The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish
Abstract
:1. Introduction
2. Results
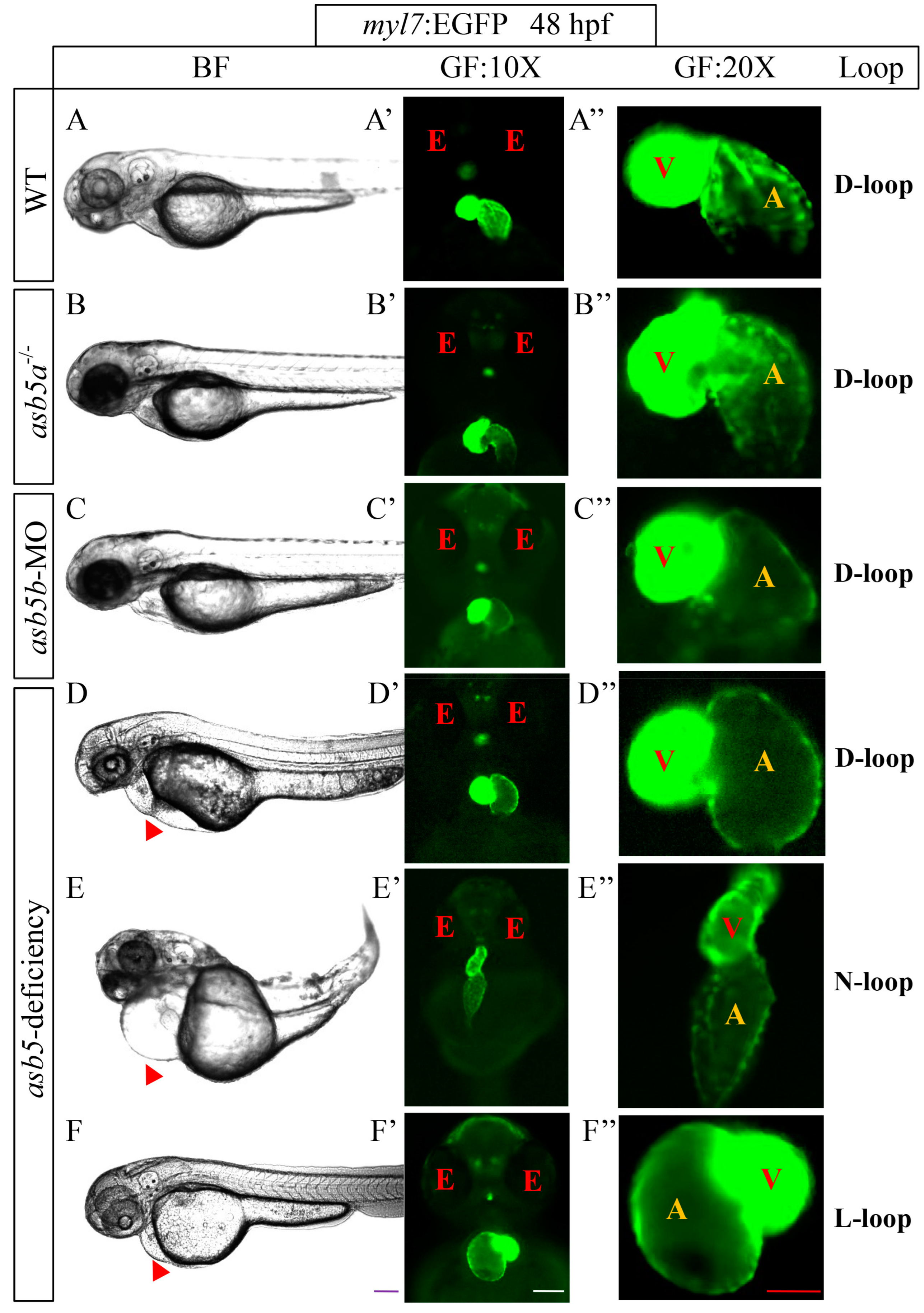
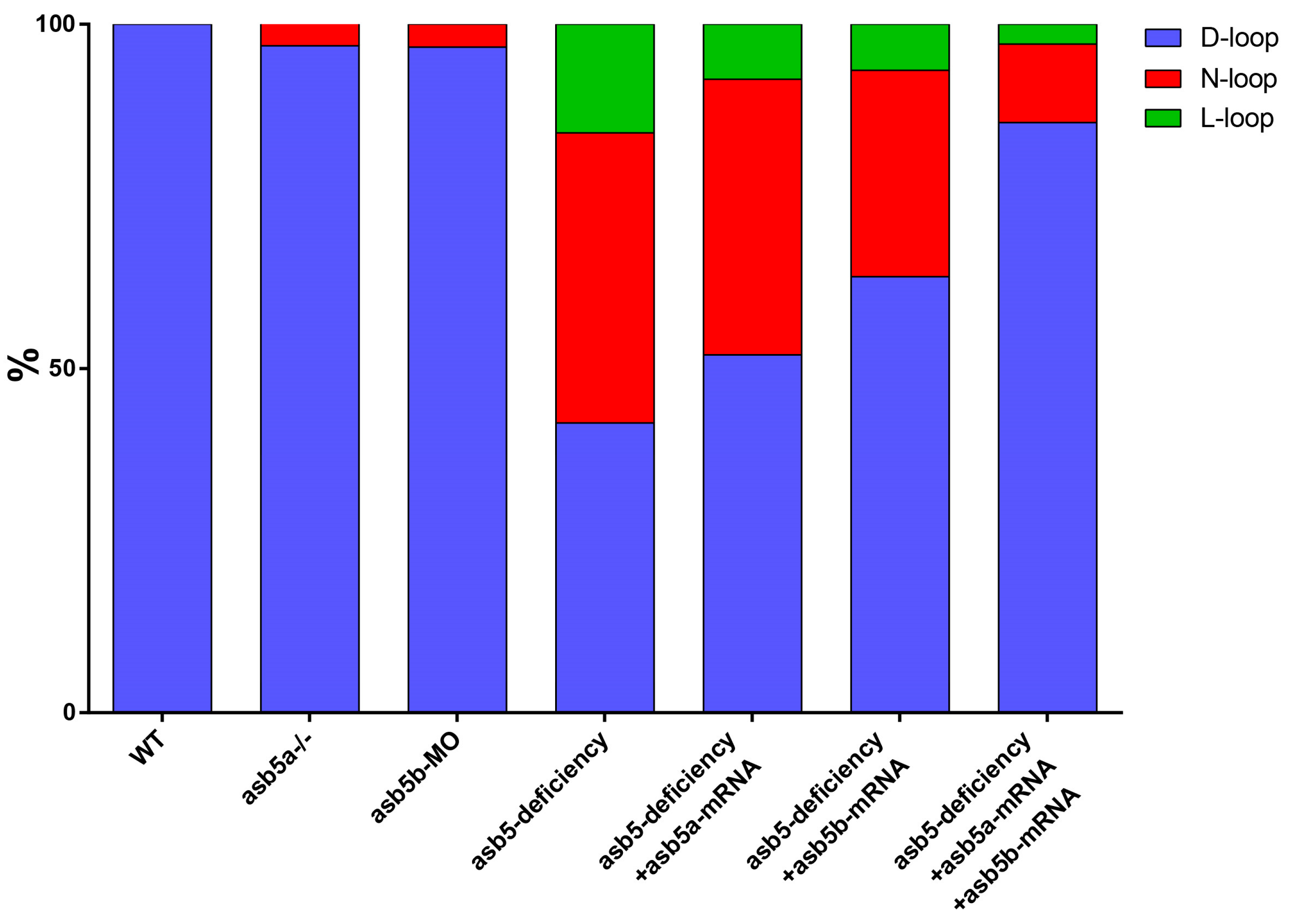
2.1. Loss of asb5 Disrupts Heart L-R Asymmetry Development in Zebrafish Embryos
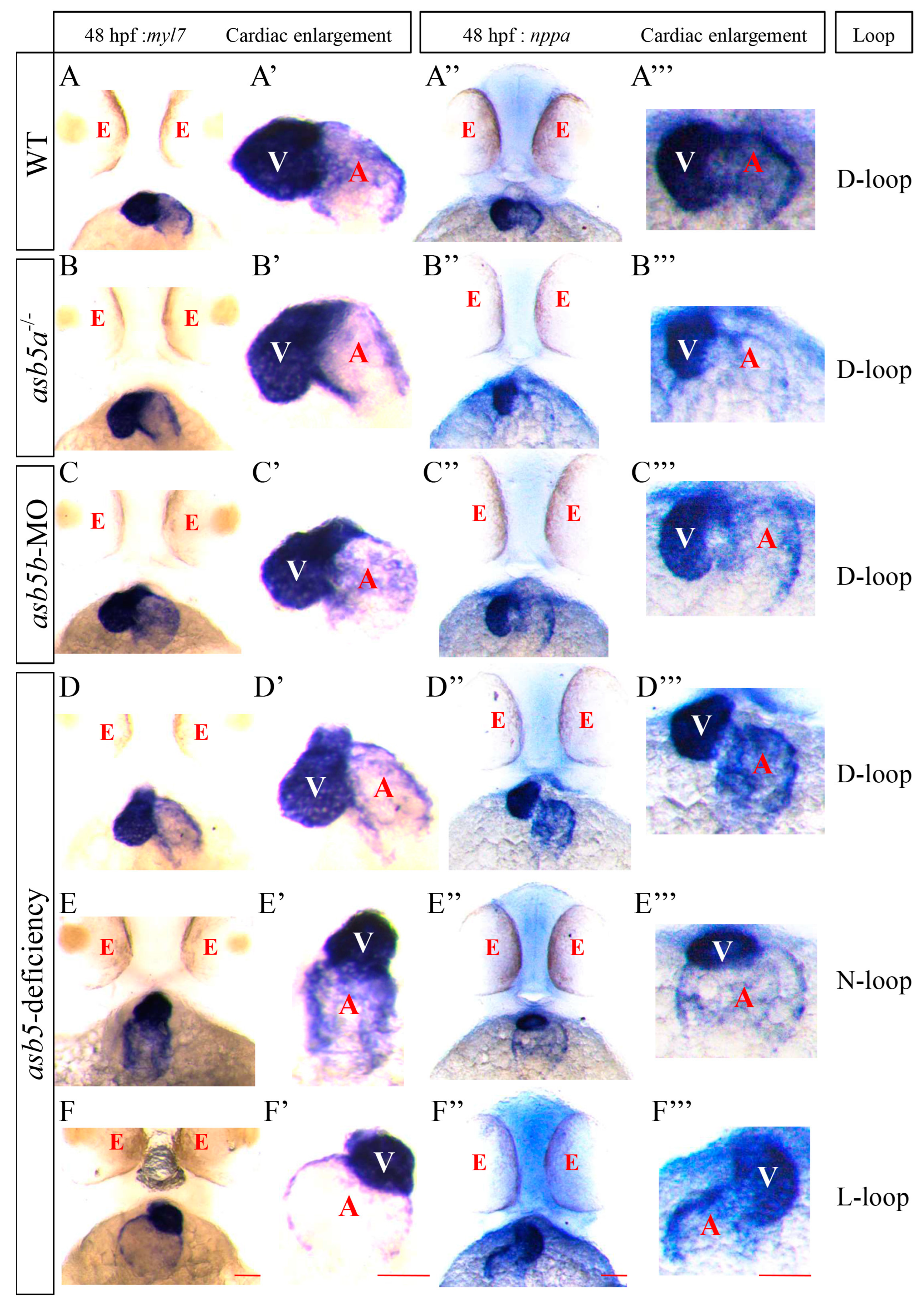
2.2. WISH Results Indicating Loss of asb5 Leads to Abnormal Cardiac Looping
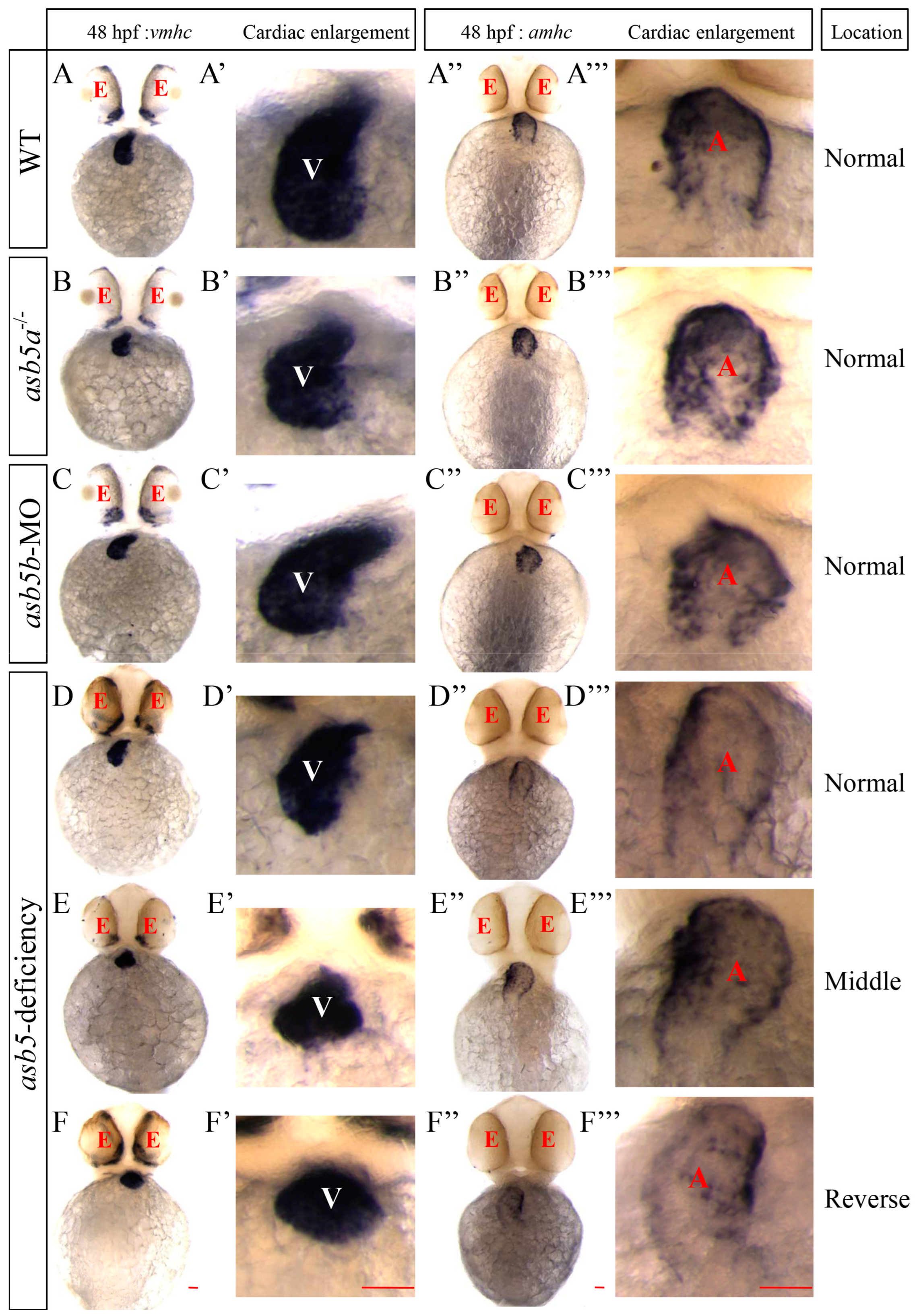
2.3. The Atria and Ventricles in the asb5-Deficiency Group Are Normally Differentiated
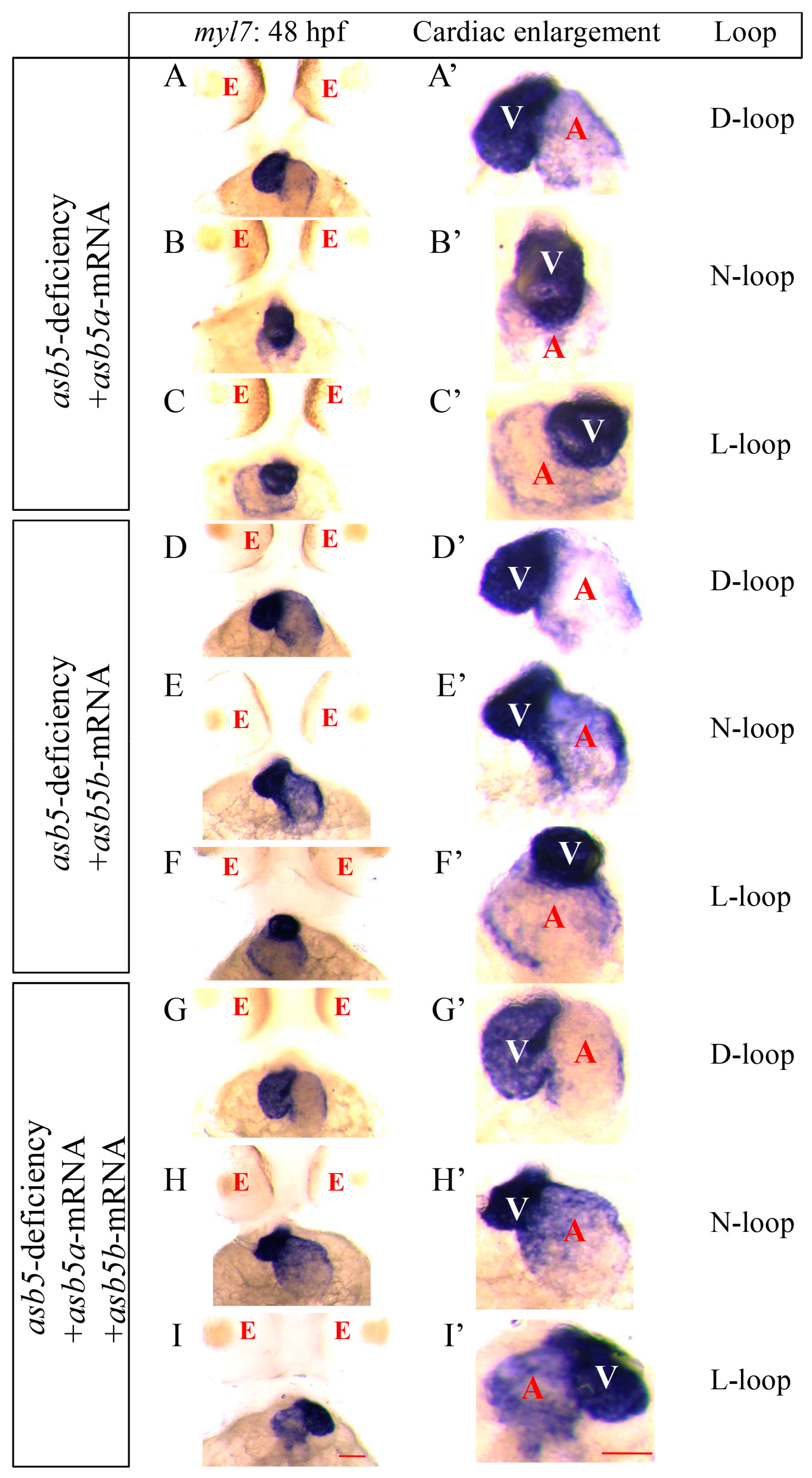
2.4. Simultaneous Injection of asb5a and asb5b mRNA Can Effectively Rescue the Circularization Abnormal Phenotype in the asb5-Deficiency Group
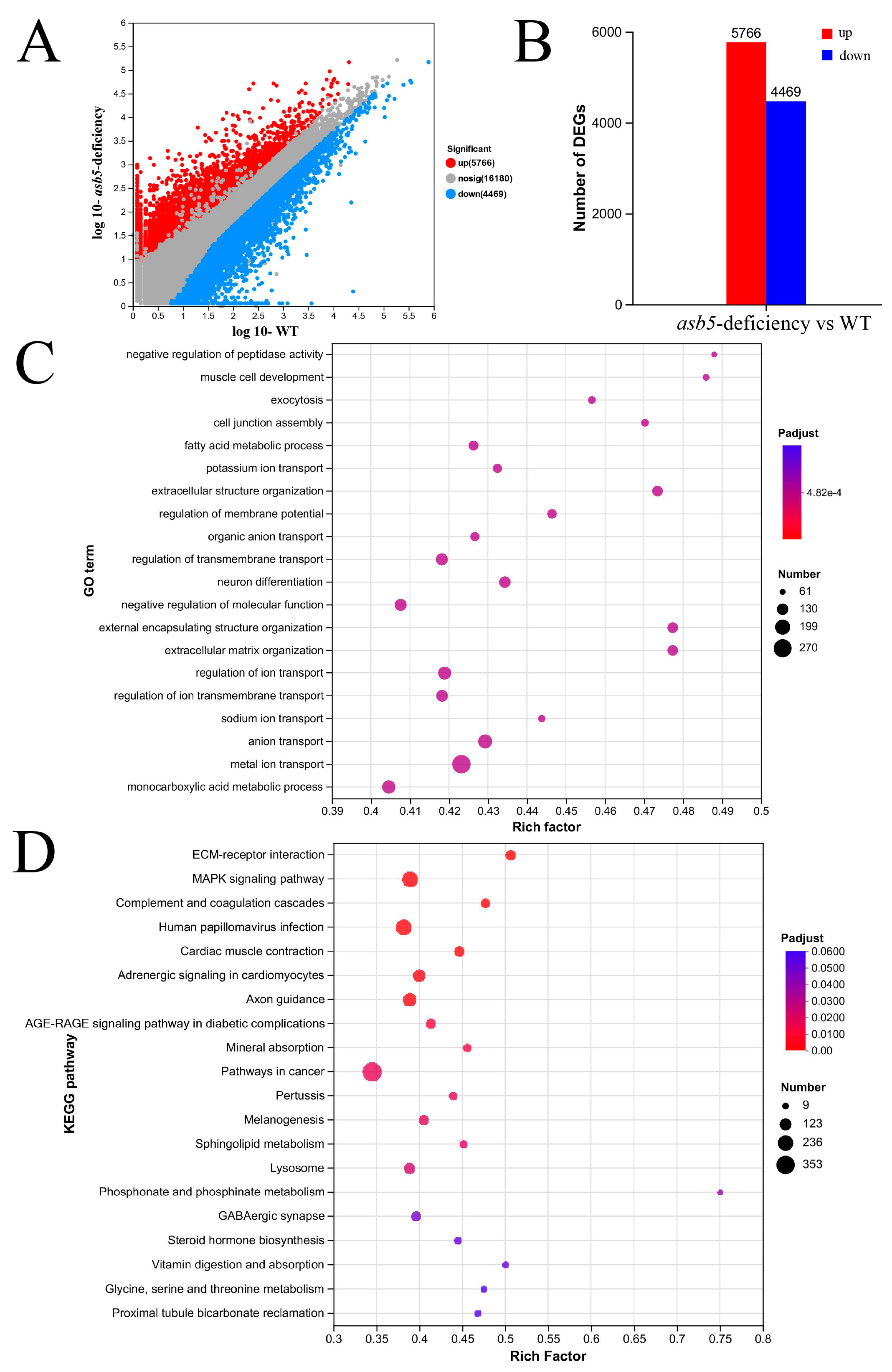
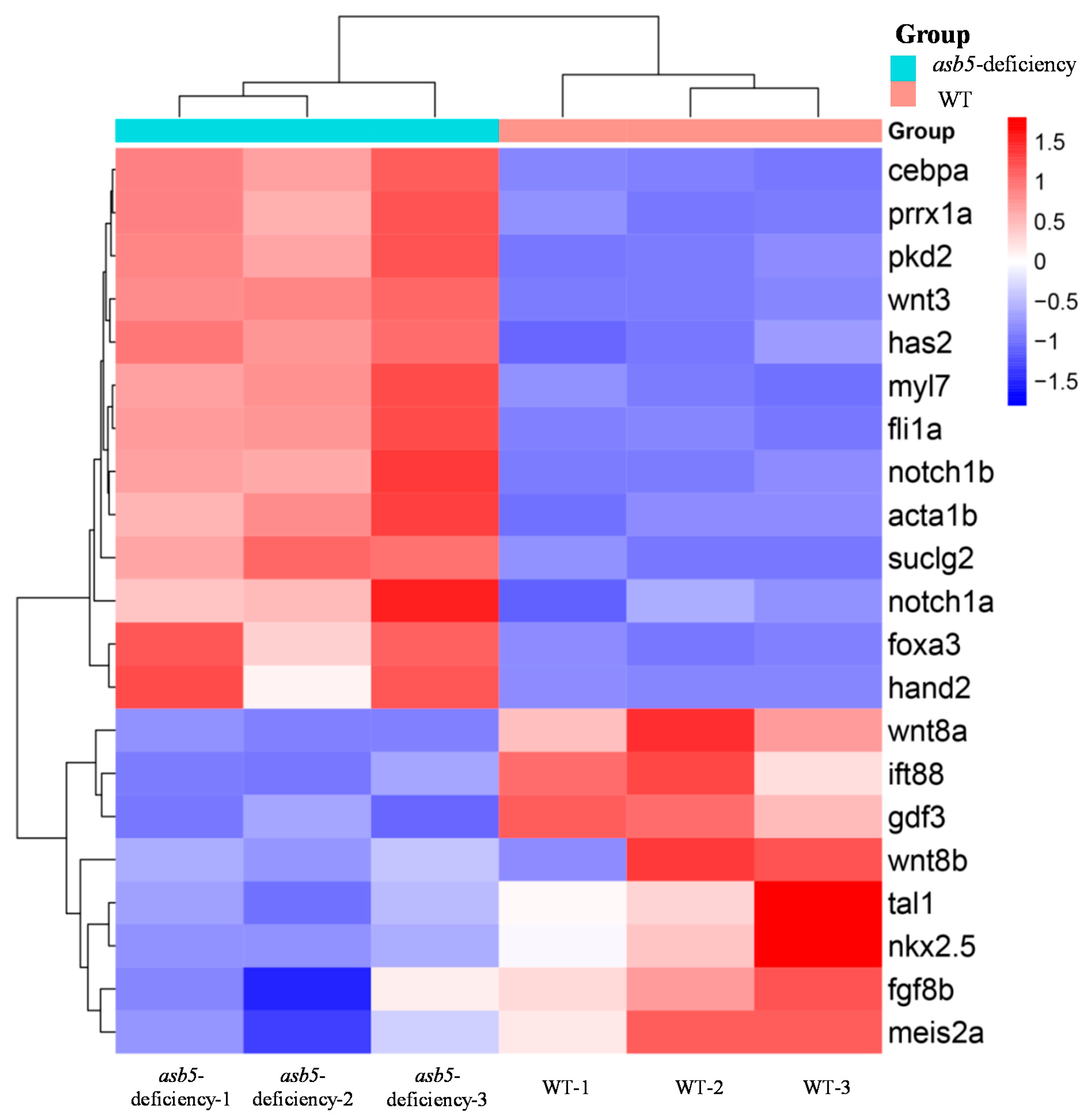
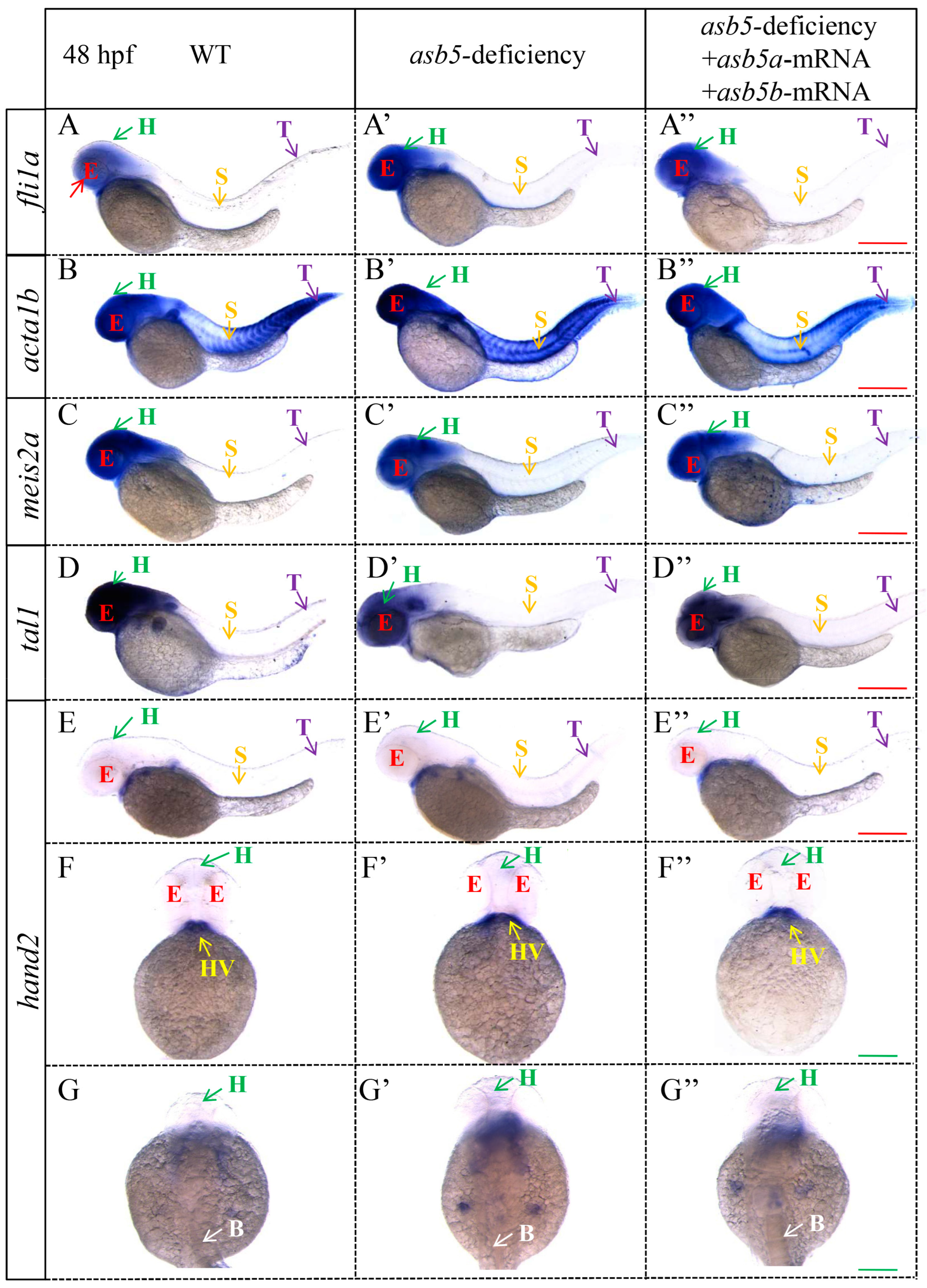
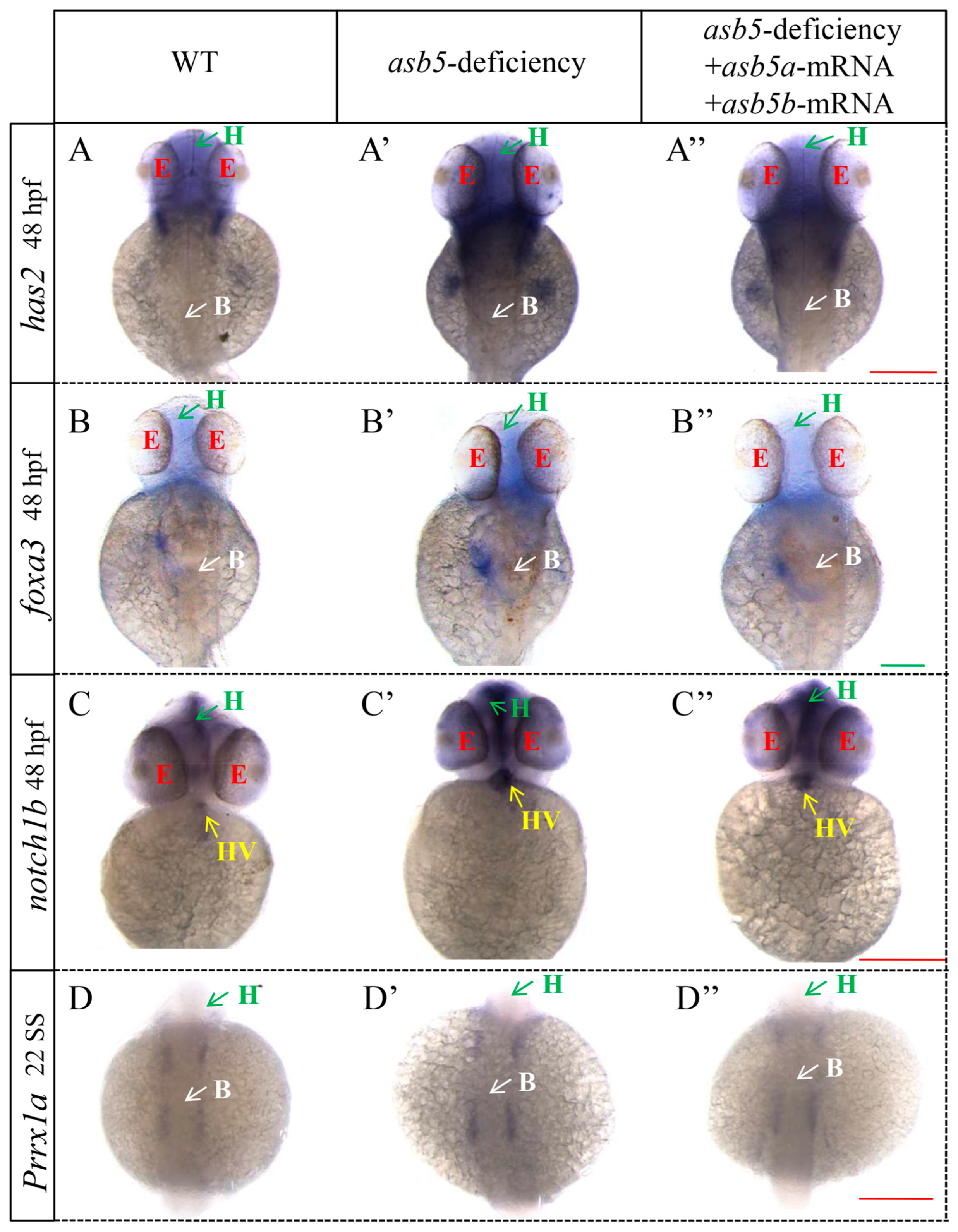
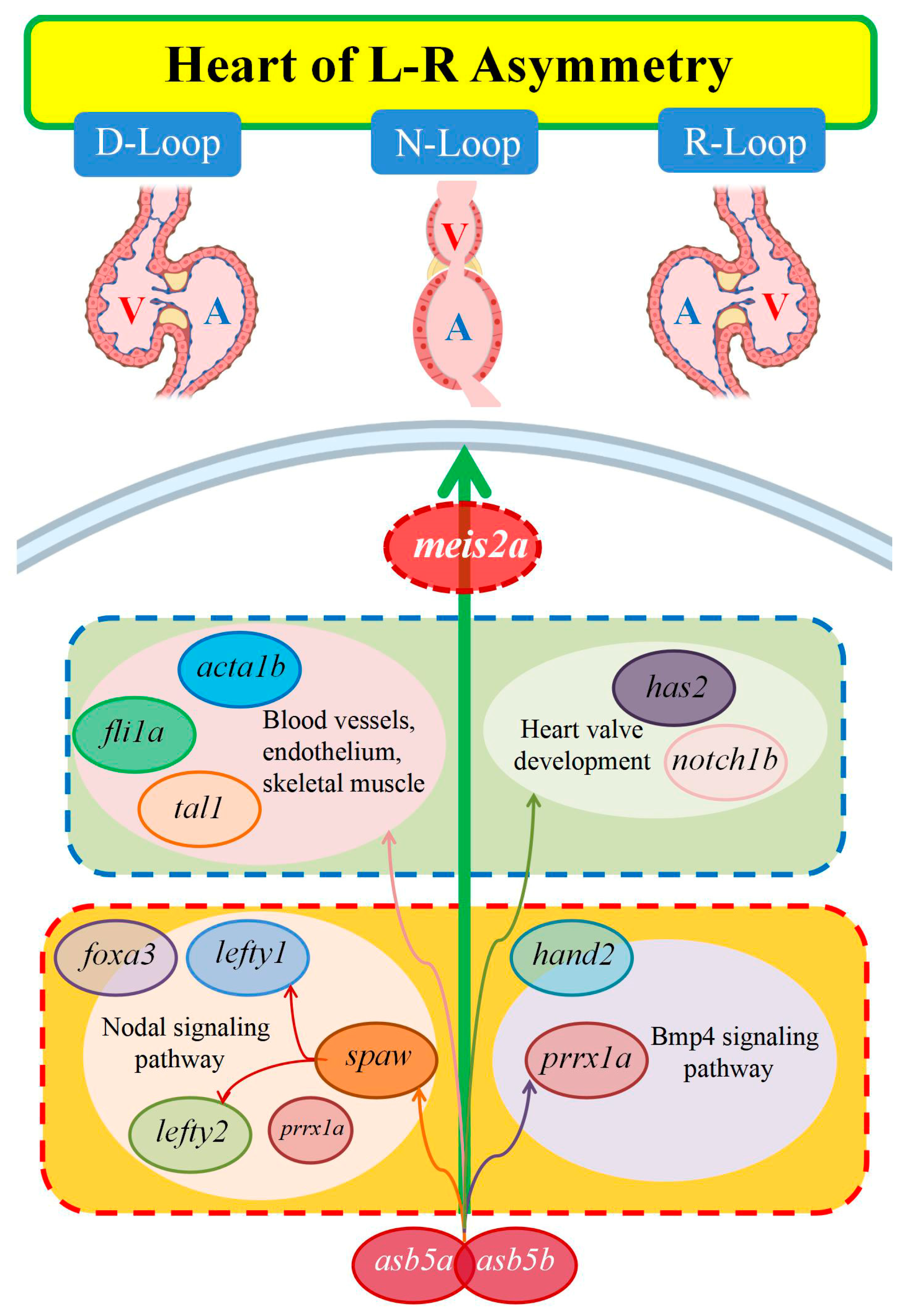
2.5. Loss of asb5 Affects the Expression of Genes Related to L-R Asymmetric Development
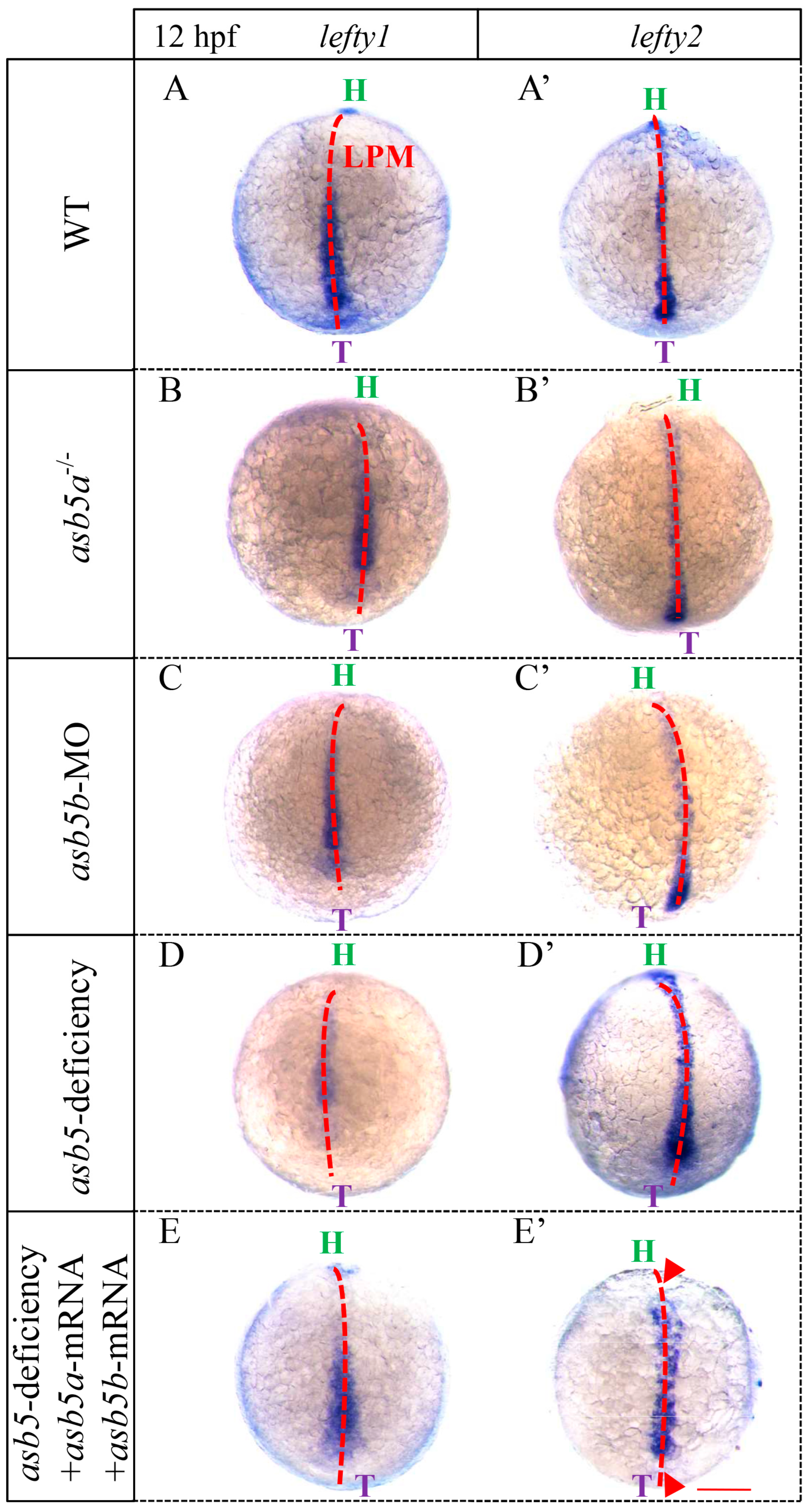
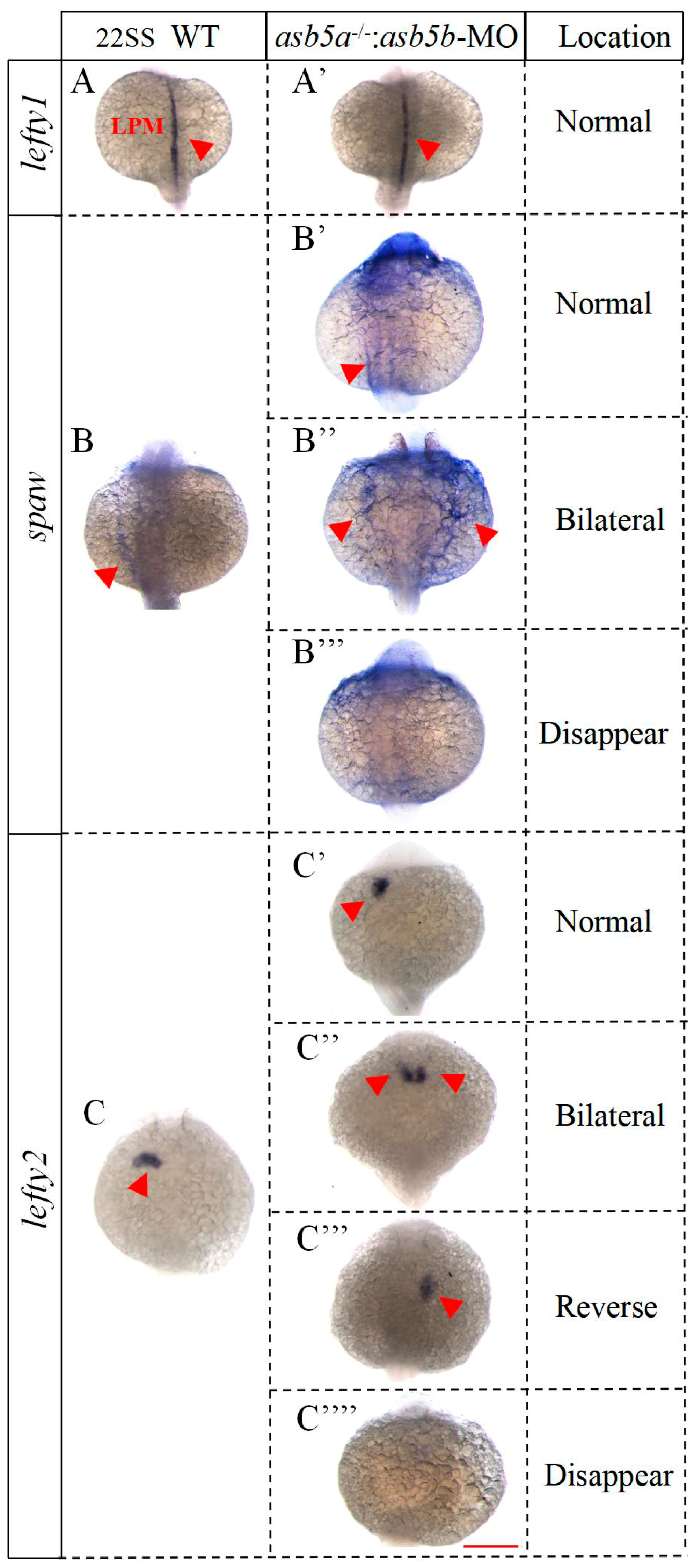
2.6. Nodal Signaling-Related Genes Are Expressed Imbalanced in the asb5-Deficiency Group
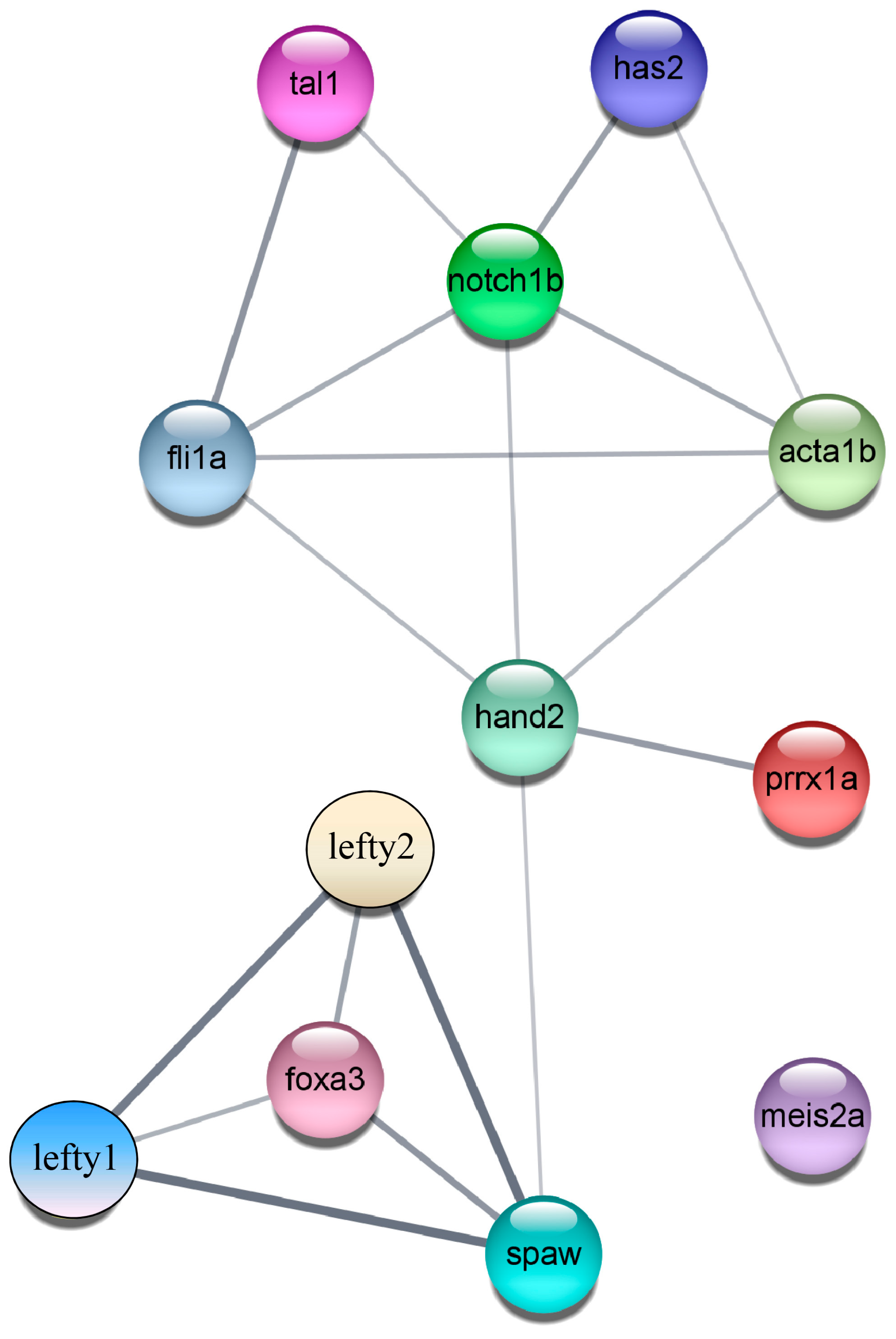
2.7. Construction of the PPI (Protein–Protein Interaction) Regulatory Network Diagram
3. Discussion
4. Materials and Methods
4.1. Zebrafish Strain Rearing and Breeding
4.2. Imaging Technique and Image Analysis
4.3. Synthesis of RNA Probes and WISH in Embryos
4.4. In Vitro Synthesis of Cap-Modified mRNA for asb5a and asb5b
4.5. RNA-Seq Transcriptome Analysis
4.6. Construction of PPI Network and Module Screening
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MO | Morpholino |
| Hpf | Hours post fertilization |
| WT | Wild type |
| WISH | Whole-mount in situ hybridization |
| LPM | Lateral plate mesoderm |
| L-R | Left–right |
| D-loop | Dextro-looping |
| N-loop | No looping |
| L-loop | Levo-looping |
| RT-PCR | Reverse transcription polymerase chain reaction |
| CDS | Coding sequence |
| DEGs | Differentially expressed genes |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SS | Somite stage |
References
- Mercola, M. Left-Right Asymmetry: Nodal Points. J. Cell Sci. 2003, 116, 3251–3257. [Google Scholar] [CrossRef] [PubMed]
- Ibañes, M.; Izpisúa Belmonte, J.C. Left-right axis determination. J. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 210–219. [Google Scholar] [CrossRef]
- Vo, B.T.; Khan, S.A. Expression of nodal and nodal receptors in prostate stem cells and prostate cancer cells: Autocrine effects on cell proliferation and migration. J. Prostate. 2011, 71, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Montague, T.G.; Gagnon, J.A.; Schier, A.F. Conserved Regulation of Nodal-Mediated Left-Right Patterning in Zebrafish and Mouse. Development 2018, 145, dev171090. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Ott, T. Animal Left-Right Asymmetry. Curr. Biol. 2018, 28, R301–R304. [Google Scholar] [CrossRef]
- Grimes, D.T.; Patterson, V.L.; Luna-Arvizu, G.; Schottenfeld-Roames, J.; Irons, Z.H.; Burdine, R.D. Left-Right Asymmetric Heart Jogging Increases the Robustness of Dextral Heart Looping in Zebrafish. Dev. Biol. 2020, 459, 79–86. [Google Scholar] [CrossRef]
- Long, S.; Ahmad, N.; Rebagliati, M. The Zebrafish Nodal-Related Gene Southpaw is Required for Visceral and Diencephalic Left-Right Asymmetry. Development 2003, 130, 2303–2316. [Google Scholar] [CrossRef]
- de Campos-Baptista, M.I.M.; Holtzman, N.G.; Yelon, D.; Schier, A.F. Nodal Signaling Promotes the Speed and Directional Movement of Cardiomyocytes in Zebrafish. Dev. Dyn. 2008, 237, 3624–3633. [Google Scholar] [CrossRef]
- Lahvic, J.L.; Ji, Y.; Marin, P.; Zuflacht, J.P.; Springel, M.W.; Wosen, J.E.; Davis, L.; Hutson, L.D.; Amack, J.D.; Marvin, M.J. Small Heat Shock Proteins are Necessary for Heart Migration and Laterality Determination in Zebrafish. Dev. Biol. 2013, 384, 166–180. [Google Scholar] [CrossRef]
- Lenhart, K.F.; Lin, S.-Y.; Titus, T.A.; Postlethwait, J.H.; Burdine, R.D. Two Additional Midline Barriers Function with Midline Lefty1 Expression to Maintain Asymmetric Nodal Signaling during Left-Right Axis Specification in Zebrafish. Development 2011, 138, 4405–4410. [Google Scholar] [CrossRef]
- Meno, C.; Shimono, A.; Saijoh, Y.; Yashiro, K.; Mochida, K.; Ohishi, S.; Noji, S.; Kondoh, H.; Hamada, H. Lefty-1 Is Required for Left-Right Determination as a Regulator of Lefty-2 and Nodal. Cell 1998, 94, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Meno, C.; Gritsman, K.; Ohishi, S.; Ohfuji, Y.; Heckscher, E.; Mochida, K.; Shimono, A.; Kondoh, H.; Talbot, W.S.; Robertson, E.J.; et al. Mouse Lefty2 and Zebrafish Antivin Are Feedback Inhibitors of Nodal Signaling during Vertebrate Gastrulation. Mol. Cell 1999, 4, 287–298. [Google Scholar] [CrossRef]
- Ruiz-Lozano, P.; Ryan, A.K.; Izpisua-Belmonte, J.C. Left-Right Determination. Trends Cardiovasc. Med. 2000, 10, 258–262. [Google Scholar] [CrossRef]
- Mercola, M.; Levin, M. Left-Right Asymmetry Determination in Vertebrates. Annu. Rev. Cell Dev. Biol. 2001, 17, 779–805. [Google Scholar] [CrossRef]
- Männer, J. On Rotation, Torsion, Lateralization, and Handedness of the Embryonic Heart Loop: New Insights from a Simulation Model for the Heart Loop of Chick Embryos. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 278, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Debrincat, M.A.; Zhang, J.-G.; Willson, T.A.; Silke, J.; Connolly, L.M.; Simpson, R.J.; Alexander, W.S.; Nicola, N.A.; Kile, B.T.; Hilton, D.J. Ankyrin Repeat and Suppressors of Cytokine Signaling Box Protein Asb-9 Targets Creatine Kinase B for Degradation. J. Biol. Chem. 2007, 282, 4728–4737. [Google Scholar] [CrossRef]
- Liu, P.; Verhaar, A.P.; Peppelenbosch, M.P. Signaling Size: Ankyrin and SOCS Box-Containing ASB E3 Ligases in Action. Trends Biochem. Sci. 2019, 44, 64–74. [Google Scholar] [CrossRef]
- Boengler, K.; Pipp, F.; Fernandez, B.; Richter, A.; Schaper, W.; Deindl, E. The Ankyrin Repeat Containing SOCS Box Protein 5: A Novel Protein Associated with Arteriogenesis. Biochem. Biophys. Res. Commun. 2003, 302, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, Y.; Luo, Y.; Gao, L.; Zhang, J.; Jiang, Z.; Fan, X.; Li, F.; Xie, Y.; Wu, X.; et al. Asb5a/Asb5b Double Knockout Affects Zebrafish Cardiac Contractile Function. Int. J. Mol. Sci. 2023, 24, 16364. [Google Scholar] [CrossRef]
- Kamachi, Y.; Kawahara, A. CRISPR-Cas9-Mediated Genome Modifications in Zebrafish. Methods Mol. Biol. 2023, 2637, 313–324. [Google Scholar] [CrossRef]
- Daniel, J.G.; Yu, X.; Ferguson, A.C.; Shavit, J.A. CRISPR/Cas9-Mediated Genome Editing in Zebrafish. Methods Mol. Biol. 2023, 2631, 371–380. [Google Scholar] [CrossRef]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A Primer for Morpholino Use in Zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Westcot, S.E.; Ekker, S.C. Lessons from Morpholino-Based Screening in Zebrafish. Brief. Funct. Genomics 2011, 10, 181–188. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, Y.; Hu, J.; Gao, M.; Hong, Y. L-Selenomethionine Induces Zebrafish Embryo Cardiovascular Defects via down-Regulating Expression of Lrp2b. Chemosphere 2022, 290, 133351. [Google Scholar] [CrossRef] [PubMed]
- Ocorr, K.; Fink, M.; Cammarato, A.; Bernstein, S.; Bodmer, R. Semi-Automated Optical Heartbeat Analysis of Small Hearts. J. Vis. Exp. 2009, 31, 1435. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, R.; Wang, H. Identification and Genetic Analysis of Rare Variants in Myosin Family Genes in 412 Han Chinese Congenital Heart Disease Patients. Mol. Genet. Genomic Med. 2022, 10, e2041. [Google Scholar] [CrossRef]
- Gong, J.; Chai, L.; Xu, G.; Ni, Y.; Liu, D. The Expression of Natriuretic Peptide Receptors in Developing Zebrafish Embryos. Gene Expr. Patterns 2018, 29, 65–71. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, H.-S.; Kim, J.-D.; Seo, J.; Chung, K.-S.; Lee, H.-S.; Huh, T.-L.; Jo, I.; Kim, Y.-O. Isolation of a Ventricle-Specific Promoter for the Zebrafish Ventricular Myosin Heavy Chain (Vmhc) Gene and Its Regulation by GATA Factors during Embryonic Heart Development. Dev. Dyn. 2009, 238, 1574–1581. [Google Scholar] [CrossRef]
- Jin, D.; Ni, T.T.; Hou, J.; Rellinger, E.; Zhong, T.P. Promoter Analysis of Ventricular Myosin Heavy Chain (Vmhc) in Zebrafish Embryos. Dev. Dyn. 2009, 238, 1760–1767. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, F.; Gu, J.; Wu, L.; Wu, W.; Ji, G. Paroxetine Induced Larva Zebrafish Cardiotoxicity through Inflammation Response. Ecotoxicol. Environ. Saf. 2023, 260, 115096. [Google Scholar] [CrossRef]
- Fujii, T.; Tsunesumi, S.; Yamaguchi, K.; Watanabe, S.; Furukawa, Y. Smyd3 Is Required for the Development of Cardiac and Skeletal Muscle in Zebrafish. PLoS ONE 2011, 6, e23491. [Google Scholar] [CrossRef] [PubMed]
- Pulina, M.V.; Hou, S.-Y.; Mittal, A.; Julich, D.; Whittaker, C.A.; Holley, S.A.; Hynes, R.O.; Astrof, S. Essential Roles of Fibronectin in the Development of the Left-Right Embryonic Body Plan. Dev. Biol. 2011, 354, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Paardekooper Overman, J.; Tessadori, F.; Noël, E.; Bakkers, J.; den Hertog, J. Noonan and LEOPARD Syndrome Shp2 Variants Induce Heart Displacement Defects in Zebrafish. Development 2014, 141, 1961–1970. [Google Scholar] [CrossRef]
- Toyoizumi, R.; Kobayashi, T.; Kikukawa, A.; Oba, J.; Takeuchi, S. Adrenergic Neurotransmitters and Calcium Ionophore-Induced Situs Inversus Viscerum in Xenopus Laevis Embryos. Dev. Growth Differ. 1997, 39, 505–514. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; Li, J.; Lian, J.; Chen, Q.; Meng, P.; Lu, T.; Xie, H.; Zhang, W.; Xu, J.; et al. Genetic and Epigenetic Orchestration of Gfi1aa-Lsd1-Cebpa in Zebrafish Neutrophil Development. Development 2021, 148, dev199516. [Google Scholar] [CrossRef] [PubMed]
- Al Qaryoute, A.; Fallatah, W.; Dhinoja, S.; Raman, R.; Jagadeeswaran, P. Role of microRNAs and Their Downstream Target Transcription Factors in Zebrafish Thrombopoiesis. Sci. Rep. 2023, 13, 16066. [Google Scholar] [CrossRef]
- Ocaña, O.H.; Coskun, H.; Minguillón, C.; Murawala, P.; Tanaka, E.M.; Galcerán, J.; Muñoz-Chápuli, R.; Nieto, M.A. A right-handed signalling pathway drives heart looping in vertebrates. J. Nature. 2017, 549, 86–90. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Fatehi, M.; Wang, Y.; Long, W.; Tian, R.; Deng, X.; Weng, Z.; Xu, Q.; Light, P.E.; et al. Regulation of PKD2 Channel Function by TACAN. J. Physiol. 2023, 601, 83–98. [Google Scholar] [CrossRef]
- Schottenfeld, J.; Sullivan-Brown, J.; Burdine, R.D. Zebrafish Curly up Encodes a Pkd2 Ortholog That Restricts Left-Side-Specific Expression of Southpaw. Development 2007, 134, 1605–1615. [Google Scholar] [CrossRef]
- Little, R.B.; Norris, D.P. Right, left and cilia: How asymmetry is established. Semin. Cell Dev. Biol. 2021, 110, 11–18. [Google Scholar] [CrossRef]
- Veerkamp, J.; Rudolph, F.; Cseresnyes, Z.; Priller, F.; Otten, C.; Renz, M.; Schaefer, L.; Abdelilah-Seyfried, S. Unilateral Dampening of Bmp Activity by Nodal Generates Cardiac Left-Right Asymmetry. Dev. Cell 2013, 24, 660–667. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F. Cautious Use of Fli1a:EGFP Transgenic Zebrafish in Vascular Research. Biochem. Biophys. Res. Commun. 2012, 427, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Carra, S.; Gaudenzi, G.; Dicitore, A.; Cantone, M.C.; Plebani, A.; Saronni, D.; Zappavigna, S.; Caraglia, M.; Candeo, A.; Bassi, A.; et al. Modeling Lung Carcinoids with Zebrafish Tumor Xenograft. Int. J. Mol. Sci. 2022, 23, 8126. [Google Scholar] [CrossRef]
- Hsu, J.J.; Vedula, V.; Baek, K.I.; Chen, C.; Chen, J.; Chou, M.I.; Lam, J.; Subhedar, S.; Wang, J.; Ding, Y.; et al. Contractile and Hemodynamic Forces Coordinate Notch1b-Mediated Outflow Tract Valve Formation. JCI Insight 2019, 5, e124460. [Google Scholar] [CrossRef] [PubMed]
- Donat, S.; Lourenço, M.; Paolini, A.; Otten, C.; Renz, M.; Abdelilah-Seyfried, S. Heg1 and Ccm1/2 Proteins Control Endocardial Mechanosensitivity during Zebrafish Valvulogenesis. eLife 2018, 7, e28939. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Zhou, Q.; Liu, Z.; Li, Q. Hexavalent Chromium Amplifies the Developmental Toxicity of Graphene Oxide during Zebrafish Embryogenesis. Ecotoxicol. Environ. Saf. 2021, 208, 111487. [Google Scholar] [CrossRef]
- Ulloa, P.E.; Rincón, G.; Islas-Trejo, A.; Araneda, C.; Iturra, P.; Neira, R.; Medrano, J.F. RNA Sequencing to Study Gene Expression and SNP Variations Associated with Growth in Zebrafish Fed a Plant Protein-Based Diet. Mar. Biotechnol. 2015, 17, 353–363. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Zhao, Y.; He, L.; Huang, Y.; Yang, X.; Zhou, Y.; Yu, L.; Zhao, Q.; Dong, X. Zebrafish Znfl1s Regulate Left-Right Asymmetry Patterning through Controlling the Expression of Fgfr1a. J. Cell Physiol. 2019, 234, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Guo, X.; Dong, X.; Ren, J.; Zu, Y.; Li, W.; Zhang, Q. Notch1a Can Widely Mediate Innate Immune Responses in Zebrafish Larvae Infected with Vibrio Parahaemolyticus. Fish. Shellfish. Immunol. 2019, 92, 680–689. [Google Scholar] [CrossRef]
- Seiliez, I.; Thisse, B.; Thisse, C. FoxA3 and Goosecoid Promote Anterior Neural Fate through Inhibition of Wnt8a Activity before the Onset of Gastrulation. Dev. Biol. 2006, 290, 152–163. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Yuan, M.; Liu, X. The Synthetic Pyrethroid Deltamethrin Impairs Zebrafish (Danio Rerio) Swim Bladder Development. Sci. Total Environ. 2020, 701, 134870. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Yamagishi, H.; Overbeek, P.A.; Olson, E.N.; Srivastava, D. The bHLH Factors, dHAND and eHAND, Specify Pulmonary and Systemic Cardiac Ventricles Independent of Left-Right Sidedness. Dev. Biol. 1998, 196, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, X. Distinct Functions of Wnt/Beta-Catenin Signaling in KV Development and Cardiac Asymmetry. Development 2009, 136, 207–217. [Google Scholar] [CrossRef]
- Sukumaran, S.; Perkins, B.D. Early Defects in Photoreceptor Outer Segment Morphogenesis in Zebrafish Ift57, Ift88 and Ift172 Intraflagellar Transport Mutants. Vision. Res. 2009, 49, 479–489. [Google Scholar] [CrossRef]
- Lunt, S.C.; Haynes, T.; Perkins, B.D. Zebrafish Ift57, Ift88, and Ift172 Intraflagellar Transport Mutants Disrupt Cilia but Do Not Affect Hedgehog Signaling. Dev. Dyn. 2009, 238, 1744–1759. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, J.L.; Jindal, G.A.; Burdine, R.D. Gdf3 Is Required for Robust Nodal Signaling during Germ Layer Formation and Left-Right Patterning. eLife 2017, 6, e28635. [Google Scholar] [CrossRef]
- Bisgrove, B.W.; Su, Y.-C.; Yost, H.J. Maternal Gdf3 Is an Obligatory Cofactor in Nodal Signaling for Embryonic Axis Formation in Zebrafish. eLife 2017, 6, e28534. [Google Scholar] [CrossRef]
- Kelly, G.M.; Greenstein, P.; Erezyilmaz, D.F.; Moon, R.T. Zebrafish Wnt8 and Wnt8b Share a Common Activity but Are Involved in Distinct Developmental Pathways. Development 1995, 121, 1787–1799. [Google Scholar] [CrossRef]
- Bussmann, J.; Bakkers, J.; Schulte-Merker, S. Early Endocardial Morphogenesis Requires Scl/Tal1. PLoS Genet. 2007, 3, e140. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, H.; Zhao, W.; Zheng, J.; Sun, L.; Luo, C. Zygotic Vsx1 Plays a Key Role in Defining V2a Interneuron Sub-Lineage by Directly Repressing Tal1 Transcription in Zebrafish. Int. J. Mol. Sci. 2020, 21, 3600. [Google Scholar] [CrossRef]
- Harrington, J.K.; Sorabella, R.; Tercek, A.; Isler, J.R.; Targoff, K.L. Nkx2.5 Is Essential to Establish Normal Heart Rate Variability in the Zebrafish Embryo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R265–R271. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.-T.; Yang, T.-C.; Tsai, H.-J. Nkx2.7 and Nkx2.5 Function Redundantly and Are Required for Cardiac Morphogenesis of Zebrafish Embryos. PLoS ONE 2009, 4, e4249. [Google Scholar] [CrossRef]
- Roussigne, M.; Blader, P.; Wilson, S.W. Breaking Symmetry: The Zebrafish as a Model for Understanding Left-Right Asymmetry in the Developing Brain. Dev. Neurobiol. 2012, 72, 269–281. [Google Scholar] [CrossRef]
- Guerra, A.; Germano, R.F.; Stone, O.; Arnaout, R.; Guenther, S.; Ahuja, S.; Uribe, V.; Vanhollebeke, B.; Stainier, D.Y.; Reischauer, S. Distinct Myocardial Lineages Break Atrial Symmetry during Cardiogenesis in Zebrafish. eLife 2018, 15, e32833. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Smith, K.A.; Uribe, V. Getting to the Heart of Left-Right Asymmetry: Contributions from the Zebrafish Model. J. Cardiovasc. Dev. Dis. 2021, 8, 64. [Google Scholar] [CrossRef]
- Malaviya, R.; Zhou, Z.; Raymond, H.; Wertheimer, J.; Jones, B.; Bunting, R.; Wilkinson, P.; Madireddy, L.; Hall, L.; Ryan, M.; et al. Repeated exposure of house dust mite induces progressive airway inflammation in mice: Differential roles of CCL17 and IL-13. Pharmacol Res Perspect. 2021, 9, e00770–e00783. [Google Scholar] [CrossRef]
- Kaestner, K.H.; Hiemisch, H.; Schütz, G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol Cell Biol 1998, 18, 4245–4251. [Google Scholar] [CrossRef] [PubMed]
- Castroviejo, N.; Ocaña, O.H.; Rago, L.; Coskun, H.; Arcas, A.; Galcerán, J.; Nieto, M.A. Reply to: Zebrafish Prrx1a Mutants Have Normal Hearts. Nature 2020, 585, E17–E19. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; de Bakker, D.E.M.; Barske, L.; Nelson, N.; Algra, H.A.; Willekers, S.; Nichols, J.T.; Crump, J.G.; Bakkers, J. Zebrafish Prrx1a Mutants Have Normal Hearts. Nature 2020, 585, E14–E16. [Google Scholar] [CrossRef] [PubMed]
- McCulley, D.J.; Kang, J.O.; Martin, J.F.; Black, B.L. BMP4 Is Required in the Anterior Heart Field and Its Derivatives for Endocardial Cushion Remodeling, Outflow Tract Septation, and Semilunar Valve Development. Dev. Dyn. 2008, 237, 3200–3209. [Google Scholar] [CrossRef]
- Milan, D.J.; Giokas, A.C.; Serluca, F.C.; Peterson, R.T.; MacRae, C.A. Notch1b and Neuregulin Are Required for Specification of Central Cardiac Conduction Tissue. Development 2006, 133, 1125–1132. [Google Scholar] [CrossRef]
- Chung, Y.S.; Zhang, W.J.; Arentson, E.; Kingsley, P.D.; Palis, J.; Choi, K. Lineage Analysis of the Hemangioblast as Defined by FLK1 and SCL Expression. Development 2002, 129, 5511–5520. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Cai, W.; Li, Y.; Gao, L.; Liu, X.; Liu, S.; Lei, J.; Zhang, J.; Wang, Y.; Jiang, Z.; et al. The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish. Int. J. Mol. Sci. 2025, 26, 2765. https://doi.org/10.3390/ijms26062765
Zhou W, Cai W, Li Y, Gao L, Liu X, Liu S, Lei J, Zhang J, Wang Y, Jiang Z, et al. The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish. International Journal of Molecular Sciences. 2025; 26(6):2765. https://doi.org/10.3390/ijms26062765
Chicago/Turabian StyleZhou, Wanbang, Wanwan Cai, Yongqing Li, Luoqing Gao, Xin Liu, Siyuan Liu, Junrong Lei, Jisheng Zhang, Yuequn Wang, Zhigang Jiang, and et al. 2025. "The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish" International Journal of Molecular Sciences 26, no. 6: 2765. https://doi.org/10.3390/ijms26062765
APA StyleZhou, W., Cai, W., Li, Y., Gao, L., Liu, X., Liu, S., Lei, J., Zhang, J., Wang, Y., Jiang, Z., Wu, X., Fan, X., Li, F., Zheng, L., & Yuan, W. (2025). The Interaction Between the asb5a and asb5b Subtypes Jointly Regulates the L-R Asymmetrical Development of the Heart in Zebrafish. International Journal of Molecular Sciences, 26(6), 2765. https://doi.org/10.3390/ijms26062765





