Central Serotonin Deficiency Impairs Recovery of Sensorimotor Abilities After Spinal Cord Injury in Rats
Abstract
1. Introduction
2. Results
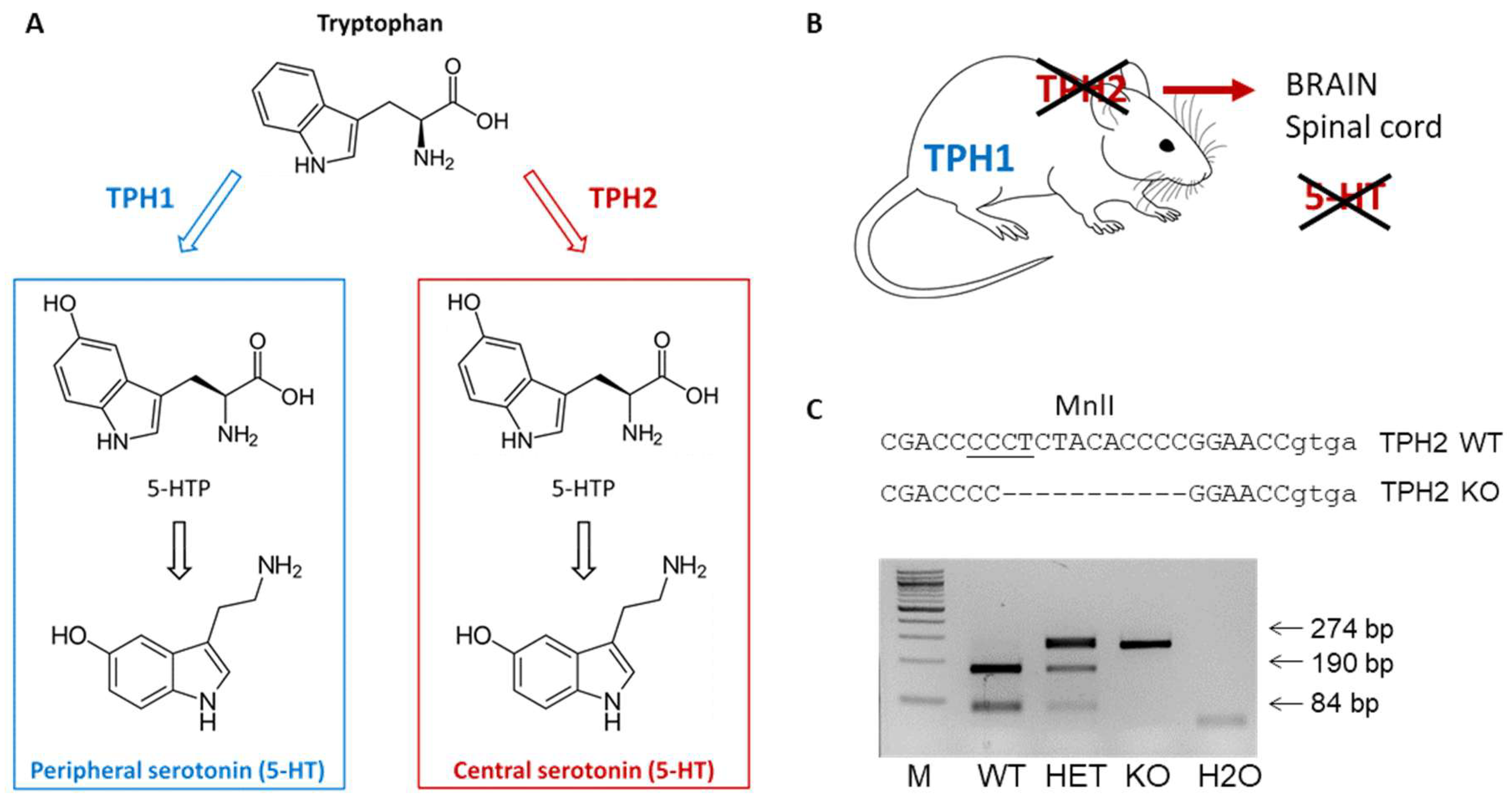
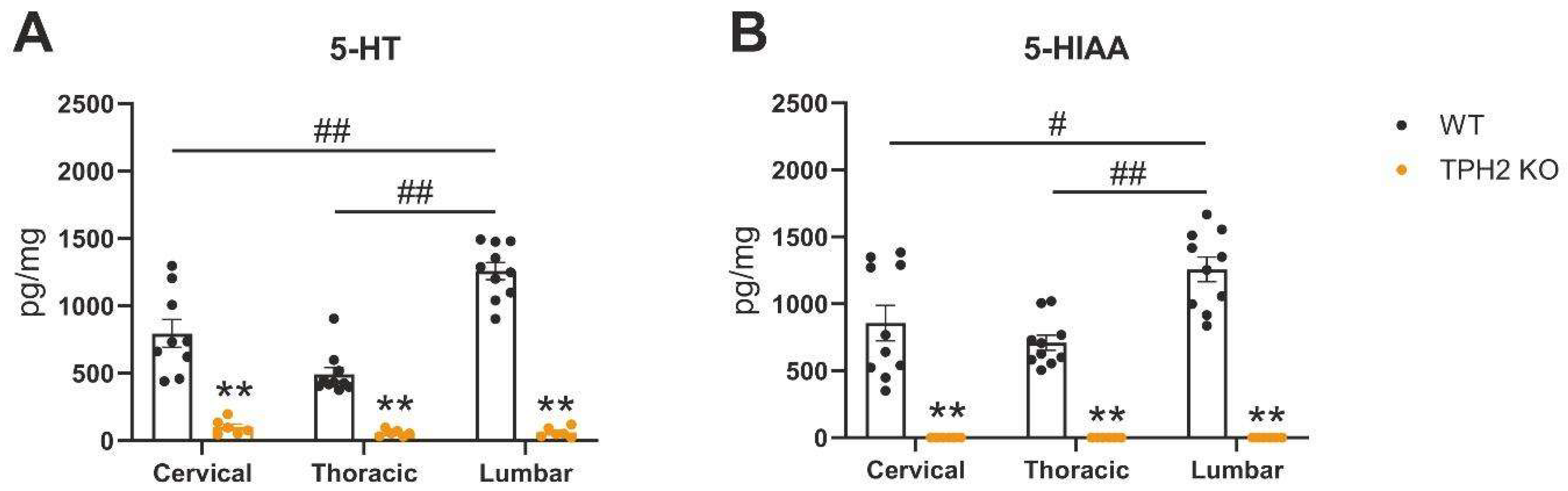
2.1. TPH2 Deficiency Results in Serotonin Depletion in Rat Spinal Cord
2.2. A Lack of Central Serotonin Affects the Recovery Dynamics of Sensorimotor Capacities After SCI
2.2.1. Rat Behavior in Tapered Beam Walking Test After SCI
2.2.2. Rat Behavior in Ladder Walking Test After SCI
2.2.3. Rat Behavior in Static Rod After SCI
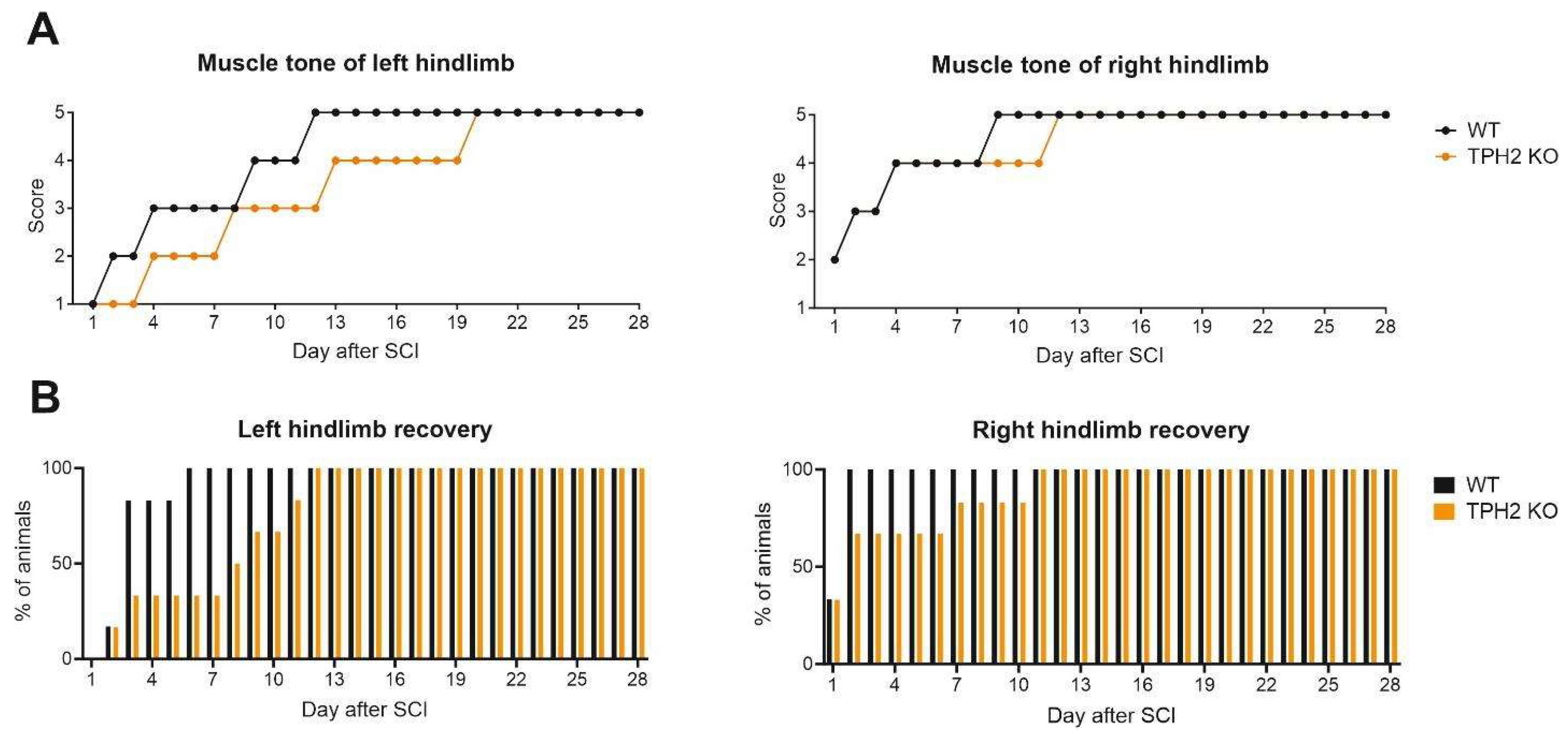
2.2.4. Muscle Tone and Voluntary Movements
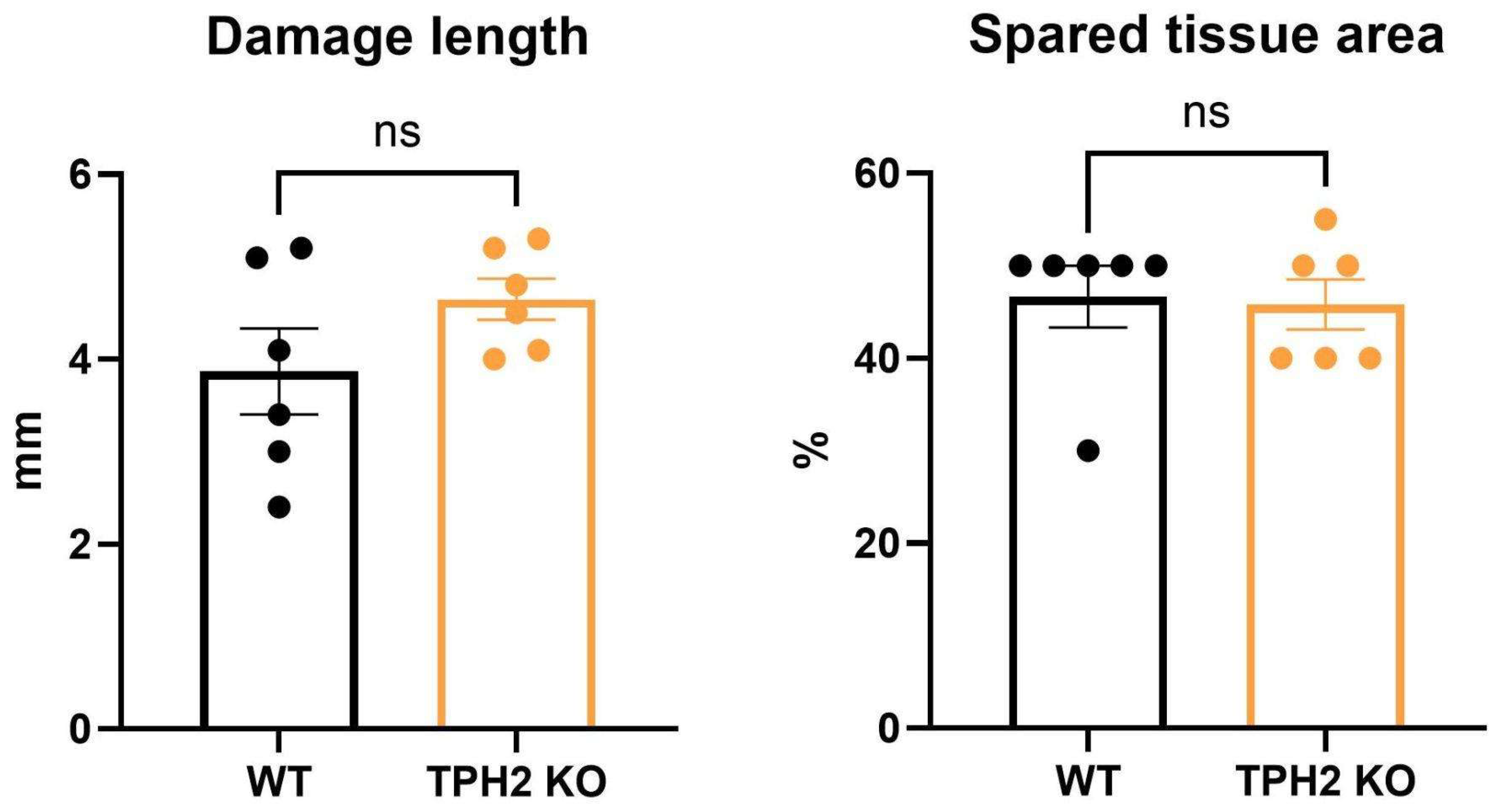
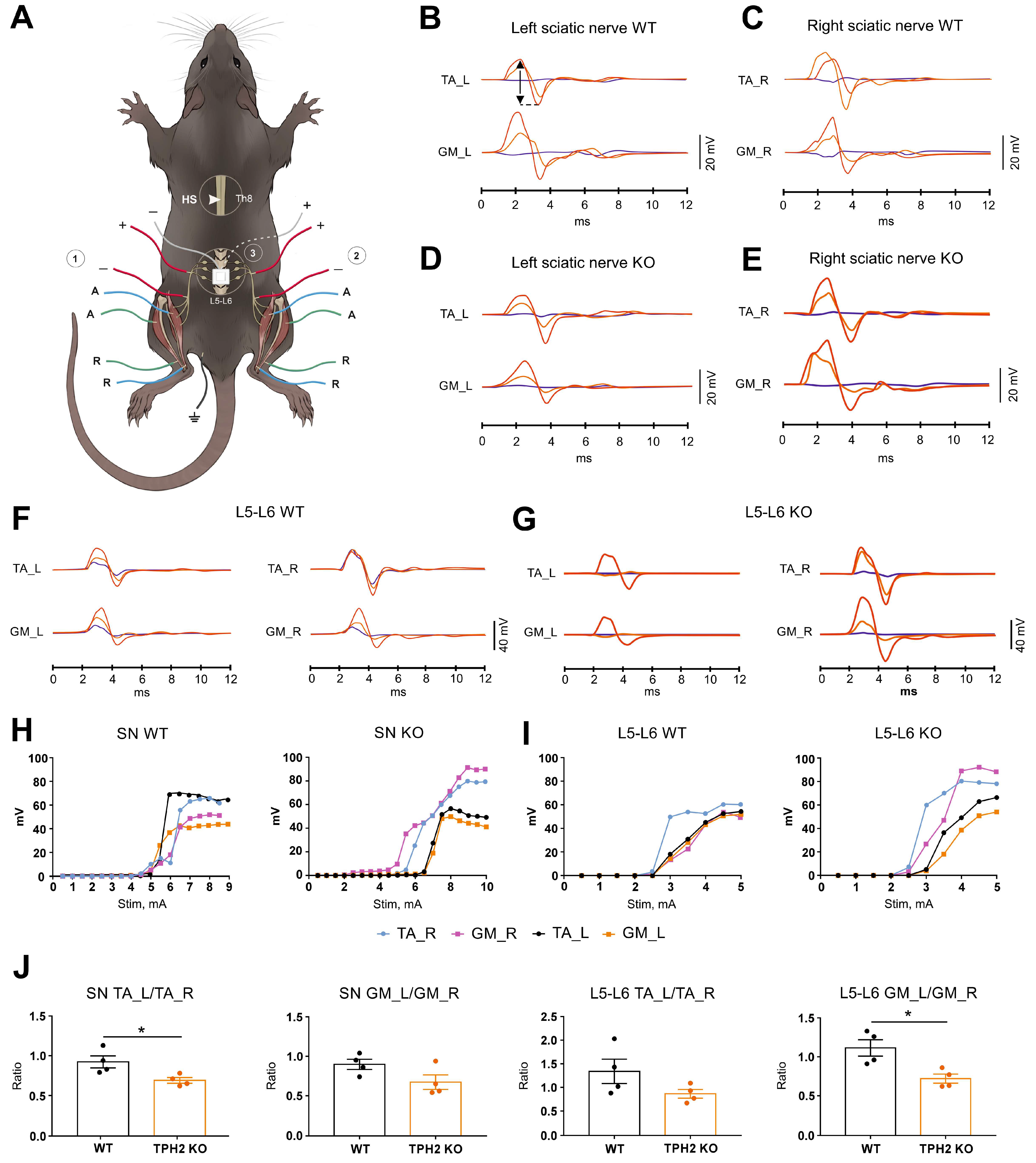
2.3. TPH2 Deficiency Slows the Recovery of Sensorimotor Pathways in Rats
3. Discussion
4. Materials and Methods
4.1. Animals
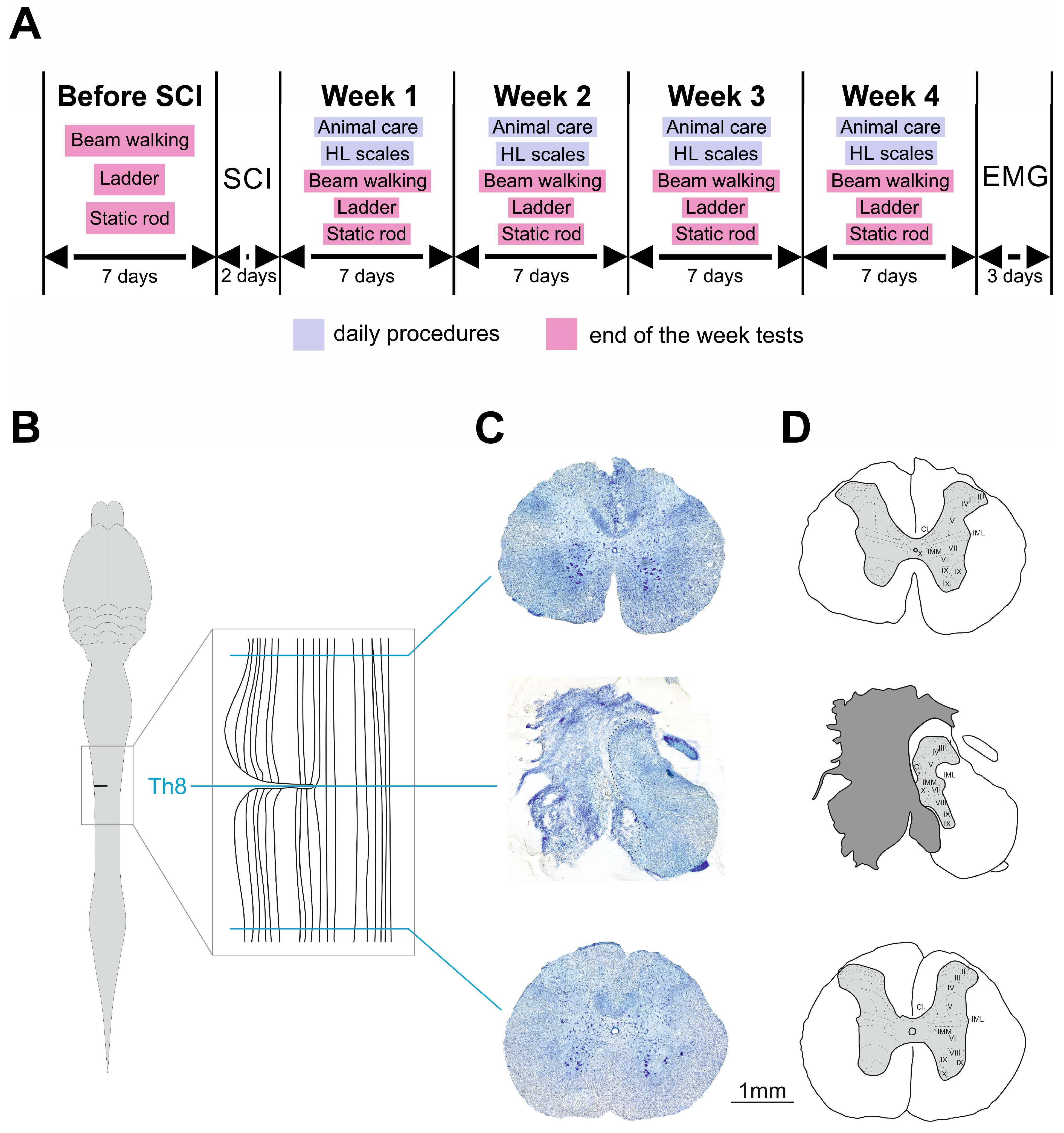
4.2. Experimental Schedule
4.3. Surgery and Animal Care
4.4. Motor Function Assessment
4.4.1. Tapered Beam Walking Test
4.4.2. Ladder Walking Test
4.4.3. Static Rod
4.5. Scales for Assessment of Hindlimb Function Recovery
4.6. Terminal Electrophysiological Recording and Histological Examination
4.7. HPLC Analysis of Serotonin Tissue Content
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | 5-hydroxytryptamine, serotonin |
| 5-HTP | 5-hydroxytryptophan |
| 8-OH-DPAT | 8-hydroxy-2-(di-n-propylamino)-tetralin |
| BBB | Basso, Beattie and Bresnahan |
| BDNF | Brain-derived neurotrophic factor |
| CMAP | Compound muscle action potentials |
| CNS | Central nervous system |
| DNA | Deoxyribonucleic acid |
| GM | Gastrocnemius |
| HPLC | High-performance liquid chromatography |
| KO | Knockout |
| LTD | Long-term depression |
| LW | Ladder walking |
| PCR | Polymerase chain reaction |
| RN-NSC | Raphe nucleus-derived neural stem cells |
| SCI | Spinal cord injury |
| TA | Tibialis anterior |
| TBW | Tapered beam walking |
| TPH | Tryptophan hydroxylase |
| TPH2 | Tryptophan hydroxylase 2 |
| WT | Wild type |
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Spinal Cord Injury: Overview of Experimental Approaches Used to Restore Locomotor Activity. Rev. Neurosci. 2015, 26, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Terson de Paleville, D.G.L.; Harkema, S.J.; Angeli, C.A. Epidural Stimulation with Locomotor Training Improves Body Composition in Individuals with Cervical or Upper Thoracic Motor Complete Spinal Cord Injury: A Series of Case Studies. J. Spinal Cord Med. 2019, 42, 32–38. [Google Scholar] [CrossRef]
- Calvert, J.S.; Grahn, P.J.; Zhao, K.D.; Lee, K.H. Emergence of Epidural Electrical Stimulation to Facilitate Sensorimotor Network Functionality After Spinal Cord Injury. Neuromodulation 2019, 22, 244–252. [Google Scholar] [CrossRef]
- Perrin, F.E.; Noristani, H.N. Serotonergic Mechanisms in Spinal Cord Injury. Exp. Neurol. 2019, 318, 174–191. [Google Scholar] [CrossRef]
- Hains, B.C.; Everhart, A.W.; Fullwood, S.D.; Hulsebosch, C.E. Changes in Serotonin, Serotonin Transporter Expression and Serotonin Denervation Supersensitivity: Involvement in Chronic Central Pain after Spinal Hemisection in the Rat. Exp. Neurol. 2002, 175, 347–362. [Google Scholar] [CrossRef]
- Courtine, G.; Gerasimenko, Y.; Van Den Brand, R.; Yew, A.; Musienko, P.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; et al. Transformation of Nonfunctional Spinal Circuits into Functional States after the Loss of Brain Input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef]
- Barbeau, H.; Rossignol, S. The Effects of Serotonergic Drugs on the Locomotor Pattern and on Cutaneous Reflexes of the Adult Chronic Spinal Cat. Brain Res. 1990, 514, 55–67. [Google Scholar] [CrossRef]
- Cristante, A.F.; Filho, T.E.P.B.; Oliveira, R.P.; Marcon, R.M.; Ferreira, R.; Santos, G.B. Effects of Antidepressant and Treadmill Gait Training on Recovery from Spinal Cord Injury in Rats. Spinal Cord 2013, 51, 501–507. [Google Scholar] [CrossRef]
- Hentall, I.D.; Gonzalez, M.M.C. Promotion of Recovery from Thoracic Spinal Cord Contusion in Rats by Stimulation of Medullary Raphe or Its Midbrain Input. Neurorehabil. Neural Repair 2012, 26, 374–384. [Google Scholar] [CrossRef]
- Feraboli-Lohnherr, D.; Barthe, J.Y.; Orsal, D. Serotonin-Induced Activation of the Network for Locomotion in Adult Spinal Rats. J. Neurosci. Res. 1999, 55, 87–98. [Google Scholar] [CrossRef]
- Kaplan, K.; Echert, A.E.; Massat, B.; Puissant, M.M.; Palygin, O.; Geurts, A.M.; Hodges, M.R. Chronic Central Serotonin Depletion Attenuates Ventilation and Body Temperature in Young but Not Adult Tph2 Knockout Rats. J. Appl. Physiol. 2016, 120, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Ko, M.L.; King, M.A.; Gordon, T.L.; Crisp, T. The Effects of Aging on Spinal Neurochemistry in the Rat. Brain Res. Bull. 1997, 42, 95–98. [Google Scholar] [CrossRef]
- Ichiyama, R.M.; Gerasimenko, Y.; Jindrich, D.L.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Dose Dependence of the 5-HT Agonist Quipazine in Facilitating Spinal Stepping in the Rat with Epidural Stimulation. Neurosci. Lett. 2008, 438, 281–285. [Google Scholar] [CrossRef]
- Musienko, P.; van den Brand, R.; Märzendorfer, O.; Roy, R.R.; Gerasimenko, Y.; Edgerton, V.R.; Courtine, G. Controlling Specific Locomotor Behaviors through Multidimensional Monoaminergic Modulation of Spinal Circuitries. J. Neurosci. 2011, 31, 9264–9278. [Google Scholar] [CrossRef]
- Barbeau, H.; Filion, M.; Bedard, P. Effects of Agonists and Antagonists of Serotonin on Spontaneous Hindlimb EMG Activity in Chronic Spinal Rats. Neuropharmacology 1981, 20, 99–107. [Google Scholar] [CrossRef]
- Cazalets, J.R.; Sqalli-Houssaini, Y.; Clarac, F. Activation of the Central Pattern Generators for Locomotion by Serotonin and Excitatory Amino Acids in Neonatal Rat. J. Physiol. 1992, 455, 187–204. [Google Scholar] [CrossRef]
- Sławínska, U.; Miazga, K.; Jordan, L.M. 5-HT2 and 5-HT7 Receptor Agonists Facilitate Plantar Stepping in Chronic Spinal Rats through Actions on Different Populations of Spinal Neurons. Front. Neural Circuits 2014, 8, 95. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Musienko, P.; Bogacheva, I.; Moshonkina, T.; Savochin, A.; Lavrov, I.; Roy, R.R.; Edgerton, V.R. Propriospinal Bypass of the Serotonergic System That Can Facilitate Stepping. J. Neurosci. 2009, 29, 5681–5689. [Google Scholar] [CrossRef]
- Jin, Y.; Dougherty, S.E.; Wood, K.; Sun, L.; Cudmore, R.H.; Abdalla, A.; Kannan, G.; Pletnikov, M.; Hashemi, P.; Linden, D.J. Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron 2016, 91, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Kajstura, T.J.; Dougherty, S.E.; Linden, D.J. Serotonin Axons in the Neocortex of the Adult Female Mouse Regrow after Traumatic Brain Injury. J. Neurosci. Res. 2018, 96, 512–526. [Google Scholar] [CrossRef]
- Hou, S.; Saltos, T.M.; Mironets, E.; Trueblood, C.T.; Connors, T.M.; Tom, V.J. Grafting Embryonic Raphe Neurons Reestablishes Serotonergic Regulation of Sympathetic Activity to Improve Cardiovascular Function after Spinal Cord Injury. J. Neurosci. 2020, 40, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Ganzer, P.D.; Moxon, K.A.; Knudsen, E.B.; Shumsky, J.S. Serotonergic Pharmacotherapy Promotes Cortical Reorganization after Spinal Cord Injury. Exp. Neurol. 2013, 241, 84–94. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after Spinal Cord Injury: A Review of the Critical Timeline of Signaling Cues and Cellular Infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef]
- Kjell, J.; Olson, L. Rat Models of Spinal Cord Injury: From Pathology to Potential Therapies. DMM Dis. Model. Mech. 2016, 9, 1125–1137. [Google Scholar] [CrossRef]
- Brivio, P.; Sbrini, G.; Peeva, P.; Todiras, M.; Bader, M.; Alenina, N.; Calabrese, F. TPH2 Deficiency Influences Neuroplastic Mechanisms and Alters the Response to an Acute Stress in a Sex Specific Manner. Front. Mol. Neurosci. 2018, 11, 389. [Google Scholar] [CrossRef]
- Migliarini, S.; Pacini, G.; Pelosi, B.; Lunardi, G.; Pasqualetti, M. Lack of Brain Serotonin Affects Postnatal Development and Serotonergic Neuronal Circuitry Formation. Mol. Psychiatry 2013, 18, 1106–1118. [Google Scholar] [CrossRef]
- Kronenberg, G.; Mosienko, V.; Gertz, K.; Alenina, N.; Hellweg, R.; Klempin, F. Increased Brain-Derived Neurotrophic Factor (BDNF) Protein Concentrations in Mice Lacking Brain Serotonin. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 281–284. [Google Scholar] [CrossRef]
- Kojima, M.; Mizui, T. BDNF Propeptide: A Novel Modulator of Synaptic Plasticity. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2017; Volume 104, pp. 19–28. [Google Scholar]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Filli, L.; Zörner, B.; Weinmann, O.; Schwab, M.E. Motor deficits and recovery in rats with unilateral spinal cord hemisection mimic the Brown-Sequard syndrome. Brain 2011, 134 Pt 8, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Feraboli-Lohnherr, D.; Orsal, D.; Yakovleff, A.; Giménez y Ribotta, M.; Privat, A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: Specific role of serotonergic neurons. Exp. Brain Res. 1997, 113, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Magnusson, T.; Rosengren, E. 5-Hydroxytryptamine of the spinal cord normally and after transection. Experientia 1963, 19, 359. [Google Scholar] [CrossRef]
- Ballion, B.; Branchereau, P.; Chapron, J.; Viala, D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res. Dev. Brain Res. 2002, 137, 81–88. [Google Scholar] [CrossRef]
- Rossignol, S.; Lund, J.P.; Drew, T. The role of sensory inputs in regulating patterns of rhythmical movements in higher vertebrates. A comparison between locomotion, respiration and mastication. In Neural Control of Rhythmic Movements in Vertebrates; Cohen, A.H., Rossignol, S., Grillner, S., Eds.; Wiley: New York, NY, USA, 1988; pp. 201–283. [Google Scholar]
- Guertin, P.A.; Steuer, I. Ionotropic 5-HT3 receptor agonist-induced motor responses in the hindlimbs of paraplegic mice. J. Neurophysiol. 2005, 94, 3397–3405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Akay, T.; Hedlund, P.B.; Pearson, K.G.; Jordan, L.M. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: In vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J. Neurophysiol. 2009, 102, 337–348. [Google Scholar] [CrossRef]
- Gimenez y Ribotta, M.G.; Roudet, C.; Sandillon, F.; Privat, A. Transplantation of embryonic noradrenergic neurons in two models of adult rat spinal cord injury: Ultrastructural immunocytochemical study. Brain Res. 1996, 707, 245–255. [Google Scholar] [CrossRef]
- Orsal, D.; Barthe, J.Y.; Antri, M.; Feraboli-Lohnherr, D.; Yakovleff, A.; Giménez y Ribotta, M.; Privat, A.; Provencher, J.; Rossignol, S. Locomotor recovery in chronic spinal rat: Long-term pharmacological treatment or transplantation of embryonic neurons? Prog. Brain Res. 2002, 137, 213–230. [Google Scholar] [CrossRef]
- Tavee, J. Nerve conduction studies: Basic concepts. Handb. Clin. Neurol. 2019, 160, 217–224. [Google Scholar] [CrossRef]
- Laliberte, A.M.; Goltash, S.; Lalonde, N.R.; Bui, T.V. Propriospinal Neurons: Essential Elements of Locomotor Control in the Intact and Possibly the Injured Spinal Cord. Front. Cell Neurosci. 2019, 13, 512. [Google Scholar] [CrossRef]
- Barkhaus, P.E.; Nandedkar, S.D.; de Carvalho, M.; Swash, M.; Stålberg, E.V. Revisiting the compound muscle action potential (CMAP). Clin. Neurophysiol. Pract. 2024, 9, 176–200. [Google Scholar] [CrossRef]
- Hassannejad, Z.; Zadegan, S.A.; Shakouri-Motlagh, A.; Mokhatab, M.; Rezvan, M.; Sharif-Alhoseini, M.; Shokraneh, F.; Moshayedi, P.; Rahimi-Movaghar, V. The fate of neurons after traumatic spinal cord injury in rats: A systematic review. Iran J. Basic Med. Sci. 2018, 21, 546–557. [Google Scholar] [CrossRef]
- Xu, X.; Talifu, Z.; Zhang, C.J.; Gao, F.; Ke, H.; Pan, Y.Z.; Gong, H.; Du, H.Y.; Yu, Y.; Jing, Y.L.; et al. Mechanism of skeletal muscle atrophy after spinal cord injury: A narrative review. Front. Nutr. 2023, 10, 1099143. [Google Scholar] [CrossRef]
- Leszczyńska, A.N.; Majczyński, H.; Wilczyński, G.M.; Sławińska, U.; Cabaj, A.M. Thoracic Hemisection in Rats Results in Initial Recovery Followed by a Late Decrement in Locomotor Movements, with Changes in Coordination Correlated with Serotonergic Innervation of the Ventral Horn. PLoS ONE 2015, 10, e0143602. [Google Scholar] [CrossRef]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated Aggression and Decreased Anxiety in Mice Deficient in Brain Serotonin. Transl. Psychiatry 2012, 2, e122. [Google Scholar] [CrossRef]
- Kästner, N.; Richter, S.H.; Urbanik, S.; Kunert, J.; Waider, J.; Lesch, K.P.; Kaiser, S.; Sachser, N. Brain Serotonin Deficiency Affects Female Aggression. Sci. Rep. 2019, 9, 1366. [Google Scholar] [CrossRef]
- Peeters, D.G.A.; de Boer, S.F.; Terneusen, A.; Newman-Tancredi, A.; Varney, M.A.; Verkes, R.J.; Homberg, J.R. Enhanced Aggressive Phenotype of Tph2 Knockout Rats Is Associated with Diminished 5-HT1A Receptor Sensitivity. Neuropharmacology 2019, 153, 134–141. [Google Scholar] [CrossRef]
- Alonso, L.; Peeva, P.; Fernández-Del Valle Alquicira, T.; Erdelyi, N.; Gil Nolskog, Á.; Bader, M.; Winter, Y.; Alenina, N.; Rivalan, M. Poor Decision Making and Sociability Impairment Following Central Serotonin Reduction in Inducible TPH2-Knockdown Rats. Int. J. Mol. Sci. 2024, 25, 5003. [Google Scholar] [CrossRef]
- Zhukov, I.S.; Alnefeesi, Y.; Krotova, N.A.; Nemets, V.V.; Demin, K.A.; Karpenko, M.N.; Budygin, E.A.; Kanov, E.V.; Kalueff, A.V.; Shabanov, P.D.; et al. Trace amine-associated receptor 1 agonist reduces aggression in brain serotonin-deficient tryptophan hydroxylase 2 knockout rats. Front. Psychiatry 2024, 19, 1484925. [Google Scholar] [CrossRef]
- Kane, M.J.; Angoa-Peréz, M.; Briggs, D.I.; Sykes, C.E.; Francescutti, D.M.; Rosenberg, D.R.; Kuhn, D.M. Mice Genetically Depleted of Brain Serotonin Display Social Impairments, Communication Deficits and Repetitive Behaviors: Possible Relevance to Autism. PLoS ONE 2012, 7, e48975. [Google Scholar] [CrossRef]
- Wang, T.; Homberg, J.R.; Boreggio, L.; Samina, M.C.F.; Castro, R.C.R.; Kolk, S.M.; Alenina, N.; Bader, M.; Dai, J.; Wöhr, M. Socio-affective communication in Tph2-deficient rat pups: Communal nesting aggravates growth retardation despite ameliorating maternal affiliation deficits. Mol. Autism. 2024, 15, 50. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Sykes, C.E.; Shah, M.M.; Francescutti, D.M.; Rosenberg, D.R.; Thomas, D.M.; Kuhn, D.M. Genetic Depletion of Brain 5HT Reveals a Common Molecular Pathway Mediating Compulsivity and Impulsivity. J. Neurochem. 2012, 121, 974–984. [Google Scholar] [CrossRef]
- Lin, C.Y.; Sparks, A.; Lee, Y.S. Improvement of Lower Urinary Tract Function by a Selective Serotonin 5-HT1A Receptor Agonist, NLX-112, after Chronic Spinal Cord Injury. Exp. Neurol. 2020, 332, 113395. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. Cortical and Subcortical Lesions Impair Skilled Walking in the Ladder Rung Walking Test: A New Task to Evaluate Fore- and Hindlimb Stepping, Placing, and Co-Ordination. J. Neurosci. Methods 2002, 115, 169–179. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Measuring Motor Coordination in Mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, N.; Tong, L.; Tong, X.J. Electrical Stimulation Enhances Peripheral Nerve Regeneration after Crush Injury in Rats. Mol. Med. Rep. 2013, 7, 1523–1527. [Google Scholar] [CrossRef]
- Xiong, G.X.; Guan, Y.; Hong, Y.; Zhang, J.W.; Guan, H. Motor Unit Number Estimation May Be a Useful Method to Evaluate Motor Function Recovery after Spinal Cord Transection in Rats. Spinal Cord 2010, 48, 363–366. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Prikhodko, V.A.; Chernyakov, R.T.; Idiyatullin, R.D.; Musienko, P.E.; Okovityi, S.V. Effects of Alpha-2 Adrenergic Agonist Mafedine on Brain Electrical Activity in Rats after Traumatic Brain Injury. Brain Sci. 2021, 11, 981. [Google Scholar] [CrossRef]
- Ward-Flanagan, R.; Dickson, C.T. Intravenous Chloral Hydrate Anesthesia Provides Appropriate Analgesia for Surgical Interventions in Male Sprague-Dawley Rats. PLoS ONE 2023, 18, e0286504. [Google Scholar] [CrossRef]
- Pollari, E.; Prior, R.; Robberecht, W.; Van Damme, P.; Van Den Bosch, L. In Vivo Electrophysiological Measurement of Compound Muscle Action Potential from the Forelimbs in Mouse Models of Motor Neuron Degeneration. J. Vis. Exp. 2018, 2018, e57741. [Google Scholar] [CrossRef]
- Mohan, R.; Tosolini, A.P.; Morris, R. Segmental Distribution of the Motor Neuron Columns That Supply the Rat Hindlimb: A Muscle/Motor Neuron Tract-Tracing Analysis Targeting the Motor End Plates. Neuroscience 2015, 307, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Shkorbatova, P.; Lyakhovetskii, V.; Pavlova, N.; Popov, A.; Bazhenova, E.; Kalinina, D.; Gorskii, O.; Musienko, P. Mapping of the Spinal Sensorimotor Network by Transvertebral and Transcutaneous Spinal Cord Stimulation. Front. Syst. Neurosci. 2020, 14, 555593. [Google Scholar] [CrossRef]
- Asboth, L.; Friedli, L.; Beauparlant, J.; Martinez-Gonzalez, C.; Anil, S.; Rey, E.; Baud, L.; Pidpruzhnykova, G.; Anderson, M.A.; Shkorbatova, P.; et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 2018, 21, 576–588. [Google Scholar] [CrossRef]
- Mordhorst, A.; Dhandapani, P.; Matthes, S.; Mosienko, V.; Rothe, M.; Todiras, M.; Self, J.; Schunck, W.H.; Schütz, A.; Bader, M.; et al. Phenylalanine Hydroxylase Contributes to Serotonin Synthesis in Mice. FASEB J. 2021, 35, e21648. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sysoev, Y.I.; Shkorbatova, P.Y.; Prikhodko, V.A.; Kalinina, D.S.; Bazhenova, E.Y.; Okovityi, S.V.; Bader, M.; Alenina, N.; Gainetdinov, R.R.; Musienko, P.E. Central Serotonin Deficiency Impairs Recovery of Sensorimotor Abilities After Spinal Cord Injury in Rats. Int. J. Mol. Sci. 2025, 26, 2761. https://doi.org/10.3390/ijms26062761
Sysoev YI, Shkorbatova PY, Prikhodko VA, Kalinina DS, Bazhenova EY, Okovityi SV, Bader M, Alenina N, Gainetdinov RR, Musienko PE. Central Serotonin Deficiency Impairs Recovery of Sensorimotor Abilities After Spinal Cord Injury in Rats. International Journal of Molecular Sciences. 2025; 26(6):2761. https://doi.org/10.3390/ijms26062761
Chicago/Turabian StyleSysoev, Yuri I., Polina Y. Shkorbatova, Veronika A. Prikhodko, Daria S. Kalinina, Elena Y. Bazhenova, Sergey V. Okovityi, Michael Bader, Natalia Alenina, Raul R. Gainetdinov, and Pavel E. Musienko. 2025. "Central Serotonin Deficiency Impairs Recovery of Sensorimotor Abilities After Spinal Cord Injury in Rats" International Journal of Molecular Sciences 26, no. 6: 2761. https://doi.org/10.3390/ijms26062761
APA StyleSysoev, Y. I., Shkorbatova, P. Y., Prikhodko, V. A., Kalinina, D. S., Bazhenova, E. Y., Okovityi, S. V., Bader, M., Alenina, N., Gainetdinov, R. R., & Musienko, P. E. (2025). Central Serotonin Deficiency Impairs Recovery of Sensorimotor Abilities After Spinal Cord Injury in Rats. International Journal of Molecular Sciences, 26(6), 2761. https://doi.org/10.3390/ijms26062761







