Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications
Abstract
1. Introduction
2. The Current Role of NGS in Solid Tumor Oncology
2.1. Challenging Established Classifications and Redefining Diagnostics with Precision Oncology
2.2. Guiding Compass for Tumor-Agnostic Tumors
2.2.1. Neurotrophic Tyrosine Receptor Kinase (NTRK) Fusion-Positive
2.2.2. Rearranged During Transfection (RET) Fusion-Positive Cancers
2.2.3. Von Hippel–Lindau Disease
2.2.4. Human Epidermal Growth Factor Receptor 2-Positive (Her2-Positive) Tumors
2.2.5. BRAF V600E-Mutated Cancers
2.2.6. High Mutational Burden Tumors
2.2.7. Mismatch Repair Deficient (dMMR)/High Microsatellite Instability (MSI-H) Cancers
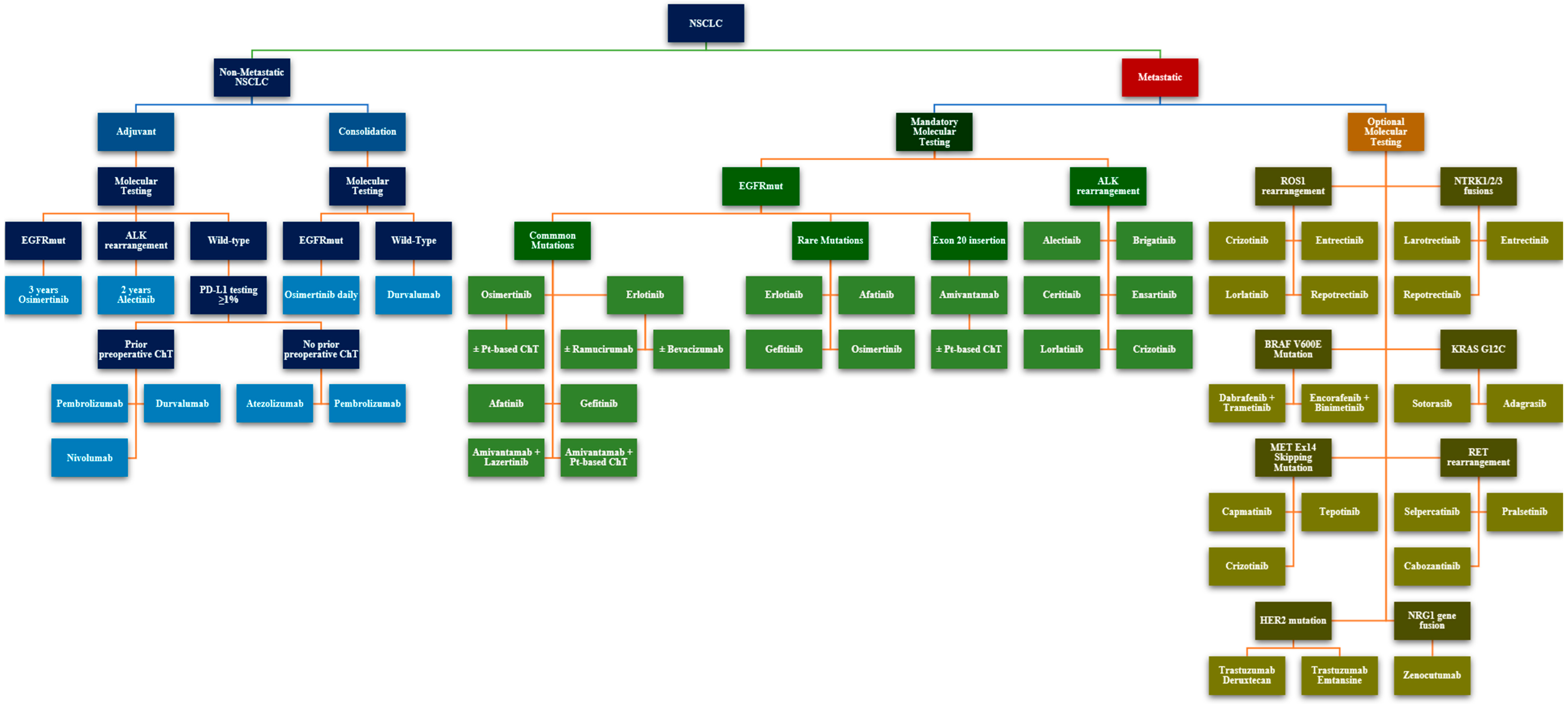
2.3. The Oncogenic Driver Landscape in NSCLC
2.4. Investigating Homologous Repair Deficiencies—Treatment Avenues and Hereditary Cancer Risk Evaluation
2.5. Bridging the Hormone–Chemotherapy Gap in HR-Positive Advanced Breast Cancer
3. Discussion
4. Conclusions
5. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Events |
| ALK | Anaplastic Lymphoma Kinase |
| BRAF | B-Raf Proto-Oncogene |
| BRCA1/2 | Breast Cancer Gene 1/2 |
| CDK4/CDK6 | Cyclin-Dependent Kinases 4 and 6 |
| cfDNA | Circulating Free DNA |
| ChT | Chemotherapy |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| CRC | Colorectal Cancer |
| DFS | Disease-Free Survival |
| dMMR | Deficient Mismatch Repair |
| DoR | Duration of Response |
| EGFR | Epidermal Growth Factor Receptor |
| ER | Estrogen Receptor |
| ERBB2 (HER2) | Erb-B2 Receptor Tyrosine Kinase 2 (also known as HER2) |
| ERBB3 | Erb-B2 Receptor Tyrosine Kinase 3 |
| ESCAT | European Society for Medical Oncology Scale for Clinical Actionability of Molecular Targets |
| ESR1 | Estrogen Receptor 1 |
| FFPE | Formalin-Fixed, Paraffin-Embedded |
| FISH | Fluorescence In Situ Hybridization |
| FGFR1/2/3 | Fibroblast Growth Factor Receptor 1, 2, and 3 |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HR | Hazard Ratio |
| HR+ | Hormone Receptor-Positive |
| HRD | Homologous Recombination Deficiency |
| HRR | Homologous Recombination Repair |
| IHC | Immunohistochemistry |
| KIT | KIT Proto-Oncogene, Receptor Tyrosine Kinase |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene |
| mDoR | Median Duration of Response |
| mDoT | Median Duration of Therapy |
| MET | MET Proto-Oncogene |
| MLH1 | MutL Homolog 1 |
| mOS | Median Overall Survival |
| mPFS | Median Progression-Free Survival |
| MPR | Major Pathological Response |
| MSI | Microsatellite Instability |
| MSI-H | Microsatellite Instability-High |
| MTC | Medullary Thyroid Carcinoma |
| mut/Mb | Mutations per Megabase |
| NCCN | National Comprehensive Cancer Network |
| NGS | Next-Generation Sequencing |
| NR | Not Reached |
| NTRK | Neurotrophic Tyrosine Receptor Kinase |
| NSCLC | Non-Small Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PARPi | Poly (ADP-ribose) Polymerase Inhibitor |
| PD | Progressive Disease |
| PD-1 | Programmed Death-1 |
| PDGFRA | Platelet-Derived Growth Factor Receptor Alpha |
| PFS | Progression-Free Survival |
| PIK3CA | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha |
| PTC | Papillary Thyroid Carcinoma |
| PTEN | Phosphatase and Tensin Homolog |
| q3w | Every 3 Weeks |
| RET | Rearranged During Transfection |
| ROS1 | ROS Proto-Oncogene 1 |
| RT | Radiotherapy |
| SCLC | Small Cell Lung Cancer |
| SoC | Standard of Care |
| TMB | Tumour Mutational Burden |
| TKI | Tyrosine Kinase Inhibitor |
| TME | Total Mesorectal Excision |
| TRKA/B/C | Tropomyosin Receptor Kinase A/B/C |
| TSC1/2 | Tuberous Sclerosis Complex 1 and 2 |
| VHL | Von Hippel–Lindau |
References
- Targeted Therapy Drug List by Cancer Type—NCI. Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/approved-drug-list (accessed on 14 December 2024).
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision Oncology: Who, How, What, When, and When Not? In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2024. [Google Scholar]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Galeș, L.N.; Păun, M.-A.; Anghel, R.M.; Trifănescu, O.G. Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening. Cancers 2024, 16, 1191. [Google Scholar] [CrossRef]
- Milbury, C.A.; Creeden, J.; Yip, W.-K.; Smith, D.L.; Pattani, V.; Maxwell, K.; Sawchyn, B.; Gjoerup, O.; Meng, W.; Skoletsky, J.; et al. Clinical and Analytical Validation of FoundationOne®CDx, a Comprehensive Genomic Profiling Assay for Solid Tumors. PLoS ONE 2022, 17, e0264138. [Google Scholar] [CrossRef]
- Takeda, M.; Takahama, T.; Sakai, K.; Shimizu, S.; Watanabe, S.; Kawakami, H.; Tanaka, K.; Sato, C.; Hayashi, H.; Nonagase, Y.; et al. Clinical Application of the FoundationOne CDx Assay to Therapeutic Decision-Making for Patients with Advanced Solid Tumors. Oncologist 2021, 26, e588–e596. [Google Scholar] [CrossRef]
- Woodhouse, R.; Li, M.; Hughes, J.; Delfosse, D.; Skoletsky, J.; Ma, P.; Meng, W.; Dewal, N.; Milbury, C.; Clark, T.; et al. Clinical and Analytical Validation of FoundationOne Liquid CDx, a Novel 324-Gene cfDNA-Based Comprehensive Genomic Profiling Assay for Cancers of Solid Tumor Origin. PLoS ONE 2020, 15, e0237802. [Google Scholar] [CrossRef]
- Bauml, J.M.; Li, B.T.; Velcheti, V.; Govindan, R.; Curioni-Fontecedro, A.; Dooms, C.; Takahashi, T.; Duda, A.W.; Odegaard, J.I.; Cruz-Guilloty, F.; et al. Clinical Validation of Guardant360 CDx as a Blood-Based Companion Diagnostic for Sotorasib. Lung Cancer Amst. Neth. 2022, 166, 270–278. [Google Scholar] [CrossRef]
- Cheng, D.T.; Prasad, M.; Chekaluk, Y.; Benayed, R.; Sadowska, J.; Zehir, A.; Syed, A.; Wang, Y.E.; Somar, J.; Li, Y.; et al. Comprehensive Detection of Germline Variants by MSK-IMPACT, a Clinical Diagnostic Platform for Solid Tumor Molecular Oncology and Concurrent Cancer Predisposition Testing. BMC Med. Genom. 2017, 10, 33. [Google Scholar] [CrossRef]
- Saito, A.; Terai, H.; Kim, T.; Emoto, K.; Kawano, R.; Nakamura, K.; Hayashi, H.; Takaoka, H.; Ogata, A.; Kinoshita, K.; et al. Clinical Utility of the Oncomine Dx Target Test multi-CDx System and the Possibility of Utilizing Those Original Sequence Data. Cancer Med. 2024, 13, e7077. [Google Scholar] [CrossRef]
- Dumur, C.I.; Krishnan, R.; Almenara, J.A.; Brown, K.E.; Dugan, K.R.; Farni, C.; Ibrahim, F.Z.; Sanchez, N.A.; Rathore, S.; Pradhan, D.; et al. Analytical Validation and Clinical Utilization of the Oncomine Comprehensive Assay Plus Panel for Comprehensive Genomic Profiling in Solid Tumors. J. Mol. Pathol. 2023, 4, 109–127. [Google Scholar] [CrossRef]
- Beaubier, N.; Tell, R.; Lau, D.; Parsons, J.R.; Bush, S.; Perera, J.; Sorrells, S.; Baker, T.; Chang, A.; Michuda, J.; et al. Clinical Validation of the Tempus xT Next-Generation Targeted Oncology Sequencing Assay. Oncotarget 2019, 10, 2384–2396. [Google Scholar] [CrossRef]
- Carter, P.; Alifrangis, C.; Cereser, B.; Chandrasinghe, P.; Del Bel Belluz, L.; Moderau, N.; Poyia, F.; Schwartzberg, L.S.; Tabassum, N.; Wen, J.; et al. Molecular Profiling of Advanced Breast Cancer Tumors Is Beneficial in Assisting Clinical Treatment Plans. Oncotarget 2018, 9, 17589–17596. [Google Scholar] [CrossRef] [PubMed]
- Mosele, M.F.; Westphalen, C.B.; Stenzinger, A.; Barlesi, F.; Bayle, A.; Bièche, I.; Bonastre, J.; Castro, E.; Dienstmann, R.; Krämer, A.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Advanced Cancer in 2024: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2024, 35, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Johnson, A.; Sklar, J.; Lindeman, N.I.; Moore, K.; Ganesan, S.; Lovly, C.M.; Perlmutter, J.; Gray, S.W.; Hwang, J.; et al. Somatic Genomic Testing in Patients with Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2022, 40, 1231–1258. [Google Scholar] [CrossRef] [PubMed]
- Trifănescu, O.G.; Trifănescu, R.A.; Mitrică, R.; Mitrea, D.; Ciornei, A.; Georgescu, M.; Butnariu, I.; Galeș, L.N.; Șerbănescu, L.; Anghel, R.M.; et al. Upstaging and Downstaging in Gliomas-Clinical Implications for the Fifth Edition of the World Health Organization Classification of Tumors of the Central Nervous System. Diagnostics 2023, 13, 197. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Nannini, M.; Rizzo, A.; Indio, V.; Schipani, A.; Astolfi, A.; Pantaleo, M.A. Targeted Therapy in SDH-Deficient GIST. Ther. Adv. Med. Oncol. 2021, 13, 17588359211023278. [Google Scholar] [CrossRef] [PubMed]
- Radu, P.; Zurzu, M.; Paic, V.; Bratucu, M.; Garofil, D.; Tigora, A.; Georgescu, V.; Prunoiu, V.; Popa, F.; Surlin, V.; et al. Interstitial Cells of Cajal—Origin, Distribution and Relationship with Gastrointestinal Tumors. Medicina 2023, 59, 63. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, L.; Xu, P.; Hu, W. An Overview of Agents and Treatments for PDGFRA-Mutated Gastrointestinal Stromal Tumors. Front. Oncol. 2022, 12, 927587. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Le, D.T.; Lemery, S. Tissue-Agnostic Drug Development. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2025. [Google Scholar]
- Subbiah, V.; Gouda, M.A.; Ryll, B.; Burris, H.A.; Kurzrock, R. The Evolving Landscape of Tissue-agnostic Therapies in Precision Oncology. CA. Cancer J. Clin. 2024, 74, 433–452. [Google Scholar] [CrossRef]
- Coquerelle, S.; Darlington, M.; Michel, M.; Durand, M.; Borget, I.; Baffert, S.; Marino, P.; Perrier, L.; Durand-Zaleski, I. Impact of Next Generation Sequencing on Clinical Practice in Oncology in France: Better Genetic Profiles for Patients Improve Access to Experimental Treatments. Value Health 2020, 23, 898–906. [Google Scholar] [CrossRef]
- Park, J.J.H.; Hsu, G.; Siden, E.G.; Thorlund, K.; Mills, E.J. An Overview of Precision Oncology Basket and Umbrella Trials for Clinicians. CA Cancer J. Clin. 2020, 70, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Ramagopalan, S.V.; Ray, J.; Roze, S.; Subbiah, V. Assessment of Tumour-Agnostic Therapies in Basket Trials. Lancet Oncol. 2022, 23, e7. [Google Scholar] [CrossRef]
- Lengliné, E.; Peron, J.; Vanier, A.; Gueyffier, F.; Kouzan, S.; Dufour, P.; Guillot, B.; Blondon, H.; Clanet, M.; Cochat, P.; et al. Basket Clinical Trial Design for Targeted Therapies for Cancer: A French National Authority for Health Statement for Health Technology Assessment. Lancet Oncol. 2021, 22, e430–e434. [Google Scholar] [CrossRef]
- Gouda, M.A.; Nelson, B.E.; Buschhorn, L.; Wahida, A.; Subbiah, V. Tumor-Agnostic Precision Medicine from the AACR GENIE Database: Clinical Implications. Clin. Cancer Res. 2023, 29, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for Paediatric Solid Tumours Harbouring NTRK Gene Fusions: A Multicentre, Open-Label, Phase 1 Study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef]
- Brose, M.S.; Westphalen, C.B.; Kehl, K.L.; Pan, X.; Bernard-Gauthier, V.; Kurtinecz, M.; Guo, H.; Aris, V.; Brett, N.R.; Majdi, A.; et al. Outcomes of Larotrectinib Compared with Real-World Data from Non-TRK Inhibitor Therapies in Patients with TRK Fusion Cancer: VICTORIA Study. J. Clin. Oncol. 2024, 42, 3105. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multi-Targeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib (RXDX-101): Combined Results from Two Phase 1 Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1–2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Desai, A.V.; Robinson, G.W.; Gauvain, K.; Basu, E.M.; Macy, M.E.; Maese, L.; Whipple, N.S.; Sabnis, A.J.; Foster, J.H.; Shusterman, S.; et al. Entrectinib in Children and Young Adults with Solid or Primary CNS Tumors Harboring NTRK, ROS1, or ALK Aberrations (STARTRK-NG). Neuro-Oncology 2022, 24, 1776–1789. [Google Scholar] [CrossRef]
- Drilon, A.; Camidge, D.R.; Lin, J.J.; Kim, S.-W.; Solomon, B.J.; Dziadziuszko, R.; Besse, B.; Goto, K.; De Langen, A.J.; Wolf, J.; et al. Repotrectinib in ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 118–131. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-Agnostic Efficacy and Safety of Selpercatinib in Patients with RET Fusion-Positive Solid Tumours Other than Lung or Thyroid Tumours (LIBRETTO-001): A Phase 1/2, Open-Label, Basket Trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Brose, M.S.; Subbiah, V.; Worden, F.; Solomon, B.; Robinson, B.; Hadoux, J.; Tomasini, P.; Weiler, D.; Deschler-Baier, B.; et al. Durability of Response with Selpercatinib in Patients with RET-Activated Thyroid Cancer: Long-Term Safety and Efficacy From LIBRETTO-001. J. Clin. Oncol. 2024, 42, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Shao-Weng Tan, D.; Alonso, G.; Wolf, J.; Park, K.; et al. Selpercatinib in Patients with RET Fusion–Positive Non–Small-Cell Lung Cancer: Updated Safety and Efficacy from the Registrational LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. 2023, 41, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-Cancer Efficacy of Pralsetinib in Patients with RET Fusion–Positive Solid Tumors from the Phase 1/2 ARROW Trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Griesinger, F.; Curigliano, G.; Thomas, M.; Subbiah, V.; Baik, C.S.; Tan, D.S.W.; Lee, D.H.; Misch, D.; Garralda, E.; Kim, D.-W.; et al. Safety and Efficacy of Pralsetinib in RET Fusion–Positive Non-Small-Cell Lung Cancer Including as First-Line Therapy: Update from the ARROW Trial. Ann. Oncol. 2022, 33, 1168–1178. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET Fusion-Positive Non-Small-Cell Lung Cancer (ARROW): A Multi-Cohort, Open-Label, Phase 1/2 Study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for Patients with Advanced or Metastatic RET-Altered Thyroid Cancer (ARROW): A Multi-Cohort, Open-Label, Registrational, Phase 1/2 Study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef]
- Lannon, C.L.; Sorensen, P.H.B. ETV6–NTRK3: A Chimeric Protein Tyrosine Kinase with Transformation Activity in Multiple Cell Lineages. Semin. Cancer Biol. 2005, 15, 215–223. [Google Scholar] [CrossRef]
- Pulciani, S.; Santos, E.; Lauver, A.V.; Long, L.K.; Aaronson, S.A.; Barbacid, M. Oncogenes in Solid Human Tumours. Nature 1982, 300, 539–542. [Google Scholar] [CrossRef]
- Ruiz-Cordero, R.; Ng, D.L. Neurotrophic Receptor Tyrosine Kinase (NTRK) Fusions and Their Role in Cancer. Cancer Cytopathol. 2020, 128, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Davis, L.E.; Vaishnavi, A.; Le, A.T.; Estrada-Bernal, A.; Keysar, S.; Jimeno, A.; Varella-Garcia, M.; Aisner, D.L.; Li, Y.; et al. An Oncogenic NTRK Fusion in a Soft Tissue Sarcoma Patient with Response to the Tropomyosin-Related Kinase (TRK) Inhibitor LOXO-101. Cancer Discov. 2015, 5, 1049–1057. [Google Scholar] [CrossRef]
- Menichincheri, M.; Ardini, E.; Magnaghi, P.; Avanzi, N.; Banfi, P.; Bossi, R.; Buffa, L.; Canevari, G.; Ceriani, L.; Colombo, M.; et al. Correction to Discovery of Entrectinib: A New 3-Aminoindazole as a Potent Anaplastic Lymphoma Kinase (ALK), c-Ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) Inhibitor. J. Med. Chem. 2019, 62, 8364. [Google Scholar] [CrossRef]
- Demetri, G.D.; De Braud, F.; Drilon, A.; Siena, S.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; Chiu, C.-H.; Lin, J.J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Patients with NTRK Fusion-Positive Solid Tumors. Clin. Cancer Res. 2022, 28, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Michos, O.; Shakya, R.; Riccio, P.; Enomoto, H.; Licht, J.D.; Asai, N.; Takahashi, M.; Ohgami, N.; Kato, M.; et al. Ret-Dependent Cell Rearrangements in the Wolffian Duct Epithelium Initiate Ureteric Bud Morphogenesis. Dev. Cell 2009, 17, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Crawford, P.A.; Gorodinsky, A.; Heuckeroth, R.O.; Johnson, E.M.; Milbrandt, J. RET Signaling Is Essential for Migration, Axonal Growth and Axon Guidance of Developing Sympathetic Neurons. Dev. Camb. Engl. 2001, 128, 3963–3974. [Google Scholar] [CrossRef]
- Enomoto, H.; Heuckeroth, R.O.; Golden, J.P.; Johnson, E.M.; Milbrandt, J. Development of Cranial Parasympathetic Ganglia Requires Sequential Actions of GDNF and Neurturin. Dev. Camb. Engl. 2000, 127, 4877–4889. [Google Scholar] [CrossRef]
- Pachnis, V.; Mankoo, B.; Costantini, F. Expression of the C-Ret Proto-Oncogene during Mouse Embryogenesis. Development 1993, 119, 1005–1017. [Google Scholar] [CrossRef]
- Trupp, M.; Rydén, M.; Jörnvall, H.; Funakoshi, H.; Timmusk, T.; Arenas, E.; Ibáñez, C.F. Peripheral Expression and Biological Activities of GDNF, a New Neurotrophic Factor for Avian and Mammalian Peripheral Neurons. J. Cell Biol. 1995, 130, 137–148. [Google Scholar]
- Regua, A.T.; Najjar, M.; Lo, H.-W. RET Signaling Pathway and RET Inhibitors in Human Cancer. Front. Oncol. 2022, 12, 932353. [Google Scholar] [CrossRef]
- Grieco, M.; Santoro, M.; Berlingieri, M.T.; Melillo, R.M.; Donghi, R.; Bongarzone, I.; Pierotti, M.A.; Della Ports, G.; Fusco, A.; Vecchiot, G. PTC Is a Novel Rearranged Form of the Ret Proto-Oncogene and Is Frequently Detected in Vivo in Human Thyroid Papillary Carcinomas. Cell 1990, 60, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-H.I.; Zhu, V.W. Catalog of 5′ Fusion Partners in RET+ NSCLC Circa 2020. JTO Clin. Res. Rep. 2020, 1, 100037. [Google Scholar] [CrossRef]
- Subbiah, V.; Velcheti, V.; Tuch, B.B.; Ebata, K.; Busaidy, N.L.; Cabanillas, M.E.; Wirth, L.J.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET Kinase Inhibition for Patients with RET-Altered Cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; Iversen, A.B.B.; et al. LITESPARK-004 (MK-6482-004) Phase 2 Study of Belzutifan, an Oral Hypoxia-Inducible Factor 2α Inhibitor (HIF-2α), for von Hippel-Lindau (VHL) Disease: Update with More than Two Years of Follow-up Data. J. Clin. Oncol. 2022, 40, 4546–4546. [Google Scholar] [CrossRef]
- Else, T.; Jonasch, E.; Iliopoulos, O.; Beckermann, K.E.; Narayan, V.; Maughan, B.L.; Oudard, S.; Maranchie, J.K.; Iversen, A.B.; Goldberg, C.M.; et al. Belzutifan for von Hippel–Lindau Disease: Pancreatic Lesion Population of the Phase 2 LITESPARK-004 Study. Clin. Cancer Res. 2024, 30, 1750–1757. [Google Scholar] [CrossRef]
- Iliopoulos, O.; Iversen, A.B.; Narayan, V.; Maughan, B.L.; Beckermann, K.E.; Oudard, S.; Else, T.; Maranchie, J.K.; Goldberg, C.M.; Fu, W.; et al. Belzutifan for Patients with von Hippel-Lindau Disease-Associated CNS Haemangioblastomas (LITESPARK-004): A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2024, 25, 1325–1336. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef]
- Curry, L.; Soleimani, M. Belzutifan: A Novel Therapeutic for the Management of von Hippel–Lindau Disease and Beyond. Future Oncol. 2024, 20, 1251–1266. [Google Scholar] [CrossRef]
- Rubin, I.; Yarden, Y. The Basic Biology of HER2. Ann. Oncol. 2001, 12, S3–S8. [Google Scholar] [CrossRef]
- Cho, H.-S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W.; Leahy, D.J. Structure of the Extracellular Region of HER2 Alone and in Complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Xia, X.; Gong, C.; Zhang, Y.; Xiong, H. The History and Development of HER2 Inhibitors. Pharmaceuticals 2023, 16, 1450. [Google Scholar] [CrossRef]
- Kallioniemi, O.P.; Kallioniemi, A.; Kurisu, W.; Thor, A.; Chen, L.C.; Smith, H.S.; Waldman, F.M.; Pinkel, D.; Gray, J.W. ERBB2 Amplification in Breast Cancer Analyzed by Fluorescence in Situ Hybridization. Proc. Natl. Acad. Sci. USA 1992, 89, 5321–5325. [Google Scholar] [PubMed]
- Citri, A.; Yarden, Y. EGF–ERBB Signalling: Towards the Systems Level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Yarden, Y. Biology of HER2 and Its Importance in Breast Cancer. Oncology 2001, 61, 1–13. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Bazley, L.A.; Gullick, W.J. The Epidermal Growth Factor Receptor Family. Endocr. Relat. Cancer 2005, 12, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the Preferred Heterodimerization Partner of All ErbB Receptors, Is a Mediator of Lateral Signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Hainsworth, J.D.; Bose, R.; Burris, H.A.; Kurzrock, R.; Swanton, C.; Friedman, C.F.; Spigel, D.R.; Szado, T.; Schulze, K.; et al. MyPathway Human Epidermal Growth Factor Receptor 2 Basket Study: Pertuzumab + Trastuzumab Treatment of a Tissue-Agnostic Cohort of Patients with Human Epidermal Growth Factor Receptor 2–Altered Advanced Solid Tumors. J. Clin. Oncol. 2024, 42, 258–265. [Google Scholar] [CrossRef]
- Gouda, M.A.; Subbiah, V. Precision Oncology for BRAF-Mutant Cancers with BRAF and MEK Inhibitors: From Melanoma to Tissue-Agnostic Therapy. ESMO Open 2023, 8, 100788. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Karasarides, M.; Marais, R. The RAF Proteins Take Centre Stage. Nat. Rev. Mol. Cell Biol. 2004, 5, 875–885. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF Alterations in Cancer: New Rational Therapeutic Strategies for Actionable Mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Corcoran, R.B. Targeting Alterations in the RAF–MEK Pathway. Cancer Discov. 2019, 9, 329–341. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Yu, Y.; Zhi, J.; Zheng, X.; Yu, J.; Gao, M. Comparison of Diagnostic Methods for the Detection of a BRAF Mutation in Papillary Thyroid Cancer. Oncol. Lett. 2019, 17, 4661–4666. [Google Scholar] [CrossRef]
- Cheng, L.; Lopez-Beltran, A.; Massari, F.; MacLennan, G.T.; Montironi, R. Molecular Testing for BRAF Mutations to Inform Melanoma Treatment Decisions: A Move toward Precision Medicine. Mod. Pathol. 2018, 31, 24–38. [Google Scholar] [CrossRef]
- Szymonek, M.; Kowalik, A.; Kopczyński, J.; Gąsior-Perczak, D.; Pałyga, I.; Walczyk, A.; Gadawska-Juszczyk, K.; Płusa, A.; Mężyk, R.; Chrapek, M.; et al. Immunohistochemistry Cannot Replace DNA Analysis for Evaluation of BRAF V600E Mutations in Papillary Thyroid Carcinoma. Oncotarget 2017, 8, 74897–74909. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Jeong, B.-K.; Yoon, Y.S.; Yu, C.S.; Kim, J. Utility of BRAF VE1 Immunohistochemistry as a Screening Tool for Colorectal Cancer Harboring BRAF V600E Mutation. J. Pathol. Transl. Med. 2018, 52, 157–163. [Google Scholar] [CrossRef]
- Chakravarty, D.; Gao, J.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus Trametinib in Patients with BRAF V600E-Mutant Anaplastic Thyroid Cancer: Updated Analysis from the Phase II ROAR Basket Study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; De Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600E-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Stein, A.; Van Den Bent, M.; De Greve, J.; Wick, A.; De Vos, F.Y.F.L.; Von Bubnoff, N.; Van Linde, M.E.; Lai, A.; Prager, G.W.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600E-Mutant Low-Grade and High-Grade Glioma (ROAR): A Multicentre, Open-Label, Single-Arm, Phase 2, Basket Trial. Lancet Oncol. 2022, 23, 53–64. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Moreau, P.; Ravandi, F.; Hutchings, M.; Gazzah, A.; Michallet, A.-S.; Wainberg, Z.A.; Stein, A.; Dietrich, S.; de Jonge, M.J.A.; et al. Dabrafenib plus Trametinib in Patients with Relapsed/Refractory BRAF V600E Mutation–Positive Hairy Cell Leukemia. Blood 2023, 141, 996–1006. [Google Scholar] [CrossRef]
- Fusco, M.J.; West, H.; Walko, C.M. Tumor Mutation Burden and Cancer Treatment. JAMA Oncol. 2021, 7, 316. [Google Scholar] [CrossRef]
- Galuppini, F.; Dal Pozzo, C.A.; Deckert, J.; Loupakis, F.; Fassan, M.; Baffa, R. Tumor Mutation Burden: From Comprehensive Mutational Screening to the Clinic. Cancer Cell Int. 2019, 19, 209. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Solit, D.B.; Chan, T.A.; Kurzrock, R. The FDA Approval of Pembrolizumab for Adult and Pediatric Patients with Tumor Mutational Burden (TMB) ≥10: A Decision Centered on Empowering Patients and Their Physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Kang, Y.-J.; O’Haire, S.; Franchini, F.; IJzerman, M.; Zalcberg, J.; Macrae, F.; Canfell, K.; Steinberg, J. A Scoping Review and Meta-Analysis on the Prevalence of Pan-Tumour Biomarkers (dMMR, MSI, High TMB) in Different Solid Tumours. Sci. Rep. 2022, 12, 20495. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; de Braud, F.; Arkenau, H.-T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients with Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef]
- Frenel, J.-S.; Le Tourneau, C.; O’Neil, B.; Ott, P.A.; Piha-Paul, S.A.; Gomez-Roca, C.; van Brummelen, E.M.J.; Rugo, H.S.; Thomas, S.; Saraf, S.; et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J. Clin. Oncol. 2017, 35, 4035–4041. [Google Scholar] [CrossRef] [PubMed]
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B.; et al. Pembrolizumab for Patients with PD-L1-Positive Advanced Gastric Cancer (KEYNOTE-012): A Multicentre, Open-Label, Phase 1b Trial. Lancet Oncol. 2016, 17, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability–High/Mismatch Repair–Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Georgescu, M.-T.; Patrascu, T.; Serbanescu, L.G.; Anghel, R.M.; Gales, L.N.; Georgescu, F.T.; Mitrica, R.I.; Georgescu, D.E. When Should We Expect Curative Results of Neoadjuvant Treatment in Locally Advanced Rectal Cancer Patients? Chirurgia 2021, 116, 16. [Google Scholar] [CrossRef]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability–High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Lent, A.U.V.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair–Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- Ludford, K.; Ho, W.J.; Thomas, J.V.; Raghav, K.P.S.; Murphy, M.B.; Fleming, N.D.; Lee, M.S.; Smaglo, B.G.; You, Y.N.; Tillman, M.M.; et al. Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors. J. Clin. Oncol. 2023, 41, 2181–2190. [Google Scholar] [CrossRef]
- Addeo, A.; Passaro, A.; Malapelle, U.; Banna, G.L.; Subbiah, V.; Friedlaender, A. Immunotherapy in Non-Small Cell Lung Cancer Harbouring Driver Mutations. Cancer Treat. Rev. 2021, 96, 102179. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Perol, M.; Banna, G.L.; Parikh, K.; Addeo, A. Oncogenic Alterations in Advanced NSCLC: A Molecular Super-Highway. Biomark. Res. 2024, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Foffano, L.; Bertoli, E.; Bortolot, M.; Torresan, S.; De Carlo, E.; Stanzione, B.; Del Conte, A.; Puglisi, F.; Spina, M.; Bearz, A. Immunotherapy in Oncogene-Addicted NSCLC: Evidence and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 583. [Google Scholar] [CrossRef]
- Corvaja, C.; Passaro, A.; Attili, I.; Aliaga, P.T.; Spitaleri, G.; Signore, E.D.; Marinis, F. de Advancements in Fourth-Generation EGFR TKIs in EGFR-Mutant NSCLC: Bridging Biological Insights and Therapeutic Development. Cancer Treat. Rev. 2024, 130, 102824. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef]
- Suda, K.; Onozato, R.; Yatabe, Y.; Mitsudomi, T. EGFR T790M Mutation: A Double Role in Lung Cancer Cell Survival? J. Thorac. Oncol. 2009, 4, 1–4. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.-H.; Yanagitani, N.; Kim, S.-W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Kato, T.; Dong, X.; Ahn, M.-J.; Quang, L.-V.; Soparattanapaisarn, N.; Inoue, T.; Wang, C.-L.; Huang, M.; Yang, J.C.-H.; et al. Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N. Engl. J. Med. 2024, 391, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients with Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the Transforming EML4-ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Solomon, B.J.; Bauer, T.M.; Mok, T.S.K.; Liu, G.; Mazieres, J.; de Marinis, F.; Goto, Y.; Kim, D.-W.; Wu, Y.-L.; Jassem, J.; et al. Efficacy and Safety of First-Line Lorlatinib versus Crizotinib in Patients with Advanced, ALK-Positive Non-Small-Cell Lung Cancer: Updated Analysis of Data from the Phase 3, Randomised, Open-Label CROWN Study. Lancet Respir. Med. 2023, 11, 354–366. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.-S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in Resected ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef]
- Vingiani, A.; Agnelli, L.; Duca, M.; Lorenzini, D.; Damian, S.; Proto, C.; Niger, M.; Nichetti, F.; Tamborini, E.; Perrone, F.; et al. Molecular Tumor Board as a Clinical Tool for Converting Molecular Data Into Real-World Patient Care. JCO Precis. Oncol. 2023, 7, e2300067. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Wu, Y.-L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.-P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus Cisplatin-Based Chemotherapy for EGFR Mutation-Positive Lung Adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of Overall Survival Data from Two Randomised, Phase 3 Trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhou, C.; Hu, C.-P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus Cisplatin plus Gemcitabine for First-Line Treatment of Asian Patients with Advanced Non-Small-Cell Lung Cancer Harbouring EGFR Mutations (LUX-Lung 6): An Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Shih, J.-Y.; Su, W.-C.; Hsia, T.-C.; Tsai, C.-M.; Ou, S.-H.I.; Yu, C.-J.; Chang, G.-C.; Ho, C.-L.; Sequist, L.V.; et al. Afatinib for Patients with Lung Adenocarcinoma and Epidermal Growth Factor Receptor Mutations (LUX-Lung 2): A Phase 2 Trial. Lancet Oncol. 2012, 13, 539–548. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Sequist, L.V.; Geater, S.L.; Tsai, C.-M.; Mok, T.S.K.; Schuler, M.; Yamamoto, N.; Yu, C.-J.; Ou, S.-H.I.; Zhou, C.; et al. Clinical Activity of Afatinib in Patients with Advanced Non-Small-Cell Lung Cancer Harbouring Uncommon EGFR Mutations: A Combined Post-Hoc Analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015, 16, 830–838. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Hirsh, V.; Smylie, M.; Findlay, B.; Santabárbara, P. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus Gefitinib as First-Line Treatment for Patients with EGFR-Mutation-Positive Non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Chawla, A.; Rosell, R.; Corral, J.; Migliorino, M.R.; et al. Updated Overall Survival in a Randomized Study Comparing Dacomitinib with Gefitinib as First-Line Treatment in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. Drugs 2021, 81, 257–266. [Google Scholar] [CrossRef]
- Inoue, A.; Kobayashi, K.; Maemondo, M.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Updated Overall Survival Results from a Randomized Phase III Trial Comparing Gefitinib with Carboplatin–Paclitaxel for Chemo-Naïve Non-Small Cell Lung Cancer with Sensitive EGFR Gene Mutations (NEJ002). Ann. Oncol. 2013, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Mok, T.S.; Han, J.-Y.; Ahn, M.-J.; Delmonte, A.; Ramalingam, S.S.; Kim, S.W.; Shepherd, F.A.; Laskin, J.; He, Y.; et al. Osimertinib versus Platinum-Pemetrexed for Patients with EGFR T790M Advanced NSCLC and Progression on a Prior EGFR-Tyrosine Kinase Inhibitor: AURA3 Overall Survival Analysis. Ann. Oncol. 2020, 31, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, K.-J.; Cho, B.C.; Liu, B.; Paz-Ares, L.; Cheng, S.; Kitazono, S.; Thiagarajan, M.; Goldman, J.W.; Sabari, J.K.; et al. Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N. Engl. J. Med. 2023, 389, 2039–2051. [Google Scholar] [CrossRef]
- Cho, B.C.; Lu, S.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.-S.; Lee, S.-H.; Ostapenko, Y.; Danchaivijitr, P.; Liu, B.; et al. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2024, 391, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.-W.; Planchard, D.; Ahn, M.-J.; Smit, E.F.; de Langen, A.J.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients with HER2-Mutant Metastatic Non–Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.-M.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus Docetaxel for Previously Treated Non-Small-Cell Lung Cancer with KRASG12C Mutation: A Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. A Phase 2 Single Arm Trial of Cabozantinib in Patients with Advanced RET-Rearranged Lung Cancers. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; Pas, T.D.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-Line Ceritinib versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Tan, D.S.W.; Geater, S.; Yu, C.-J.; Tsai, C.-M.; Hsia, T.-C.; Chen, J.; Lin, M.-C.; Lu, Y.; Sriuranpong, V.; Yang, C.-T.; et al. Ceritinib Efficacy and Safety in Treatment-Naive Asian Patients with Advanced ALK-Rearranged NSCLC: An ASCEND-4 Subgroup Analysis. JTO Clin. Res. Rep. 2021, 2, 100131. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus Chemotherapy in Patients with ALK-Rearranged Non-Small-Cell Lung Cancer Previously given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Ahn, M.J.; Kim, H.R.; Yang, J.C.H.; Han, J.-Y.; Li, J.Y.-C.; Hochmair, M.J.; Chang, G.-C.; Delmonte, A.; Lee, K.H.; Campelo, R.G.; et al. Efficacy and Safety of Brigatinib Compared with Crizotinib in Asian vs. Non-Asian Patients with Locally Advanced or Metastatic ALK-Inhibitor-Naive ALK+ Non-Small Cell Lung Cancer: Final Results From the Phase III ALTA-1L Study. Clin. Lung Cancer 2022, 23, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Garcia Campelo, M.R.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Tiseo, M.; Reckamp, K.L.; West, H.L.; Groen, H.J.; Langer, C.J.; Reichmann, W.; Kerstein, D.; Kim, D.-W.; Camidge, D.R. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: Updates from the Pivotal Randomized Phase 2 Trial (ALTA). Ann. Oncol. 2017, 28, ii35–ii36. [Google Scholar] [CrossRef]
- Huber, R.M.; Hansen, K.H.; Rodríguez, L.P.-A.; West, H.L.; Reckamp, K.L.; Leighl, N.B.; Tiseo, M.; Smit, E.F.; Kim, D.-W.; Gettinger, S.N.; et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J. Thorac. Oncol. 2020, 15, 404–415. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Mok, T.; Camidge, D.R.; Gadgeel, S.M.; Rosell, R.; Dziadziuszko, R.; Kim, D.-W.; Pérol, M.; Ou, S.-H.I.; Ahn, J.S.; Shaw, A.T.; et al. Updated Overall Survival and Final Progression-Free Survival Data for Patients with Treatment-Naive Advanced ALK-Positive Non-Small-Cell Lung Cancer in the ALEX Study. Ann. Oncol. 2020, 31, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase−Positive Non–Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Bauer, T.M.; De Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK -Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Liu, G.; Felip, E.; Mok, T.S.K.; Soo, R.A.; Mazieres, J.; Shaw, A.T.; de Marinis, F.; Goto, Y.; Wu, Y.-L.; et al. Lorlatinib Versus Crizotinib in Patients with Advanced ALK-Positive Non–Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J. Clin. Oncol. 2024, 42, 3400–3409. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer (NSCLC): Updated Results, Including Overall Survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef]
- Drilon, A.; Chiu, C.-H.; Fan, Y.; Cho, B.C.; Lu, S.; Ahn, M.-J.; Krebs, M.G.; Liu, S.V.; John, T.; Otterson, G.A.; et al. Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion–Positive NSCLC. JTO Clin. Res. Rep. 2022, 3, 100332. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; Soo, R.A.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in Advanced ROS1-Positive Non-Small-Cell Lung Cancer: A Multicentre, Open-Label, Single-Arm, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; De Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET -Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Wolf, J.; Hochmair, M.; Han, J.-Y.; Reguart, N.; Souquet, P.-J.; Smit, E.F.; Orlov, S.V.; Vansteenkiste, J.; Nishio, M.; Jonge, M. de; et al. Capmatinib in MET Exon 14-Mutated Non-Small-Cell Lung Cancer: Final Results from the Open-Label, Phase 2 GEOMETRY Mono-1 Trial. Lancet Oncol. 2024, 25, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients with MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-Term Follow-up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.-H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor Activity of Crizotinib in Lung Cancers Harboring a MET Exon 14 Alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Hashemi, S.M.S.; Mazieres, J.; Kim, T.M.; Quoix, E.; Souquet, P.-J.; Barlesi, F.; Baik, C.; et al. Phase 2 Study of Dabrafenib Plus Trametinib in Patients with BRAF V600E-Mutant Metastatic NSCLC: Updated 5-Year Survival Rates and Genomic Analysis. J. Thorac. Oncol. 2022, 17, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus Trametinib in Patients with Previously Untreated BRAFV600E-Mutant Metastatic Non-Small-Cell Lung Cancer: An Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef]
- Riely, G.J.; Smit, E.F.; Ahn, M.-J.; Felip, E.; Ramalingam, S.S.; Tsao, A.; Johnson, M.; Gelsomino, F.; Esper, R.; Nadal, E.; et al. Phase II, Open-Label Study of Encorafenib Plus Binimetinib in Patients with BRAFV600-Mutant Metastatic Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 3700–3711. [Google Scholar] [CrossRef]
- Drilon, A.; Tan, D.S.W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Patel, J.D.; Rosen, L.; Solomon, B.; Norenberg, R.; Dima, L.; et al. Efficacy and Safety of Larotrectinib in Patients with Tropomyosin Receptor Kinase Fusion-Positive Lung Cancers. JCO Precis. Oncol. 2022, 6, e2100418. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Doebele, R.C.; Farago, A.F.; Liu, S.V.; Chawla, S.P.; Tosi, D.; Blakely, C.M.; Krauss, J.C.; Sigal, D.; Bazhenova, L.; et al. Entrectinib in NTRK Fusion-Positive Non-Small Cell Lung Cancer (NSCLC): Integrated Analysis of Patients (Pts) Enrolled in STARTRK-2, STARTRK-1 and ALKA-372-001. Ann. Oncol. 2019, 30, ii48–ii49. [Google Scholar] [CrossRef]
- Cho, B.C.; Chiu, C.-H.; Massarelli, E.; Buchschacher, G.L.; Goto, K.; Overbeck, T.R.; Loong, H.H.F.; Chee, C.E.; Garrido, P.; Dong, X.; et al. Updated Efficacy and Safety of Entrectinib in NTRK Fusion-Positive Non-Small Cell Lung Cancer. Lung Cancer 2024, 188, 107442. [Google Scholar] [CrossRef]
- Kim, D.-W.; Schram, A.M.; Hollebecque, A.; Nishino, K.; Macarulla, T.; Rha, S.Y.; Duruisseaux, M.; Liu, S.V.; Al Hallak, M.N.; Umemoto, K.; et al. The Phase I/II eNRGy Trial: Zenocutuzumab in Patients with Cancers Harboring NRG1 Gene Fusions. Future Oncol. Lond. Engl. 2024, 20, 1057–1067. [Google Scholar] [CrossRef]
- Simion, L.; Chitoran, E.; Cirimbei, C.; Stefan, D.-C.; Neicu, A.; Tanase, B.; Ionescu, S.O.; Luca, D.C.; Gales, L.; Gheorghe, A.S.; et al. A Decade of Therapeutic Challenges in Synchronous Gynecological Cancers from the Bucharest Oncological Institute. Diagnostics 2023, 13, 2069. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Tasca, G.; Dieci, M.V.; Baretta, Z.; Faggioni, G.; Montagna, M.; Nicoletto, M.O.; Peccatori, F.A.; Guarneri, V.; Colombo, N. Synchronous and Metachronous Breast and Ovarian Cancer: Experience From Two Large Cancer Center. Front. Oncol. 2020, 10, 608783. [Google Scholar] [CrossRef]
- Concolino, P.; Gelli, G.; Rizza, R.; Costella, A.; Scambia, G.; Capoluongo, E. BRCA1 and BRCA2 Testing through Next Generation Sequencing in a Small Cohort of Italian Breast/Ovarian Cancer Patients: Novel Pathogenic and Unknown Clinical Significance Variants. Int. J. Mol. Sci. 2019, 20, 3442. [Google Scholar] [CrossRef]
- den Brok, W.D.; Schrader, K.A.; Sun, S.; Tinker, A.V.; Zhao, E.Y.; Aparicio, S.; Gelmon, K.A. Homologous Recombination Deficiency in Breast Cancer: A Clinical Review. JCO Precis. Oncol. 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Haupts, A.; Kloth, M.; Roth, W.; Hartmann, N. A Novel Targeted NGS Panel Identifies Numerous Homologous Recombination Deficiency (HRD)-Associated Gene Mutations in Addition to Known BRCA Mutations. Diagn. Pathol. 2024, 19, 9. [Google Scholar] [CrossRef]
- Tsantikidi, A.; Papadopoulou, E.; Metaxa-Mariatou, V.; Kapetsis, G.; Tsaousis, G.; Meintani, A.; Florou-Chatzigiannidou, C.; Gazouli, M.; Papadimitriou, C.; Timotheadou, E.; et al. The Utility of NGS Analysis in Homologous Recombination Deficiency Tracking. Diagnostics 2023, 13, 2962. [Google Scholar] [CrossRef]
- Prophylactic Interventions for Hereditary Breast and Ovarian Cancer Risks and Mortality in BRCA1/2 Carriers. Available online: https://www.mdpi.com/2072-6694/16/1/103 (accessed on 30 January 2025).
- Bono, M.; Fanale, D.; Incorvaia, L.; Cancelliere, D.; Fiorino, A.; Calò, V.; Dimino, A.; Filorizzo, C.; Corsini, L.R.; Brando, C.; et al. Impact of Deleterious Variants in Other Genes beyond BRCA1/2 Detected in Breast/Ovarian and Pancreatic Cancer Patients by NGS-Based Multi-Gene Panel Testing: Looking over the Hedge. ESMO Open 2021, 6, 100235. [Google Scholar] [CrossRef]
- Kurian, A.W.; Hughes, E.; Handorf, E.A.; Gutin, A.; Allen, B.; Hartman, A.-R.; Hall, M.J. Breast and Ovarian Cancer Penetrance Estimates Derived From Germline Multiple-Gene Sequencing Results in Women. JCO Precis. Oncol. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Akbari, M.R.; Wallis, C.J.D.; Toi, A.; Trachtenberg, J.; Sun, P.; Narod, S.A.; Nam, R.K. The Impact of a BRCA2 Mutation on Mortality from Screen-Detected Prostate Cancer. Br. J. Cancer 2014, 111, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association Between BRCA1 and BRCA2 Mutations and Survival in Women with Invasive Epithelial Ovarian Cancer. JAMA J. Am. Med. Assoc. 2012, 307, 382–390. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Zhang, C.; Sun, S.; Zhang, J.; Liu, W.; Huang, J.; Zhang, Z. BRCA Mutations and Survival in Breast Cancer: An Updated Systematic Review and Meta-Analysis. Oncotarget 2016, 7, 70113–70127. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab First-Line Maintenance in Ovarian Cancer: Final Overall Survival Results from the PAOLA-1/ENGOT-Ov25 Trial. Ann. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Geyer, C.E.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall Survival in the OlympiA Phase III Trial of Adjuvant Olaparib in Patients with Germline Pathogenic Variants in BRCA1/2 and High-Risk, Early Breast Cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD Final Overall Survival and Tolerability Results: Olaparib versus Chemotherapy Treatment of Physician’s Choice in Patients with a Germline BRCA Mutation and HER2-Negative Metastatic Breast Cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.E.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Tung, N.; Armstrong, A.; Dymond, M.; et al. OlympiAD Extended Follow-up for Overall Survival and Safety: Olaparib versus Chemotherapy Treatment of Physician’s Choice in Patients with a Germline BRCA Mutation and HER2-Negative Metastatic Breast Cancer. Eur. J. Cancer 2023, 184, 39–47. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; de Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Clarke, N.W.; Oya, M.; Shore, N.; Procopio, G.; Guedes, J.D.; Arslan, C.; Mehra, N.; Parnis, F.; Brown, E.; et al. Olaparib plus Abiraterone versus Placebo plus Abiraterone in Metastatic Castration-Resistant Prostate Cancer (PROpel): Final Prespecified Overall Survival Results of a Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1094–1108. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Cutsem, E.V.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Kindler, H.L.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J. Clin. Oncol. 2022, 40, 3929–3939. [Google Scholar] [CrossRef]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.-Y.; Pepin, J.T.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab with or without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Christensen, R.D.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Monk, B.J.; Barretina-Ginesta, M.P.; Pothuri, B.; Vergote, I.; Graybill, W.; Mirza, M.R.; McCormick, C.C.; Lorusso, D.; Moore, R.G.; Freyer, G.; et al. Niraparib First-Line Maintenance Therapy in Patients with Newly Diagnosed Advanced Ovarian Cancer: Final Overall Survival Results from the PRIMA/ENGOT-OV26/GOG-3012 Trial. Ann. Oncol. 2024, 35, 981–992. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Pereira de Santana Gomes, A.J.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib Maintenance Treatment for Recurrent Ovarian Carcinoma after Response to Platinum Therapy (ARIEL3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. Rucaparib for Patients with Platinum-Sensitive, Recurrent Ovarian Carcinoma (ARIEL3): Post-Progression Outcomes and Updated Safety Results from a Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2020, 21, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus Chemotherapy in Patients with Germline BRCA1/2-Mutated HER2-Negative Advanced Breast Cancer: Final Overall Survival Results from the EMBRACA Trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; Giorgi, U.D.; Joung, J.Y.; Fong, P.C.C.; et al. Talazoparib plus Enzalutamide in Men with First-Line Metastatic Castration-Resistant Prostate Cancer (TALAPRO-2): A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef]
- Fizazi, K.; Azad, A.A.; Matsubara, N.; Carles, J.; Fay, A.P.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; Voog, E.; Jones, R.J.; et al. First-Line Talazoparib with Enzalutamide in HRR-Deficient Metastatic Castration-Resistant Prostate Cancer: The Phase 3 TALAPRO-2 Trial. Nat. Med. 2024, 30, 257–264. [Google Scholar] [CrossRef]
- Zgura, A.; Gales, L.; Bratila, E.; Mehedintu, C.; Haineala, B.; Barac, R.I.; Popa, A.R.; Buhas, C.; Berceanu, C.; Andreescu, C.V.; et al. Variation of the T Lymphocytes According to Treatment in Breast Cancer. Rev. Chim. 2019, 70, 1649–1654. [Google Scholar] [CrossRef]
- Cardoso, F.; Hirshfield, K.M.; Kraynyak, K.A.; Tryfonidis, K.; Bardia, A. Immunotherapy for Hormone Receptor‒positive HER2-Negative Breast Cancer. Npj Breast Cancer 2024, 10, 104. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.-L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An Integrative Genomic and Proteomic Analysis of PIK3CA, PTEN, and AKT Mutations in Breast Cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef] [PubMed]
- Raei, M.; Heydari, K.; Tabarestani, M.; Razavi, A.; Mirshafiei, F.; Esmaeily, F.; Taheri, M.; Hoseini, A.; Nazari, H.; Shamshirian, D.; et al. Diagnostic Accuracy of ESR1 Mutation Detection by Cell-Free DNA in Breast Cancer: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy. BMC Cancer 2024, 24, 908. [Google Scholar] [CrossRef]
- Turner, N.C.; Im, S.-A.; Saura, C.; Juric, D.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Sunpaweravong, P.; Musolino, A.; et al. Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer. N. Engl. J. Med. 2024, 391, 1584–1596. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus Fulvestrant for PIK3CA-Mutated, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor-2-Negative Advanced Breast Cancer: Final Overall Survival Results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.-A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results from the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Sebastião, M.M.; Ho, R.S.; de Carvalho, J.P.V.; Nussbaum, M. Diagnostic Accuracy of Next Generation Sequencing Panel Using Circulating Tumor DNA in Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. J. Health Econ. Outcomes Res. 2020, 7, 158–163. [Google Scholar] [CrossRef]
- Gašperšič, J.; Videtič Paska, A. Potential of Modern Circulating Cell-Free DNA Diagnostic Tools for Detection of Specific Tumour Cells in Clinical Practice. Biochem. Medica 2020, 30, 030504. [Google Scholar] [CrossRef]
- Gu, W.; Zhuang, W.; Zhuang, M.; He, M.; Li, Z. DNA Damage Response and Repair Gene Mutations Are Associated with Tumor Mutational Burden and Outcomes to Platinum-Based Chemotherapy/Immunotherapy in Advanced NSCLC Patients. Diagn. Pathol. 2023, 18, 119. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Sang, Y.; Yang, X.; Tian, G.; Wang, Z.; Fang, J.; Sun, W.; Zhou, L.; Jia, L.; et al. A Study of ALK-Positive Pulmonary Squamous-Cell Carcinoma: From Diagnostic Methodologies to Clinical Efficacy. Lung Cancer Amst. Neth. 2019, 130, 135–142. [Google Scholar] [CrossRef]
- Byron, S.A.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA Sequencing into Clinical Diagnostics: Opportunities and Challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.N.; Peneva, D.; Cuyun Carter, G.; Palomares, M.R.; Thakkar, S.; Hall, D.W.; Dalglish, H.; Campos, C.; Yermilov, I. Comprehensive Review on the Clinical Impact of Next-Generation Sequencing Tests for the Management of Advanced Cancer. JCO Precis. Oncol. 2023, 7, e2200715. [Google Scholar] [CrossRef]
- Mirza, M.; Goerke, L.; Anderson, A.; Wilsdon, T. Assessing the Cost-Effectiveness of Next-Generation Sequencing as a Biomarker Testing Approach in Oncology and Policy Implications: A Literature Review. Value Health 2024, 27, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Trifanescu, O.G.; Gales, L.; Bacinschi, X.; Serbanescu, L.; Georgescu, M.; Sandu, A.; Michire, A.; Anghel, R. Impact of the COVID-19 Pandemic on Treatment and Oncologic Outcomes for Cancer Patients in Romania. Vivo 2022, 36, 934–941. [Google Scholar] [CrossRef]
- Schluckebier, L.; Caetano, R.; Garay, O.U.; Montenegro, G.T.; Custodio, M.; Aran, V.; Gil Ferreira, C. Cost-Effectiveness Analysis Comparing Companion Diagnostic Tests for EGFR, ALK, and ROS1 versus next-Generation Sequencing (NGS) in Advanced Adenocarcinoma Lung Cancer Patients. BMC Cancer 2020, 20, 875. [Google Scholar] [CrossRef]
- Ferreira-Gonzalez, A.; Ko, G.; Fusco, N.; Stewart, F.; Kistler, K.; Appukkuttan, S.; Hocum, B.; Allen, S.M.; Babajanyan, S. Barriers and Facilitators to Next-Generation Sequencing Use in United States Oncology Settings: A Systematic Review. Future Oncol. 2024, 20, 2765–2777. [Google Scholar] [CrossRef]
- Rehm, H.L.; Page, A.J.H.; Smith, L.; Adams, J.B.; Alterovitz, G.; Babb, L.J.; Barkley, M.P.; Baudis, M.; Beauvais, M.J.S.; Beck, T.; et al. GA4GH: International Policies and Standards for Data Sharing across Genomic Research and Healthcare. Cell Genom. 2021, 1, 100029. [Google Scholar] [CrossRef]
- Mulder, N.; Abimiku, A.; Adebamowo, S.N.; de Vries, J.; Matimba, A.; Olowoyo, P.; Ramsay, M.; Skelton, M.; Stein, D.J. H3Africa: Current Perspectives. Pharmacogenom. Pers. Med. 2018, 11, 59–66. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Araujo, D.M.; Razak, A.R.A.; Agulnik, M.; Attia, S.; Blay, J.-Y.; Garcia, I.C.; Charlson, J.A.; Choy, E.; Demetri, G.D.; et al. Afamitresgene Autoleucel for Advanced Synovial Sarcoma and Myxoid Round Cell Liposarcoma (SPEARHEAD-1): An International, Open-Label, Phase 2 Trial. Lancet 2024, 403, 1460–1471. [Google Scholar] [CrossRef]
- Gulilat, M.; Lamb, T.; Teft, W.A.; Wang, J.; Dron, J.S.; Robinson, J.F.; Tirona, R.G.; Hegele, R.A.; Kim, R.B.; Schwarz, U.I. Targeted next Generation Sequencing as a Tool for Precision Medicine. BMC Med. Genom. 2019, 12, 81. [Google Scholar] [CrossRef] [PubMed]
| NGS Test | Technology Used | Sample Type | Key Notes | Time | Molecular Alterations | Relevant Literature |
|---|---|---|---|---|---|---|
| FoundationOne CDx | Hybrid Capture NGS | Tumor Tissue | Comprehensive solid tumor profiling includes >300 genes and detects fusions and amplifications. | 14 days | EGFR, ALK, BRAF, BRCA1/2, MET, NTRK, ROS1, PIK3CA, RET, ERBB2 (HER2), KRAS, IDH1, FGFR1/2/3, CDK4, CDK6, KIT, PDGFRA, TSC1/2, ERBB3 | [5,6] |
| FoundationOne Liquid CDx | cfDNA Sequencing | Blood (Liquid Biopsy) | Liquid biopsy test; sensitivity decreases for variants with allele frequency <0.1%. | 10–14 days | EGFR, ALK, BRAF, BRCA1/2, MET, NTRK, ROS1, PIK3CA, RET, ERBB2 (HER2), KRAS, IDH1, FGFR2/3 | [7] |
| Guardant360 CDx | cfDNA Sequencing | Blood (Liquid Biopsy) | High sensitivity for detecting rare cfDNA variants; optimized for minimal input samples. | 7 days | EGFR, ALK, BRAF, BRCA1/2, MET, RET, ERBB2 (HER2), PIK3CA, KRAS, IDH1 | [8] |
| MSK-IMPACT | Hybrid Capture NGS | Tumor Tissue | Highly validated for solid tumors; includes 468 cancer-relevant genes. | 21 days | EGFR, ALK, BRAF, BRCA1/2, MET, NTRK, ROS1, PIK3CA, RET, ERBB2 (HER2), KRAS, IDH1, FGFR1/2/3, CDK4, CDK6, KIT, PDGFRA, TSC1/2, ERBB3 | [9] |
| Oncomine Dx Target Test | AmpliSeq NGS | Tumor Tissue | Focused on NSCLC; detects EGFR, ALK, ROS1, BRAF, and other actionable mutations. | 7–10 days | EGFR, ALK, BRAF, ROS1, RET, MET, KRAS | [10] |
| Oncomine Comprehensive Assay | AmpliSeq NGS | Tumor Tissue | Comprehensive tumor profiling with >500 genes, including fusion detection. | 7–14 days | EGFR, ALK, BRAF, ROS1, RET, MET, KRAS | [11] |
| Tempus xT | Hybrid Capture NGS | Tumor Tissue | The broad panel covering >600 genes; includes TMB and MSI analysis. | 10–14 days | EGFR, ALK, BRAF, BRCA1/2, MET, NTRK, ROS1, PIK3CA, RET, ERBB2 (HER2), KRAS, IDH1, FGFR1/2/3, CDK4, CDK6, KIT, PDGFRA, TSC1/2, ERBB3 | [12] |
| Caris Molecular Intelligence | Multiple NGS Platforms | Tumor Tissue | Uses multiple platforms; integrates NGS, IHC, and other molecular tests for a holistic profile. | 10–14 days | EGFR, ALK, BRAF, BRCA1/2, MET, NTRK, ROS1, PIK3CA, RET, ERBB2 (HER2), KRAS, IDH1, FGFR1/2/3, CDK4, CDK6, KIT, PDGFRA, TSC1/2, ERBB3 | [13] |
| Com.Pl.it DX Colon | Hybrid Capture NGS | Tumor Tissue | Focused on colorectal cancer profiling; including MSI detection and fusion analysis. | 10–14 days | EGFR, ALK, BRAF, KRAS, MET, RET, PIK3CA, ERBB2 (HER2), MSI detection | |
| Com.Pl.it DX Liquid Biopsy | cfDNA Sequencing | Blood (Liquid Biopsy) | Designed for liquid biopsy; slightly lower sensitivity than tissue-based assays for rare variants. | 10–14 days | EGFR, ALK, BRAF, KRAS, MET, RET, PIK3CA, ERBB2 (HER2), FGFR1/2/3, MSI |
| Molecular Marker | Biological Function of Affected Genes | Diagnostic Role | Therapeutic Value |
|---|---|---|---|
| IDH1 (R132) or IDH2 (R172) mutation | Gain-of-function mutation | Distinguishes diffuse gliomas with IDH mutation from IDH-wild-type glioblastomas or other gliomas | Predictive biomarker for treatment with Vorasidenib in IDH-1 or IDH-2-mutant low-grade gliomas |
| 1p/19q codeletion | Inactivation of putative tumor suppressor genes on 1p (such as FUBP1) and 19q (such as CIC) | Distinguishes IDH-mutant oligodendroglioma and 1p/19q-codeleted from IDH-mutant astrocytoma | Different therapeutic management |
| Loss of nuclear ATRX | Cell proliferation and longevity promoter with telomere lengthening activity | Characteristic of tumors of astrocytic lineage | Specific therapeutic management of astrocytic tumors |
| Histone H3 K27M | Missense mutations affect the epigenetic regulation of gene expression | Characteristic of the particular diffuse midline glioma, H3 K27M-mutant | Specific therapeutic management, radiotherapy alone as adjuvant treatment permitted |
| Histone H3.3 G34R/V mutation | Mutation that affects the epigenetic regulation of gene expression | Characteristic of the particular diffuse hemispheric glioma, H3.3 G34-mutant | Specific adjuvant management with Temozolomide chemoradiotherapy as standard |
| MGMT promoter methylation | DNA repair | None | Predictive biomarker for the benefit of alkylating ChT agents in IDH-wt glioblastoma (very important in frail or elderly patients) |
| Homozygous deletion of CDKN2A/CDKN2B | Encode kinase inhibitors and tumor suppressors functioning as regulators of Rb1 and p53-dependent signaling | Marker of poor outcome and WHO Grade 4 disease in IDH-mutant astrocytoma. | Specific adjuvant management with Temozolomide chemoradiotherapy |
| EGFR amplification | Impact on cellular proliferation, invasion, and resistance to induction of apoptosis | Molecular marker characteristic of glioblastoma, IDH wild type, WHO Grade 4 | Specific therapeutic management–Stupp protocol, with adjuvant Temozolomide for 6 months following Temozolomide chemoradiotherapy |
| TERT promotor alteration | Cell proliferation marker, which promotes cellular longevity via increased TERT expression | Molecular marker of glioblastoma, IDH wild type, WHO Grade 4 | Specific therapeutic management–Stupp protocol, with adjuvant Temozolomide for 6 months following Temozolomide chemoradiotherapy |
| +7/−10 cytogenetic signature | Gain of chromosome 7 combined with loss of chromosome 10, meaning gain of genes such as PDGFA and EGFR simultaneous with the loss of PTEN and MGMT | Molecular marker of glioblastoma, IDH wild type, WHO Grade 4 | Specific therapeutic management–Stupp protocol, with adjuvant Temozolomide for 6 months following Temozolomide chemoradiotherapy |
| BRAF V600E mutation | Oncogenic driver mutation that leads to the activation of the MAPK pathway | Rare in adult diffuse gliomas | Amenable to pharmacological intervention with BRAF inhibitors (Dabrafenib ± Trametinib, Vemurafenib ± Cobimetinib) |
| Therapy | Targeted Molecular Alteration(s) | ESCAT | Key Clinical Trial(s) and Relevant Literature | Molecular Tests Performed | Tumors Included | Trial Design | Trial Outcomes | Toxicity and AEs From |
|---|---|---|---|---|---|---|---|---|
| Larotrectinib (First-Generation NTRKi) | TRKA/B/C | IC | NAVIGATE [28] (NCT02576431 NCT02122913) | NGS, FISH | Soft tissue sarcomas, salivary gland tumors, thyroid, lung, colorectal, melanoma, breast, pancreatic, and primary CNS tumors. | Phase 2 basket trial that enrolled 55 patients, both adults and children, with TRK fusion-positive tumors. | The most common are fatigue, dizziness, and nausea. Serious (Grade ≥ 3): 13% of patients, primarily elevated liver enzymes. No treatment-related deaths. | |
| SCOUT [29] (NCT02637687) | NGS, FISH | Infantile fibrosarcoma, thyroid, CNS tumors, and other solid tumors in children. | Phase 1/2 trial enrolled 24 pediatric patients with locally advanced or metastatic solid or CNS tumors, 17 of which had NTRK fusion-positive tumors. Larotrectinib was administered orally, twice daily, on a continuous 28 day cycle in increasing dose levels. | Demonstrated antitumor activity for all patients with TRK fusion-positive tumors at a dose of 100 mg/m2 (cap of 100 mg), ORR 93%. | ||||
| VICTORIA [30] | NA | Protocol-driven, an exact-matching analysis comparing 82 patients with TRK fusion-positive cancers treated with Larotrectinib in clinical trials with TRK fusion-positive treated with other non-TRK inhibitor therapies in a real-world setting. | Improved OS (NR vs. 37.2 months, HR 0.44, 95% CI 0.23–0.83), longer time to next therapy, duration of therapy, and PFS (36.8 vs. 5.2 months, HR 0.29, 95% CI 0.18–0.46). | |||||
| Entrectinib (First-Generation NTRKi) | TRKA/B/C, ROS1, ALK | IC | ALKA-372-001 (EudraCT 2012-000148-88) and STARTRK-1 (NCT02097810) [31] | NGS, FISH | NSCLC, colorectal, breast, pancreatic, thyroid, and salivary gland tumors. | Two Phase I trials assessed the safety, tolerability, and preliminary efficacy in 119 adults with advanced or metastatic tumors harboring fusions in the NTRK1/2/3, ROS1, or ALK genes. | Demonstrated a favorable safety profile with preliminary antitumor activity and established the recommended dose of 600 mg of Entrectinib daily. | Most common: fatigue, dysgeusia, dizziness. Serious (Grade ≥ 3): eosinophilic myocarditis, cognitive disturbance, fatigue—resolved with dose interruption. No treatment-related deaths. |
| STARTRK-2 [32] (NCT02568267) | NGS, FISH | NSCLC, colorectal, breast, pancreatic, thyroid, soft tissue sarcomas, and salivary gland tumors. | Phase II, basket trial, evaluating the efficacy and safety of Entrectinib 600 mg daily in patients with tumors with NTRK1/2/3, ROS1, and ALK gene functions. | ORR for NTRK fusion-positive tumors: 57.4% (95% CI 43.2–70.8); mDoR 10.4 months; mPFS 11.2 months; mOS 20.9 months. | Most common: Grade 1–2 dysgeusia (47.1%), constipation (27.9%), fatigue (27.9%). Serious (Grade ≥ 3): anemia (11.8%), weight increase (10.3%). No treatment-related deaths. | |||
| STARTRK-NG [33] (NCT02650401) | NGS, FISH | Neuroblastoma, infantile fibrosarcoma, glioblastoma, high-grade gliomas, medulloblastoma, soft tissue sarcomas, Ewing sarcoma, rhabdomyosarcoma. | Phase I/II, open-label, dose-escalation, and expansion study in 47 pediatric and young adult patients with solid tumors or primary CNS tumors harboring NTRK1/2/3, ROS1, or ALK gene fusions. | Established the recommended phase II dose for pediatric patients; observed objective responses in NTRK fusion-positive tumors (ORR 57.7%, 95% CI 36.9–76.7, mDoR NR, mDoT 10.6 months, 4.2–18.4 months), indicating potential efficacy in the pediatric population. | Safety profile consistent with adult studies; common AEs included fatigue, gastrointestinal symptoms, and increased liver enzymes; no new safety signals were identified in the pediatric population. | |||
| Repotrectinib (Second-Generation NTRKi) | TRKA/B/C, ROS1, ALK | IC | (ongoing) TRIDENT-1 [34] (NCT03093116) | NGS | NSCLC, colorectal, breast, pancreatic, thyroid, soft tissue sarcomas, and salivary gland tumors. | Phase I/II, open-label, multicenter study in patients with advanced solid tumors harboring specific gene fusions. Includes both treatment-naïve and pretreated patients. | Current data are available only for ROS1 fusion-positive advanced NSCLC [34]. The trial determined a recommended dose of 160 mg daily Repotrectinib for 14 days, followed by 160 mg twice daily. For treatment-naïve tumors: ORR 79% (95% CI, 68–88%), mDoR 34.1 months, mPFS 35.7 months. For ROS1 TKI-pretreated patients: ORR 38% (95% CI, 25–52%), mDoR 14.8 months, mPFS 9 months. Data are immature for NTRK-positive tumor agnostics. | Most common: dizziness (58%), dysgeusia (50%), paresthesia (30%). Serious (Grade ≥ 3): reported in 29% of patients—anemia, increased blood creatine kinase levels, dizziness. No treatment-related deaths. |
| Selpercatinib | RET gene fusions and mutations | IC | LIBRETTO-001 [35,36,37] (NCT03157128) | NGS, FISH | NSCLC, MTC, and PTC thyroid cancer, pancreatic cancer, and colorectal cancer. | Ongoing Phase I/II, open-label trial in patients with RET fusion-positive or RET-mutant cancers. | NSCLC (n = 105): ORR 85%; mDoR: 18.4 months. Thyroid (MTC, n = 143; PTC, n = 19): MTC: ORR 69%; mPFS: 23.6 months. PTC: ORR 79%. Agnostic non-thyroid, non-NSCLC, n = 45: ORR 57%; mDoR: 13.0 months. | Most common: dry mouth, diarrhea, hypertension, fatigue. Serious (Grade ≥ 3): hypertension, increased liver enzymes. No treatment-related deaths. |
| Pralsetinib | RET gene fusions and mutations | IC | ARROW [38,39,40,41] (NCT03037385) | NGS, FISH | NSCLC, MTC, PTC, pancreatic cancer, cholangiocarcinoma, neuroendocrine, SCLC, unknown primary, thymic, ovarian, head and neck, colorectal. | Phase I/II, open-label study evaluating RET fusion-positive or RET-mutant cancers. | NSCLC (n = 216): ORR 70% (95% CI: 62–77); mPFS: 17.1 months. Thyroid (RET-mutant MTC and RET fusion-positive thyroid cancer, n = 29): ORR 60%; mPFS: not reached. Agnostic (non-thyroid, non-NSCLC, n = 38): ORR 57% (95% CI: 42–71). | Most common: constipation (30%), dry mouth (26%), hypertension (24%), fatigue (23%). Serious (Grade ≥ 3): hypertension (15%), neutropenia (10%), anemia (8%). No treatment-related deaths. |
| Trial Name | Immunotherapy | Indication | Neoadjuvant Regimen | Design | MSI-H (No. of Patients) | Clinical Outcome |
|---|---|---|---|---|---|---|
| NEONIPIGA [103] (NCT04006262) | Nivolumab plus Ipilimumab | Localized esophageal and gastric adenocarcinoma | Nivolumab 240 mg once every 2 weeks ×6 and ipilimumab 1 mg/kg once every 6 weeks ×2, followed by surgery and adjuvant nivolumab 480 mg once every 4 weeks (nine injections). | Phase II, prospective-single-arm, open-label | 32 | 29/32 patients underwent surgery, 17/29 had pCR. |
| NICHE-1 [104] (NCT03026140) | Nivolumab plus Ipilimumab | Colon cancer | 1 mg/kg Ipilimumab (1 dose) and 3 mg/kg Nivolumab (2 doses), followed by surgery. | Phase II, prospective-single-arm, open-label | 20 | All patients responded, with 19/20 having an MPR and 12/20 achieving pCR. |
| NICHE-2 [105] (NCT03026140) | Nivolumab plus Ipilimumab | Colon cancer | 1 mg/kg Ipilimumab (1 dose) and 3 mg/kg Nivolumab (2 doses), followed by surgery. | Phase II, prospective-single-arm, open-label | 115 | 113/115 had surgery, 109/111 had a response, 105 had MPR, and 75 achieved pCR. |
| Cercek et al. [101] (NCT04165772) | Dostarlimab | Rectal cancer | 500 mg q3w for 6 months (9 cycles), followed by RT (50.4 Gy/28 fractions with concurrent Capecitabine at standard doses) and then TME if no cCR. | Phase II, prospective-single-arm | 12 | 100% cCR |
| Ludford et al. [106] (NCT04082572) | Pembrolizumab | Colorectal cancer | 200 mg q3w for 6 months, followed by surgical resection, with an option to continue therapy for 1 year. | Phase II, open-label, single-center trial | 35 (27 CRC, 8 non-CRC) | 17/35 underwent surgery. Of these, 14 had CRC, and 11/14 had pCR. |
| Targetable Oncogenic Alteration | Drug | Setting | Trial | Design | ORR | mPFS/DFS in mo. (HR) | mOS in mo. (HR) |
|---|---|---|---|---|---|---|---|
| EGFR typical and atypical mutations | Afatinib * | First line | LUX-Lung 3/6 [122,123,124] (NCT00949650; NCT01121393) | Phase 3 open-label trial comparing Afatinib to platinum-based ChT in Asian and Western patients. | 66% | 11.0 vs. 6.9 (HR: 0.58) | 27.3 vs. 24.3 (HR: 0.81) |
| Subsequent post-ChT | LUX-Lung 2 [125,126] (NCT00525148) | Phase 2 single-arm trial | 58% | 4.4 | 24.8 | ||
| Erlotinib * | First line | EURTAC [127] (NCT00446225) | Phase 3 open-label trial comparing Erlotinib to ChT | 58% | 9.7 vs. 5.2 (HR: 0.37) | 19.3 (NR for chemo) | |
| Subsequent post-ChT | NCIC-CTG [128] | Phase 3 open-label trial comparing Erlotinib to ChT | 8.9% | 2.2 vs. 1.8 (HR: 0.61) | 6.7 vs. 4.7 (HR: 0.70) | ||
| Dacomitinib * | First line | ARCHER1050 [129,130] (NCT01774721) | Phase 3 double-blind trial comparing Dacomitinib to Gefitinib | 74.9% | 14.7 vs. 9.2 (HR: 0.59) | 34.1 vs. 26.8 (HR: 0.76) | |
| Gefitinib * | First line | NEJ002 [131,132] (UMIN-CTR, C000000376) | Phase 3 open-label trial comparing Gefitinib to ChT | 73.7% | 10.8 vs. 5.4 (HR: 0.30) | 30.5 vs. 23.6 (HR: 0.72) | |
| Osimertinib * | Adjuvant | ADAURA [115,133] (NCT02511106) | Phase 3 double-blind trial comparing Osimertinib to placebo as adjuvant 3 year-treatment after surgical resection of Stage IB-IIIA NSCLC | NA | NR vs. 27.5 months (HR: 0.20) | 5-yr OS: 88% vs. 78% (HR: 0.49) | |
| Post-RT consolidation | LAURA [116] (NCT03521154) | Phase 3 double-blind trial comparing Osimertinib to placebo as consolidation treatment | NA | 39.1 vs. 5.6 (HR: 0.16) | Data not yet mature | ||
| First line | FLAURA [113,134] (NCT02296125) | Phase 3 double-blind trial comparing Osimertinib to first-generation EGFR TKI in first-line metastatic EGFR-positive (exon 19 deletion or L858R) NSCLC | 80% | 18.9 vs. 10.2 (HR: 0.46) | 38.6 vs. 31.8 (HR: 0.80) | ||
| First line + ChT | FLAURA2 [114] (NCT04035486) | Ongoing Phase 3 trial investigating the addition of chemotherapy to first-line Osimertinib | 83% | 29.4 vs. 19.9 (HR 0.62) | Data not yet mature | ||
| Subsequent post-ChT | AURA3 [135,136] (NCT02151981) | Phase 3 double-blind trial comparing Osimertinib to platinum-pemetrexed chemotherapy for treatment of T790M EGFR-mutant metastatic NSCLC. | 71% | 10.1 vs. 4.4 (HR: 0.30) | 26.8 vs. 22.5 (HR: 0.87) | ||
| Amivantamab | First line + ChT | PAPILLON [137] (NCT04538664) | Ongoing Phase 3 trial investigating the addition of Amivantamab to platinum ChT in EGFR exon 20 insertion mutations | 73% | 11.4 vs. 6.7 (HR: 0.40) | Data not yet mature | |
| First line + Lazertinib | MARIPOSA [138] (NCT04487080) | Ongoing Phase 3 trial investigating the addition of Amivantamab to Lazertinib, in comparison with Osimertinib | 86% | 23.7 vs. 16.6 (HR: 0.70) | Data not yet mature | ||
| HER2 mutation (IHC3+) | T-DXd | Subsequent lines | DESTINY-Lung02 [139] (NCT04644237) | Phase 2 single-arm trial investigating T-DXd for previously treated (platinum-ChT) patients with Her2 mutation-positive patients | 49% | 9.9 | 19.5 |
| KRAS G12C | Sotorasib | Subsequent lines | CodeBreak200 [140] (NCT04303780) | Phase 3 double-blind trial comparing Sotorasib to Docetaxel in patients with previously treated metastatic NSCLC with KRAS G12C mutation | 28% | 5.6 vs. 4.5 (HR: 0.66) | 10.6 vs. 11.3 months (HR: 1.01) |
| Adagrasib | Subsequent lines | KRYSTAL-1 [141] (NCT03785249) | Phase 2 single-arm trial | 42.9% | 6.5 | 12.6 | |
| RET rearrangement | Selpercatinib | First line | LIBRETTO-001 [37] (NCT03157128) | Phase 1/2 basket trial | 84% | 20.3 | Not available |
| Pralsetinib | First line | ARROW [40] (NCT03037385) | Phase 1/2 basket trial | 70% | 16.5 | Not available | |
| Cabozantinib | Subsequent lines | Drilon et al, 2016 [142] NCT01639508 | Phase 2, single-center, open-label, Simon two-stage trial investigating the effectiveness of Cabozantinib in RET rearranged metastatic NSCLC | 31% | 5.5 | 9.9 | |
| ALK rearrangement | Crizotinib | First line | PROFILE 1014 [143] (NCT01154140) | Phase 3 double-blind trial comparing Crizotinib to ChT in the first line setting for ALK fusion-positive metastatic NSCLC | 74% | 10.9 vs. 7 (HR 0.45) | 47.5 vs. 47.7 (HR 1.00) |
| Subsequent post-ChT | Shaw et al, 2013 [144] (NCT00932893) | Phase 3 open-label trial comparing Crizotinib to ChT in previously ChT-treated ALK fusion-positive metastatic NSCLC patients | 65% | 7.7 vs. 3.0 (HR 0.49) | 20.3 vs. 22.8 (HR 1.02) | ||
| Ceritinib | First line | ASCEND-4 [145,146] (NCT01828099) | Phase 3 open-label trial comparing Ceritinib to ChT | 72.5% | 16.6 vs. 8.1 (HR: 0.55) | NR vs. 26.2 (HR 0.73) | |
| Subsequent post-ChT | ASCEND-5 [147] (NCT01828112) | Phase 3 double-blind trial comparing Ceritinib to ChT | 39.1% | 5.4 vs. 1.6 (HR: 0.49) | NR vs. 20.0 | ||
| Brigatinib | First line | ALTA-1L [148,149,150] (NCT02737501) | Phase 3 open-label trial comparing Brigatinib to Crizotinib | 74% | 24.0 vs. 11.0 (HR: 0.49) | NR vs. 52.2 | |
| Subsequent lines | ALTA [151,152] (NCT02094573) | Phase 2 single-arm trial investigating Brigatinib in Crizotinib-refractory ALK fusion-positive metastatic NSCLC | 53% | 15.6 | 34.1 | ||
| Alectinib | First/subsequent lines | ALEX [153,154] (NCT02075840) | Phase 3 open-label trial, compared with Crizotinib | 82.9% | 25.8 vs. 12.7 (HR: 0.51) | 5-yr OS: 62.5% vs. 45.5% (HR 0.67) | |
| Ensartinib | First line | eXalt3 [155] (NCT02767804) | Phase 3 open-label trial, compared with Crizotinib | 75% | 25.8 vs. 12.7 (HR: 0.51) | 5-yr OS: 78% vs. 78% | |
| Lorlatinib | First/subsequent lines | CROWN [119,156,157] (NCT03052608) | Phase 3 double-blind trial, compared with Crizotinib | 81% | NR vs. 9.1 (HR: 0.19) | Data not yet mature | |
| ROS1 rearrangement | Crizotinib | First line | PROFILE 1001 [158,159] (NCT00585195) | Phase 1/2 single-arm trial | 72% | 19.2 | 51.4 |
| Entrectinib | First line | STARTRK-1, -2 and ALKA-001 [160,161] (NCT02097810; NCT02568267; EudraCT, 2012–000148–88 | Integrated analysis of three Phase 1/2 trials investigating the efficiency of Entrectinib in ROS1 rearrangement-positive tumors | 77% | 19 | 47.8 | |
| Repotrectinib | First/subsequent lines | TRIDENT-1 [34] (NCT03093116) | Phase 1/2 basket trial, investigating first line or subsequent line Repotrectinib in tumors harboring ROS1, NTRK1-3, ALK gene fusions, including metastatic NSCLC. | 79% in the first line | 35.7 in the first line | Not estimable in the first line | |
| 38% post-TKI | 9 post-TKI | 25.1 post-TKI | |||||
| Lorlatinib | First/subsequent lines | Shaw et al, 2019 [162] (NCT01970865) | Open-label, single-arm Phase 1/2 trial investigating Lorlatinib in ROS1-positive NSCLC | 62% in the first line | 21.0 in the first line | Not available | |
| 35% post-TKI | 8.5 post-TKI | Not available | |||||
| MET exon 14 skipping mutation | Capmatinib | First/subsequent lines | GEOMETRY mono-1 [163,164] (NCT02414139) | Phase 2 single-arm trial | 41% in first line | 12.5 in first line | 21.4 in first line |
| 44% post-TKI | 5.5 post-TKI | 16.8 in first line | |||||
| Tepotinib | First/subsequent lines | VISION [165,166] (NCT02864992) | Phase 2 single-arm trial | 57.3% in first line | 12.6 in first line | 21.3 in first line | |
| 45.0% in pretreated | 11 in pretreated | 19.3 in pretreated | |||||
| Crizotinib | First/subsequent lines | Drilon et al., 2021 [167] (NCT00585195) | Phase 2 single-arm trial | 32% | 7.3 | 20.5 | |
| BRAF V600E | Dabrafenib + Trametinib | First/subsequent lines | Planchard et al, 2017 [168,169] (NCT01336634) | Phase 2 single-arm | 63.9% in first line | 10.8 in first line | 17.3 in first line |
| 68.4% in pretreated | 10.2 in pretreated | 18.2 in pretreated | |||||
| Encorafenib + Binimetinib | First/subsequent lines | Riely et al, 2023 [170] (NCT03915951) | Phase 2 single-arm | 75% in first line | NE in first line | Not available | |
| 46% in pretreated | 9.3 in pretreated | ||||||
| NTRK1/2/3 gene fusion | Larotrectinib | First/subsequent lines | Drilon et al, 2018 [171] (NCT02576431 NCT02122913) | Retrospective analysis of multiple Phase 1/2 single-arm trials | 73% | 35.4 | 40.7 |
| Entrectinib | First/subsequent lines | Paz-Ares et al, 2019 [172,173] ALKA (EudraCT 2012-000148-88), STARTRK-1, -2 (NCT02097810; NCT02568267) | Integrated analysis of multiple Phase 1/2 single-arm trials enrolling patients treated with Entrectinib for NTRK fusion-positive tumors, including metastatic NSCLC | 62.7% | 27.3 | 41.5 | |
| Repotrectinib | First/subsequent lines | TRIDENT-1 (NCT03093116) | Ongoing Phase 1/2 basket trial, results published for patients with ROS1 fusion (see above). | Not Available | Not Available | Not Available | |
| NRG1 gene fusion | Zenocutuzumab | Subsequent lines | eNRGy [174] (NCT02912949) | Ongoing Phase 2 single-arm trial | Not Available | Not Available | Not Available |
| PARP inhibitor | Cancer Treated | Clinical Setting | Clinical Trial | Trial Design | mPFS/DFS in mo. (HR) | mOS in mo. (HR) |
|---|---|---|---|---|---|---|
| Olaparib | Ovarian | Maintenance post-ChT | SOLO-1 [188,189] (NCT01844986) | Phase 3 double-blind trial vs. placebo, which included 391 patients with gBRCA1/2m, treated until PD/unacceptable toxicity or no evidence of disease after 2 years of treatment with Olaparib 300 mg bid. | 56.0 vs. 13.8 (0.33) | NR vs. 75.2 (0.55) |
| Maintenance post-ChT (with Bevacizumab) | PAOLA-1 [190,191] (NCT02477644) | Phase 3 double-blind trial vs. placebo, which included 806 patients with HRD-positive tumors, treated with Bevacizumab 15 mg/kg IV q3w until PD/unacceptable toxicity, or up to 15 months of treatment (including the period when Bevacizumab was offered as primary treatment), and Olaparib 300 mg bid as maintenance until PD/unacceptable toxicity or up to 2 years. | 22.1 vs. 16.6 (0.59) | 75.2 vs. 57.3 (0.62) | ||
| Breast | Adjuvant | OlympiA [192,193] (NCT02032823) | Phase 3 double-blind trial vs. placebo, which included 1836 patients with gBRCA1/2m high-risk, HER2-negative early breast cancer for 1 year of treatment with adjuvant 300 mg bid Olaparib. | NR (Δ4yr-iDFS = 7.3%, HR: 0.63) | NR (Δ4yr-OS = 3.4%, HR: 0.68) | |
| Metastatic | OlympiAD [194,195,196] (NCT02000622) | Phase 3 open-label trial vs. chemotherapy (physician’s choice), which included 302 patients with gBRCA1/2m and HER2-negative advanced breast cancer who received Olaparib 300 mg bid until PD/unacceptable toxicities. | 7.0 vs. 4.2 (0.58) | 22.6 vs. 14.7 (0.55) | ||
| Prostate | Second line mCRPC (monotherapy) | PROfound [197] (NCT02987543) | In a Phase 3 open-label trial, Cohort A included 245 patients with alterations in BRCA1, BRCA2, or ATM, previously treated with Enzalutamide or Abiraterone. Patients were randomized 2:1 to receive Olaparib 300 mg bid or a novel hormonal agent (physician’s choice between Enzalutamide or Abiraterone). | 7.4 vs. 3.6 (0.34) | 18.5 vs. 15.1 (0.64) | |
| First line mCRPC (with Abiraterone) | PROpel [198,199] (NCT03732820) | Phase 3 double-blind trial vs. placebo, which included 796 patients with mCRPC who received Olaparib 300 mg bid plus Abiraterone 1000 mg daily vs. placebo plus Abiraterone as first-line treatment, regardless of HRD status. | 24.8 vs. 16.6 (HR: 0.66) | 42.1 vs. 34.7 (HR: 0.81) | ||
| Pancreatic | Maintenance | POLO [200,201] (NCT02184195) | Phase 3 double-blind trial vs. placebo. Patients with gBRCA1/2m pancreatic adenocarcinoma who did not progress after 16 weeks of first-line platinum-based ChT received maintenance Olaparib 300 mg bid until PD/unacceptable toxicity. | 7.4 vs. 3.8 (HR: 0.53) | 19.0 vs. 19.2 (HR: 0.83; not significant) | |
| Endometrial | Advance/Recurrent | DUO-E/GOG-3041/ENGOT-EN10 [202] (NCT04269200) | Phase 3 double-blind trial evaluating Olaparib in combination with Durvalumab as maintenance therapy after primary ChT plus Durvalumab for 718 patients with advanced endometrial cancer. | 15.1 vs. 9.6 (HR: 0.55) | NR vs. 25.9 (HR: 0.59) | |
| Niraparib | Ovarian | Maintenance post-ChT | PRIMA [203,204] (NCT02655016) | Phase 3 double-blind trial vs. placebo, investigating maintenance Niraparib 300 mg daily for up to 36 months in patients who responded to primary platinum-based ChT, regardless of HRD status. | 13.8 vs. 8.2 (HR: 0.62 in the overall population) 21.9 vs. 10.4 (HR: 0.43 in patients with HRD-positive) | No significant difference in OS in the overall population. |
| Prostate | mCRPC (with Abiraterone) | MAGNITUDE [205] (NCT03748641) | Phase 3 double-blind trial vs. placebo, investigating the efficiency of Niraparib added to Abiraterone in 423 patients with HRD-positive and 247 with no HRD alterations. | 16.5 vs. 13.7 (HR: 0.73 in the HRD-positive group, while HRD-negative was closed for futility) | NA | |
| Rucaparib | Ovarian | Maintenance post-ChT | ARIEL3 [206,207] (NCT01968213) | Phase 3 open-label trial vs. physician’s choice, which investigated the efficiency of Rucaparib 600mg bid for up to 24 months as maintenance in 564 patients who responded to primary platinum-based ChT. | 10.8 vs. 5.4 (HR: 0.36 in the overall population, regardless of BRCA1/2m or HRD status) | No significant difference in OS in the overall population. |
| Prostate | mCRPC monotherapy | TRITON3 [208] (NCT02975934) | Phase 3 open-label trial vs. physician’s choice in patients with mCRPC who progressed after first-line treatment (novel hormonal agent or docetaxel). Patients (405) with BRCA1/2m or ATM alteration were randomized 2:1. | 10.2 vs. 6.4 (HR: 0.61 in the overall population) 11.2 vs. 6.4 (HR: 0.50 in the BRCA1/2m population) | NA | |
| Talazoparib | Breast | Metastatic | EMBRACA [209,210] (NCT01945775) | Phase 3 open-label trial, which enrolled 431 patients with gBRCA1/2m advanced breast cancer to compare Talazoparib 1 mg/day to physician’s choice single-agent ChT. | 8.6 vs. 5.6 (HR: 0.55) | No significant difference in mOS. |
| Prostate | mCRPC (with Enzalutamide) | TALAPRO-2 [211,212] (NCT03395197) | Phase 3 open-label trial, enrolling 805 patients to investigate the efficiency of Talazoparib and Enzalutamide in first-line treatment of mCRPC, regardless of HRD status. Another analysis presented data for the HRD-positive cohort, 399 patients, investigating the efficiency of Talazoparib 1mg/day added to Enzalutamide as first-line therapy of mCRPC. | rPFS: NR vs. 13.8 (HR: 0.45 for HRD-positive) rPFS: NR vs. 21.9 (HR: 0.63 for all participants) | Data are immature. |
| Biomarker | FDA Approved Therapies | Clinical Trial | Trial Design | Trial Outcomes |
|---|---|---|---|---|
| PIK3CA activating mutation | Inavolisib + Palbociclib + Fulvestrant | INAVO120 [217] (NCT04191499) | The 325 patients with ER-positive, HER2-negative ABC, who progressed within 12 months after the completion of adjuvant endocrine therapy, were randomized to receive Inavolisib 9mg daily or placebo, with Fulvestrant and Palbociclib. | In the ongoing study, mPFS was improved (15 months vs. 7.3 months, HR 0.43, 95% CI 0.32–0.59 p < 0.001), but with higher toxicities associated. Data for OS are still immature. |
| Alpelisib + Fulvestrant | SOLAR-1 [218,219] (NCT02437318) | A randomized, Phase 3 trial enrolled 572 patients with ER-positive, HER2-negative ABC, who progressed on previous endocrine therapy. | Improved PFS (11.0 vs. 5.7 months; HR = 0.65, p < 0.001) in PIK3CA-mutated tumors. There is no PFS/OS benefit for patients without PIK3CA mutation. | |
| Capivasertib + Fulvestrant (active on PIK3CA or AKT1 activating mutations, as well as PTEN alterations) | CAPItello-291 [220] (NCT04305496) | The 708 patients with HER2-negative, ER-positive, and ABC who progressed on first-line CDK4/6 inhibitor were enrolled in a Phase 3, randomized, double-blind trial comparing the addition of Capivasertib to Fulvestrant with placebo. | Significant improvement in PFS (7.2 vs. 3.6 months; HR = 0.60, p < 0.001); OS data pending. | |
| ESR1 mutation | Elacestrant | EMERALD [221] (NCT03778931) | The 477 patients with ER-positive/HER2-negative ABC, who had 1–2 line(s) of endocrine therapy (CDK4/6 inhibitor, and ≤1 ChT) were randomized in an open-label, Phase III trial to receive Elacestrant 400 mg/day or SoC endocrine monotherapy. | PFS benefit both for ESR1-positive (3.8 vs. 1.9 months; HR = 0.55, p < 0.001) and for the general population (2.8 vs. 1.9 months; HR = 0.70); OS trend favoring Elacestrant only for ESR1-positive. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeș, L.N.; Păun, M.-A.; Butnariu, I.; Simion, L.; Manolescu, L.S.C.; Trifănescu, O.G.; Anghel, R.M. Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications. Int. J. Mol. Sci. 2025, 26, 3123. https://doi.org/10.3390/ijms26073123
Galeș LN, Păun M-A, Butnariu I, Simion L, Manolescu LSC, Trifănescu OG, Anghel RM. Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications. International Journal of Molecular Sciences. 2025; 26(7):3123. https://doi.org/10.3390/ijms26073123
Chicago/Turabian StyleGaleș, Laurenția Nicoleta, Mihai-Andrei Păun, Ioana Butnariu, Laurentiu Simion, Loredana Sabina Cornelia Manolescu, Oana Gabriela Trifănescu, and Rodica Maricela Anghel. 2025. "Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications" International Journal of Molecular Sciences 26, no. 7: 3123. https://doi.org/10.3390/ijms26073123
APA StyleGaleș, L. N., Păun, M.-A., Butnariu, I., Simion, L., Manolescu, L. S. C., Trifănescu, O. G., & Anghel, R. M. (2025). Next-Generation Sequencing in Oncology—A Guiding Compass for Targeted Therapy and Emerging Applications. International Journal of Molecular Sciences, 26(7), 3123. https://doi.org/10.3390/ijms26073123







