The Role of Puccinia polysora Underw Effector PpEX in Suppressing Plant Defenses and Facilitating Pathogenicity
Abstract
1. Introduction
2. Results
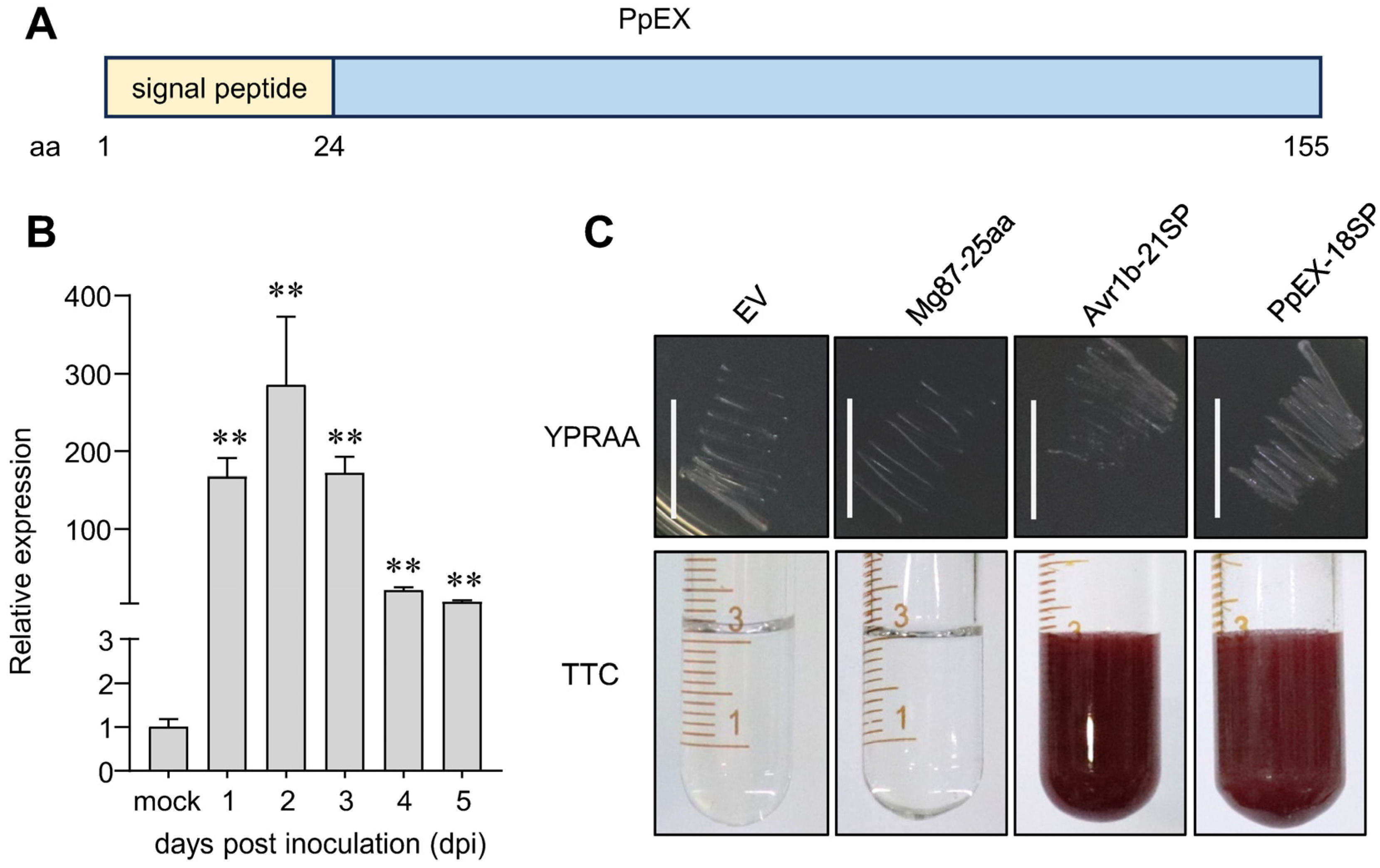
2.1. Characteristics of the Candidate Effector PpEX
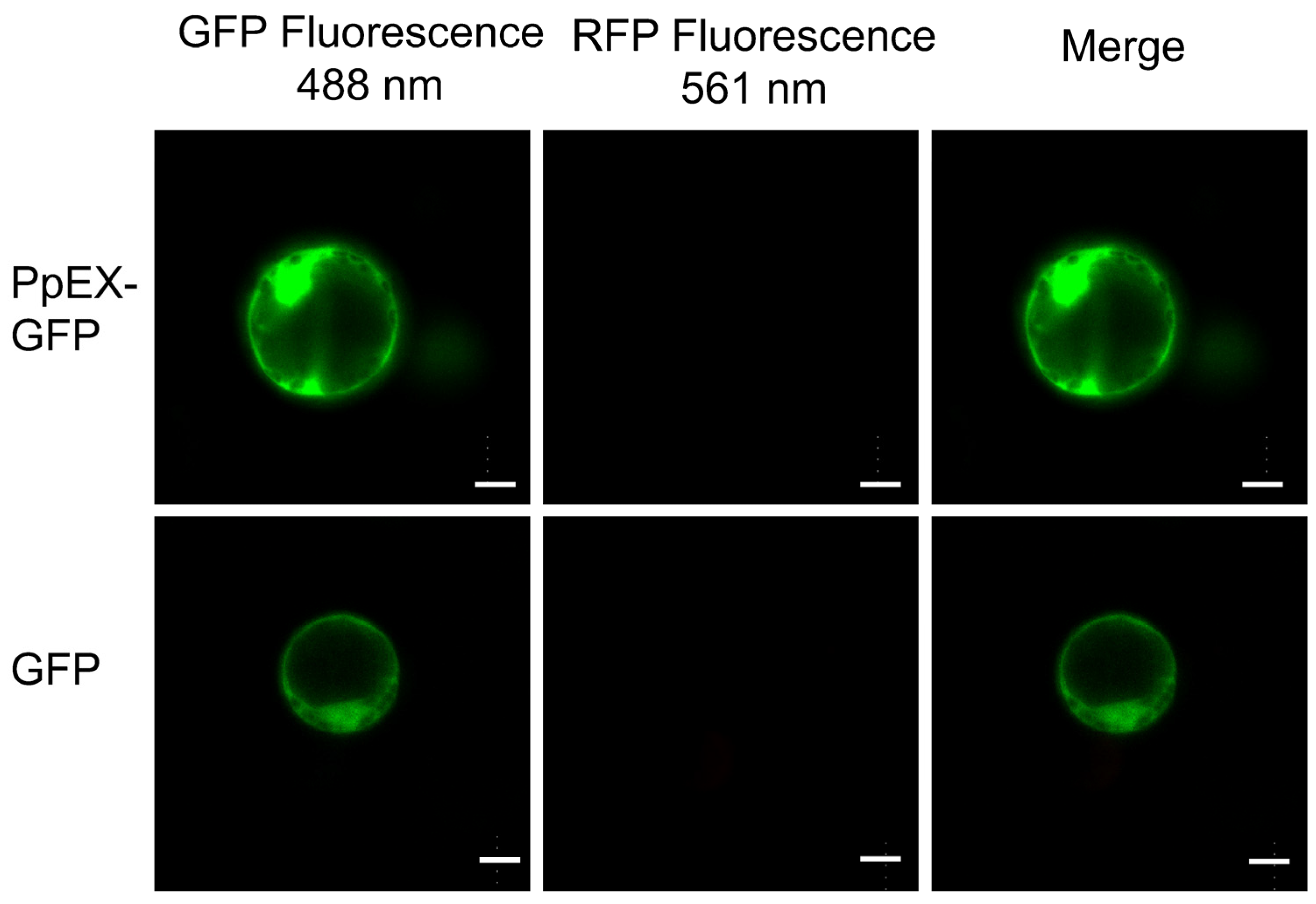
2.2. Subcellular Localization of PpEX in Maize
2.3. Overexpression of PpEX in Nicotiana benthamiana Suppresses Programmed Cell Death
2.4. PpEX Suppresses Maize PTI Immunity
2.5. Overexpression of PpEX Enhances P. polysora Pathogenicity in Maize
2.6. PpEX Interacts with the Maize ZmMPK3
2.7. ZmMPK3 Positively Regulates Plant Resistance to SCR
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Virus Inoculation
4.2. Agrobacterium-Mediated Transient Expression in N. benthamiana
4.3. Gene Expression Analysis
4.4. Subcellular Localization
4.5. Western Blot Analysis
4.6. TurboID Proximity Labeling and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
4.7. Yeast Secretion Signal Trapping System
4.8. Protein Interaction Analysis
4.9. ROS Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aime, M.C. Toward resolving family-level relationships in rust fungi (Uredinales). Mycoscience 2006, 47, 112–122. [Google Scholar] [CrossRef]
- Mei, J.; Zhou, S.; Liu, W. Gene-for-gene-mediated resistance to southern corn rust in maize. Trends Plant Sci. 2023, 28, 255–258. [Google Scholar] [CrossRef]
- Sun, Q.; Li, L.; Guo, F.; Zhang, K.; Dong, J.; Luo, Y.; Ma, Z. Southern corn rust caused by Puccinia polysora Underw: A review. Phytopathol. Res. 2021, 3, 25. [Google Scholar] [CrossRef]
- Deng, C.; Leonard, A.; Cahill, J.; Lv, M.; Li, Y.; Thatcher, S.; Li, X.; Zhao, X.; Du, W.; Li, Z.; et al. The RppC-AvrRppC NLR-effector interaction mediates the resistance to southern corn rust in maize. Mol. Plant 2022, 15, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Espinoza, C.; Liang, Y.; Stacey, G. Chitin receptor CERK1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant J. 2017, 89, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yamaguchi, K.; Shirakawa, T.; Nakagami, H.; Mine, A.; Ishikawa, K.; Fujiwara, M.; Narusaka, M.; Narusaka, Y.; Ichimura, K.; et al. The Arabidopsis CERK1-associated kinase PBL27 connects chitin perception to MAPK activation. EMBO J. 2016, 35, 2468–2483. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yamada, K.; Ishikawa, K.; Yoshimura, S.; Hayashi, N.; Uchihashi, K.; Ishihama, N.; Kishi-Kaboshi, M.; Takahashi, A.; Tsuge, S.; et al. A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe 2013, 13, 347–357. [Google Scholar] [CrossRef]
- Lamb, C.J.; Lawton, M.A.; Dron, M.; Dixon, R.A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell 1989, 56, 215–224. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Staskawicz, B.J.; Dangl, J.L. The plant immune system: From discovery to deployment. Cell 2024, 187, 2095–2116. [Google Scholar] [CrossRef]
- Garnica, D.P.; Nemri, A.; Upadhyaya, N.M.; Rathjen, J.P.; Dodds, P.N. The ins and outs of rust haustoria. PLoS Pathog. 2014, 10, e1004329. [Google Scholar] [CrossRef] [PubMed]
- McCombe, C.L.; Catanzariti, A.M.; Greenwood, J.R.; Desai, A.M.; Outram, M.A.; Yu, D.S.; Ericsson, D.J.; Brenner, S.E.; Dodds, P.N.; Kobe, B.; et al. A rust-fungus Nudix hydrolase effector decaps mRNA in vitro and interferes with plant immune pathways. New Phytol. 2023, 239, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Liu, X.; Wang, X.; Zheng, Q.; Kang, L.; Gao, X.; Wei, Y.; Wu, W.; Zhao, H.; Shan, W. Susceptibility factor RTP1 negatively regulates Phytophthora parasitica resistance via modulating UPR regulators bZIP60 and bZIP28. Plant Physiol. 2021, 186, 1269–1287. [Google Scholar] [CrossRef]
- Ortiz, D.; Chen, J.; Outram, M.A.; Saur, I.M.L.; Upadhyaya, N.M.; Mago, R.; Ericsson, D.J.; Cesari, S.; Chen, C.; Williams, S.J.; et al. The stem rust effector protein AvrSr50 escapes Sr50 recognition by a substitution in a single surface-exposed residue. New Phytol. 2022, 234, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.B.; Liu, M.X.; Chen, T.T.; Ma, X.; Li, Z.K.; Zheng, Z.; Zheng, S.R.; Chen, L.; Li, Y.Z.; Tang, L.R.; et al. Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism. Sci. Adv. 2022, 8, eabq5108. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, K.; Yao, J.; Li, S.; Wang, X.; Huang, L.; Kang, Z. PSTha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp. tritici, is involved in plant defense suppression and rust pathogenicity. Environ. Microbiol. 2017, 19, 1717–1729. [Google Scholar]
- Qi, T.; Guo, J.; Liu, P.; He, F.; Wan, C.; Islam, M.A.; Tyler, B.M.; Kang, Z.; Guo, J. Stripe rust effector PstGSRE1 disrupts nuclear localization of ROS-Promoting transcription factor TaLOL2 to defeat ROS-Induced defense in wheat. Mol Plant. 2019, 12, 1624–1638. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Hu, Z.; Wang, X.; Wang, J.; Wang, J.; Huang, X.; Kang, Z.; Tang, C. The Puccinia striiformis effector Hasp98 facilitates pathogenicity by blocking the kinase activity of wheat TaMAPK4. J. Integr. Plant Biol. 2023, 65, 249–264. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Du, Y.; Cai, K.; Wang, Y.; Guo, J.; Bai, X.; Kang, Z.; Guo, J. A stripe rust fungal effector PstSIE1 targets TaSGT1 to facilitate pathogen infection. Plant J. 2022, 112, 1413–1428. [Google Scholar] [CrossRef]
- Wang, N.; Tang, C.; Fan, X.; He, M.; Gan, P.; Zhang, S.; Hu, Z.; Wang, X.; Yan, T.; Shu, W.; et al. Inactivation of a wheat protein kinase gene confers broad-spectrum resistance to rust fungi. Cell 2022, 185, 2961–2974.e19. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Ding, J.; Wang, H.; Deng, C.; Wang, J.; Yang, Q.; Pi, Q.; Zhang, R.; Zhai, H.; et al. Cloning southern corn rust resistant gene RppK and its cognate gene AvrRppK from Puccinia polysora. Nat. Commun. 2022, 13, 4392. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, Y.; Dodds, P.N.; Figueroa, M.; Sperschneider, J.; Han, S.; Tsui, C.K.M.; Zhang, K.; Li, L.; Ma, Z.; et al. Haplotype-phased and chromosome-level genome assembly of Puccinia polysora, a giga-scale fungal pathogen causing southern corn rust. Mol. Ecol. Resour. 2023, 23, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pruitt, R.N.; Nürnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Thulasi Devendrakumar, K.; Li, X.; Zhang, Y. MAP kinase signalling: Interplays between plant PAMP- and effector-triggered immunity. Cell. Mol. Life Sci. 2018, 75, 2981–2989. [Google Scholar] [CrossRef]
- Meng, X.; Xu, J.; He, Y.; Yang, K.Y.; Mordorski, B.; Liu, Y.; Zhang, S. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 2013, 25, 1126–1142. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Huang, H.; Gao, M.; Wu, D.; Kong, Q.; Zhang, Y. The NLR protein SUMM2 senses the disruption of an immune signaling MAP kinase cascade via CRCK3. EMBO Rep. 2017, 18, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Hou, S.; Wang, X.; Li, Y.; Ren, D.; Chen, S.; Tang, X.; Zhou, J.M. A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell 2010, 22, 2033–2044. [Google Scholar] [CrossRef]
- Jacobs, K.A.; Collins-Racie, L.A.; Colbert, M.; Duckett, M.; Golden-Fleet, M.; Kelleher, K.; Kriz, R.; LaVallie, E.R.; Merberg, D.; Spaulding, V.; et al. A genetic selection for isolating cDNAs encoding secreted proteins. Gene 1997, 198, 289–296. [Google Scholar] [CrossRef]
- Oh, S.K.; Young, C.; Lee, M.; Oliva, R.; Bozkurt, T.O.; Cano, L.M.; Win, J.; Bos, J.I.; Liu, H.Y.; van Damme, M.; et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell 2009, 21, 2928–2947. [Google Scholar] [CrossRef]
- Tian, M.; Win, J.; Savory, E.; Burkhardt, A.; Held, M.; Brandizzi, F.; Day, B. 454 Genome sequencing of Pseudoperonospora cubensis reveals effector proteins with a QXLR translocation motif. Mol. Plant Microbe Interact. 2011, 24, 543–553. [Google Scholar] [CrossRef]
- Gu, B.; Kale, S.D.; Wang, Q.; Wang, D.; Pan, Q.; Cao, H.; Meng, Y.; Kang, Z.; Tyler, B.M.; Shan, W. Rust secreted protein Ps87 is conserved in diverse fungal pathogens and contains a RXLR-like motif sufficient for translocation into plant cells. PLoS ONE 2011, 6, e27217. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Cao, M.; Leung, D.; Tyler, B.M. The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Mol. Plant Microbe Interact. 2004, 17, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013, 11, 800–814. [Google Scholar] [CrossRef]
- Kamoun, S.; van West, P.; de Jong, A.J.; de Groot, K.E.; Vleeshouwers, V.G.; Govers, F. A gene encoding a protein elicitor of Phytophthora infestans is down-regulated during infection of potato. Mol. Plant Microbe Interact. 1997, 10, 13–20. [Google Scholar] [CrossRef]
- Kanzaki, H.; Saitoh, H.; Ito, A.; Fujisawa, S.; Kamoun, S.; Katou, S.; Yoshioka, H.; Terauchi, R. Cytosolic HSP90 and HSP70 are essential components of INF1-mediated hypersensitive response and non-host resistance to Pseudomonas cichorii in Nicotiana benthamiana. Mol. Plant Pathol. 2003, 4, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, C.; Ferreira, A.O.; Yu, X.; Ye, W.; Tripathy, S.; Kale, S.D.; Gu, B.; Sheng, Y.; Sui, Y.; et al. Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell 2011, 23, 2064–2086. [Google Scholar] [CrossRef]
- Du, K.; Peng, D.; Wu, J.; Zhu, Y.; Jiang, T.; Wang, P.; Chen, X.; Jiang, S.; Li, X.; Cao, Z.; et al. Maize splicing-mediated mRNA surveillance impeded by sugarcane mosaic virus-coded pathogenic protein NIa-Pro. Sci. Adv. 2024, 10, eadn3010. [Google Scholar] [CrossRef]
- Sharma, P.C.; Ito, A.; Shimizu, T.; Terauchi, R.; Kamoun, S.; Saitoh, H. Virus-induced silencing of WIPK and SIPK genes reduces resistance to a bacterial pathogen, but has no effect on the INF1-induced hypersensitive response (HR) in Nicotiana benthamiana. Mol. Genet. Genom. 2003, 269, 583–591. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Hu, L.; Zhang, T.; Zhang, G.; Lou, Y. OsMPK3 positively regulates the JA signaling pathway and plant resistance to a chewing herbivore in rice. Plant Cell Rep. 2013, 32, 1075–1084. [Google Scholar] [CrossRef]
- Speth, E.B.; Lee, Y.N.; He, S.Y. Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr. Opin. Plant Biol. 2007, 10, 580–586. [Google Scholar] [CrossRef]
- Ning, N.; Xie, X.; Yu, H.; Mei, J.; Li, Q.; Zuo, S.; Wu, H.; Liu, W.; Li, Z. Plant peroxisome-targeting effector MoPtep1 is required for the virulence of Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 2515. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.J.; Wu, X.Q.; Wen, T.Y.; Qiu, Y.J.; Rui, L.; Zhang, Y.; Ye, J.R. A Bursaphelenchus xylophilus Effector, BxSCD3, suppresses plant defense and contributes to virulence. Int. J. Mol. Sci. 2022, 23, 6417. [Google Scholar] [CrossRef]
- Wang, M.Y.; Chen, J.B.; Wu, R.; Guo, H.L.; Chen, Y.; Li, Z.J.; Wei, L.Y.; Liu, C.; He, S.F.; Du, M.D.; et al. The plant immune receptor SNC1 monitors helper NLRs targeted by a bacterial effector. Cell Host Microbe 2023, 31, 1792–1803.e7. [Google Scholar]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef]
- Park, C.H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Shirsekar, G.; Bellizzi, M.; Chen, S.; Songkumarn, P.; Xie, X.; Shi, X.; Ning, Y.; Zhou, B.; Suttiviriya, P.; et al. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice. PLoS Pathog. 2016, 12, e1005529. [Google Scholar] [CrossRef]
- Zhang, F.; Fang, H.; Wang, M.; He, F.; Tao, H.; Wang, R.; Long, J.; Wang, J.; Wang, G.L.; Ning, Y. APIP5 functions as a transcription factor and an RNA-binding protein to modulate cell death and immunity in rice. Nucleic. Acids Res. 2022, 50, 5064–5079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, H.; Shi, X.; He, F.; Wang, R.; Fan, J.; Bai, P.; Wang, J.; Park, C.H.; Bellizzi, M.; et al. A fungal effector and a rice NLR protein have antagonistic effects on a Bowman-Birk trypsin inhibitor. Plant Biotechnol. J. 2020, 18, 2354–2363. [Google Scholar] [CrossRef]
- Shi, X.; Long, Y.; He, F.; Zhang, C.; Wang, R.; Zhang, T.; Wu, W.; Hao, Z.; Wang, Y.; Wang, G.L.; et al. The fungal pathogen Magnaporthe oryzae suppresses innate immunity by modulating a host potassium channel. PLoS Pathog. 2018, 14, e1006878. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Xiang, T.; Liu, Z.; Laluk, K.; Ding, X.; Zou, Y.; Gao, M.; Zhang, X.; Chen, S.; et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 2010, 7, 290–301. [Google Scholar] [CrossRef]
- Liu, T.; Ji, J.; Cheng, Y.; Zhang, S.; Wang, Z.; Duan, K.; Wang, Y. CRISPR/Cas9-mediated editing of GmTAP1 confers enhanced resistance to Phytophthora sojae in soybean. J. Integr. Plant Biol. 2023, 65, 1609–1612. [Google Scholar] [CrossRef]
- Xu, G.; Zhong, X.; Shi, Y.; Liu, Z.; Jiang, N.; Liu, J.; Ding, B.; Li, Z.; Kang, H.; Ning, Y.; et al. A fungal effector targets a heat shock-dynamin protein complex to modulate mitochondrial dynamics and reduce plant immunity. Sci. Adv. 2020, 6, eabb7719. [Google Scholar] [CrossRef] [PubMed]
- Zuo, N.; Bai, W.Z.; Wei, W.Q.; Yuan, T.L.; Zhang, D.; Wang, Y.Z.; Tang, W.H. Fungal CFEM effectors negatively regulate a maize wall-associated kinase by interacting with its alternatively spliced variant to dampen resistance. Cell Rep. 2022, 41, 111877. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, D.; Xie, K.; Wang, Y.; Liao, Q.; Hong, Y.; Liu, Y. Efficient and high-throughput pseudorecombinant-chimeric Cucumber mosaic virus-based VIGS in maize. Plant Physiol. 2021, 187, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gao, L.; Pan, L.; Shi, Y.; Zeng, R.; Li, M.; Li, Z.; Zhang, X.; Zhao, X.; Gong, X.; Huang, W.; et al. Genetic variation in a heat shock transcription factor modulates cold tolerance in maize. Mol. Plant 2024, 17, 1423–1438. [Google Scholar] [CrossRef]
- Su, Q.; Wang, K.; Zhang, Z. Ecotopic expression of the antimicrobial peptide DmAMP1W improves resistance of transgenic wheat to two diseases: Sharp eyespot and common root rot. Int. J. Mol. Sci. 2020, 21, 647. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, G.; Lal, N.K.; Nagalakshmi, U.; Li, Y.; Zheng, W.; Huang, P.J.; Branon, T.C.; Ting, A.Y.; Walley, J.W.; et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat. Commun. 2019, 10, 3252. [Google Scholar] [CrossRef]
- Chen, H.; Cao, Y.; Li, Y.; Xia, Z.; Xie, J.; Carr, J.P.; Wu, B.; Fan, Z.; Zhou, T. Identification of differentially regulated maize proteins conditioning Sugarcane mosaic virus systemic infection. New Phytol. 2017, 215, 1156–1172. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H.; Willems, A.R.; Woods, R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 1995, 11, 355–360. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Shang, Y.; Lin, H.; Wang, Y.; Cai, R.; Tang, X.; Zhou, J.M. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008, 146, 368–376. [Google Scholar] [CrossRef]
- Bi, G.; Hu, M.; Fu, L.; Zhang, X.; Zuo, J.; Li, J.; Yang, J.; Zhou, J.M. The cytosolic thiol peroxidase PRXIIB is an intracellular sensor for H2O2 that regulates plant immunity through a redox relay. Nat. Plants 2022, 8, 1160–1175. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Qi, X.; Li, K.; Zou, W. The Role of Puccinia polysora Underw Effector PpEX in Suppressing Plant Defenses and Facilitating Pathogenicity. Int. J. Mol. Sci. 2025, 26, 3159. https://doi.org/10.3390/ijms26073159
Su Q, Qi X, Li K, Zou W. The Role of Puccinia polysora Underw Effector PpEX in Suppressing Plant Defenses and Facilitating Pathogenicity. International Journal of Molecular Sciences. 2025; 26(7):3159. https://doi.org/10.3390/ijms26073159
Chicago/Turabian StyleSu, Qiang, Xiaofan Qi, Kunyu Li, and Wenli Zou. 2025. "The Role of Puccinia polysora Underw Effector PpEX in Suppressing Plant Defenses and Facilitating Pathogenicity" International Journal of Molecular Sciences 26, no. 7: 3159. https://doi.org/10.3390/ijms26073159
APA StyleSu, Q., Qi, X., Li, K., & Zou, W. (2025). The Role of Puccinia polysora Underw Effector PpEX in Suppressing Plant Defenses and Facilitating Pathogenicity. International Journal of Molecular Sciences, 26(7), 3159. https://doi.org/10.3390/ijms26073159




