mortimer, K.; Lesosky, M.; García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; Sony, A.E.; Ellwood, P.; Marks, G.B.; et al. Global Asthma Network Phase I Study Group. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur. Respir. J. 2022, 60, 2102865. [Google Scholar] [CrossRef] [PubMed]
- Savouré, M.; Bousquet, J.; Jaakkola, J.J.K.; Jaakkola, M.S.; Jacquemin, B.; Nadif, R. Worldwide prevalence of rhinitis in adults: A review of definitions and temporal evolution. Clin. Transl. Allergy 2022, 12, e12130. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Zuberbier, T.; Lötvall, J.; Simoens, S.; Subramanian, S.V.; Church, M.K. Economic burden of inadequate management of allergic diseases in the European Union: A GA(2) LEN review. Allergy 2014, 69, 1275–1279. [Google Scholar] [CrossRef]
- Fong, A.T.; Ahlstedt, S.; Golding, M.A.; Protudjer, J.L.P. The Economic Burden of Food Allergy: What We Know and What We Need to Learn. Curr. Treat. Options Allergy 2022, 9, 169–186. [Google Scholar] [CrossRef]
- Durham, S.R.; Shamji, M.H. Allergen immunotherapy: Past, present and future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Incorvaia, C.; Al-Ahmad, M.; Ansotegui, I.J.; Arasi, S.; Bachert, C.; Bos, C.; Bousquet, J.; Bozek, A.; Caimmi, D.; Calderón, M.A.; et al. Personalized medicine for allergy treatment: Allergen immunotherapy still a unique and unmatched model. Allergy 2021, 76, 1041–1052. [Google Scholar] [CrossRef]
- Ojeda, P.; Barjau, M.C.; Subiza, J.; Moreno, A.; Ojeda, I.; Solano, E.; Alonso, A.; Caballero, R.; Del Pozo, S.; Gómez-Perosanz, M.; et al. Grass pollen allergoids conjugated with mannan for subcutaneous and sublingual immunotherapy: A dose-finding study. Front. Immunol. 2024, 15, 1431351. [Google Scholar] [CrossRef]
- Komatsuzaki, K.; Kageshima, H.; Sekino, Y.; Suzuki, Y.; Ugajin, T.; Tamaoka, M.; Hanazawa, R.; Hirakawa, A.; Miyazaki, Y. Local nasal immunotherapy with birch pollen-galactomannan conjugate-containing ointment in mice and humans. Allergol. Int. 2024, 73, 290–301. [Google Scholar] [CrossRef]
- Maggi, E. The TH1/TH2 paradigm in allergy. Immunotechnology 1998, 3, 233–244. [Google Scholar] [CrossRef]
- Strobl, M.R.; Demir, H.; Acosta, G.S.; Drescher, A.; Kitzmüller, C.; Möbs, C.; Pfützner, W.; Bohle, B. The role of IgG(1) and IgG(4) as dominant IgE-blocking antibodies shifts during allergen immunotherapy. J. Allergy Clin. Immunol. 2023, 151, 1371–1378.e5. [Google Scholar] [CrossRef] [PubMed]
- Bohle, B. The role of IgG1 and IgG4 as dominant IgE-blocking antibodies during allergen immunotherapy. Allergo J. Int. 2024, 33, 282–288. [Google Scholar] [CrossRef]
- Rivas, M.N.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Benamar, M.; Chen, Q.; Martinez-Blanco, M.; Chatila, T.A. Regulatory T cells in allergic inflammation. Semin. Immunol. 2023, 70, 101847. [Google Scholar] [CrossRef]
- Nahm, D.H. Regulatory T Cell-Targeted Immunomodulatory Therapy for Long-Term Clinical Improvement of Atopic Dermatitis: Hypotheses and Perspectives. Life 2023, 13, 1674. [Google Scholar] [CrossRef]
- Schülke, S. Induction of Interleukin-10 Producing Dendritic Cells as a Tool to Suppress Allergen-Specific T Helper 2 Responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Giovannini, M.; Escribese, M.M.; Paoletti, G.; Heffler, E.; Alvaro Lozano, M.; Barber, D.; Canonica, G.W.; Pfaar, O. Mechanisms of Allergen Immunotherapy and Potential Biomarkers for Clinical Evaluation. J. Pers. Med. 2023, 13, 845. [Google Scholar] [CrossRef]
- Lao-Araya, M. Novel Approaches to Allergen Immunotherapy for Respiratory Allergies. Pharmaceuticals 2024, 17, 1510. [Google Scholar] [CrossRef]
- Penagos, M.; Durham, S.R. Allergen immunotherapy for long-term tolerance and prevention. J. Allergy Clin. Immunol. 2022, 149, 802–811. [Google Scholar] [CrossRef]
- Penagos, M.; Durham, S.R. Long-term efficacy of the sublingual and subcutaneous routes in allergen immunotherapy. Allergy Asthma Proc. 2022, 43, 292–298. [Google Scholar] [CrossRef]
- Boursiquot, J.N.; Gagnon, R.; Quirt, J.; Ellis, A.K. Allergen immunotherapy. Allergy Asthma Clin. Immunol. 2024, 20 (Suppl. 3), 66. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, S.; Junker, A.C.; He, C.; Schülke, S.; Toda, M. The role of IgA in the manifestation and prevention of allergic immune responses. Curr. Allergy Asthma Rep. 2023, 23, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tang, L.F.; Cheng, L.; Wang, H.Y. The clinical significance of allergen-specific IgG4 in allergic diseases. Front. Immunol. 2022, 13, 1032909. [Google Scholar] [CrossRef]
- Nikolov, G.; Todordova, Y.; Emilova, R.; Hristova, D.; Nikolova, M.; Petrunov, B. Allergen-Specific IgE and IgG4 as Biomarkers for Immunologic Changes during Subcutaneous Allergen Immunotherapy. Antibodies 2021, 10, 49. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Rispens, T.; Meesters, J.; Rose, R.J.; den Bleker, T.H.; Loverix, S.; Bremer, E.T.J.v.D.; Neijssen, J.; Vink, T.; Lasters, I.; et al. Species-specific determinants in the IgG CH3 domain enable Fab-arm exchange by affecting the noncovalent CH3-CH3 interaction strength. J. Immunol. 2011, 187, 3238–3246. [Google Scholar] [CrossRef]
- Van der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Bleker, T.H.D.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef]
- Beneduce, C.; Kurtagic, E.; Bosques, C.J. Anti-Inflammatory Activity of IgG-Fc. In Fc Mediated Activity of Antibodies: Structural and Functional Diversity; Ravetch, J.V., Nimmerjahn, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 35–62. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Vogel, M.; Speiser, D.E. Successful Allergen-Specific Immunotherapy: Induction of Unresponsiveness by “Vaccination”. Vaccines 2023, 11, 1852. [Google Scholar] [CrossRef]
- Liu, H.; Perugino, C.A.; Ghebremichael, M.; Wallace, Z.S.; Montesi, S.B.; Stone, J.H.; Pillai, S. Disease Severity Linked to Increase in Autoantibody Diversity in IgG4-Related Disease. Arthritis Rheumatol. 2020, 72, 687–693. [Google Scholar] [CrossRef]
- Culver, E.L.; Vermeulen, E.; Makuch, M.; van Leeuwen, A.; Sadler, R.; Cargill, T.; Klenerman, P.; Aalberse, R.C.; van Ham, S.M.; Barnes, E.; et al. Increased IgG4 responses to multiple food and animal antigens indicate a polyclonal expansion and differentiation of pre-existing B cells in IgG4-related disease. Ann. Rheum. Dis. 2015, 74, 944–947. [Google Scholar] [CrossRef]
- Motta, R.V.; Culver, E.L. IgG4 autoantibodies and autoantigens in the context of IgG4-autoimmune disease and IgG4-related disease. Front. Immunol. 2024, 15, 1272084. [Google Scholar] [CrossRef]
- Quinn, L.; Nguyen, B.; Menard-Katcher, C.; Spencer, L. IgG4+ cells are increased in the gastrointestinal tissue of pediatric patients with active eosinophilic gastritis and duodenitis and decrease in remission. Dig. Liver Dis. 2023, 55, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.; Würtzen, P.; van Neerven, R.J. Invited review: The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef] [PubMed]
- Firer, M. Might Selective B-Cell Depletion have a Place in Targeted Allergy Therapy? J. Hematol. Res. 2014, 1, 11–15. [Google Scholar] [CrossRef]
- Fiyouzi, T.; Pelaez-Prestel, H.F.; Reyes-Manzanas, R.; Lafuente, E.M.; Reche, P.A. Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 7797. [Google Scholar] [CrossRef]
- Komlósi, Z.I.; van de Veen, W.; Kovács, N.; Szűcs, G.; Sokolowska, M.; O’Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and molecular mechanisms of allergic asthma. Mol. Asp. Med. 2022, 85, 100995. [Google Scholar] [CrossRef]
- Yamazaki, S. Diverse roles of dendritic cell and regulatory T cell crosstalk in controlling health and disease. Int. Immunol. 2024, 37, 5–14. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; Angelina, A.; Palomares, O.; Akdis, M. Mechanisms in AIT: Insights 2021. Allergol. Sel. 2022, 6, 259–266. [Google Scholar] [CrossRef]
- Baris, S.; Benamar, M.; Chen, Q.; Catak, M.C.; Martínez-Blanco, M.; Wang, M.; Fong, J.; Massaad, M.J.; Sefer, A.P.; Kara, A.; et al. Severe allergic dysregulation due to a gain of function mutation in the transcription factor STAT6. J. Allergy Clin. Immunol. 2023, 152, 182–194.e7. [Google Scholar] [CrossRef]
- León, B. Understanding the development of Th2 cell-driven allergic airway disease in early life. Front. Allergy 2022, 3, 1080153. [Google Scholar] [CrossRef]
- Pan, L.; Wang, J.; Liu, J.; Guo, L.; Yang, S. Deficiency in the frequency and function of Tr1 cells in IgAV and the possible role of IL-27. Rheumatology 2021, 60, 3432–3442. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Tocut, M.; Tampa, M.; Georgescu, S.R.; Matei, C.; Leulescu, I.M.; Nicolae, I.; Ene, C. Changes in Serum IL-12 Levels following the Administration of H1-Antihistamines in Patients with Chronic Spontaneous Urticaria. J. Pers. Med. 2024, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, L.; Wang, L.; Song, J.; Fan, J.; Lai, C.; Bao, J.; Weng, C.; Wang, Y.; Shuai, J.; et al. Allergen immunotherapy combined with Notch pathway inhibitors improves HDM-induced allergic airway inflammation and inhibits ILC2 activation. Front. Immunol. 2023, 14, 1264071. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Wangorsch, A.; Gomez, F.; Schülke, S.; Torres, M.J.; Vieths, S.; Scheurer, S.; Toda, M.; Mayorga, C. Immunotherapy with Native Molecule rather than Hypoallergenic Variant of Pru p 3, the Major Peach Allergen, Shows Beneficial Effects in Mice. J. Immunol. Res. 2018, 2018, 3479185. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Fonseca, B.P.F.; Barboza, B.A.; Viola, J.P.B. The role of interferon-gamma on immune and allergic responses. Mem. Inst. Oswaldo Cruz 2005, 100 (Suppl. 1), 137–144. [Google Scholar] [CrossRef]
- Von Bubnoff, D.; Bieber, T. The indoleamine 2,3-dioxygenase (IDO) pathway controls allergy. Allergy 2012, 67, 718–725. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, H.; Zeng, X.; Guo, L.; Sun, X.; He, S. Subsets of regulatory T cells and their roles in allergy. J. Transl. Med. 2014, 12, 125. [Google Scholar] [CrossRef]
- Riaz, F.; Huang, Z.; Pan, F. Targeting post-translational modifications of Foxp3: A new paradigm for regulatory T cell-specific therapy. Front. Immunol. 2023, 14, 1280741. [Google Scholar] [CrossRef]
- Revenko, A.; Carnevalli, L.S.; Sinclair, C.; Johnson, B.; Peter, A.; Taylor, M.; Hettrick, L.; Chapman, M.; Klein, S.; Solanki, A.; et al. Direct targeting of FOXP3 in Tregs with AZD8701, a novel antisense oligonucleotide to relieve immunosuppression in cancer. J. Immunother. Cancer 2022, 10, e003892. [Google Scholar] [CrossRef]
- Saxena, V.; Lakhan, R.; Iyyathurai, J.; Bromberg, J.S. Mechanisms of exTreg induction. Eur. J. Immunol. 2021, 51, 1956–1967. [Google Scholar] [CrossRef]
- Su, Y.; Romeu-Bonilla, E.; Anagnostou, A.; Fitz-Patrick, D.; Hearl, W.; Heiland, T. Safety and long-term immunological effects of CryJ2-LAMP plasmid vaccine in Japanese red cedar atopic subjects: A phase I study. Hum. Vaccines Immunother. 2017, 13, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Briede, K.; Ugenskiene, R.; Valiukeviciene, S.; Gradauskiene, B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. Int. J. Mol. Sci. 2024, 25, 9021. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Abraham, S.N. Mast cell-sensory neuron crosstalk in allergic diseases. J. Allergy Clin. Immunol. 2024, 153, 939–953. [Google Scholar] [CrossRef]
- López-Sanz, C.; Jiménez-Saiz, R.; Esteban, V.; Delgado-Dolset, M.I.; Perales-Chorda, C.; Villaseñor, A.; Barber, D.; Escribese, M.M. Mast Cell Desensitization in Allergen Immunotherapy. Front. Allergy 2022, 3, 898494. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Woodfolk, J.A.; Schuyler, A.J.; Stillman, L.C.; Chapman, M.D.; Platts-Mills, T.A.E. Half-life of IgE in serum and skin: Consequences for anti-IgE therapy in patients with allergic disease. J. Allergy Clin. Immunol. 2017, 139, 422–428.e4. [Google Scholar] [CrossRef]
- Arinobu, Y.; Iwasaki, H.; Akashi, K. Origin of basophils and mast cells. Allergol. Int. 2009, 58, 21–28. [Google Scholar] [CrossRef]
- Poto, R.; Cristinziano, L.; Criscuolo, G.; Strisciuglio, C.; Palestra, F.; Lagnese, G.; Di Salvatore, A.; Marone, G.; Spadaro, G.; Loffredo, S.; et al. The JAK1/JAK2 inhibitor ruxolitinib inhibits mediator release from human basophils and mast cells. Front. Immunol. 2024, 15, 1443704. [Google Scholar] [CrossRef]
- Morales, J.K.; Falanga, Y.T.; Depcrynski, A.; Fernando, J.; Ryan, J.J. Mast cell homeostasis and the JAK-STAT pathway. Genes. Immun. 2010, 11, 599–608. [Google Scholar] [CrossRef]
- Morales, A.R.; Shah, N.; Castells, M. Antigen-IgE desensitization in signal transducer and activator of transcription 6-deficient mast cells by suboptimal doses of antigen. Ann. Allergy Asthma Immunol. 2005, 94, 575–580. [Google Scholar] [CrossRef]
- Shalit, M.; Levi-Schaffer, F. Challenge of mast cells with increasing amounts of antigen induces desensitization. Clin. Exp. Allergy J. 1995, 25, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, R.; Karagiannis, S.N.; Jordakieva, G.; Jensen-Jarolim, E. The Role of IgG4 in the Fine Tuning of Tolerance in IgE-Mediated Allergy and Cancer. Int. J. Mol. Sci. 2020, 21, 5017. [Google Scholar] [CrossRef] [PubMed]
- Bossi, G.; Brazzelli, V.; De Amici, M.; Pietra, D.; Raviola, C.; Naso, M.; Regalbuto, C.; Boselli, F.; Fortina, V.; Marseglia, G.L. Successful treatment with Omalizumab of a child affected by Systemic Mastocytosis: Clinical and biological implications. Ital. J. Pediatr. 2023, 49, 6. [Google Scholar] [CrossRef] [PubMed]
- Slapnicar, C.; Trinkaus, M.; Hicks, L.; Vadas, P. Efficacy of Omalizumab in Indolent Systemic Mastocytosis. Case Rep. Hematol. 2019, 2019, 3787586. [Google Scholar] [CrossRef]
- Gülsen, A.; Ruëff, F.; Jappe, U. Omalizumab ensures compatibility to bee venom immunotherapy (VIT) after VIT-induced anaphylaxis in a patient with systemic mastocytosis. Allergol. Sel. 2021, 5, 128–132. [Google Scholar] [CrossRef]
- Meucci, E.; Radice, A.; Fassio, F.; Iorno, M.L.C.; Macchia, D. Omalizumab for prevention of anaphylactic episodes in a patient with severe mosquito allergy. Clin. Case Rep. 2021, 9, e04935. [Google Scholar] [CrossRef]
- Alnabulsi, R.; Pak, Y.; Pasha, M. Efficacy of concurrent treatment with omalizumab in a patient with anaphylaxis to venom immunotherapy. Ann. Allergy Asthma Immunol. 2022, 129, S107. [Google Scholar] [CrossRef]
- Buono, E.V.; Giannì, G.; Scavone, S.; Esposito, S.; Caffarelli, C. Omalizumab and Oral Immunotherapy in IgE-Mediated Food Allergy in Children: A Systematic Review and a Meta-Analysis. Pharmaceuticals 2025, 18, 437. [Google Scholar] [CrossRef]
- Moffa, A.; Iafrati, F.; Giorgi, L.; Nardelli, D.; Carnuccio, L.; Baptista, P.; Olszewska, E.; Casale, M. Clinical Evidence of the Use of Mepolizumab in the Treatment of Chronic Rhinosinusitis with Nasal Polyps: A Prospective Observational Study. Healthcare 2025, 13, 419. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Vatrella, A.; Tinello, C.; Terracciano, R.; Pelaia, G. Molecular Targets for Biological Therapies of Severe Asthma. Front. Immunol. 2020, 11, 603312. [Google Scholar] [CrossRef]
- Deng, S.; Wang, H.; Chen, S.; Kong, M.; Yang, X.; Song, Z.; Chen, Q. Dupilumab and subcutaneous immunotherapy for the treatment of refractory moderate to severe atopic dermatitis: A preliminary report. Int. Immunopharmacol. 2023, 125 Pt A, 111137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Boo, J.; Jang, H.; Jung, Y.W.; Kim, J.; Zhang, K.; Park, C.O. Combined Dupilumab and Allergen-Specific Immunotherapy in Severe Refractory Atopic Dermatitis. Allergy Asthma Immunol. Res. 2024, 16, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Larson, D.; Altman, M.C.; Segnitz, R.M.; Avila, P.C.; Greenberger, P.A.; Baroody, F.; Moss, M.H.; Nelson, H.; Burbank, A.J.; et al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: A randomized controlled trial. J. Allergy Clin. Immunol. 2023, 151, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Kappen, J.H.; Akdis, M.; Jensen-Jarolim, E.; Knol, E.F.; Kleine-Tebbe, J.; Bohle, B.; Chaker, A.M.; Till, S.J.; Valenta, R.; et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: An EAACI Position Paper. Allergy 2017, 72, 1156–1173. [Google Scholar] [CrossRef]
- Pongracic, J.A.; Gagnon, R.; Sussman, G.; Siri, D.; Oriel, R.C.; Brown-Whitehorn, T.F.; Anvari, S.; Berger, W.E.; Bird, J.A.; Chan, E.S.; et al. Long-term safety of epicutaneous immunotherapy in peanut-allergic children: An open-label active treatment (REALISE study). J. Allergy Clin. Immunol. Pract. 2025, 17, 1510. [Google Scholar] [CrossRef]
- Greenhawt, M.; Albright, D.; Anvari, S.; Arends, N.; Arkwright, P.D.; Bégin, P.; Blümchen, K.; Brown-Whitehorn, T.; Cassell, H.; Chan, E.S.; et al. Efficacy and safety of epicutaneous immunotherapy in peanut-allergic toddlers: Open-label extension to EPITOPE. J. Allergy Clin. Immunol. Pract. 2025. [Google Scholar] [CrossRef]
- Fu, Y.; Song, Y.L.; Liu, Z.G. Recent developments in immunotherapy approaches for allergic rhinitis. World J. Clin. Cases 2024, 12, 6451–6461. [Google Scholar] [CrossRef]
- Flory, S.; Hviid-Vyff, B.; Šošić, L.; Schmid, J.M.; Ahlbeck, L.; Widmer, E.C.J.; Lang, C.C.V.; Ikenberg, K.; Kündig, T.M.; Hoffmann, H.J.; et al. How to hit the allergy target: A critical appraisal of intralymphatic immunotherapy with practical recommendations on ultrasound-guided injections. Allergy 2024, 79, 2222–2234. [Google Scholar] [CrossRef]
| Altered Component | Mechanism | Potential Influence on Allergy/AIT |
|---|---|---|
| STAT3 | ↑ STAT3 → Th17 differentiation and ILC3 cytokine production (IL-17, IL-22) | Exacerbated Th17/ILC3-mediated inflammation, particularly in mucosal tissues |
| STAT5 | ↑ STAT5 activation via IL-2 → ILC2 expansion and can suppress Treg stability | Amplified type 2 immunity via ILC2 expansion, impaired regulation via unstable Tregs |
| STAT6 | ↑ STAT6 via IL-4/IL-13 → enhanced Th2 polarization and ILC2 activation | Heightened Th2/ILC2 responses in asthma, atopic dermatitis, eosinophilia |
| STAT1 | ↓ STAT1 via impaired IFN-γ signaling, weakened Tr1 tolerance induction and Th1 response | Loss of Th1-mediated counterbalance to Th2 with enhanced allergic inflammation |
| STAT4 | ↓ STAT4 → deficient IL-12-mediated Th1 polarization and IFN-γ production | Failure to suppress Th2 expansion with increased allergic sensitivity |
| Notch Ligands (e.g., Jagged1, Delta-like 4) | ↑ Jagged1 on dendritic cells promotes Th2/ILC2 polarization via Notch2 → enhanced type 2 immunity | Bias toward Th2 differentiation with enhanced allergic sensitization and airway inflammation |
| IFN-γ/IFN-γ Receptor Signaling | ↓ IFN-γR expression or STAT1 signaling → impaired Th1 differentiation, reduced antagonism of Th2/Th17 responses | Loss of immune counter-regulation with unchecked Th2/Th17-driven allergic inflammation |
| GATA3, IL-4 signaling | ↑ Th2 signaling, resistance to Treg suppression | Enhanced eosinophilia, IgE-skewed class switching, asthma |
| RORγt, IL-23 | ↑ Th17 RORγt, IL-23 responsiveness, GM-CSF co-expression | Neutrophilic inflammation, steroid-resistant airway disease |
| IL-33/IL-25 | ↑ ILC2 IL-33/IL-25 sensitivity, loss of PD-1/IL-10R regulation | Exaggerated airway reactivity, fibrosis, atopic dermatitis |
| IL-23R | ↑ ILC3 IL-23R signaling, plasticity toward IL-17+ ILC3s | Chronic intestinal inflammation, epithelial hyperreactivity |
| T-bet, IL-12 | ↓ Th1 T-bet or IL-12 signaling, impaired IFN-γ response | Failure to counterbalance Th2 response with worsened allergic inflammation |
| IL-10 | ↓ Tr1 IL-10 production → failure to suppress Th2/Th17 responses | Insufficient control of effector T cells with sustained allergic responses |
| IL-18 | ↑ IL-18 in allergic tissues → Th2/ILC2 activation in synergy with IL-33 | Amplification of type 2 inflammation, eosinophilia, and airway hyperreactivity |
| IL-27 | ↓ IL-27 → diminished inhibition of Th2/Th17 and reduced IL-10 induction | Loss of immune regulation with sustained allergic inflammation, reduced tolerance |
| Trial ID | Title | Status |
|---|---|---|
| 2023-508013-16-00 | A prospective, randomized, double-blind placebo-controlled multicenter study with mannan-conjugated birch pollen allergoids administered subcutaneously to adolescent and adult patients with birch pollen-induced allergic rhinitis or rhinoconjunctivitis. | Ended |
| 2022-502366-25-00 | A three-year, multi-center, double-blind, extension study to evaluate the long-term safety and efficacy of ligelizumab in patients who completed ligelizumab’s Phase III studies in food allergy | Ended |
| 2022-502984-39-00 | A multicenter, randomized, double-blind, parallel-group placebo-controlled, Phase III, efficacy and safety study of tezepelumab in 5-to-<12-year old children with severe uncontrolled asthma (HORIZON) | Ongoing, recruiting |
| 2024-511383-88-00 | A Phase II–III study to assess the efficacy and safety of subcutaneous cluster-immunotherapy in patients suffering from Olea europaea pollen allergy | Ongoing, recruitment ended |
| 2023-505567-37-00 | A Phase II–III study to assess the efficacy and safety of sublingual immunotherapy in patients suffering from birch pollen allergy | Ended |
| 2023-505880-35-00 | A Phase II–III study to assess efficacy and safety of sublingual immunotherapy in patients suffering from grass pollen allergy | Ended |
| 2023-504942-75-01 | A randomized, double-blind, placebo-controlled, multi-center study to assess the efficacy of PURETHAL Mites mixture 50,000 AUeq/mL subcutaneous immunotherapy in adult subjects with moderate to severe allergic rhinitis/rhinoconjunctivitis with or without asthma induced by house dust mite (HDM) allergy | Ongoing, recruitment ended |
| 2022-502110-85-00 | A Phase III, double-blind, placebo-controlled, randomized study to assess the efficacy and safety of epicutaneous immunotherapy with DBV712 250 μg in 4–7-year-old children with peanut allergy (VITESSE) | Ongoing, recruitment ended |
| 2022-503053-19-00 | A clinical study investigating OM-85-IN safety and tolerability in healthy volunteers and mild allergic asthma patients | Ended |
| 2024-515717-17-00 | A prospective, randomized, double-blind placebo-controlled multicenter trial with mannan-conjugated birch pollen allergoids administered subcutaneously to adolescents and adults with birch pollen-induced allergic rhinitis or rhinoconjunctivitis. | Ongoing, recruitment ended |
| 2023-508520-36-00 | A long-term, double-blind, randomized, placebo-controlled clinical trial to investigate the efficacy and safety of PQ Grass 27,600 SU in children and adolescents with seasonal allergic rhinitis/rhinoconjunctivitis induced by grass pollen exposure | Ongoing, recruiting |
| 2023-508817-18-00 | A Phase III, multicenter, randomized, double-blind, placebo-controlled, parallel group study to evaluate the efficacy and safety of lebrikizumab/LY3650150 in adult participants with perennial allergic rhinitis | authorized, recruiting |
| 2023-509833-38-00 | A multi-national Phase IIIb, double-blind, placebo-controlled trial to determine the safety and efficacy of STALORAL® Birch 300 IR in children and ddolescents of 5 to 17 years old with birch pollen-induced allergic rhinoconjunctivitis with or without asthma | Ongoing, recruitment ended |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaș, T.P.; Ciurariu, E. Allergen Immunotherapy: Pitfalls, Perks and Unexpected Allies. Int. J. Mol. Sci. 2025, 26, 3535. https://doi.org/10.3390/ijms26083535
Tamaș TP, Ciurariu E. Allergen Immunotherapy: Pitfalls, Perks and Unexpected Allies. International Journal of Molecular Sciences. 2025; 26(8):3535. https://doi.org/10.3390/ijms26083535
Chicago/Turabian StyleTamaș, Tudor Paul, and Elena Ciurariu. 2025. "Allergen Immunotherapy: Pitfalls, Perks and Unexpected Allies" International Journal of Molecular Sciences 26, no. 8: 3535. https://doi.org/10.3390/ijms26083535
APA StyleTamaș, T. P., & Ciurariu, E. (2025). Allergen Immunotherapy: Pitfalls, Perks and Unexpected Allies. International Journal of Molecular Sciences, 26(8), 3535. https://doi.org/10.3390/ijms26083535






