Crosstalk Between Wnt/β-Catenin and Hedgehog Supports Gli1+ Lineage Osteogenesis in Cranial Sutures
Abstract
1. Introduction
2. Results
2.1. β-Catenin Overlaps with Gli1+ Progeny at the Osteogenic Front
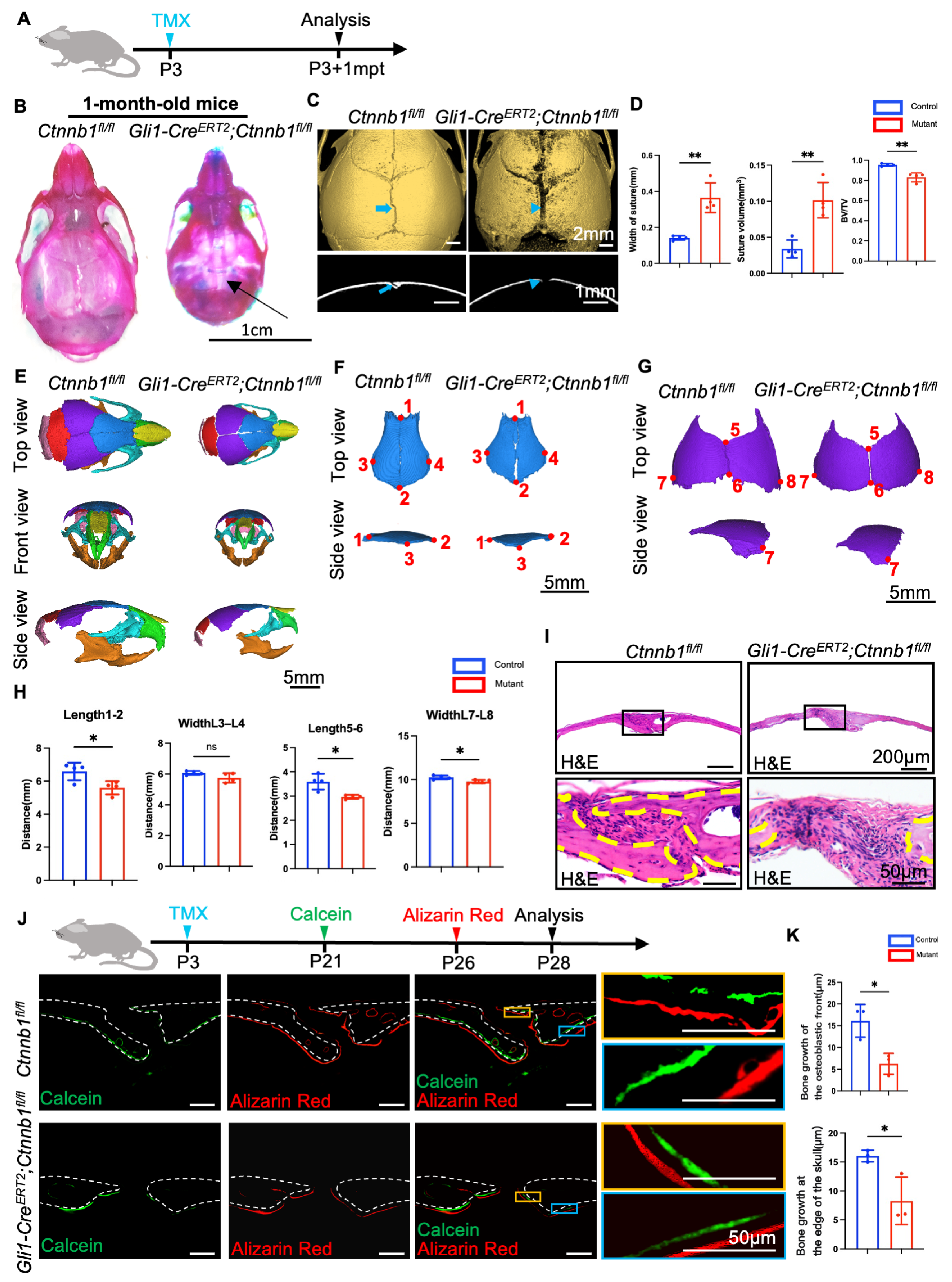
2.2. Loss of Ctnnb1 in Gli1+ Lineage Impairs Craniofacial Bone Formation
2.3. Knockout of Ctnnb1 in Gli1+ MSCs Impairs Proliferation and Osteoblast Differentiation Activities
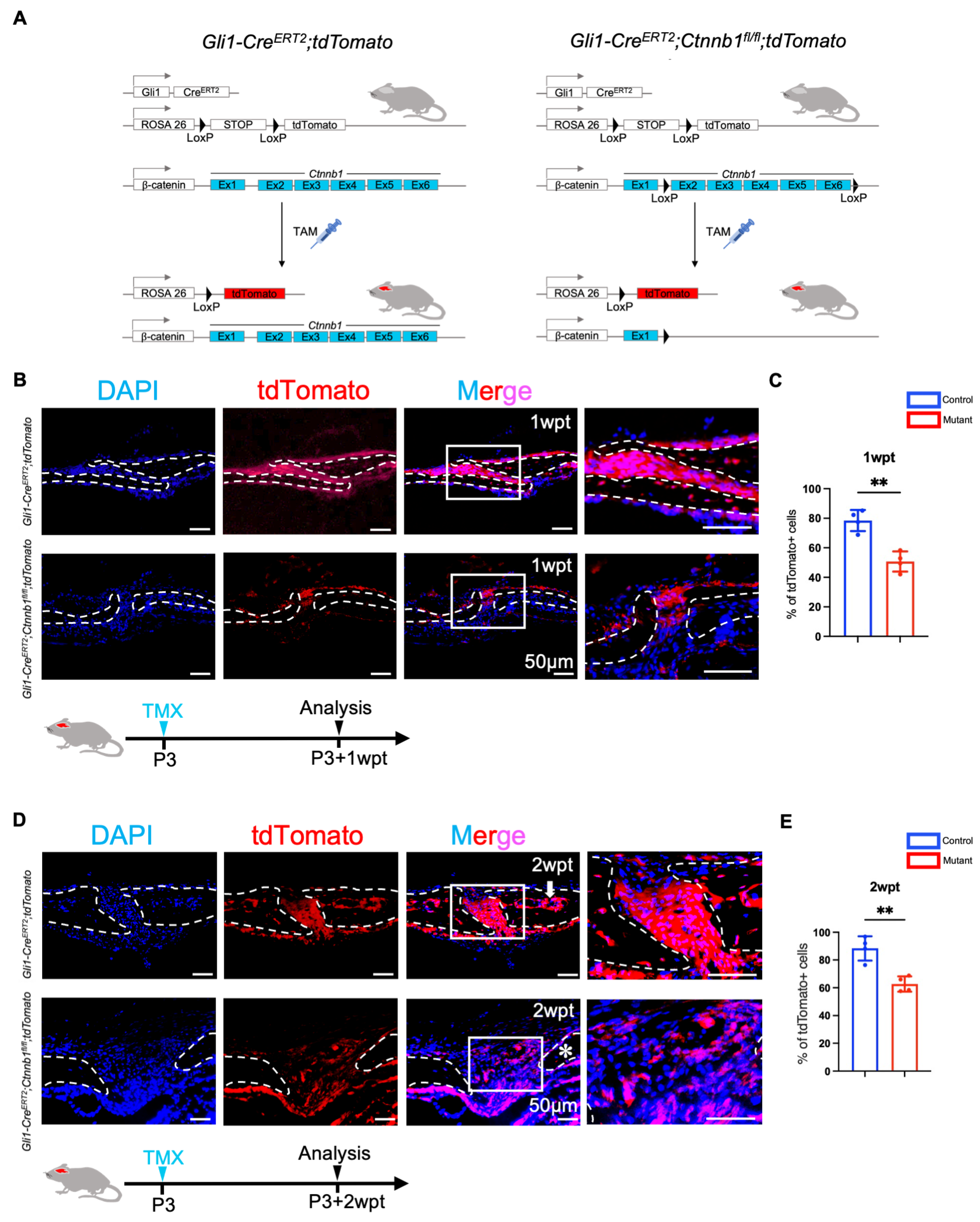
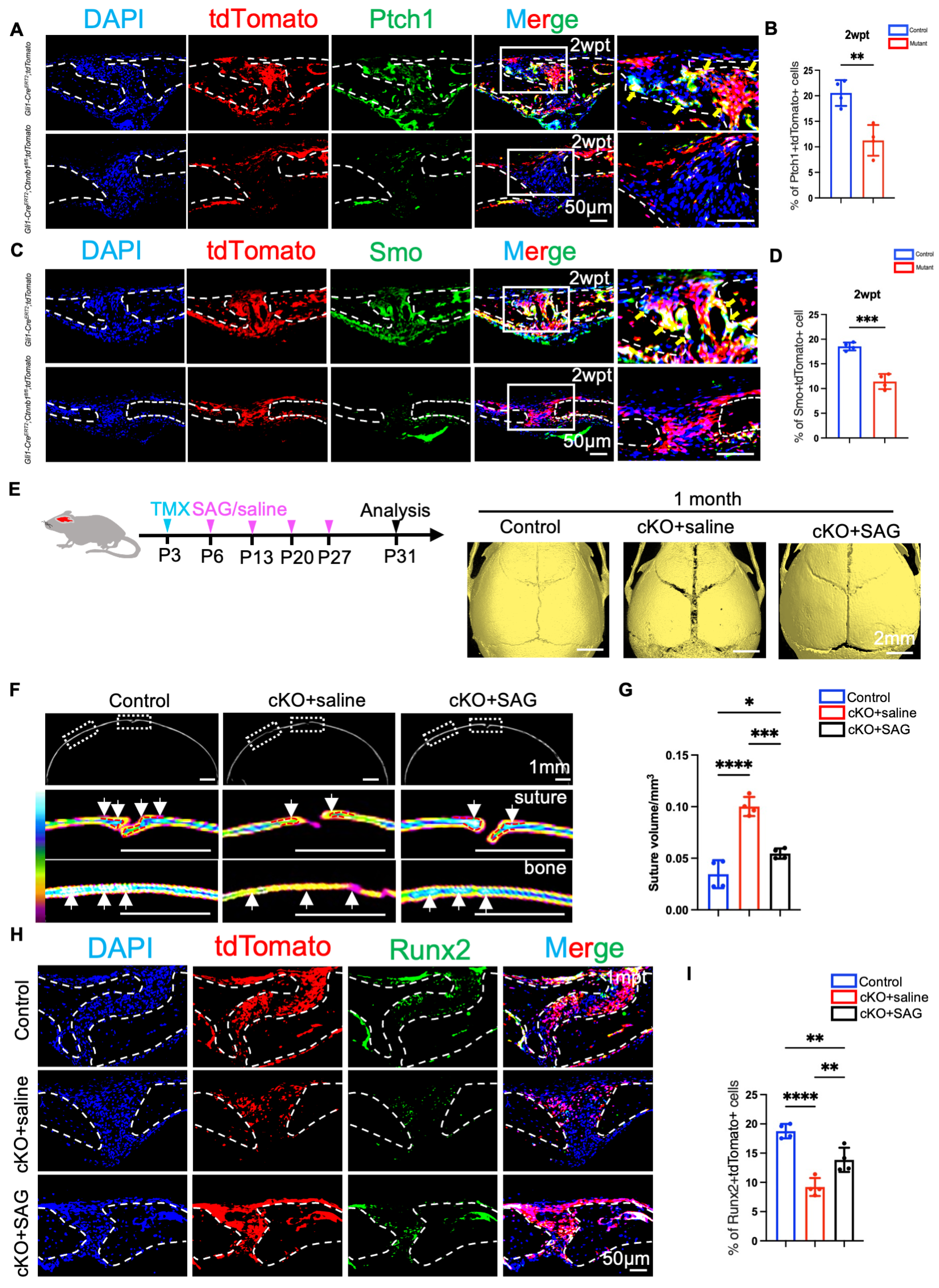
2.4. Canonical Wnt Signaling Is Essential for the Activation of Gli1+ Cells Within Sutures
2.5. Hh Signaling Upregulation Promotes the Activation of Gli1+ Cells and Partially Rescues the Craniofacial Bone Deformity
3. Discussion
4. Materials and Methods
4.1. The Animals
4.2. Tamoxifen Administration
4.3. Micro-CT Analysis and Three-Dimensional Reconstruction
4.4. Alcian Blue and Alizarin Red S Staining of the Skeleton
4.5. Histologic Preparation and Staining
4.6. Immunohistochemical Staining
4.7. Immunofluorescence Staining
4.8. Double Labeling
4.9. SAG or Vehicle Treatment
4.10. Quantification and Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells; |
| Hh | Hedgehog; |
| BMP | Bone morphogenetic protein; |
| ARE | Alizarin Red S; |
| CT | Computed tomography; |
| HE | Hematoxylin and eosin; |
| Smo | Smoothened membrane protein; |
| Ptc | Patched receptor. |
References
- Minoux, M.; Rijli, F.M. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 2010, 137, 2605–2621. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.N.; Jones, R.E.; Borrelli, M.R.; Robertson, K.; Salhotra, A.; Wan, D.C.; Longaker, M.T. Craniofacial and Long Bone Development in the Context of Distraction Osteogenesis. Plast. Reconstr. Surg. 2021, 147, 54E–65E. [Google Scholar] [CrossRef]
- Opperman, L.A. Cranial sutures as intramembranous bone growth sites. Developmental dynamics: An official publication of the American Association of Anatomists. Dev. Dyn. 2000, 219, 472–485. [Google Scholar] [CrossRef]
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922. [Google Scholar] [CrossRef]
- Ono, N.; Balani, D.H.; Kronenberg, H.M. Stem and progenitor cells in skeletal development. In Vertebrate Skeletal Development; Olsen, B.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 133, pp. 1–24. [Google Scholar]
- Zhao, H.; Feng, J.; Ho, T.-V.; Grimes, W.; Urata, M.; Chai, Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol. 2015, 17, 386–396. [Google Scholar] [CrossRef]
- Doro, D.; Liu, A.; Lau, J.S.; Rajendran, A.K.; Healy, C.; Krstic, M.; Grigoriadis, A.E.; Iseki, S.; Liu, K.J. Cranial suture lineage and contributions to repair of the mouse skull. Development 2024, 151, dev202116. [Google Scholar] [CrossRef]
- Doro, D.H.; Grigoriadis, A.E.; Liu, K.J. Calvarial Suture-Derived Stem Cells and Their Contribution to Cranial Bone Repair. Front. Physiol. 2017, 8, 956. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Z.; Xiao, L.; Ai, M.; Cao, Y.; Mao, J.; Song, K. The Role of Gli1+ Mesenchymal Stem Cells in Osteogenesis of Craniofacial Bone. Biomolecules 2023, 13, 1351. [Google Scholar] [CrossRef]
- Luo, W.; Yi, Y.; Jing, D.; Zhang, S.; Men, Y.; Ge, W.-P.; Zhao, H. Investigation of postnatal craniofacial bone development with tissue clearing-based three-dimensional imaging. Stem Cells Dev. 2019, 28, 1310–1321. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, J.; Tian, J.J.; Lv, C.H.; Li, Y.H.; Yu, S.Y.; Liu, J.; Zhang, J. Key Roles of Gli1 and Ihh Signaling in Craniofacial Development. Stem Cells Dev. 2024, 33, 251–261. [Google Scholar]
- Neben, C.L.; Merrill, A.E. Signaling Pathways in Craniofacial Development: Insights from Rare Skeletal Disorders. Curr. Top. Dev. Biol. 2015, 115, 493–542. [Google Scholar] [PubMed]
- Guo, Y.; Yuan, Y.; Wu, L.; Ho, T.-V.; Jing, J.; Sugii, H.; Li, J.; Han, X.; Feng, J.; Guo, C. BMP-IHH-mediated interplay between mesenchymal stem cells and osteoclasts supports calvarial bone homeostasis and repair. Bone Res. 2018, 6, 30. [Google Scholar] [PubMed]
- Graf, D.; Malik, Z.; Hayano, S.; Mishina, Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev. 2016, 27, 129–139. [Google Scholar] [PubMed]
- Yu, H.M.I.; Jerchow, B.; Sheu, T.J.; Liu, B.; Costantini, F.; Puzas, J.E.; Birchmeier, W.; Hsu, W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 2005, 132, 1995–2005. [Google Scholar] [CrossRef]
- Pietro, L.D.; Barba, M.; Prampolini, C.; Ceccariglia, S.; Frassanito, P.; Vita, A.; Guadagni, E.; Bonvissuto, D.; Massimi, L.; Tamburrini, G.; et al. GLI1 and AXIN2 Are Distinctive Markers of Human Calvarial Mesenchymal Stromal Cells in Nonsyndromic Craniosynostosis. Int. J. Mol. Sci. 2020, 21, 4356. [Google Scholar] [CrossRef]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar]
- Mirando, A.J.; Maruyama, T.; Fu, J.; Yu, H.M.; Hsu, W. β-catenin/cyclin D1 mediated development of suture mesenchyme in calvarial morphogenesis. BMC Dev. Biol. 2010, 10, 116. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Miyazaki, T.; Komori, T. Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt and Pthlh signaling pathway gene expressions in suture mesenchymal cells. Hum. Mol. Genet. 2019, 28, 896–911. [Google Scholar] [CrossRef]
- Veistinen, L.K.; Mustonen, T.; Hasan, M.R.; Takatalo, M.; Kobayashi, Y.; Kesper, D.A.; Vortkamp, A.; Rice, D.P. Regulation of Calvarial Osteogenesis by Concomitant De-repression of GLI3 and Activation of IHH Targets. Front. Physiol. 2017, 8, 1036. [Google Scholar]
- Lenton, K.; James, A.W.; Manu, A.; Brugmann, S.A.; Birker, D.; Nelson, E.R.; Leucht, P.; Helms, J.A.; Longaker, M.T. Indian Hedgehog Positively Regulates Calvarial Ossification and Modulates Bone Morphogenetic Protein Signaling. Genesis 2011, 49, 784–796. [Google Scholar] [CrossRef]
- Feng, W.; Choi, I.; Clouthier, D.E.; Niswander, L.; Williams, T. The Ptch1DL Mouse: A New Model to Study Lambdoid Craniosynostosis and Basal Cell Nevus Syndrome-Associated Skeletal Defects. Genesis 2013, 51, 677–689. [Google Scholar] [PubMed]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.-P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024, 12, 39. [Google Scholar] [PubMed]
- Kobayashi, Y.; Iwamoto, R.; He, Z.F.; Udagawa, N. Wnt family members regulating osteogenesis and their origins. J. Bone Miner. Metab. 2024, 43, 39–45. [Google Scholar] [PubMed]
- Shi, Y.; He, G.; Lee, W.-C.; McKenzie, J.A.; Silva, M.J.; Long, F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat. Commun. 2017, 8, 2043. [Google Scholar]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar]
- Wu, Z.B.; Li, W.M.; Jiang, K.L.; Lin, Z.X.; Qian, C.; Wu, M.Z.; Xia, Y.; Li, N.; Zhang, H.T.; Xiao, H.X.; et al. Regulation of bone homeostasis: Signaling pathways and therapeutic targets. Medcomm 2024, 5, e657. [Google Scholar]
- Jiang, Q.; Nagano, K.; Moriishi, T.; Komori, H.; Sakane, C.; Matsuo, Y.; Zhang, Z.; Nishimura, R.; Ito, K.; Qin, X.; et al. Roles of Sp7 in osteoblasts for the proliferation, differentiation, and osteocyte process formation. J. Orthop. Transl. 2024, 47, 161–175. [Google Scholar]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.P.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef]
- Ingham, P.W. Hedgehog signaling. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 149, pp. 1–58. [Google Scholar]
- Pan, A.; Chang, L.; Nguyen, A.; James, A.W. A review of hedgehog signaling in cranial bone development. Front. Physiol. 2013, 4, 61. [Google Scholar]
- Slavkin, H.; Nuckolls, G.; Shum, L. Craniofacial development and patterning. Methods Mol. Biol. 2000, 136, 45–54. [Google Scholar] [PubMed]
- Thilander, B. Basic Mechanisms in Craniofacial Growth. Acta Odontol. Scand. 1995, 53, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Ouchi, T.; Shibata, S.; Fujimura, T.; Kawana, H.; Okano, H.; Nakagawa, T. Applications of Mesenchymal Stem Cells and Neural Crest Cells in Craniofacial Skeletal Research. Stem Cells Int. 2016, 2016, 2849879. [Google Scholar] [PubMed]
- Zheng, Z.; Liu, H.; Liu, S.; Luo, E.; Liu, X. Mesenchymal stem cells in craniofacial reconstruction: A comprehensive review. Front. Mol. Biosci. 2024, 11, 1362338. [Google Scholar]
- Wu, X.; Zhou, X.M.; Zhu, Y.F.; Zhang, Z.Z.; Dong, Y.H.; Wang, J.; Liu, J. Insights into Suture Stem Cells: Distributions, Characteristics, and Applications. Curr. Stem Cell Res. Ther. 2024. Early View. [Google Scholar] [CrossRef]
- Kléber, M.; Sommer, L. Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 2004, 16, 681–687. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. WNT signaling pathway and stem cell signaling network. Clin. Cancer Res. 2007, 13, 4042–4045. [Google Scholar] [CrossRef]
- Long, F.X. Sequential roles of hedgehog and Wnt signaling in osteoblast development. Faseb J. 2005, 19, A1364. [Google Scholar]
- Rodda, S.J.; McMahon, A.P. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 2006, 133, 3231–3244. [Google Scholar]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar]
- Nishimura, R.; Wakabayashi, M.; Hata, K.; Matsubara, T.; Honma, S.; Wakisaka, S.; Kiyonari, H.; Shioi, G.; Yamaguchi, A.; Tsumaki, N.; et al. Osterix Regulates Calcification and Degradation of Chondrogenic Matrices through Matrix Metalloproteinase 13 (MMP13) Expression in Association with Transcription Factor Runx2 during Endochondral Ossification. J. Biol. Chem. 2012, 287, 33179–33190. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Signaling networks in RUNX2-dependent bone development. J. Cell. Biochem. 2011, 112, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Naruse, M.; Chiba, Y.; Komori, T.; Sasaki, K.; Iwamoto, M.; Fukumoto, S. Novel hedgehog agonists promote osteoblast differentiation in mesenchymal stem cells. J. Cell. Physiol. 2015, 230, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Beachy, P.A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687. [Google Scholar] [CrossRef]
- Ho, T.-V.; Iwata, J.; Ho, H.A.; Grimes, W.C.; Park, S.; Sanchez-Lara, P.A.; Chai, Y. Integration of comprehensive 3D microCT and signaling analysis reveals differential regulatory mechanisms of craniofacial bone development. Dev. Biol. 2015, 400, 180–190. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Xue, H.; Liu, W.; Guo, Y.; Xu, R.; Shao, B.; Yuan, Q. Ubiquitin-specific protease 34 inhibits osteoclast differentiation by regulating NF-κB signaling. J. Bone Miner. Res. 2020, 35, 1597–1608. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wang, J.; Chen, S.; He, Y. Crosstalk Between Wnt/β-Catenin and Hedgehog Supports Gli1+ Lineage Osteogenesis in Cranial Sutures. Int. J. Mol. Sci. 2025, 26, 3508. https://doi.org/10.3390/ijms26083508
Sun L, Wang J, Chen S, He Y. Crosstalk Between Wnt/β-Catenin and Hedgehog Supports Gli1+ Lineage Osteogenesis in Cranial Sutures. International Journal of Molecular Sciences. 2025; 26(8):3508. https://doi.org/10.3390/ijms26083508
Chicago/Turabian StyleSun, Lin, Jie Wang, Shuo Chen, and Yang He. 2025. "Crosstalk Between Wnt/β-Catenin and Hedgehog Supports Gli1+ Lineage Osteogenesis in Cranial Sutures" International Journal of Molecular Sciences 26, no. 8: 3508. https://doi.org/10.3390/ijms26083508
APA StyleSun, L., Wang, J., Chen, S., & He, Y. (2025). Crosstalk Between Wnt/β-Catenin and Hedgehog Supports Gli1+ Lineage Osteogenesis in Cranial Sutures. International Journal of Molecular Sciences, 26(8), 3508. https://doi.org/10.3390/ijms26083508





