Effects of Treatment with a DNA Methyltransferase Inhibitor 5-aza-dC on Sex Differentiation in Medaka (Oryzias latipes)
Abstract
1. Introduction
2. Results
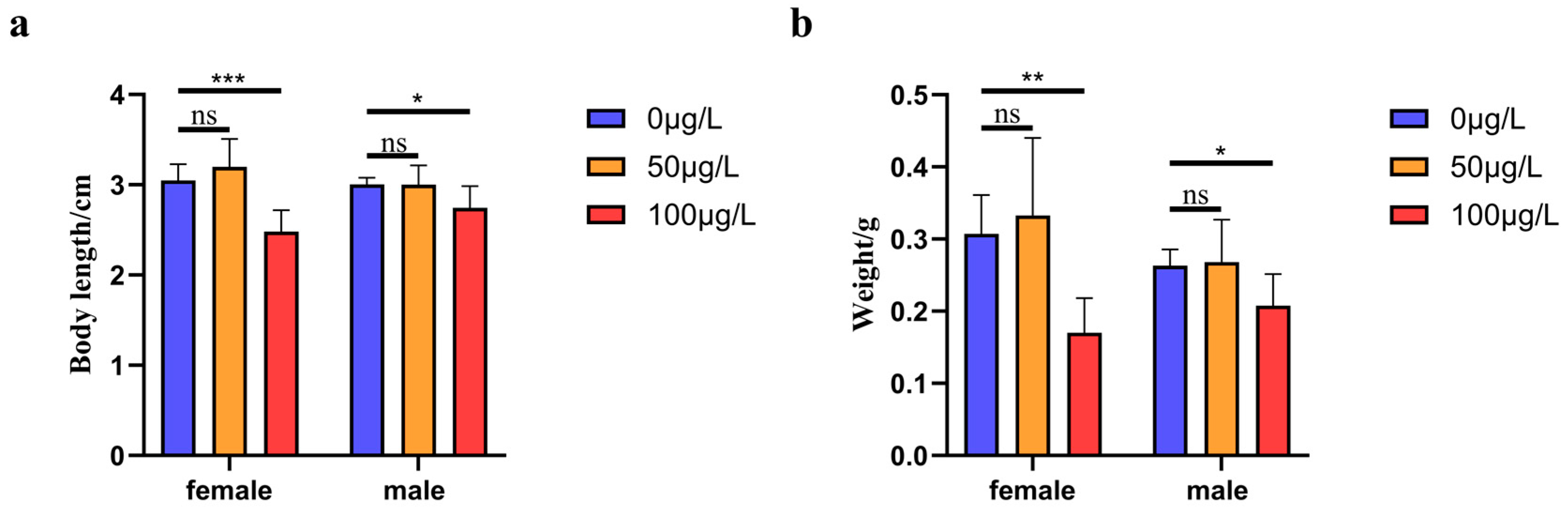
2.1. The Effect of Treatment with Different Concentrations of 5-aza-dC on the Growth Indicators of Medaka Fish
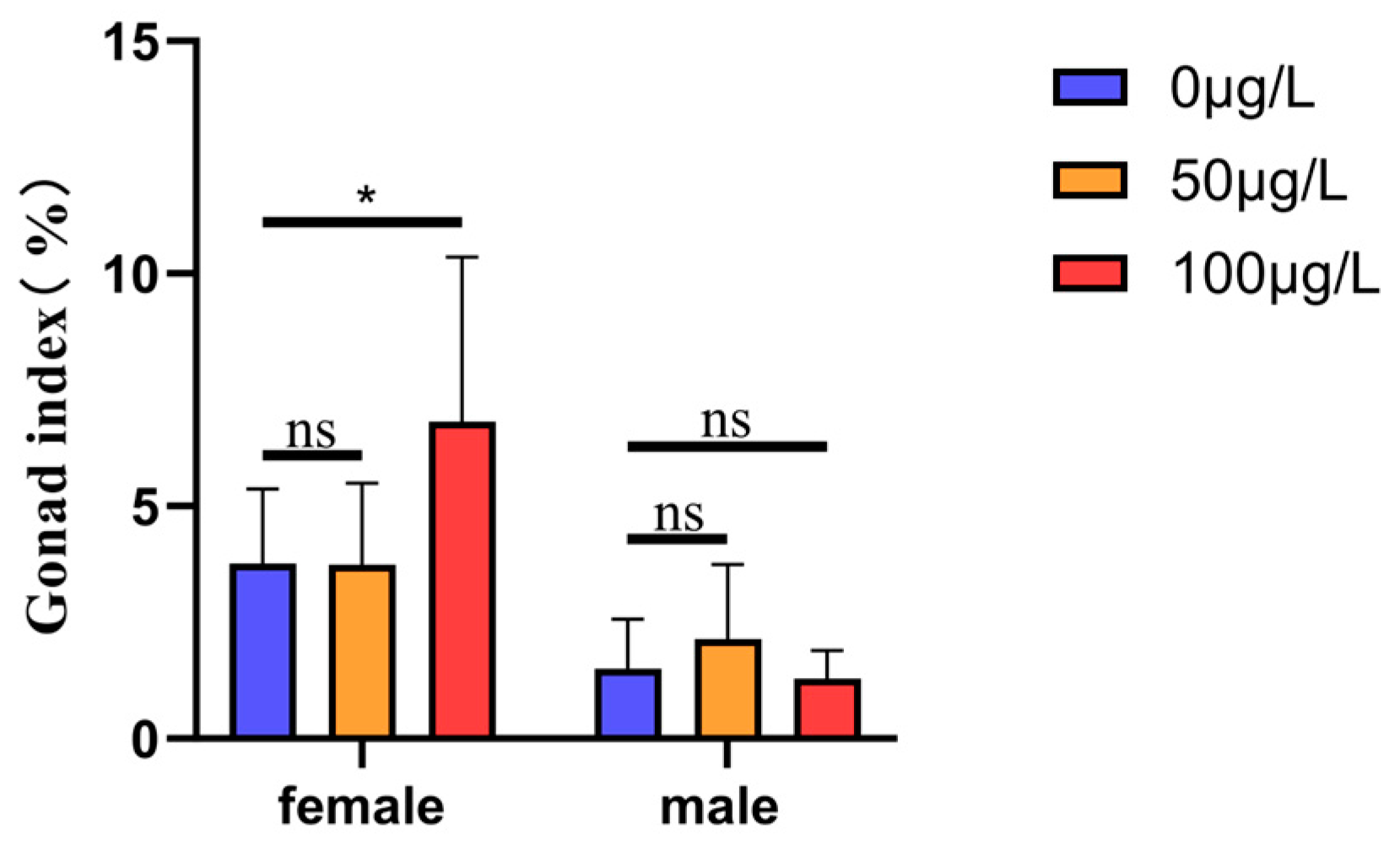
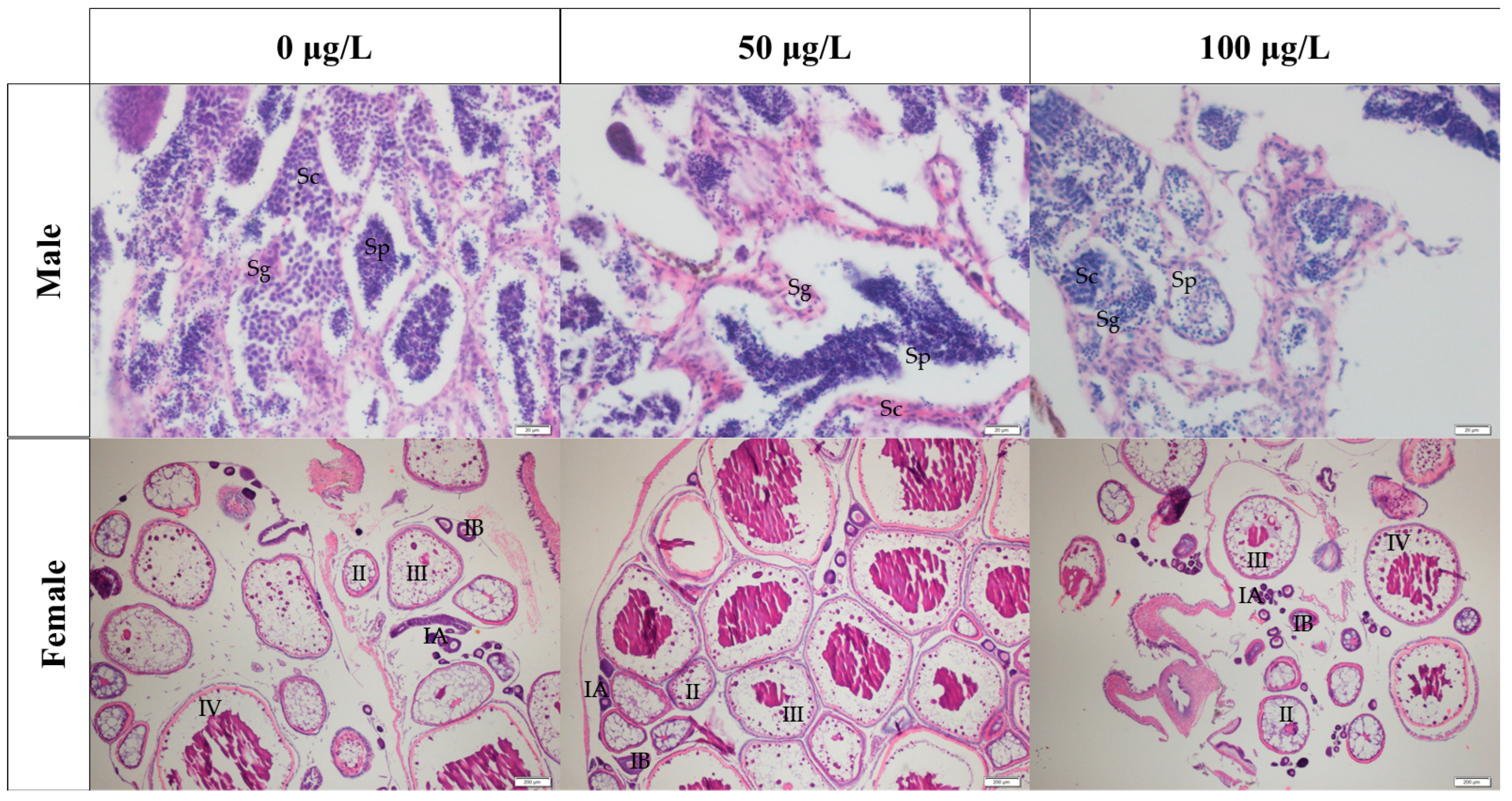
2.2. Effects of Different Concentrations of 5-aza-dC Treatment on the Gonad Development of Medaka Fish
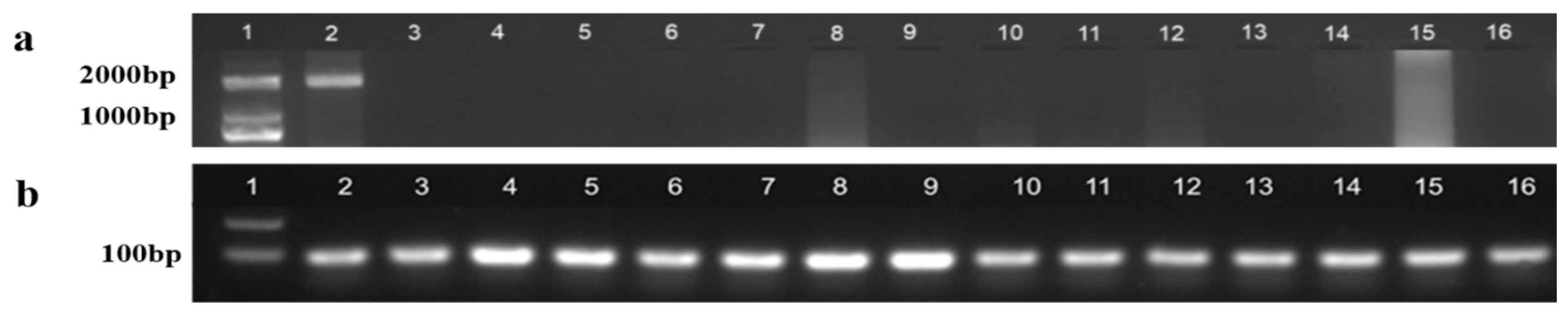
2.3. Sex Determination at the Genetic Level
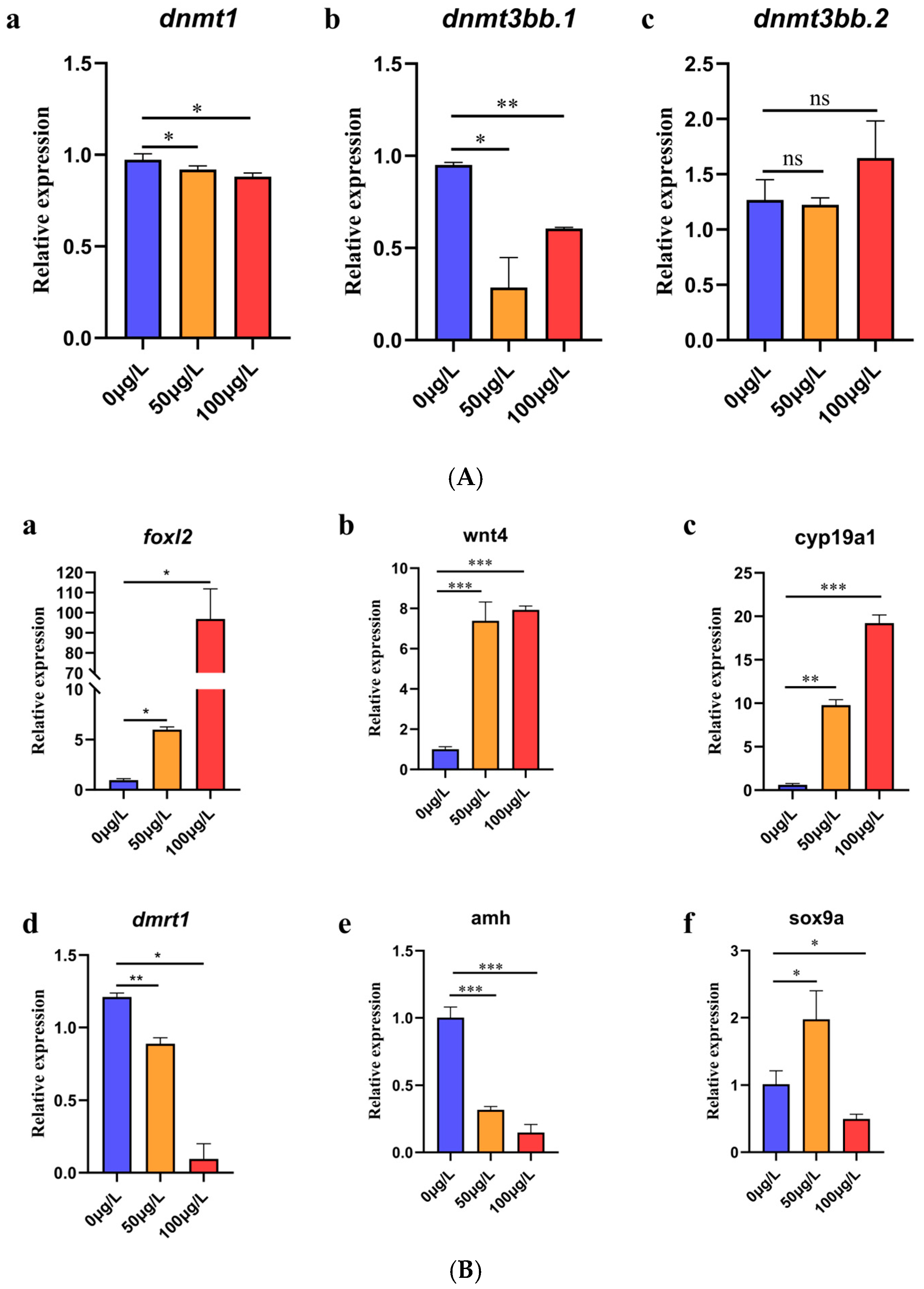
2.4. Analysis of Expression Levels of DNA Methyltransferase Gene and Genes Related to Sex Differentiation
2.4.1. DNA Methyltransferase Gene Expression Level Analysis
2.4.2. Analysis of Expression Levels of Genes Related to Sex Differentiation
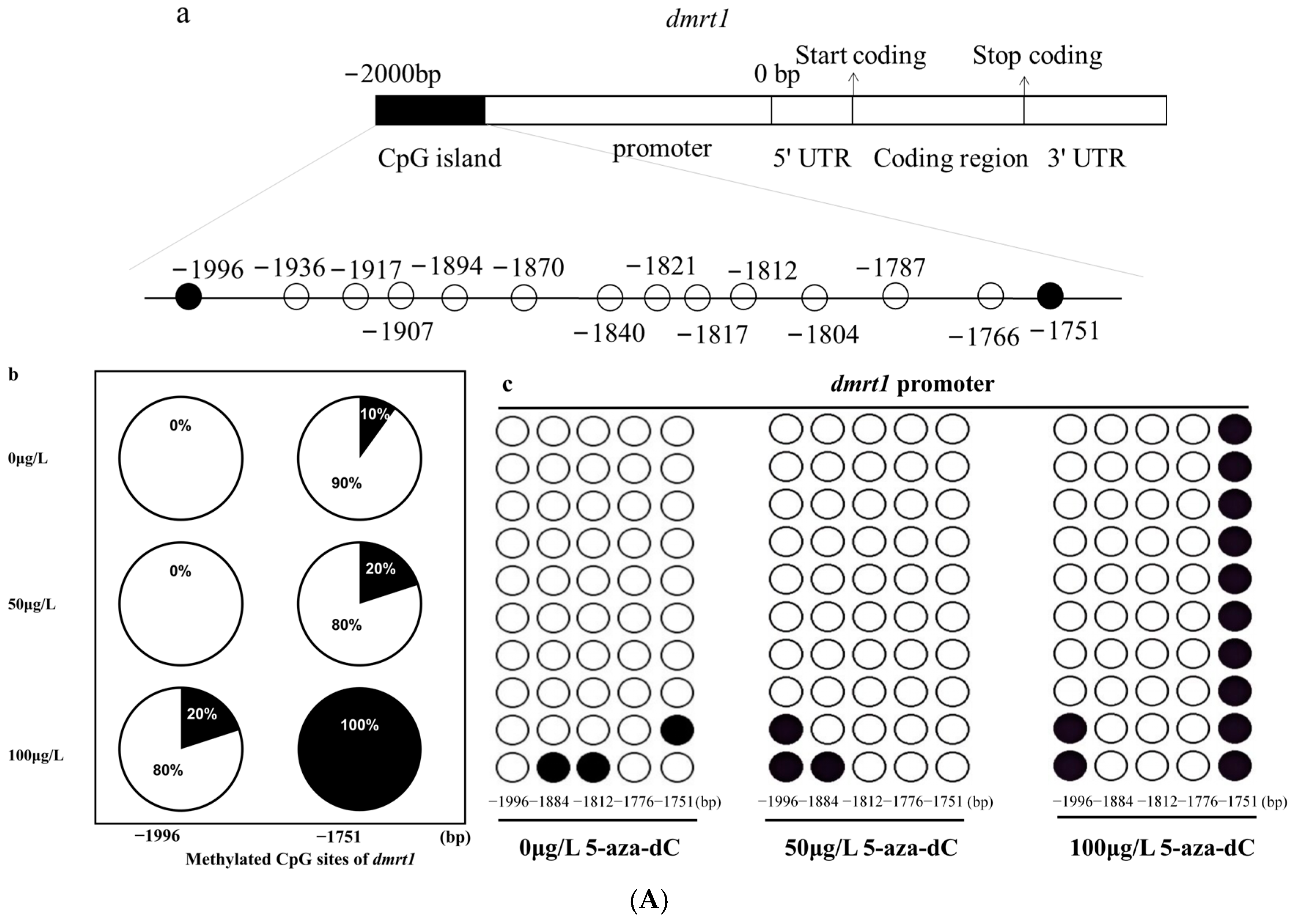
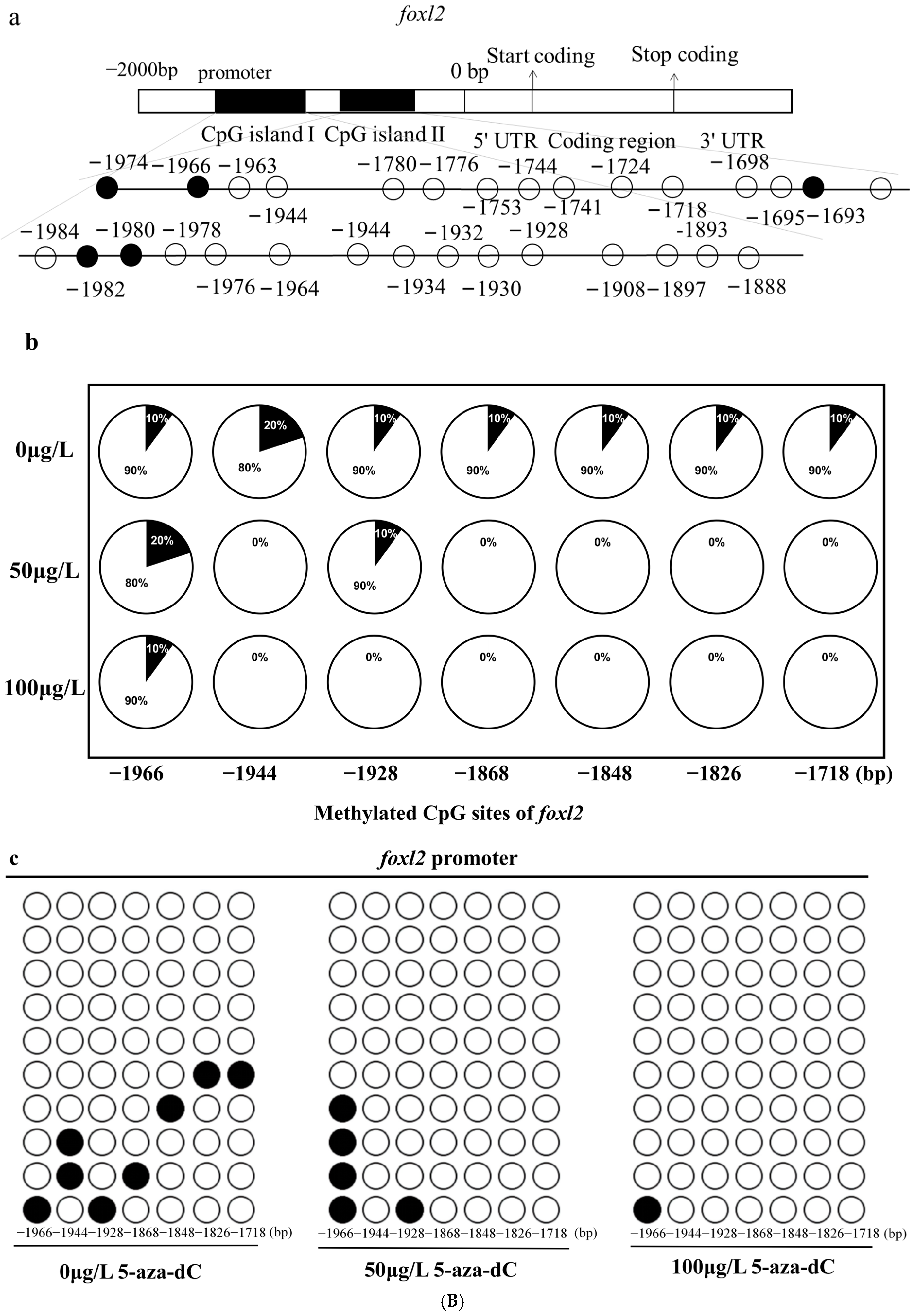
2.5. Methylation Levels of dmrt1 and foxl2 in Response to 5-aza-dC Inhibitors
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Ethics Statement
4.2. Embryos Were Collected and Treated with a 5-aza-dC Gradient Concentration
4.3. DNA Extraction and PCR Amplification
4.4. Paraffin Sections of Gonadal Tissue
4.5. RNA Isolation, cDNA Synthesis, Primer Strategy, and RT-qPCR
4.6. Bisulfite Sequencing PCR (BSP)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirasawa, R.; Chiba, H.; Kaneda, M.; Tajima, S.; Li, E.; Jaenisch, R.; Sasaki, H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008, 22, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Spaziano, A.; Cantone, D.I. X-chromosome reactivation: A concise review. Biochem. Soc. Trans. 2021, 49, 2797–2805. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Cheng, X. DNA methylation mode and biological effect. China Agric. Sci. Technol. Her. 2010, 12, 10–16. [Google Scholar]
- Zhang, Y.; Zhang, S.; Liu, Z.; Zhang, L.; Zhang, W. Epigenetic modifications during sex change repress gonadotropin stimulation of cyp19a1a in a teleost ricefield eel (Monopterus albus). Endocrinology 2013, 154, 2881–2890. [Google Scholar] [CrossRef]
- Kang, Y.; Guan, G.; Hong, Y. An overview of sex determination and sexual differentiation of bony fish using model organisms. Inheritance 2017, 39, 441–454. [Google Scholar] [CrossRef]
- Hervouet, E.; Peixoto, P.; Delage-Mourroux, R.; Boyer-Guittaut, M.; Cartron, P.F. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenet. 2018, 10, 17. [Google Scholar] [CrossRef]
- Wang, F. Function of DNA Methylase in Sexual Differentiation and Gonad Development of Nile Tilapia. Ph.D. Thesis, Southwest University, Chongqing, China, 2022. [Google Scholar] [CrossRef]
- Brandeis, M.; Frank, D.; Keshet, I.; Siegfried, Z.; Mendelsohn, M.; Nemes, A.; Temper, V.; Razin, A.; Cedar, H. Sp1 elements protect a CpG island from de novo methylation. Nature 1994, 371, 435–438. [Google Scholar] [CrossRef]
- Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Tao, B.; Hu, W. Research progress on sex control breeding of fish. China Agric. Sci. Technol. Her. 2022, 24, 1–10. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kobira, H.; Yamaguchi, T.; Shiraishi, E.; Yazawa, T.; Hirai, T.; Kamei, Y.; Kitano, T. High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol. Reprod. Dev. 2010, 77, 679–686. [Google Scholar] [CrossRef]
- Hattori, R.S.; Gould, R.J.; Fujioka, T.; Saito, T.; Kurita, J.; Strüssmann, C.A.; Yokota, M.; Watanabe, S. Temperature-dependent sex determination in Hd-rR medaka Oryzias latipes: Gender sensitivity, thermal threshold, critical period, and DMRT1 expression profile. Sex. Dev. 2007, 1, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Endo, T.; Yamahira, K.; Hamaguchi, S.; Sakaizumi, M. Induction of female-to-male sex reversal by high temperature treatment in Medaka, Oryzias latipes. Zool. Sci. 2005, 22, 985–988. [Google Scholar] [CrossRef]
- Yamamoto, T.O. 3 Sex differentiation. Fish Physiol. 1969, 3, 117–175. [Google Scholar]
- Wang, X.; Bhandari, R.K. The dynamics of DNA methylation during epigenetic reprogramming of primordial germ cells in medaka (Oryzias latipes). Epigenetics 2020, 15, 483–498. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Li, X. Advances in research on genes related to sex determination and differentiation in telrost fish. J. Henan Norm. Univ. 2017, 45, 72–78. [Google Scholar] [CrossRef]
- Rajendiran, P.; Jaafar, F.; Kar, S.; Sudhakumari, C.; Senthilkumaran, B.; Parhar, I.S. Sex Determination and Differentiation in Teleost: Roles of Genetics, Environment, and Brain. Biology 2021, 10, 973. [Google Scholar] [CrossRef]
- Yan, H.; Liang, L.; Chang, Y.; Sun, B.; Su, B. Effects of genetic and temperature factors on the expression and sex ratio of genes related to sex differentiation in fish. J. Dalian Ocean Univ. 2017, 32, 111–118. [Google Scholar]
- Wang, X.; Bhandari, R.K. DNA methylation reprogramming in medaka fish, a promising animal model for environmental epigenetics research. Environ. Epigenet. 2020, 6, dvaa008. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, C.; Qi, Y.; Li, D.; Hu, Y.; Huang, D. 2,4-Dichlorophenol induced feminization of zebrafish by down-regulating male-related genes through DNA methylation. Ecotoxicol. Environ. Saf. 2020, 189, 110042. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Viñas, J.; Ribas, L.; Díaz, N.; Gutiérrez, A.; Di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef]
- Raynal, N.J.; Charbonneau, M.; Momparler, L.F.; Momparler, R.L. Synergistic effect of 5-Aza-2′-deoxycytidine and genistein in combination against leukemia. Oncol. Res. 2008, 17, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Gabbara, S.; Bhagwat, A.S. The mechanism of inhibition of DNA (cytosine-5) methyltransferases by 5-azacytosine is likely to involve methyl transfer to the inhibitor. Biochem. J. 1995, 307, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ribas, L.; Vanezis, K.; Imués, M.A.; Piferrer, F. Treatment with a DNA methyltransferase inhibitor feminizes zebrafish and induces long term expression changes in the gonads. Epigenet. Chromatin. 2017, 10, 59–66. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Wei, G.; Ma, W.; Zhang, Z.; Chen, X.; Gao, L.; Liu, Z.; Yuan, Y.; Yi, L.; et al. The role of DNA methylation reprogramming during sex determination and transition in zebrafish. Genom. Proteom. Bioinform. 2021, 19, 48–63. [Google Scholar] [CrossRef]
- Han, J.; Hu, Y.; Qi, Y.; Yuan, C.; Naeem, S.; Huang, D. High temperature induced masculinization of zebrafish by down-regulation of sox9b and esr1 via DNA methylation. J. Environ. Sci. 2021, 107, 160–170. [Google Scholar] [CrossRef]
- Kasahara, M.; Naruse, K.; Sasaki, S.; Nakatani, Y.; Qu, W.; Ahsan, B.; Yamada, T.; Nagayasu, Y.; Doi, K.; Kasai, Y.; et al. The medaka draft genome and insights into vertebrate genome evolution. Nature 2007, 447, 714–719. [Google Scholar] [CrossRef]
- Wittbrodt, J.; Shima, A.; Schartl, M. Medaka—A model organism from the far East. Nat. Rev. Genet. 2002, 3, 53–64. [Google Scholar] [CrossRef]
- Dasmahapatra, A.K.; Khan, I.A. DNA methyltransferase expressions in Japanese rice fish (Oryzias latipes) embryogenesis is developmentally regulated and modulated by ethanol and 5-azacytidine. Comput. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 176, 1–9. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Uda, M.; Ottolenghi, C.; Crisponi, L.; Garcia, J.E.; Deiana, M.; Kimber, W.; Forabosco, A.; Cao, A.; Schlessinger, D.; Pilia, G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 2004, 13, 1171–1181. [Google Scholar] [CrossRef]
- Diotel, N.; Le Page, Y.; Mouriec, K.; Tong, S.K.; Pellegrini, E.; Vaillant, C.; Anglade, I.; Brion, F.; Pakdel, F.; Chung, B.C.; et al. Aromatase in the brain of teleost fish: Expression, regulation and putative functions. Front. Neuroendocrinol. 2010, 31, 172–192. [Google Scholar] [CrossRef]
- Boyer, A.; Lapointe, E.; Zheng, X.; Cowan, R.G.; Li, H.; Quirk, S.M.; DeMayo, F.J.; Richards, J.S.; Boerboom, D. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010, 24, 3010–3025. [Google Scholar] [CrossRef] [PubMed]
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011, 476, 101–104. [Google Scholar] [CrossRef] [PubMed]
- De Santa Barbara, P.; Bonneaud, N.; Boizet, B.; Desclozeaux, M.; Moniot, B.; Sudbeck, P.; Scherer, G.; Poulat, F.; Berta, P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol. Cell. Biol. 1998, 18, 6653–6665. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef]
- Jones, P.A.; Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 2009, 10, 805–811. [Google Scholar] [CrossRef]
| Primers | Primer Sequence (5′-3′) | Base Number (bp) | TM (°C) |
|---|---|---|---|
| PG19 | GAACCACAGCTTGAAGACCCCGCTGA | 26 | 64.3 |
| PG20 | GCATCTGCTGGTACTGCTGGTAGTTG | 26 | 62.8 |
| obB-actin4F | CTCTGGTCGTACCACTGGTATCG | 23 | 61.3 |
| obB-actin4R | GCAGAGCGTAGCCTTCATAGATG | 23 | 59.6 |
| Gene Name | Transcript ID | Primer Sequence (5′-3′) | Fragment Size (bp) | TM (°C) |
|---|---|---|---|---|
| dnmt1 | 00000040050 | F: AAGAGAAGAAGCGCCTCAAAGT R: TGAAACTCCGCGAAGAAGAGAA | 255 bp | 61.01/61.01 |
| dnmt3bb.1 | 00000036223 | F: ATGACAACAAAGGCTTCTGCAT R: TACGTCTTCGGAAACAACAGTA | 261 bp | 61.04/60.88 |
| dnmt3bb.2 | 00000036293 | F: GGACGAGTACACAGACCACTCC R: CCTCACCAGACACATGAGCAGG | 149 bp | 61.00/61.02 |
| foxl2 | 001104888.1 | F: CCTCGTCCTACAACCCCTACTC R: CTCATCCCCAACATCCTGCTCC | 242 bp | 61.25/60.93 |
| wnt4 | 001160439.1 | F: ACCGCCGATGGAACTGCTCT R: CAGGCCCTTGTGACCGCAAA | 216 bp | 62.20/61.70 |
| cyp19a1 | 001278879.1 | F: CCCTCATCCTGCTCGTCTGT R: AGGACATAAGCGGGCCCAAA | 178 bp | 59.70/59.70 |
| dmrt1 | 001104680.2 | F: AGGAGGAGCTTGGGATTTGTAG R: GATGTTTAGGGTTCGAGGAGGA | 265 bp | 60.95/60.99 |
| amh | 001360941.1 | F: CTGGCAGAGCAGGAAACGGT R: CACCGTCTTCAGCGCCTTCA | 209 bp | 60.90/61.00 |
| sox9a | 001105086.1 | F: CGCACGATCCTCAGCAGTCA R: AGGGCGCACAGTCTGATTGA | 180 bp | 60.50/59.50 |
| β-actin | 001104808.1 | F: AAAAGGGGCTCATTCTCAACTC R: CTCAACTCTTACTCGGGGAAAA | 203 bp | 60.95/60.99 |
| Primers | Primer Sequence (5′-3′) | Fragment Size (bp) |
|---|---|---|
| foxl2-1-F | AAAAGTTTATTTAGATGAATATTTTTGA | 172 bp |
| foxl2-1-R | AACATATTTATTTTCTACAACCCTACAA | |
| foxl2-2-F | GTATATTGAAGGATGTTGTGTTTTATAT | 378 bp |
| foxl2-2-R | CCACCCATATAAATCCCCCT | |
| Dmrt1-F | GTTTGATTTGTATGTATTTGTGTTTAGA | 323 bp |
| Dmrt1-R | CTTTCCRATCAAAACRAATACCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Xu, L.; Tian, N.; Peng, J. Effects of Treatment with a DNA Methyltransferase Inhibitor 5-aza-dC on Sex Differentiation in Medaka (Oryzias latipes). Int. J. Mol. Sci. 2025, 26, 3280. https://doi.org/10.3390/ijms26073280
Cui X, Xu L, Tian N, Peng J. Effects of Treatment with a DNA Methyltransferase Inhibitor 5-aza-dC on Sex Differentiation in Medaka (Oryzias latipes). International Journal of Molecular Sciences. 2025; 26(7):3280. https://doi.org/10.3390/ijms26073280
Chicago/Turabian StyleCui, Xiaojuan, Liumeiyang Xu, Nan Tian, and Jianjun Peng. 2025. "Effects of Treatment with a DNA Methyltransferase Inhibitor 5-aza-dC on Sex Differentiation in Medaka (Oryzias latipes)" International Journal of Molecular Sciences 26, no. 7: 3280. https://doi.org/10.3390/ijms26073280
APA StyleCui, X., Xu, L., Tian, N., & Peng, J. (2025). Effects of Treatment with a DNA Methyltransferase Inhibitor 5-aza-dC on Sex Differentiation in Medaka (Oryzias latipes). International Journal of Molecular Sciences, 26(7), 3280. https://doi.org/10.3390/ijms26073280




