Myotube Formation and Cellular Fusion Are Diminished Due to Low Birth Weight in Piglets
Abstract
:1. Introduction
2. Results
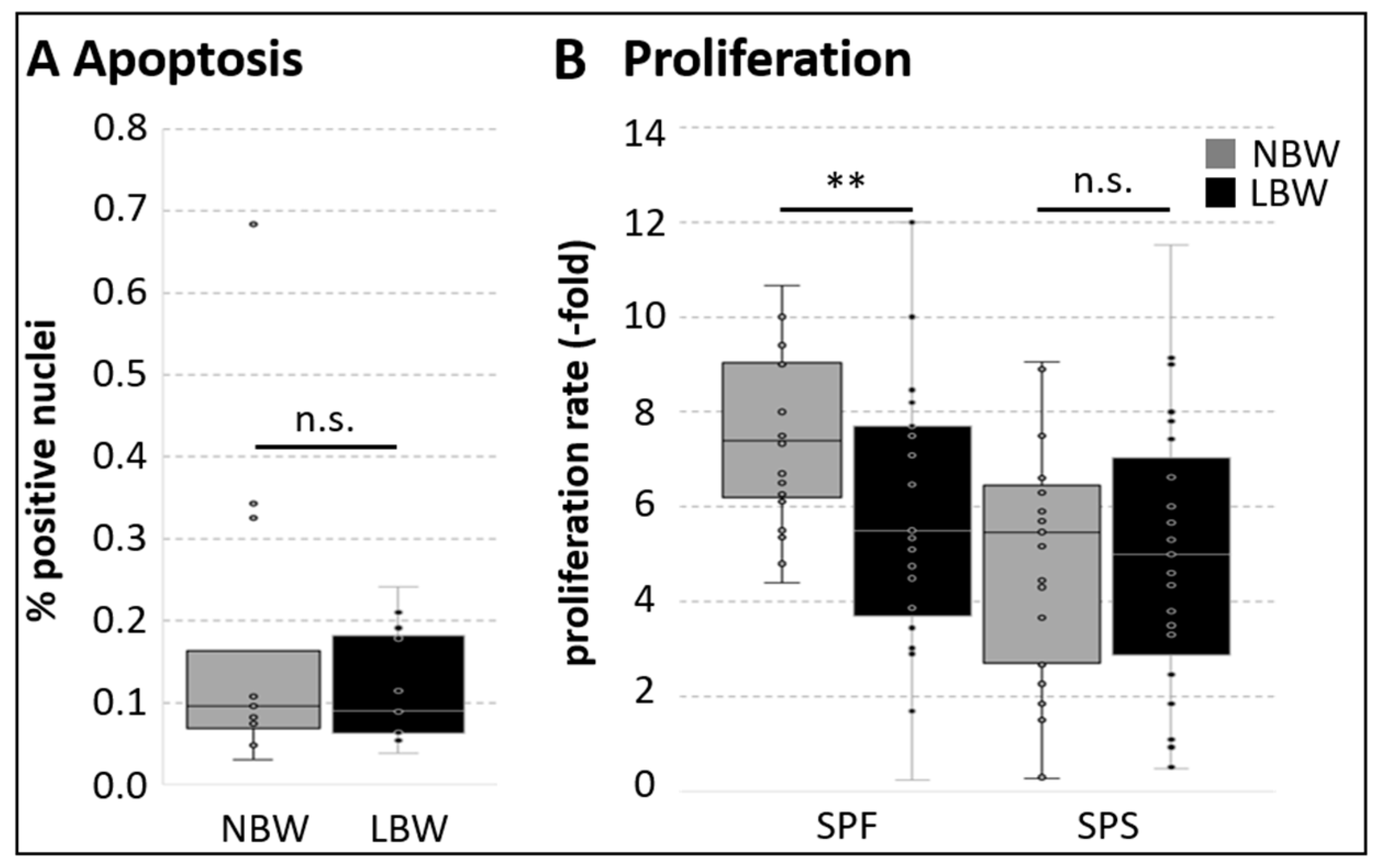
2.1. Growth and Myogenic Marker Expression of Cell Subpopulations from NBW and LBW Piglets During Proliferation
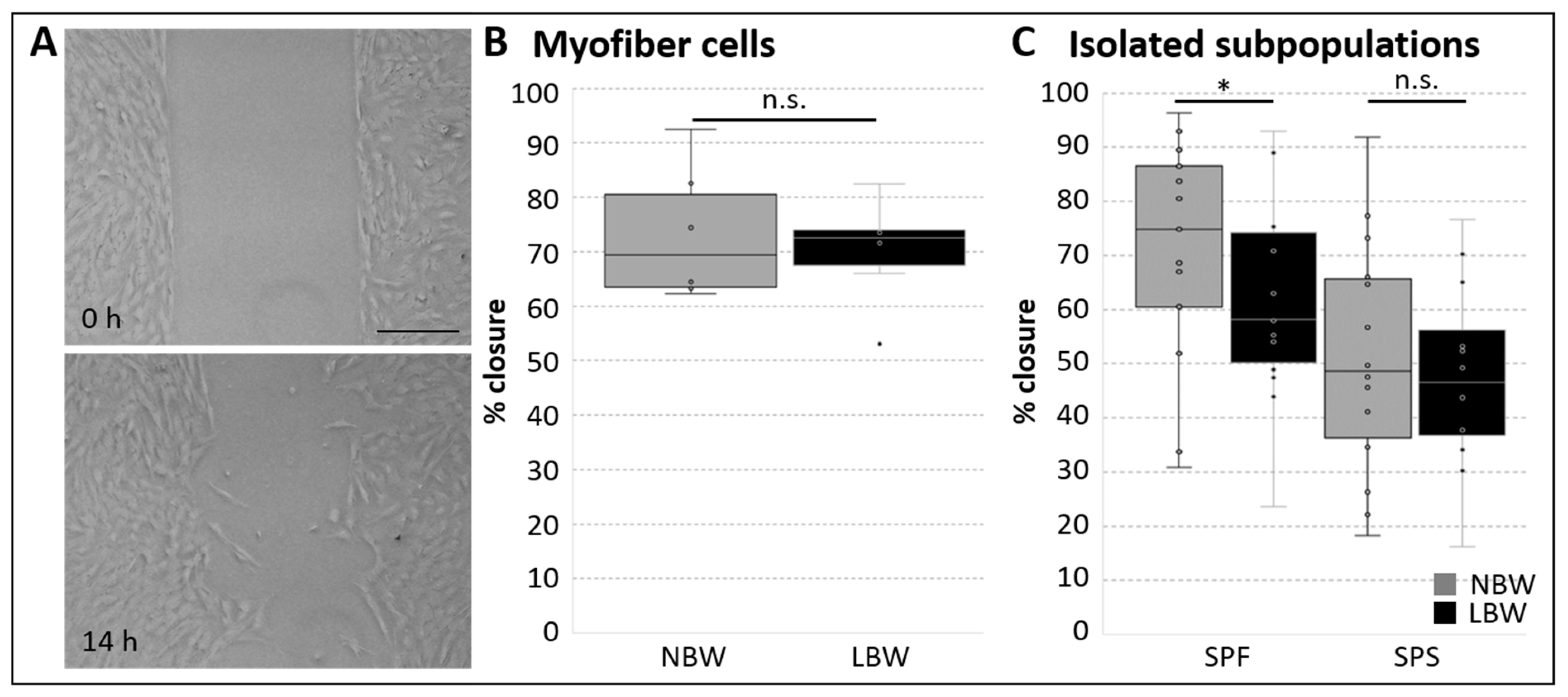
2.2. Migration of Cell Subpopulations and Myofiber-Derived Cells from NBW and LBW Piglets During Proliferation
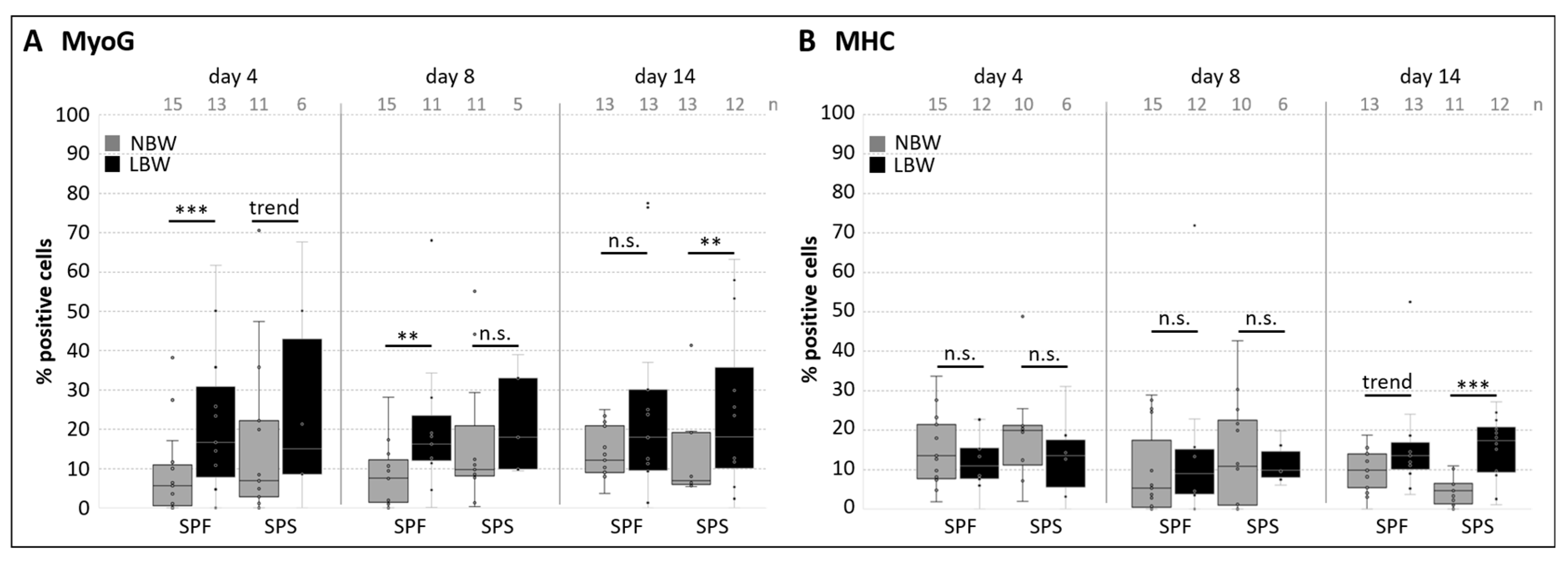
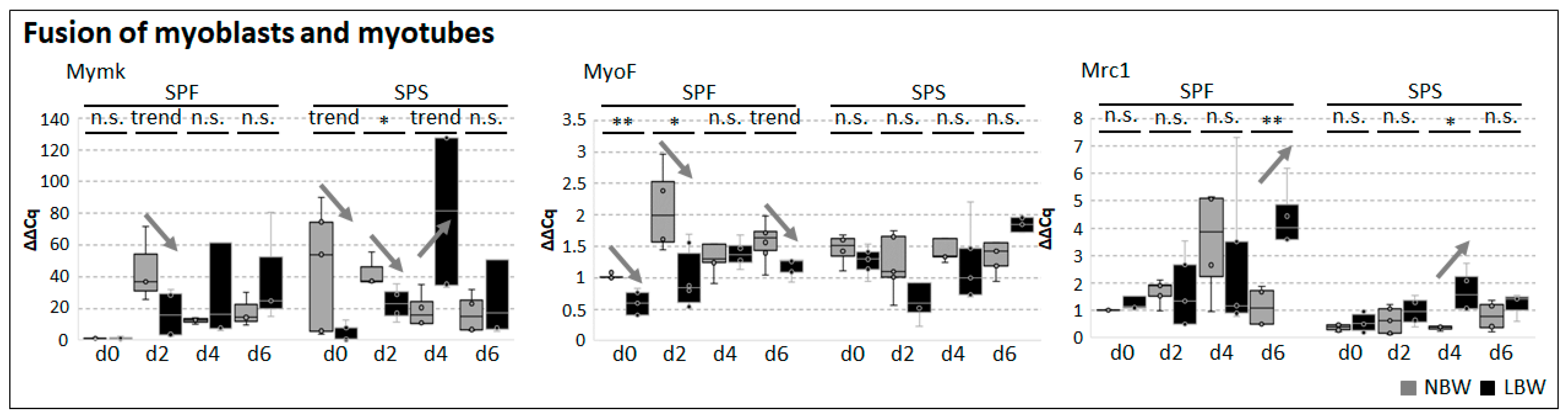
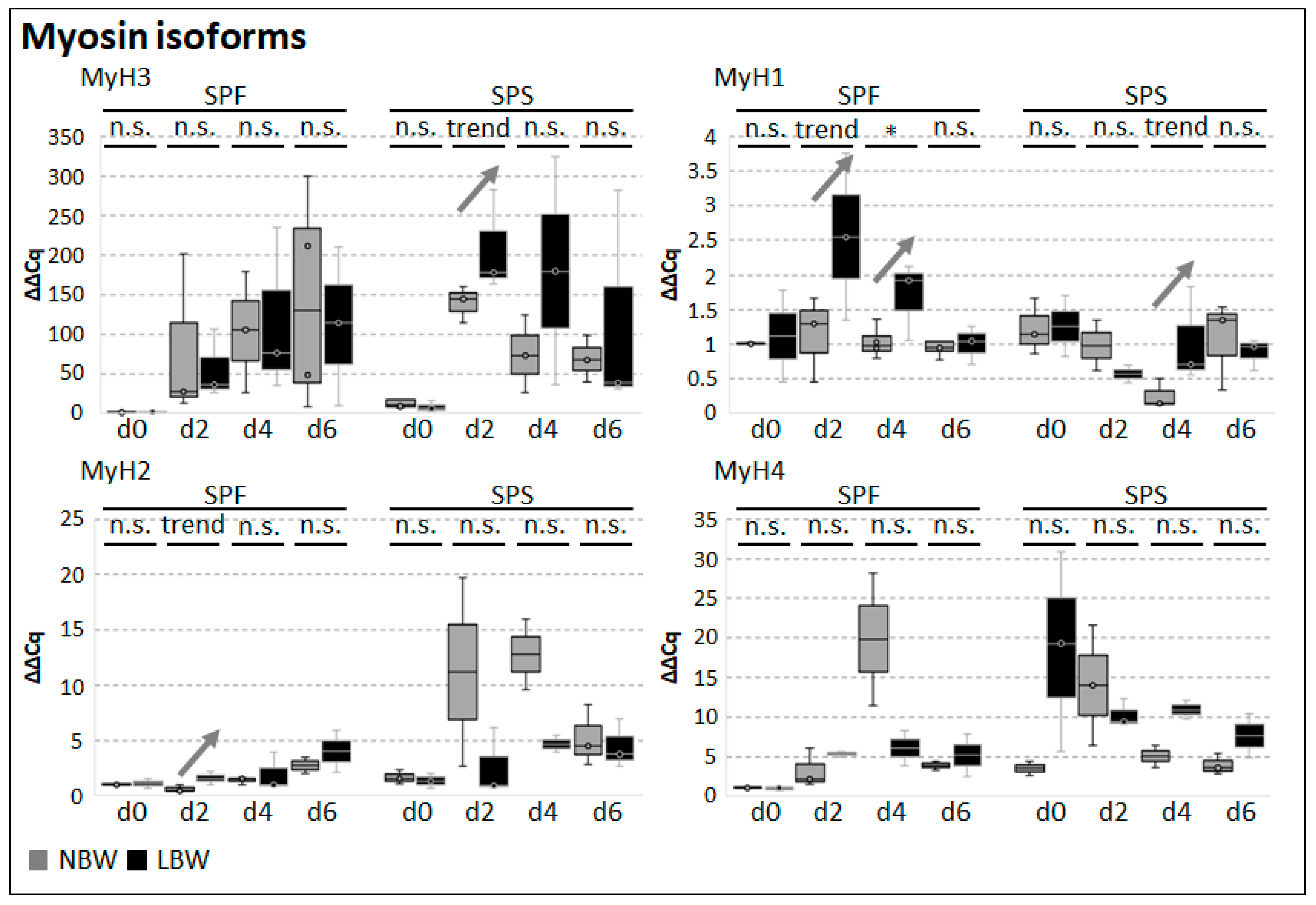
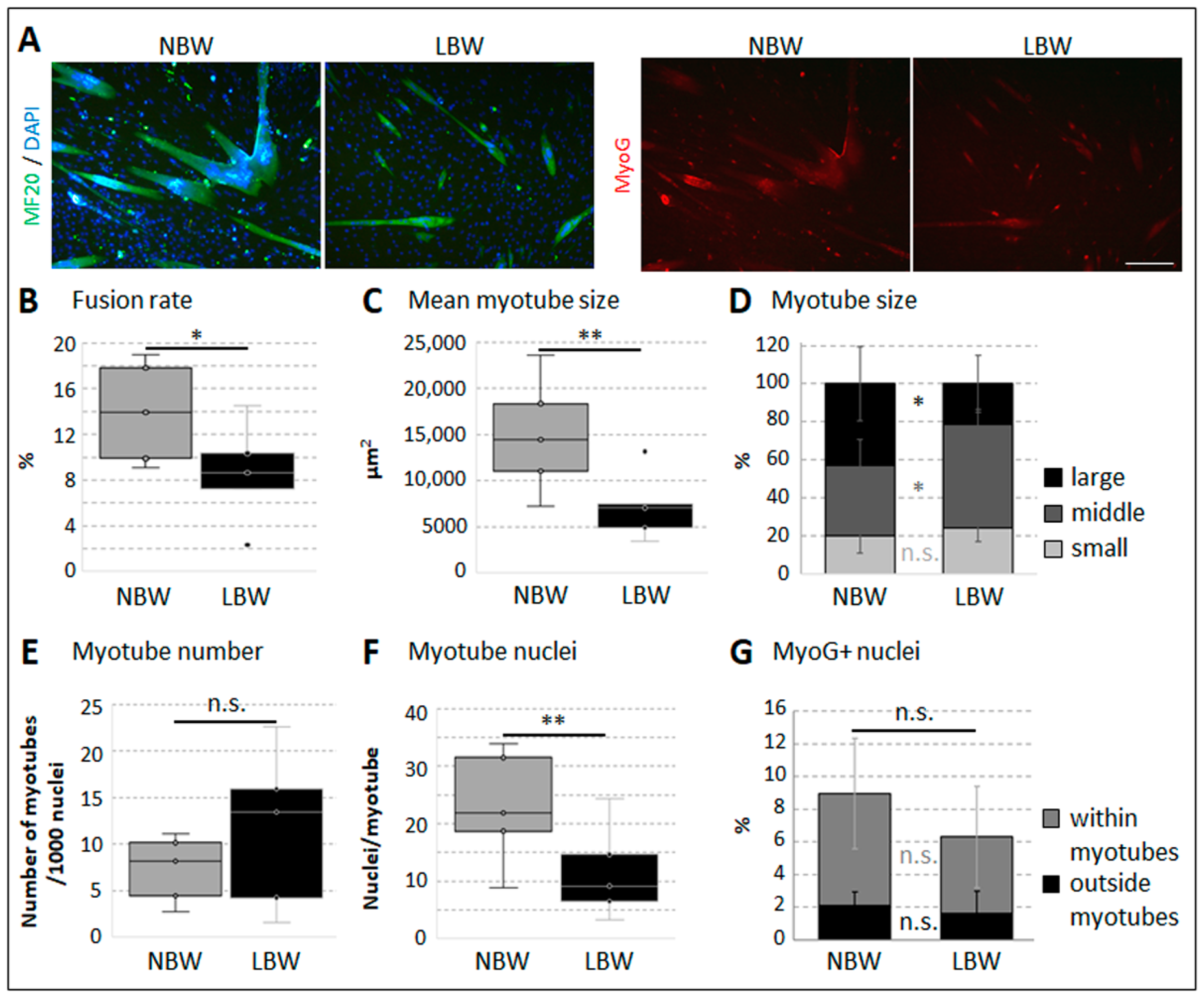
2.3. Differentiation Capacity of Muscle-Derived Cell Subpopulations
2.4. Differentiation Capacity of Myofiber-Derived Cells
3. Discussion
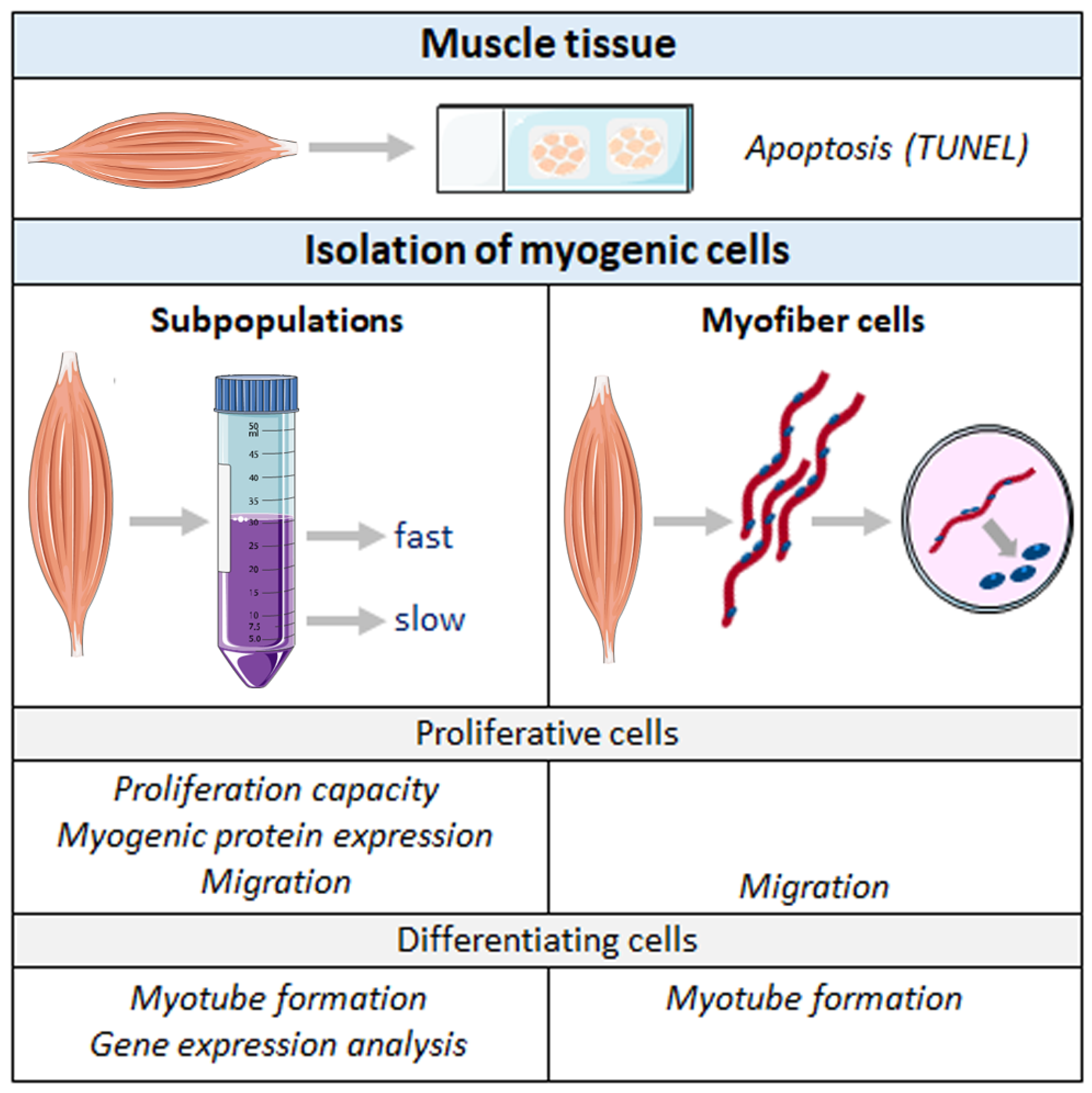
4. Materials and Methods
4.1. Animals and Muscle Dissection
4.2. TUNEL Assay
4.3. Isolation of Cell Subpopulations SPF and SPS
4.4. Isolation of Cells from Single Myofibers
4.5. Differentiation Assay
4.6. Flow Cytometry Analysis
4.7. Immunofluorescence Staining of Differentiating Cells
4.8. Migration Assay
4.9. Gene Expression Analyses
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FBS | Fetal bovine serum |
| FT | Fibularis tertius |
| LBW | Low birth weight |
| LD | Longissimus dorsi |
| MHC | Myosin heavy chain |
| MMC | Mitomycin C |
| MPC | Myogenic progenitor cells |
| Mrc1 | Mannose receptor 1 |
| MyoF | Myoferlin |
| MyoG | Myogenin |
| Mymk | Myomaker |
| NBW | Normal birth weight |
| SC | Satellite cells |
| SM | Semimembranosus |
| SPF | Subpopulation fast |
| SPS | Subpopulation slow |
References
- Rehfeldt, C.; Kuhn, G. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J. Anim. Sci. 2006, 84 (Suppl. S13), E113–E123. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal nutrition and fetal development. J. Nutr. 2004, 134, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.T.; Macko, A.R.; Nearing, M.; Chen, X.; Rhoads, R.P.; Limesand, S.W. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J. Pregnancy 2012, 2012, 631038. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Lefaucheur, L.; Louveau, I.; Lebret, B.; Pichodo, X.; Le Cozler, Y. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest. Prod. Sci. 2005, 93, 137–146. [Google Scholar] [CrossRef]
- Varona, L.; Sorensen, D.; Thompson, R. Analysis of litter size and average litter weight in pigs using a recursive model. Genetics 2007, 177, 1791–1799. [Google Scholar] [CrossRef]
- Pardo, C.E.; Kreuzer, M.; Bee, G. Effect of average litter weight in pigs on growth performance, carcass characteristics and meat quality of the offspring as depending on birth weight. Animal 2013, 7, 1884–1892. [Google Scholar] [CrossRef]
- Ramsay, T.G.; Stoll, M.J.; Shannon, A.E.; Blomberg, L.A. Metabolomic analysis of longissimus from underperforming piglets relative to piglets with normal preweaning growth. J. Anim. Sci. Biotechnol. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Milligan, B.N.; Fraser, D.; Kramer, D.L. Within-litter birth weight variation in the domestic pig and its relation to pre-weaning survival, weight gain, and variation in weaning weights. Livest. Prod. Sci. 2002, 76, 181–191. [Google Scholar] [CrossRef]
- Rutherford, K.M.D.; Baxter, E.M.; D’Eath, R.B.; Turner, S.P.; Arnott, G.; Roehe, R.; Ask, B.; Sandoe, P.; Moustsen, V.A.; Thorup, F.; et al. The welfare implications of large litter size in the domestic pig I: Biological factors. Anim. Welf. 2013, 22, 199–218. [Google Scholar] [CrossRef]
- Douglas, S.L.; Edwards, S.A.; Sutcliffe, E.; Knap, P.W.; Kyriazakis, I. Identification of risk factors associated with poor lifetime growth performance in pigs. J. Anim. Sci. 2013, 91, 4123–4132. [Google Scholar] [CrossRef]
- Gieling, E.T.; Park, S.Y.; Nordquist, R.E.; van der Staay, F.J. Cognitive performance of low- and normal-birth-weight piglets in a spatial hole-board discrimination task. Pediatr. Res. 2012, 71, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Litten, J.C.; Drury, P.C.; Corson, A.M.; Lean, I.J.; Clarke, L. The influence of piglet birth weight on physical and behavioural development in early life. Biol. Neonate 2003, 84, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Li, D.; Yin, Y.; Wang, X.; Li, P.; Dangott, L.J.; Hu, W.; Wu, G. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J. Nutr. 2008, 138, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, G.R.; Dixon, W.T.; Novak, S.; Putman, C.T.; Town, S.C.; Vinsky, M.D. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 2006, 84 (Suppl. S13), E105–E112. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Da Costa, N.; McGillivray, C.; Chang, K.C. Postnatal myosin heavy chain isoforms in prenatal porcine skeletal muscles: Insights into temporal regulation. Anat. Rec. Part A 2003, 273, 731–740. [Google Scholar] [CrossRef]
- Beermann, D.H.; Cassens, R.G.; Hausman, G.J. A second look at fiber type differentiation in porcine skeletal muscle. J. Anim. Sci. 1978, 46, 125–132. [Google Scholar] [CrossRef]
- Suzuki, A.; Cassens, R.G. A histochemical study of myofiber types in muscle of the growing pig. J. Anim. Sci. 1980, 51, 1449–1461. [Google Scholar] [CrossRef]
- Town, S.C.; Putman, C.T.; Turchinsky, N.J.; Dixon, W.T.; Foxcroft, G.R. Number of conceptuses in utero affects porcine fetal muscle development. Reproduction 2004, 128, 443–454. [Google Scholar] [CrossRef]
- Stange, K.; Miersch, C.; Sponder, G.; Rontgen, M. Low birth weight influences the postnatal abundance and characteristics of satellite cell subpopulations in pigs. Sci. Rep. 2020, 10, 6149. [Google Scholar] [CrossRef]
- Nissen, P.M.; Oksbjerg, N. In vitro primary satellite cell growth and differentiation within litters of pigs. Anim. Int. J. Anim. Biosci. 2009, 3, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Yang, W.J.; Yang, Z.; Shu, G.; Wang, S.B.; Jiang, Q.Y.; Yuan, L.; Wu, T.S. The differential proliferative ability of satellite cells in Lantang and Landrace pigs. PLoS ONE 2012, 7, e32537. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Kuang, S. Meat Science and Muscle Biology Symposium: Stem cell niche and postnatal muscle growth. J. Anim. Sci. 2012, 90, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Swatland, H.J. Accumulation of Myofiber Nuclei in Pigs with Normal and Arrested Development. J. Anim. Sci. 1977, 44, 759–764. [Google Scholar] [CrossRef]
- Campion, D.R.; Richardson, R.L.; Reagan, J.O.; Kraeling, R.R. Changes in the satellite cell population during postnatal growth of pig skeletal muscle. J. Anim. Sci. 1981, 52, 1014–1018. [Google Scholar] [CrossRef]
- Lepper, C.; Conway, S.J.; Fan, C.M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 2009, 460, 627–631. [Google Scholar] [CrossRef]
- Keefe, A.C.; Lawson, J.A.; Flygare, S.D.; Fox, Z.D.; Colasanto, M.P.; Mathew, S.J.; Yandell, M.; Kardon, G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun. 2015, 6, 7087. [Google Scholar] [CrossRef]
- Schultz, E.; Gibson, M.C.; Champion, T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radioautographic study. J. Exp. Zool. 1978, 206, 451–456. [Google Scholar] [CrossRef]
- Zammit, P.S.; Golding, J.P.; Nagata, Y.; Hudon, V.; Partridge, T.A.; Beauchamp, J.R. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J. Cell Biol. 2004, 166, 347–357. [Google Scholar] [CrossRef]
- Mesires, N.T.; Doumit, M.E. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am. J. Physiol.-Cell Physiol. 2002, 282, C899–C906. [Google Scholar] [CrossRef]
- Sampath, S.C.; Sampath, S.C.; Millay, D.P. Myoblast fusion confusion: The resolution begins. Skelet. Muscle 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Rouger, K.; Brault, M.; Daval, N.; Leroux, I.; Guigand, L.; Lesoeur, J.; Fernandez, B.; Cherel, Y. Muscle satellite cell heterogeneity: In vitro and in vivo evidences for populations that fuse differently. Cell Tissue Res. 2004, 317, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Schoneich, C.; Dremina, E.; Galeva, N.; Sharov, V. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis 2014, 19, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.; Freer, M.; Mei, Y.; Weyman, C.M. Apoptosis coincident with the differentiation of skeletal myoblasts is delayed by caspase 3 inhibition and abrogated by MEK-independent constitutive Ras signaling. Cell Death Differ. 2002, 9, 209–218. [Google Scholar] [CrossRef]
- Wang, J.; Walsh, K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 1996, 273, 359–361. [Google Scholar] [CrossRef]
- Hindi, S.M.; Tajrishi, M.M.; Kumar, A. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 2013, 6, re2. [Google Scholar] [CrossRef]
- Rochlin, K.; Yu, S.; Roy, S.; Baylies, M.K. Myoblast fusion: When it takes more to make one. Dev. Biol. 2010, 341, 66–83. [Google Scholar] [CrossRef]
- Doherty, K.R.; Cave, A.; Davis, D.B.; Delmonte, A.J.; Posey, A.; Earley, J.U.; Hadhazy, M.; McNally, E.M. Normal myoblast fusion requires myoferlin. Development 2005, 132, 5565–5575. [Google Scholar] [CrossRef]
- Lek, A.; Evesson, F.J.; Sutton, R.B.; North, K.N.; Cooper, S.T. Ferlins: Regulators of vesicle fusion for auditory neurotransmission, receptor trafficking and membrane repair. Traffic 2012, 13, 185–194. [Google Scholar] [CrossRef]
- Jansen, K.M.; Pavlath, G.K. Mannose receptor regulates myoblast motility and muscle growth. J. Cell Biol. 2006, 174, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buckingham, M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006, 172, 91–102. [Google Scholar] [CrossRef]
- Ono, Y.; Masuda, S.; Nam, H.S.; Benezra, R.; Miyagoe-Suzuki, Y.; Takeda, S. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J. Cell Sci. 2012, 125, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.B.; Cohen, R.; Blom, J.; van Heemst, D.; Westendorp, R.G. Marked heterogeneity in growth characteristics of myoblast clonal cultures and myoblast mixed cultures obtained from the same individual. Gerontology 2012, 58, 150–155. [Google Scholar] [CrossRef]
- Lindstrom, M.; Pedrosa-Domellof, F.; Thornell, L.E. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: Application to a cohort of power lifters and sedentary men. Histochem. Cell Biol. 2010, 134, 371–385. [Google Scholar] [CrossRef]
- Rouger, K.; Fornasari, B.; Armengol, V.; Jouvion, G.; Leroux, I.; Dubreil, L.; Feron, M.; Guevel, L.; Cherel, Y. Progenitor cell isolation from muscle-derived cells based on adhesion properties. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2007, 55, 607–618. [Google Scholar] [CrossRef]
- McFarland, D.C.; Pesall, J.E.; Gilkerson, K.K. The influence of growth factors on turkey embryonic myoblasts and satellite cells in vitro. Gen. Comp. Endocrinol. 1993, 89, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E. Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 1996, 175, 84–94. [Google Scholar] [CrossRef]
- Baroffio, A.; Bochaton-Piallat, M.L.; Gabbiani, G.; Bader, C.R. Heterogeneity in the progeny of single human muscle satellite cells. Differ. Res. Biol. Divers. 1995, 59, 259–268. [Google Scholar] [CrossRef]
- Chakkalakal, J.V.; Christensen, J.; Xiang, W.; Tierney, M.T.; Boscolo, F.S.; Sacco, A.; Brack, A.S. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development 2014, 141, 1649–1659. [Google Scholar] [CrossRef]
- Cossu, G.; Molinaro, M. Cell heterogeneity in the myogenic lineage. Curr. Top. Dev. Biol. 1987, 23, 185–208. [Google Scholar] [PubMed]
- Li, J.; Gonzalez, J.M.; Walker, D.K.; Hersom, M.J.; Ealy, A.D.; Johnson, S.E. Evidence of heterogeneity within bovine satellite cells isolated from young and adult animals. J. Anim. Sci. 2011, 89, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.; Hurme, T.; Lukka, R.; Heino, J.; Kalimo, H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: Evidence for two different populations of satellite cells. Lab. Investig. J. Tech. Methods Pathol. 1995, 72, 341–347. [Google Scholar]
- Yablonka-Reuveni, Z.; Nameroff, M. Skeletal muscle cell populations. Separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry 1987, 87, 27–38. [Google Scholar] [CrossRef]
- Miersch, C.; Stange, K.; Hering, S.; Kolisek, M.; Viergutz, T.; Rontgen, M. Molecular and functional heterogeneity of early postnatal porcine satellite cell populations is associated with bioenergetic profile. Sci. Rep. 2017, 7, 45052. [Google Scholar] [CrossRef] [PubMed]
- Miersch, C.; Stange, K.; Rontgen, M. Separation of functionally divergent muscle precursor cell populations from porcine juvenile muscles by discontinuous Percoll density gradient centrifugation. BMC Cell Biol. 2018, 19, 2. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z.; Rivera, A.J. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 1994, 164, 588–603. [Google Scholar] [CrossRef]
- Stuelsatz, P.; Keire, P.; Yablonka-Reuveni, Z. Isolation, Culture, and Immunostaining of Skeletal Muscle Myofibers from Wildtype and Nestin-GFP Mice as a Means to Analyze Satellite Cell. Methods Mol. Biol. 2017, 1556, 51–102. [Google Scholar] [CrossRef]
- Stange, K.; Ahrens, H.E.; von Maltzahn, J.; Rontgen, M. Isolation and ex vivo cultivation of single myofibers from porcine muscle. Vitr. Cell Dev. Biol. Anim. 2020, 56, 585–592. [Google Scholar] [CrossRef]
- Poujade, M.; Grasland-Mongrain, E.; Hertzog, A.; Jouanneau, J.; Chavrier, P.; Ladoux, B.; Buguin, A.; Silberzan, P. Collective migration of an epithelial monolayer in response to a model wound. Proc. Natl. Acad. Sci. USA 2007, 104, 15988–15993. [Google Scholar] [CrossRef]
- Glenn, H.L.; Messner, J.; Meldrum, D.R. A simple non-perturbing cell migration assay insensitive to proliferation effects. Sci. Rep. 2016, 6, 31694. [Google Scholar] [CrossRef]
- Shikatani, E.A.; Trifonova, A.; Mandel, E.R.; Liu, S.T.; Roudier, E.; Krylova, A.; Szigiato, A.; Beaudry, J.; Riddell, M.C.; Haas, T.L. Inhibition of proliferation, migration and proteolysis contribute to corticosterone-mediated inhibition of angiogenesis. PLoS ONE 2012, 7, e46625. [Google Scholar] [CrossRef]
- Shefer, G.; Yablonka-Reuveni, Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 2005, 290, 281–304. [Google Scholar] [CrossRef]
- Huttner, S.S.; Ahrens, H.E.; Schmidt, M.; Henze, H.; Jung, M.J.; Schuler, S.C.; von Maltzahn, J. Isolation and Culture of Individual Myofibers and Their Adjacent Muscle Stem Cells from Aged and Adult Skeletal Muscle. Methods Mol. Biol. 2019, 2045, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.; Derno, M.; Goers, S.; Metzler-Zebeli, B.U.; Nuernberg, G.; Martens, K.; Pfuhl, R.; Nebendahl, C.; Zeyner, A.; Hammon, H.M.; et al. Higher body fatness in intrauterine growth retarded juvenile pigs is associated with lower fat and higher carbohydrate oxidation during ad libitum and restricted feeding. Eur. J. Nutr. 2014, 53, 583–597. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Tuchscherer, A.; Hartung, M.; Kuhn, G. A second look at the influence of birth weight on carcass and meat quality in pigs. Meat Sci. 2008, 78, 170–175. [Google Scholar] [CrossRef]
- Voss, A.K.; Strasser, A. The essentials of developmental apoptosis. F1000Research 2020, 9, 148. [Google Scholar] [CrossRef]
- Nossing, C.; Ryan, K.M. 50 years on and still very much alive: ‘Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics’. Br. J. Cancer 2023, 128, 426–431. [Google Scholar] [CrossRef]
- Schumacher, T.; Reyer, H.; Maak, S.; Rontgen, M. Homer 1 genotype AA variant relates to congenital splay leg syndrome in piglets by repressing Pax7 in myogenic progenitors. Front. Vet. Sci. 2023, 10, 1028879. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.; McCauley, S.R.; Zhao, L.; Johnson, S.E.; Rhoads, R.P.; El-Kadi, S.W. Diminished satellite cell fusion and S6K1 expression in myotubes derived from skeletal muscle of low birth weight neonatal pigs. Physiol. Rep. 2017, 5, e13075. [Google Scholar] [CrossRef]
- Massenet, J.; Gardner, E.; Chazaud, B.; Dilworth, F.J. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skelet. Muscle 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; Sutherland, L.B.; Bassel-Duby, R.; Olson, E.N. Myomaker is essential for muscle regeneration. Genes Dev. 2014, 28, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.; Masaki, T.; Fischman, D.A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 1982, 95, 763–770. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.H.; Li, C.Y.; Lee, C.F.; Chang, C.K.; Fang, S.H. Scratch wound closure of myoblasts and myotubes is reduced by inflammatory mediators. Int. Wound J. 2016, 13, 680–685. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.; Moorman, A.F. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Pfaffl, M.W.; Zhao, S.; Spiess, A.N.; Boggy, G.; Blom, J.; Rutledge, R.G.; Sisti, D.; Lievens, A.; De Preter, K.; et al. Evaluation of qPCR curve analysis methods for reliable biomarker discovery: Bias, resolution, precision, and implications. Methods 2013, 59, 32–46. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Lorenz, P.; Tuomi, J.M.; Hecker, M.; van den Hoff, M.J. Fluorescent-increase kinetics of different fluorescent reporters used for qPCR depend on monitoring chemistry, targeted sequence, type of DNA input and PCR efficiency. Mikrochim. Acta 2014, 181, 1689–1696. [Google Scholar] [CrossRef]
- da Costa, N.; Blackley, R.; Alzuherri, H.; Chang, K.C. Quantifying the temporospatial expression of postnatal porcine skeletal myosin heavy chain genes. J. Histochem. Cytochem. 2002, 50, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.L.; Clark, D.I.; Beever, J.E.; Dilger, A.C. Increased prenatal IGF2 expression due to the porcine intron3-G3072A mutation may be responsible for increased muscle mass. J. Anim. Sci. 2015, 93, 2546–2558. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Forward (5′-3′) | Reverse(5′-3′) |
|---|---|---|
| Pax7 | gacccctgcccaaccacatc | acatccggagtcgccacct |
| Myf5 | tgtaccaaatgtatatgccacggat | atcggtgctggcaactggag |
| MyoD1 | actgttccgacggcatgatgga | gctcgacaccgcagcattctt |
| MyoG | cgcagcgccatccagtacat | gcagatgatcccctgggttgg |
| Desmin | tttgctagtgaggccagcgg | ggatagggaggttgatccggc |
| Mrc1 | tggagcagatggaaggtttatgg | acttgaatggaaacgcacagg |
| MyoF | gggagaatttaagattgatgtcgg | ctggtatcttctgggtcattgag |
| Mymk | tttctgcgccttgacatcct | acgccaaacatcacgaaagtc |
| MyH1 [81] | gggtctacgcaaacacgagaga | cagatcctggagcctgagaatg |
| MyH2 [82] | gcaaaagcgtaatgctgaagct | cctcttccgtctggtaggtgagt |
| MyH3 [81] | tgtccaaggcgaaggccaac | ggtcagctcgctcatgctcc |
| MyH4 [81] | cactttaagtagttgtctgccttgag | ggcagcagggcactagatggt |
| Ppia | cgcgtctccttcgagctgttt | gaagtcaccaccctggcacat |
| Csnk2A2 | agtctcacgtcccgagctg | tgttccaccacgaaggttctcc |
| Yhwaz | gaactccccagagaaagcctgc | gggtatccgatgtccacaatgtc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stange, K.; Röntgen, M. Myotube Formation and Cellular Fusion Are Diminished Due to Low Birth Weight in Piglets. Int. J. Mol. Sci. 2025, 26, 2847. https://doi.org/10.3390/ijms26072847
Stange K, Röntgen M. Myotube Formation and Cellular Fusion Are Diminished Due to Low Birth Weight in Piglets. International Journal of Molecular Sciences. 2025; 26(7):2847. https://doi.org/10.3390/ijms26072847
Chicago/Turabian StyleStange, Katja, and Monika Röntgen. 2025. "Myotube Formation and Cellular Fusion Are Diminished Due to Low Birth Weight in Piglets" International Journal of Molecular Sciences 26, no. 7: 2847. https://doi.org/10.3390/ijms26072847
APA StyleStange, K., & Röntgen, M. (2025). Myotube Formation and Cellular Fusion Are Diminished Due to Low Birth Weight in Piglets. International Journal of Molecular Sciences, 26(7), 2847. https://doi.org/10.3390/ijms26072847





